Impact of Postoperative Naples Prognostic Score to Predict Survival in Patients with Stage II–III Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Definition of NPS
2.3. Evaluation Parameters
2.4. Surgical Procedures and Adjuvant Chemotherapy
2.5. Statistical Methods
3. Results
3.1. Baseline Patient Characteristics
3.2. Perioperative Clinical Outcomes
3.3. Pathologic Outcomes According to NPS Groups
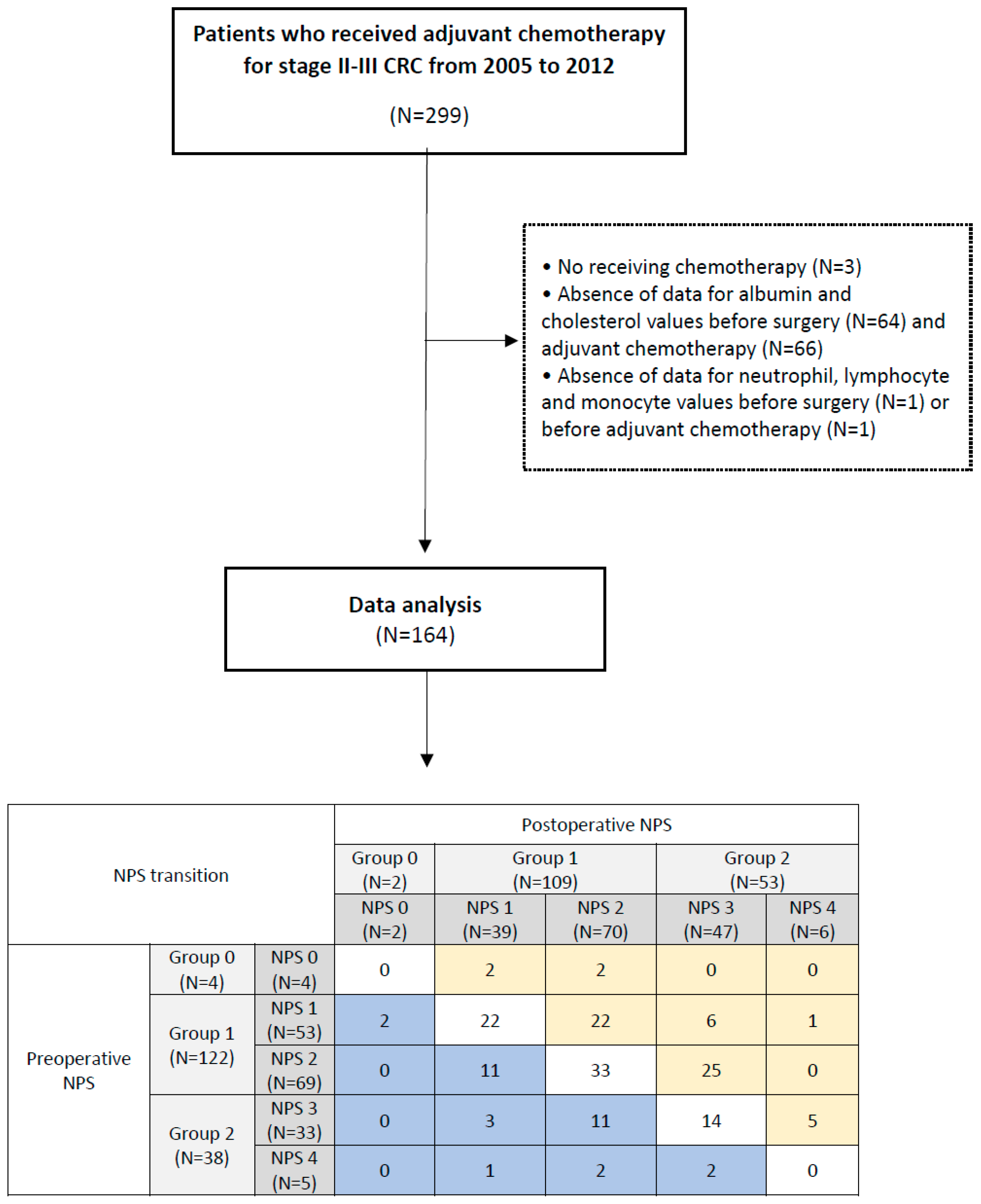
3.4. Postoperative NPS Transition
3.5. Comparison of Survival According to NPS Group
3.6. Logistic Regression for Survival Outcomes
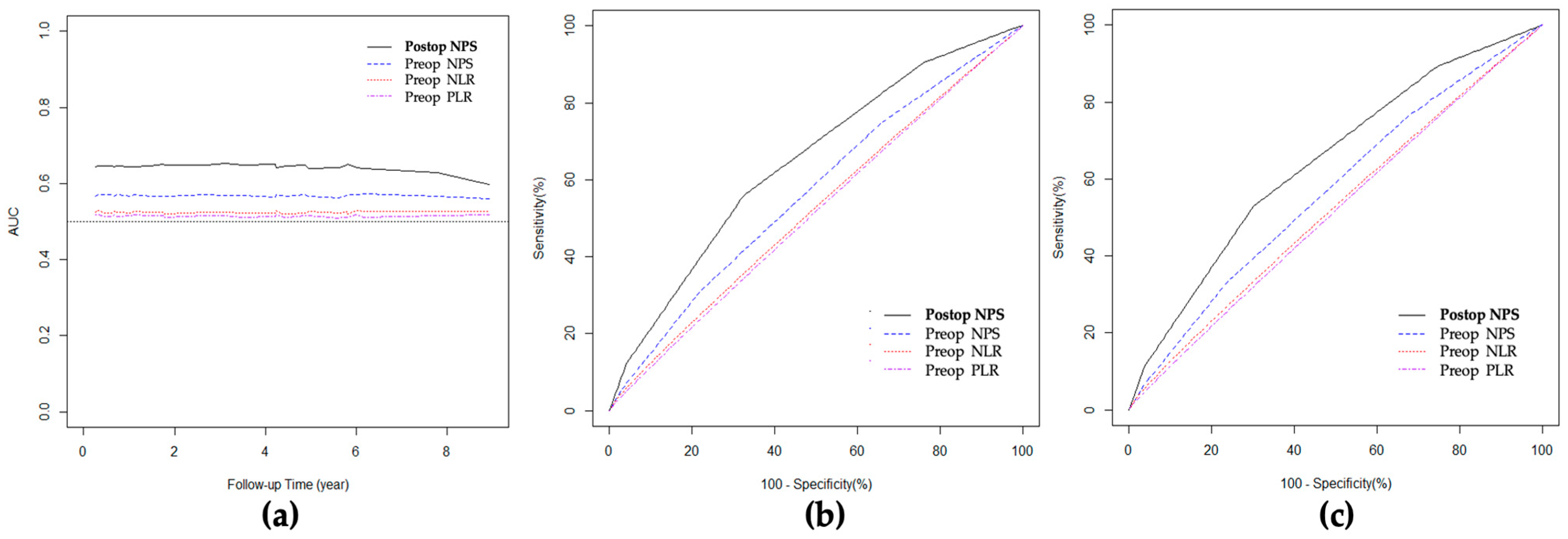
3.7. ROC Curve and AUC for Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuomisto, A.E.; Makinen, M.J.; Vayrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Shim, H.; Kim, K.; Kim, B.; Bang, H.-J.; Do, H.; Lee, H.-R.; Kim, Y. Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer. Ann. Coloproctol. 2022, 38, 97–108. [Google Scholar] [CrossRef]
- An, S.H.; Kim, I.Y. Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer? Ann. Coloproctol. 2022, 38, 253–261. [Google Scholar] [CrossRef]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sugano, K.; Ikeya, T.; Muguruma, K.; Tanaka, H.; Toyokawa, T.; et al. Impact of the Preoperative Controlling Nutritional Status (CONUT) Score on the Survival after Curative Surgery for Colorectal Cancer. PLoS ONE 2015, 10, e0132488. [Google Scholar] [CrossRef]
- Kritchevsky, S.; Kritchevsky, D. Serum cholesterol and cancer risk: An epidemiologic perspective. Annu. Rev. Nutr. 1992, 12, 391–416. [Google Scholar] [CrossRef]
- Tokunaga, R.; Sakamoto, Y.; Nakagawa, S.; Miyamoto, Y.; Yoshida, N.; Oki, E.; Watanabe, M.; Baba, H. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Dis. Colon. Rectum 2015, 58, 1048–1057. [Google Scholar] [CrossRef]
- Tokunaga, R.; Sakamoto, Y.; Nakagawa, S.; Ohuchi, M.; Izumi, D.; Kosumi, K.; Taki, K.; Higashi, T.; Miyamoto, Y.; Yoshida, N.; et al. CONUT: A novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int. J. Colorectal Dis. 2017, 32, 99–106. [Google Scholar] [CrossRef]
- Joo, J.I.; Lim, S.W.; Oh, B.Y. Prognostic Impact of Carcinoembryonic Antigen Levels in Rectal Cancer Patients Who Had Received Neoadjuvant Chemoradiotherapy. Ann. Coloproctol. 2021, 37, 179–185. [Google Scholar] [CrossRef]
- Kang, S. Carcinoembryonic Antigen, the Most Accessible Test for Predicting Colorectal Cancer Prognosis: Exploring Alternative Roles. Ann. Coloproctol. 2021, 37, 129–130. [Google Scholar] [CrossRef]
- Guo, J.; Chok, A.Y.; Lim, H.J.; Tay, W.X.; Lye, W.K.; Samarakoon, L.B.; Tan, E.J.; Mathew, R. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Obstructing Colorectal Cancer Treated by Endoscopic Stenting as a Bridge to Surgery. Ann. Coloproctol. 2021, 37, 159–165. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Hiyoshi, Y.; Daitoku, N.; Okadome, K.; Sakamoto, Y.; Yamashita, K.; Kuroda, D.; Sawayama, H.; Iwatsuki, M.; Baba, Y.; et al. Naples Prognostic Score Is a Useful Prognostic Marker in Patients With Metastatic Colorectal Cancer. Dis. Colon. Rectum 2019, 62, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples Prognostic Score, Based on Nutritional and Inflammatory Status, is an Independent Predictor of Long-term Outcome in Patients Undergoing Surgery for Colorectal Cancer. Dis. Colon. Rectum 2017, 60, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Lee, J.L.; Yu, C.S.; Kim, J.B.; Lim, S.-B.; Park, I.J.; Yoon, Y.S.; Kim, C.W.; Yang, S.-K.; Ye, B.D.; et al. Clinicopathological Characteristics and Surgical Outcomes of Crohn Disease-Associated Colorectal Malignancy. Ann. Coloproctol. 2021, 37, 101–108. [Google Scholar] [CrossRef]
- Mizuuchi, Y.; Tanabe, Y.; Sada, M.; Tamura, K.; Nagayoshi, K.; Nagai, S.; Watanabe, Y.; Tamiya, S.; Nakata, K.; Ohuchida, K.; et al. Cross-sectional area of psoas muscle as a predictive marker of anastomotic failure in male rectal cancer patients: Japanese single institutional retrospective observational study. Ann. Coloproctol. 2022, 38, 353–361. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B. AJCC Cancer Staging Manual, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Guadagni, F.; Ferroni, P.; Palmirotta, R.; Portarena, I.; Formica, V.; Roselli, M. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: An early target in anticancer therapeutic strategy. In Vivo 2007, 21, 147–161. [Google Scholar] [PubMed]
- Turner, N.; Tran, B.; Tran, P.V.; Sinnathamby, M.; Wong, H.L.; Jones, I.; Croxford, M.; Desai, J.; Tie, J.; Field, K.M.; et al. Primary Tumor Resection in Patients With Metastatic Colorectal Cancer Is Associated With Reversal of Systemic Inflammation and Improved Survival. Clin. Colorectal Cancer 2015, 14, 185–191. [Google Scholar] [CrossRef]
- Lawler, J.; Choynowski, M.; Bailey, K.; Bucholc, M.; Johnston, A.; Sugrue, M. Meta-analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. BJS Open 2020, 4, 737–747. [Google Scholar] [CrossRef]
- Arnarson, O.; Butt-Tuna, S.; Syk, I. Postoperative complications following colonic resection for cancer are associated with impaired long-term survival. Colorectal Dis. 2019, 21, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Oba, K.; Honda, M.; Sadahiro, S.; Hamada, C.; Mayanagi, S.; Kanda, M.; Maeda, H.; Kashiwabara, K.; Sakamoto, J.; et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: Analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med. 2017, 6, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Hong, Y.K.; Choi, Y.J.; Kang, J.G. Clinicopathologic characteristics of early-onset colorectal cancer. Ann. Coloproctol. 2022, 38, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Artinyan, A.; Orcutt, S.T.; Anaya, D.A.; Richardson, P.; Chen, G.J.; Berger, D.H. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann. Surg. 2015, 261, 497–505. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.J. Upregulation of prostaglandin E2 by inducible microsomal prostaglandin E synthase-1 in colon cancer. Ann. Coloproctol. 2022, 38, 153–159. [Google Scholar] [CrossRef]
- Shakeyev, K.; Turgunov, Y.; Ogizbayeva, A.; Avdiyenko, O.; Mugazov, M.; Grigolashvili, S.; Azizov, I. Presepsin (soluble CD14 subtype) as a risk factor for the development of infectious and inflammatory complications in operated colorectal cancer patients. Ann. Coloproctol. 2022, 38, 442–448. [Google Scholar] [CrossRef]
- Clark, D.A.; Yeoh, E.; Edmundson, A.; Harris, C.; Stevenson, A.; Steffens, D.; Solomon, M. A development study of drain fluid gastrografin as a biomarker of anastomotic leak. Ann. Coloproctol. 2022, 38, 124–132. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Ito, M. Optimal anastomotic technique in rectal surgery to prevent anastomotic leakage. Ann. Coloproctol. 2023, 39, 97–105. [Google Scholar] [CrossRef]
- Alekseev, M.; Rybakov, E.; Khomyakov, E.; Zarodnyuk, I.; Shelygin, Y. Intraoperative fluorescence angiography as an independent factor of anastomotic leakage and a nomogram for predicting leak for colorectal anastomoses. Ann. Coloproctol. 2022, 38, 380–386. [Google Scholar] [CrossRef]
- Suzuki, Y.; Okabayashi, K.; Hasegawa, H.; Tsuruta, M.; Shigeta, K.; Kondo, T.; Kitagawa, Y. Comparison of Preoperative Inflammation-based Prognostic Scores in Patients With Colorectal Cancer. Ann. Surg. 2018, 267, 527–531. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Oliver, M. Serum cholesterol: The knave of hearts and the joker. Lancet 1981, 2, 1090–1095. [Google Scholar] [CrossRef]
- Kataoka, M.; Gomi, K.; Ichioka, K.; Iguchi, T.; Shirota, T.; Makino, A.; Shimada, K.; Maruyama, K.; Mihara, M.; Kajikawa, S. Clinical impact of C-reactive protein to albumin ratio of the 7th postoperative day on prognosis after laparoscopic colorectal cancer surgery. Ann. Coloproctol. 2023, 39, 315–325. [Google Scholar] [CrossRef]
- Bulut, G.; Ozdemir, Z.N. Prognostic Significance of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Metastatic Colorectal Cancer. J. Gastrointest. Cancer 2022, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.W.T.; Cecire, J.; Hitos, K.; Shedden, K.; Gavegan, F.; Pathmanathan, N.; El Khoury, T.; Di Re, A.; Cocco, A.; Limmer, A.; et al. The impact of variations in care and complications within a colorectal Enhanced Recovery After Surgery program on length of stay. Ann. Coloproctol. 2022, 38, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Velkoski, J.; Grimaldi, F.; Mion, F.; Pravisani, R.; Marino, M.; Calandra, S.; Cherchi, V.; Terrosu, G. Immunonutrition in elective colorectal surgery and early inflammatory response. Minerva Surg. 2021, 76, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kampman, S.; Smalbroek, B.; Dijksman, L.; Smits, A. Postoperative inflammatory response in colorectal cancer surgery: A meta-analysis. Int. J. Colorectal Dis. 2023, 38, 233. [Google Scholar] [CrossRef]
- Crippa, J.; Calini, G.; Santambrogio, G.; Sassun, R.; Siracusa, C.; Maggioni, D.; Mari, G. ERAS Protocol Applied to Oncological Colorectal Mini-invasive Surgery Reduces the Surgical Stress Response and Improves Long-term Cancer-specific Survival. Surgl Laparosc. Endosc. Percutan Tech. 2023, 33, 297–301. [Google Scholar] [CrossRef]
- Uehara, H.; Yamazaki, T.; Iwaya, A.; Kameyama, H.; Komatsu, M.; Hirai, M. Comparison of the oncological outcomes of stenting as a bridge to surgery and surgery alone in stages II to III obstructive colorectal cancer: A retrospective study. Ann. Coloproctol. 2022, 38, 235–243. [Google Scholar] [CrossRef]
- Biondo, S.; Gálvez, A.; Ramírez, E.; Frago, R.; Kreisler, E. Emergency surgery for obstructing and perforated colon cancer: Patterns of recurrence and prognostic factors. Tech. Coloproctol. 2019, 23, 1141–1161. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.L.; Kim, T.W.; Lee, J.; Yoon, Y.S.; Lee, K.Y.; Song, K.-h.; Yu, C.S.; Cho, Y.B. Molecular characterization of dysplasia-initiated colorectal cancer with assessing matched tumor and dysplasia samples. Ann. Coloproctol. 2022, 38, 72–81. [Google Scholar] [CrossRef]
- Luo, X.J.; Zhao, Q.; Liu, J.; Zheng, J.B.; Qiu, M.Z.; Ju, H.Q.; Xu, R.H. Novel Genetic and Epigenetic Biomarkers of Prognostic and Predictive Significance in Stage II/III Colorectal Cancer. Mol. Ther. 2021, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-W.; Kim, J.-S.; Kim, J.-Y.; Lee, K.-h. Prognostic Factor and Survival Benefit of Adjuvant Chemotherapy in Stage IIA Colon Cancer. Ann. Coloproctol. 2021, 37, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Applications of propensity score matching: A case series of articles published in Annals of Coloproctology. Ann. Coloproctol. 2022, 38, 398–402. [Google Scholar] [CrossRef]
- Takasu, C.; Nishi, M.; Yoshikawa, K.; Tokunaga, T.; Kashihara, H.; Yoshimoto, T.; Shimada, M. Impact of sidedness of colorectal cancer on tumor immunity. PLoS ONE 2020, 15, e0240408. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hokuto, D.; Koyama, F.; Matsuo, Y.; Nomi, T.; Yoshikawa, T.; Kamitani, N.; Sadamitsu, T.; Takei, T.; Matsumoto, Y.; et al. The Prognosis and Recurrence Pattern of Right- and Left-Sided Colon Cancer in Stage II, Stage III, and Liver Metastasis After Curative Resection. Ann. Coloproctol. 2021, 37, 326–336. [Google Scholar] [CrossRef]
- Sugimoto, A.; Fukuoka, T.; Shibutani, M.; Kasashima, H.; Kitayama, K.; Ohira, M.; Maeda, K. Prognostic significance of the Naples prognostic score in colorectal cancer patients undergoing curative resection: A propensity score matching analysis. BMC Gastroenterol. 2023, 23, 88. [Google Scholar] [CrossRef]

| Variables | Preoperative NPS | Postoperative NPS | ||||
|---|---|---|---|---|---|---|
| Group 0–1 (N = 126) | Group 2 (N = 38) | p Value | Group 0–1 (N = 111) | Group 2 (N = 53) | p Value | |
| Age, years † | 0.248 | 0.487 | ||||
| <65 | 86 (68.3) | 22 (57.9) | 71 (64.0) | 37 (69.8) | ||
| ≥65 | 40 (31.7) | 16 (42.1) | 40 (36.0) | 16 (30.2) | ||
| Sex † | 0.189 | 0.091 | ||||
| Male | 69 (54.8) | 26 (68.4) | 59 (53.2) | 36 (67.9) | ||
| Female | 57 (45.2) | 12 (31.6) | 52 (46.8) | 17 (32.1) | ||
| BMI | 23.2 ± 2.9 (16.5–29.9) | 22.9 ± 3.0 (15.3–28.6) | 0.633 | 23.2 ± 3.0 (15.3–29.9) | 23.0 ± 2.9 (16.5–28.6) | 0.653 |
| Tumor location ‡ | 0.153 | 0.811 | ||||
| Ascending colon cancer | 24 (19.1) | 11 (28.9) | 26 (23.4) | 9 (17.0) | ||
| Transverse colon cancer | 3 (2.4) | 2 (5.3) | 3 (2.7) | 2 (3.8) | ||
| Descending colon cancer | 9 (7.1) | 4 (10.5) | 7 (6.3) | 6 (11.3) | ||
| Sigmoid colon cancer | 50 (39.7) | 17 (44.8) | 44 (39.6) | 23 (43.4) | ||
| Rectosigmoid junction cancer | 20 (15.9) | 1 (2.6) | 15 (13.5) | 6 (11.3) | ||
| Rectal cancer | 20 (15.9) | 3 (7.9) | 16 (14.4) | 7 (13.2) | ||
| ASA ‡ | 0.043 | 0.225 | ||||
| 1 | 77 (61.1) | 19 (50.0) | 70 (63.1) | 26 (49.1) | ||
| 2 | 42 (33.3) | 12 (31.6) | 33 (29.7) | 21 (39.6) | ||
| 3 | 7 (5.6) | 7 (18.4) | 8 (7.2) | 6 (11.3) | ||
| Total numbers of co-morbidity ‡ | 0.174 | 0.008 | ||||
| 0 | 59 (46.8) | 15 (39.5) | 56 (50.5) | 18 (34.0) | ||
| 1 | 46 (36.5) | 13 (34.2) | 42 (37.8) | 17 (32.1) | ||
| 2 | 18 (14.3) | 6 (15.8) | 10 (9.0) | 14 (26.4) | ||
| ≥3 | 3 (2.4) | 4 (10.5) | 3 (2.7) | 4 (7.5) | ||
| Previous abdominal operations ‡ | 0.105 | 0.911 | ||||
| Yes | 55 (43.7) | 11 (28.9) | 45 (40.5) | 21 (39.6) | ||
| No | 71 (56.3) | 27 (71.1) | 66 (59.5) | 32 (60.4) | ||
| Preoperative CEA, ng/mL | 8.4 ± 15.8 (0.4–138.3) | 7.9 ± 12.6 (0.5–72.6) | 0.863 | 7.4 ± 15.3 (0.4–138.3) | 10.1 ± 14.5 (0.5–72.6) | 0.298 |
| Serum albumin, mg/dL | 4.4 ± 0.3 (3.2–5.1) | 3.8 ± 0.6 (2.6–4.8) | <0.001 | 4.3 ± 0.5 (3.1–5.1) | 4.1 ± 0.5 (2.6–4.8) | 0.056 |
| Total cholesterol, mg/dL | 181.4 ± 37.2 (100–295) | 141.0 ± 32.4 (74–224) | <0.001 | 179.9 ± 39.7 (75–275) | 155.5 ± 35.1 (74–295) | <0.001 |
| Neutrophil: lymphocyte ratio (NLR) | 2.6 ± 1.8 (0.9–14.1) | 3.1 ± 2.1 (0.8–11.7) | 0.155 | 2.1 ± 1.4 (0.5–11.3) | 2.4 ± 1.0 (0.9–5.7) | 0.110 |
| Lymphocyte: monocyte ratio (LMR) | 6.0 ± 2.2 (1.6–14.8) | 3.9 ± 1.9 (1.1–9.3) | <0.001 | 6.02 ± 2.15 (2.1–13.7) | 4.4 ± 1.6 (1.7–8.9) | <0.001 |
| Platelet: lymphocyte ratio (PLR) | 171.6 ± 94.6 (0.3–883.5) | 207.3 ± 100.4 (83.5–541.2) | 0.046 | 161.1 ± 75.7 (36.0–470.5) | 189.8 ± 77.3 (87.8–373.9) | 0.026 |
| Prognostic nutritional index (PNI) | 43.9 ± 3.4 (32.0–51.0) | 37.7 ± 5.6 (26.0–48.0) | <0.001 | 42.2 ± 3.6 (32.01–50.01) | 39.9 ± 4.3 (30.01–49.01) | <0.001 |
| Variables | Preoperative NPS | Postoperative NPS | ||||
|---|---|---|---|---|---|---|
| Group 0–1 (N = 126) | Group 2 (N = 38) | p Value | Group 0–1 (N = 111) | Group 2 (N = 53) | p Value | |
| Operation names ‡ | 0.168 | 0.734 | ||||
| Rt. Hemicolectomy | 26 (20.6) | 12 (31.6) | 28 (25.2) | 10 (18.9) | ||
| Lt. hemicolectomy | 10 (7.9) | 7 (18.4) | 10 (9.0) | 7 (13.2) | ||
| Anterior resection | 48 (38.1) | 12 (31.6) | 41 (36.9) | 19 (35.8) | ||
| LAR | 38 (20.2) | 5 (13.2) | 29 (26.1) | 14 (26.4) | ||
| Ultra LAR with ISR | 1 (0.8) | 0 (0) | 1 (0.9) | 0 (0) | ||
| Segmental resection of colon | 1 (0.8) | 0 (0) | 0 (0) | 1 (1.9) | ||
| Hartmann’s operation | 1 (0.8) | 1 (2.6) | 1 (0.9) | 1 (1.9) | ||
| Extended resection of colon | 1 (0.8) | 1 (2.6) | 1 (0.9) | 1 (1.9) | ||
| Operation method ‡ | 0.774 | 0.825 | ||||
| Open | 45 (35.7) | 16 (42.1) | 40 (36.0) | 21 (39.6) | ||
| Laparoscopy | 63 (50.0) | 17 (44.7) | 56 (50.5) | 24 (45.3) | ||
| Robot | 18 (14.3) | 5 (13.2) | 15 (13.5) | 8 (15.1) | ||
| Operation time, min | 235.3 ± 76.3 (87–473) | 228.1 ± 92.9 (97–608) | 0.627 | 225.9 ± 71.5 (87–412) | 249.9 ± 94.6 (120–608) | 0.073 |
| Emergency operation † | 2 (1.6) | 1 (2.6) | 0.549 | 2 (1.8) | 1 (1.9) | 1.000 |
| Intestinal obstruction † | 28 (22.2) | 14 (36.8) | 0.090 | 31 (27.9) | 11(20.8) | 0.347 |
| Intestinal perforation † | 2 (1.6) | 2 (5.3) | 0.230 | 2 (1.8) | 2 (3.8) | 0.595 |
| Protective stoma formation † | 1 (0.8) | 2 (5.3) | 0.134 | 2(1.8) | 1 (1.9) | 1.000 |
| Intraoperative transfusion † | 4 (3.2) | 0 (0) | 0.574 | 3 (2.7) | 1 (1.9) | 1.000 |
| Hospital stay, day | 10.9 ± 6.8 (5–42) | 13.7 ± 11.7 (4–53) | 0.070 | 10.7 ± 6.7 (4–35) | 13.4 ± 10.7 (4–53) | 0.057 |
| Postoperative complications ‡ | 0.508 | 0.402 | ||||
| Grade I | 9 (7.1) | 2 (5.3) | 10 (9.0) | 1 (1.9) | ||
| Grade II | 5 (4.0) | 0 (0.0) | 3 (2.7) | 2 (3.8) | ||
| Grade IIIa | 7 (5.6) | 2 (5.3) | 5 (4.5) | 4 (7.5) | ||
| Wound dehiscence | ||||||
| Grade IIIb | 2 (1.6) | 2 (5.3) | 2 (1.8) | 2 (3.8) | ||
| Anastomosis leakage | ||||||
| Grade IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Grade V | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Days to beginning adjuvant chemotherapy after surgery | 26.6 ± 8.0 (10–50) | 31.5 ± 11.6 (12–55) | 0.003 | 28.1 ± 8.5 (10–52) | 26.9 ± 10.5 (11–55) | 0.469 |
| Chemotherapy completeness † | 0.331 | 0.828 | ||||
| Yes | 106 (84.1) | 29 (76.3) | 92 (82.9) | 43 (81.1) | ||
| No (Early cessation) | 20 (15.9) | 9 (23.7) | 19 (17.1) | 10 (18.9) | ||
| Chemotherapy regimen ‡ | 0.012 | 0.677 | ||||
| FOLFOX | 103 (81.7) | 24 (63.2) | 87 (78.4) | 40 (75.5) | ||
| Capcitabine | 23 (18.3) | 14 (36.8) | 24(21.6) | 13 (24.5) | ||
| 5-FU/Leucovorin | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Others | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Variables | Preoperative NPS | Postoperative NPS | ||||
|---|---|---|---|---|---|---|
| Group 0–1 (N = 126) | Group 2 (N = 38) | p Value | Group 0–1 (N = 111) | Group 2 (N = 53) | p Value | |
| Tumor size, cm | 4.4 ± 2.2 (0–14.0) | 5.8 ± 3.0 (0.50–13.0) | 0.002 | 4.6 ± 2.5 (0–14.0) | 5.0 ± 2.4 (0–13.0) | 0.408 |
| Depth of tumor ‡ | 0.757 | 0.613 | ||||
| pT1 | 8 (6.3) | 2 (5.3) | 7 (6.3) | 3 (5.7) | ||
| pT2 | 8 (6.3) | 1 (2.6) | 7 (6.3) | 2 (3.8) | ||
| pT3 | 83 (65.9) | 28 (73.7) | 77 (69.4) | 34 (64.2) | ||
| pT4 | 27 (21.4) | 7 (18.4) | 20 (18.0) | 14 (26.4) | ||
| Lymph node metastasis ‡ | 0.168 | 0.103 | ||||
| pN0 | 22 (17.5) | 12 (31.6) | 23 (20.7) | 11 (20.8) | ||
| pN1 | 66 (52.4) | 16 (42.1) | 61 (55.0) | 21 (39.6) | ||
| pN2 | 38 (30.2) | 10 (26.3) | 27 (24.3) | 21 (39.6) | ||
| TNM stage † | 0.112 | 1.000 | ||||
| Stage II | 23 (18.3) | 12 (31.6) | 24 (7.4) | 11 (20.8) | ||
| Stage III | 103 (81.7) | 26 (68.4) | 87 (15.3) | 42 (79.2) | ||
| Numbers for harvested lymph nodes | 24.8 ± 14.0 (1–71) | 28.3 ± 18.2 (0–87) | 0.276 | 26.9 ± 15.6 (0–87) | 22.9 ± 13.8 (6–67) | 0.118 |
| Proximal resection margin, cm | 11.7 ± 7.7 (2–60) | 13.9 ± 9.1 (2.5–44) | 0.178 | 12.1 ± 7.9 (2–60) | 12.4 ± 8.4 (3–44) | 0.801 |
| Distal resection margin, cm | 7.2 ± 6.6 (0.5–32) | 7.9 ± 5.3 (1–25) | 0.495 | 7.2 ± 6.3 (0.5–32) | 7.2 ± 6.5 (0.5–31) | 0.759 |
| Pathologic grade ‡ | 0.020 | 0.674 | ||||
| Well-differentiated | 9 (7.1) | 3 (7.9) | 9 (8.1) | 3 (5.7) | ||
| Moderate differentiated | 101 (80.2) | 27 (71.0) | 87 (78.4) | 41 (77.3) | ||
| Poor differentiated | 5 (4.0) | 3 (7.9) | 6 (5.4) | 2 (3.8) | ||
| Mucinous differentiated | 11 (8.7) | 2 (5.3) | 8 (7.2) | 5 (9.4) | ||
| Undifferentiated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Signet ring cell | 0 (0.0) | 3 (7.9) | 1 (0.9) | 2 (3.8) | ||
| Lymphovascular invasion † | 43 (34.1) | 17 (44.7) | 0.253 | 39 (35.1) | 21 (39.6) | 0.606 |
| Perineural invasion † | 21 (16.7) | 6 (15.8) | 1.000 | 14 (12.6) | 13 (24.5) | 0.071 |
| No Changes NPS (N = 69) | Upstaging NPS (N = 63) | Downstaging NPS (N = 32) | p Value | |
|---|---|---|---|---|
| Age, years ‡ | 0.019 | |||
| <65 | 43 (62.3) | 49 (77.8) | 16 (50.0) | |
| ≥65 | 26 (37.7) | 14 (22.2) | 16 (50.0) | |
| Gender ‡ | 0.974 | |||
| Male | 40 (58.0) | 37 (58.7) | 18 (56.3) | |
| Female | 29 (42.0) | 26 (41.3) | 14 (43.8) | |
| ASA ‡ | 0.990 | |||
| 1 | 40 (58.0) | 37 (58.7) | 19 (59.4) | |
| ≥2 | 29 (42.0) | 26 (41.3) | 13 (40.6) | |
| Preoperative CEA level | 7.3 ± 11.7 (0.4–72.6) | 10.5 ± 20.1 (0.5–138.3) | 6.0 ± 8.2 (0.5–42.7) | 0.326 |
| Operation method | 0.900 | |||
| Open | 27 (39.1) | 22 (34.9) | 12 (37.5) | |
| Laparoscopy | 34 (49.3) | 30 (47.6) | 16 (50.0) | |
| Robot | 8 (11.6) | 11 (17.5) | 4 (12.5) | |
| Operation time, min | 232.1 ± 86.4 (87–608) | 240.7 ± 78.0 (130–473) | 223.2 ± 70.9 (97–370) | 0.595 |
| Emergency operation ‡ | 0.829 | |||
| Yes | 1 (1.4) | 1 (1.6) | 1 (3.1) | |
| No | 68 (98.6) | 62 (98.4) | 31 (96.9) | |
| Intraoperative transfusion ‡ | 0.359 | |||
| Yes | 3 (4.3) | 1 (1.6) | 0 (0.0) | |
| No | 66 (95.7) | 62 (98.4) | 32 (100.0) | |
| Hospital stay, day | 11.4 ± 9.3 (4–53) | 12.4 ± 7.9 (4–35) | 10.4 ± 6.4 (4–31) | 0.547 |
| Postoperative complications | 0.617 | |||
| Yes | 12 (17.4) | 11 (17.5) | 8 (25.0) | |
| No | 57 (82.6) | 52 (82.5) | 24 (75.0) | |
| Days to beginning adjuvant chemotherapy after surgery | 29.8 ± 8.9 (11–55) | 24.7 ± 8.4 (10–50) | 29.1 ± 9.8 (12–52) | 0.249 |
| Tumor size, cm | 4.4 ± 2.2 (0.5–10.0) | 4.8 ± 2.5 (0.5–14.0) | 5.3 ± 2.9 (0.5–12.0) | 0.201 |
| TNM stage ‡ | 0.562 | |||
| Stage II | 13 (18.8) | 13 (20.6) | 9 (28.1) | |
| Stage III | 56 (81.2) | 50 (79.4) | 23 (71.9) | |
| T stage ‡ | 0.562 | |||
| pT1-2 | 9 (13.0) | 5 (8.0) | 5 (15.6) | |
| pT3-4 | 60 (87.0) | 58 (92.0) | 27 (84.4) | |
| Nodal involvement ‡ | 0.465 | |||
| pN0 | 12 (17.4) | 13 (20.6) | 9 (28.1) | |
| pN1-2 | 57 (82.6) | 50 (79.4) | 23 (71.9) |
| Variables | Event/N (%) | Overall Survival | Event/N (%) | Disease-Free Survival | ||
|---|---|---|---|---|---|---|
| HR(95%CI) | p Value | HR(95% CI) | p Value | |||
| Sex | ||||||
| Male | 22/95 (23.2) | Ref. | 14/95 (14.7) | Ref. | ||
| Female | 8/69 (11.6) | 0.545 (0.237–1.209) | 0.133 | 9/69 (13.0) | 0.921 (0.398–2.127) | 0.846 |
| Age, yr | ||||||
| <65 | 16/108 (14.8) | Ref. | 16/108 (14.8) | Ref. | ||
| ≥65 | 14/56 (25.0) | 1.844 (0.890–3.820) | 0.099 | 7/56 (12.5) | 0.841 (0.346–2.046) | 0.702 |
| ASA | ||||||
| 1 | 16/90 (17.8) | Ref. | 16/90 (17.8) | Ref. | ||
| ≥2 | 10/68 (14.7) | 0.816 (0.370–1.799) | 0.614 | 4/68 (5.9) | 0.312 (0.104–0.934) | 0.037 |
| Tumor site | ||||||
| Right | 9/40 (22.5) | Ref. | 7/40 (17.5) | Ref. | ||
| Left | 16/101 (15.8) | 0.679 (0.297–1.553) | 0.359 | 13/101 (12.9) | 0.748(0.298–1.874) | 0.535 |
| Rectum | 5/23 (21.7) | 1.055 (0.353–3.153) | 0.923 | 3/23 (13.0) | 0.767(0.198–2.967) | 0.700 |
| Adjuvant chemotherapy | ||||||
| Completeness | 17/133 (12.8) | Ref. | 16/133 (12.0) | Ref. | ||
| Incompleteness | 11/28 (39.3) | 5.321 (2.451–11.548) | <0.0001 | 6/28 (21.4) | 2.399 (0.938–6.140) | 0.068 |
| Preoperative NLR | ||||||
| <2.96 | 19/112 (17.0) | Ref. | 13/112 (11.6) | Ref. | ||
| ≥2.96 | 11/52 (21.2) | 1.173 (0.545–2.524) | 0.683 | 10/52 (19.2) | 1.718 (0.753–3.919) | 0.198 |
| Preoperative PNI | ||||||
| <49 | 28/154 (18.2) | Ref. | 23/154 (14.9) | Ref. | ||
| ≥49 | 2/10 (20.0) | 1.045 (0.248–4.394) | 0.953 | 0/10 (0.0) | 0.294 (0.017–5.147) | 0.402 |
| Postoperative NLR | ||||||
| <2.96 | 26/140 (18.6) | Ref. | 21/140 (15.0) | Ref. | ||
| ≥2.96 | 4/24 (16.7) | 0.562 (0.170–1.861) | 0.345 | 2/24 (8.3) | 0.491 (0.115–2.099) | 0.337 |
| Postoperative PNI | 0.601 | |||||
| <49 | 30/159 (18.9) | Ref. | 23/159(14.5) | Ref. | ||
| ≥49 | 0/5 (0.0) | 0.468 (0.027–8.067) | 0/5(0.0) | 0.624(0.036–10.979) | 0.748 | |
| Preoperative NPS | ||||||
| 0/1 | 7/57 (12.3) | Ref. | 6/57 (10.5) | Ref. | ||
| ≥2 | 23/107 (21.5) | 1.860 (0.757–4.573) | 0.176 | 17/107 (15.9) | 1.462 (0.576–3.711) | 0.425 |
| Postoperative NPS | ||||||
| 0/1 | 3/41 (7.3) | Ref. | 3/41 (7.3) | Ref. | ||
| ≥2 | 27/123 (22.0) | 2.744 (0.830–9.076) | 0.098 | 20/123 (16.3) | 2.110 (0.627–7.105) | 0.228 |
| NPS transition | ||||||
| Yes | 12/55 (21.8) | Ref. | 9/55 (16.4) | Ref. | ||
| No | 18/109 (16.5) | 0.704 (0.336–1.475) | 0.352 | 14/109 (12.8) | 0.814 (0.352–1.881) | 0.630 |
| NPS transition | ||||||
| No change | 18/109 (16.5) | Ref. | 14/109 (12.8) | Ref. | ||
| Upstaging | 3/19 (15.9) | 1.020 (0.299–3.483) | 0.975 | 3/19 (15.8) | 1.219 (0.350–4.248) | 0.756 |
| Downstaging | 9/36 (25.0) | 1.636 (0.728–3.676) | 0.234 | 6/36 (16.7) | 1.234 (0.474–3.212) | 0.667 |
| Variables | AUC (95% CI) | Pairwise Comparison of p Value | ||
|---|---|---|---|---|
| vs. Preop NPS | vs. Postop NPS | |||
| Heagerty’s iAUC | Preop NPS | 0.57 (0.50–0.65) | Ref. | |
| Postop NPS | 0.64 (0.54–0.72) | 0.136 | Ref. | |
| Preop NLR | 0.52 (0.50–0.62) | 0.360 | 0.032 | |
| Preop PLR | 0.51 (0.50–0.62) | 0.279 | 0.027 | |
| Heagerty’s incident/ Dynamic AUC (2 year) | Preop NPS | 0.57 (0.51–0.65) | Ref. | |
| Postop NPS | 0.65 (0.55–0.73) | 0.114 | Ref. | |
| Preop NLR | 0.52 (0.50–0.62) | 0.338 | 0.027 | |
| Preop PLR | 0.51 (0.50–0.62) | 0.279 | 0.022 | |
| Heagerty’s incident/ Dynamic AUC (5 year) | Preop NPS | 0.57 (0.51–0.65) | Ref. | |
| Postop NPS | 0.64 (0.55–0.73) | 0.113 | Ref. | |
| Preop NLR | 0.52 (0.50–0.61) | 0.339 | 0.021 | |
| Preop PLR | 0.51 (0.50–0.62) | 0.261 | 0.018 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Woo, H.S.; Hong, I.K.; Park, E.J. Impact of Postoperative Naples Prognostic Score to Predict Survival in Patients with Stage II–III Colorectal Cancer. Cancers 2023, 15, 5098. https://doi.org/10.3390/cancers15205098
Park SH, Woo HS, Hong IK, Park EJ. Impact of Postoperative Naples Prognostic Score to Predict Survival in Patients with Stage II–III Colorectal Cancer. Cancers. 2023; 15(20):5098. https://doi.org/10.3390/cancers15205098
Chicago/Turabian StylePark, Su Hyeong, Hye Seung Woo, In Kyung Hong, and Eun Jung Park. 2023. "Impact of Postoperative Naples Prognostic Score to Predict Survival in Patients with Stage II–III Colorectal Cancer" Cancers 15, no. 20: 5098. https://doi.org/10.3390/cancers15205098
APA StylePark, S. H., Woo, H. S., Hong, I. K., & Park, E. J. (2023). Impact of Postoperative Naples Prognostic Score to Predict Survival in Patients with Stage II–III Colorectal Cancer. Cancers, 15(20), 5098. https://doi.org/10.3390/cancers15205098






