The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods of Literature Research
3. Epidemiological Studies
3.1. Effect of TSH on the Prevalence of TC
3.2. The Effect of TSH on the Invasiveness of TC
3.3. The Combined Effects of TSH and Other Factors on TC
3.4. The Effect of TSH Inhibition on TC
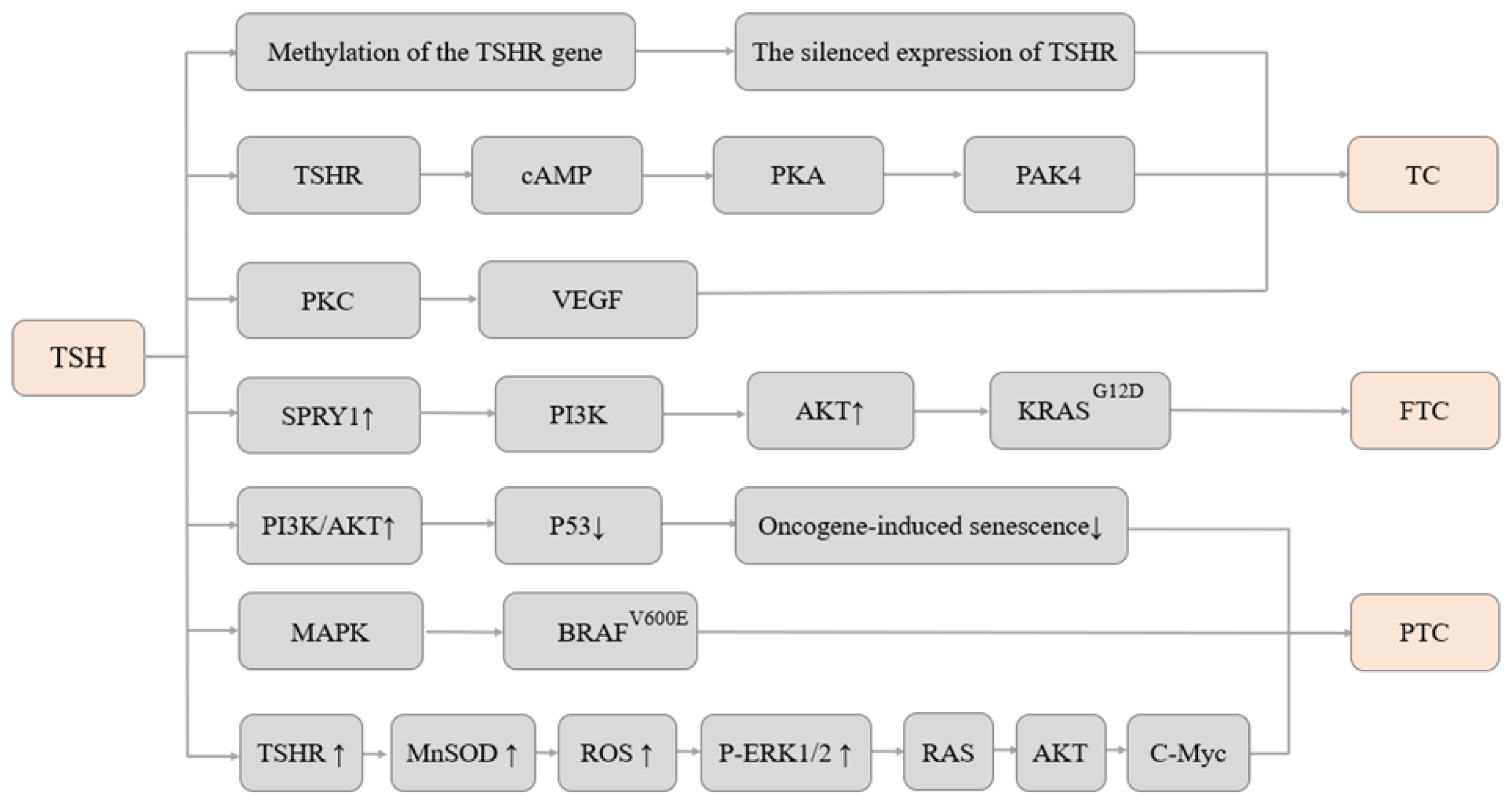
4. Research on Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gubbi, S.; Araque, K.A.; Klubo-Gwiezdzinska, J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020, 52, 562–577. [Google Scholar]
- Bogovic Crncic, T.; Ilic Tomas, M.; Girotto, N.; Grbac Ivankovic, S. Risk Factors for Thyroid Cancer: What Do We Know So Far? Acta Clin. Croat. 2020, 59 (Suppl. S1), 66–72. [Google Scholar] [CrossRef] [PubMed]
- Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated Thyroid Cancer-Treatment: State of the Art. Int. J. Mol. Sci. 2017, 18, 1292. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, T.M.; Papaleontiou, M.; Sosa, J.A. Current Controversies in Low-Risk Differentiated Thyroid Cancer: Reducing Overtreatment in an Era of Overdiagnosis. J. Clin. Endocrinol. Metab. 2023, 108, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Hoang, J.K. Thyroid cancer in the USA: Current trends and outstanding questions. Lancet Diabetes Endocrinol. 2021, 9, 11–12. [Google Scholar] [CrossRef]
- Vaccarella, S.; Dal Maso, L.; Laversanne, M.; Bray, F.; Plummer, M.; Franceschi, S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Vuong, H.G.; Le, M.K.; Hassell, L.; Kondo, T.; Kakudo, K. The differences in distant metastatic patterns and their corresponding survival between thyroid cancer subtypes. Head Neck 2022, 44, 926–932. [Google Scholar] [CrossRef]
- Morris, L.G.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The increasing incidence of thyroid cancer: The influence of access to care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, R.; Zhang, Y. The epidemic of thyroid cancer in the United States: The role of endocrinologists and ultrasounds. Thyroid 2014, 24, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Parad, M.T.; Fararouei, M.; Mirahmadizadeh, A.R.; Afrashteh, S. Thyroid cancer and its associated factors: A population-based case-control study. Int. J. Cancer 2021, 149, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, N.; Zhu, C.; Ni, X.; Ko, J.; Huang, H.; Ma, S.; Udelsman, R.; Zhang, Y. Dietary patterns and thyroid cancer risk: A population-based case-control study. Am. J. Transl. Res. 2020, 12, 180–190. [Google Scholar]
- Zhang, X.; Zhang, F.; Li, Q.; Aihaiti, R.; Feng, C.; Chen, D.; Zhao, X.; Teng, W. The relationship between urinary iodine concentration and papillary thyroid cancer: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1049423. [Google Scholar] [CrossRef]
- Peterson, E.; De, P.; Nuttall, R. BMI, diet and female reproductive factors as risks for thyroid cancer: A systematic review. PLoS ONE 2012, 7, e29177. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Lise, M.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Guillas, G.; Overvad, K.; Tjønneland, A.; Halkjaer, J.; Lukanova, A.; Kaaks, R.; et al. Body size and risk of differentiated thyroid carcinomas: Findings from the EPIC study. Int. J. Cancer 2012, 131, E1004–E1014. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, R.S.; Dora, J.M.; Maia, A.L. BRAF mutations in thyroid cancer. Curr. Opin. Oncol. 2022, 34, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Toda, K.; Yamada, K.; Yamamoto, N.; Ikenaga, M.; Fujimoto, Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: Our treatment strategies and outcomes. World J. Surg. 2010, 34, 1222–1231. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Higashiyama, T.; Kobayashi, K.; Miya, A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014, 24, 27–34. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef]
- Williams, E.D. Mechanisms and pathogenesis of thyroid cancer in animals and man. Mutat. Res. 1995, 333, 123–129. [Google Scholar] [CrossRef]
- Jonklaas, J.; Nsouli-Maktabi, H.; Soldin, S.J. Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid 2008, 18, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, K. The association between serum TSH concentration and thyroid cancer. Endocr.-Relat. Cancer 2009, 16, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Rago, T.; A Provenzale, M.; Scutari, M.; Ugolini, C.; Basolo, F.; Di Coscio, G.; Berti, P.; Grasso, L.; Elisei, R.; et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: Thyroid autonomy may play a protective role. Endocr.-Relat. Cancer 2009, 16, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.L.D.; Baek, K.H.; Lee, J.M.; Kim, M.K.; Kwon, H.S.; Song, K.H.; Kang, M.I.; Cha, B.Y.; Lee, K.W.; Son, H.Y. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 2010, 20, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Zafon, C.; Obiols, G.; Baena, J.A.; Castellví, J.; Dalama, B.; Mesa, J. Preoperative Thyrotropin Serum Concentrations Gradually Increase from Benign Thyroid Nodules to Papillary Thyroid Microcarcinomas Then to Papillary Thyroid Cancers of Larger Size. J. Thyroid Res. 2012, 2012, 530721. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Guan, H.; Li, C.; Shi, L.; Shan, Z.; Teng, W. Usefulness of serum thyrotropin for risk prediction of differentiated thyroid cancers does not apply to microcarcinomas: Results of 1,870 Chinese patients with thyroid nodules. Endocr. J. 2012, 59, 973–980. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Gu, D.N.; Hu, H.Y.; Zhou, Y.L.; Hu, X.Q.; Zhang, X.H. Hashimoto’s thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J. Surg. Oncol. 2013, 5, 56. [Google Scholar] [CrossRef]
- Mussa, A.; Salerno, M.C.; Bona, G.; Wasniewska, M.; Segni, M.; Cassio, A.; Vigone, M.C.; Gastaldi, R.; Iughetti, L.; Santanera, A.; et al. Serum Thyrotropin Concentration in Children with Isolated Thyroid Nodules. J. Pediatr. 2013, 163, 1465–1470. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, J.; Zhu, J.; Hu, G. Association between preoperative serum thyroid-stimulating hormone level and nonfunctioning malignant nodule thyroid disease. J. Clin. Otorhinolaryngol. Head Neck Surg. 2014, 28, 1931–1933. [Google Scholar]
- Wang, Z.; Lin, Y.; Jiang, Y.; Fu, R.; Wang, Y.; Zhang, Q. The associations between thyroid-related hormones and the risk of thyroid cancer: An overall and dose-response meta-analysis. Front. Endocrinol. 2022, 13, 992566. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Hsieh, A.-T.; Lee, T.-W.; Lee, T.-I.; Chien, Y.-M. The Association of Thyrotropin and Autoimmune Thyroid Disease in Developing Papillary Thyroid Cancer. Int. J. Endocrinol. 2017, 2017, 5940367. [Google Scholar] [CrossRef]
- Zheng, J.; Li, C.; Lu, W.; Wang, C.; Ai, Z. Quantitative assessment of preoperative serum thyrotropin level and thyroid cancer. Oncotarget 2016, 7, 34918–34929. [Google Scholar] [CrossRef]
- Rianto, B.U.D.; Wibowo, A.S.; Herdini, C. The Difference in Thyroid Stimulating Hormone Levels between Differentiated Carcinoma and Benign Enlargement. Int. Arch. Otorhinolaryngol. 2020, 24, e73–e79. [Google Scholar] [CrossRef]
- Kim, H.I.; Jang, H.W.; Ahn, H.S.; Ahn, S.; Park, S.Y.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Kim, J.-H.; Kim, J.S.; et al. High Serum TSH Level Is Associated With Progression of Papillary Thyroid Microcarcinoma During Active Surveillance. J. Clin. Endocrinol. Metab. 2018, 103, 446–451. [Google Scholar] [CrossRef]
- Lun, Y.; Wu, X.; Xia, Q.; Han, Y.; Zhang, X.; Liu, Z.; Wang, F.; Duan, Z.; Xin, S.; Zhang, J. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol. Head Neck Surg. 2013, 148, 396–402. [Google Scholar] [CrossRef]
- Fiore, E.; Rago, T.; A Provenzale, M.; Scutari, M.; Ugolini, C.; Basolo, F.; Di Coscio, G.; Miccoli, P.; Grasso, L.; Pinchera, A.; et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: Results of a cross-sectional study on 27914 patients. Endocr. Relat. Cancer 2010, 17, 231–239. [Google Scholar] [CrossRef]
- Hu, N.; Li, Z.M.; Liu, J.F.; Zhang, Z.Z.; Wang, L.S. An overall and dose-response meta-analysis for thyrotropin and thyroid cancer risk by histological type. Oncotarget 2016, 7, 47750–47759. [Google Scholar] [CrossRef]
- Golbert, L.; de Cristo, A.P.; Faccin, C.S.; Farenzena, M.; Folgierini, H.; Graudenz, M.S.; Maia, A.L. Serum TSH levels as a predictor of malignancy in thyroid nodules: A prospective study. PLoS ONE 2017, 12, e0188123. [Google Scholar] [CrossRef]
- Hurtado-López, L.-M.; Monroy-Lozano, B.-E.; Martínez-Duncker, C. TSH alone is not sufficient to exclude all patients with a functioning thyroid nodule from undergoing testing to exclude thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Rusiecki, J.; Zhao, N.; Chen, Y.; Ma, S.; Yu, H.; Ward, M.H.; Udelsman, R.; Zhang, Y. Thyroid-Stimulating Hormone, Thyroid Hormones, and Risk of Papillary Thyroid Cancer: A Nested Case–Control Study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Plummer, M.; Biessy, C.; Tsilidis, K.K.; Østergaard, J.N.; Overvad, K.; Tjønneland, A.; Halkjær, J.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; et al. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: The EPIC study. J. Natl. Cancer Inst. 2014, 106, dju097. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.Y.; Kim, H.J.; Jang, H.W.; Kim, S.W.; Chung, J.H. Lack of association between high serum thyroid-stimulating hormone level and risk of papillary thyroid microcarcinomas. Head Neck 2014, 36, 43–46. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, M.Y.; Jin, S.M.; Lee, S.H. The association between serum concentration of thyroid hormones and thyroid cancer: A cohort study. Endocr. Relat. Cancer 2022, 29, 635–644. [Google Scholar] [CrossRef]
- Negro, R.; Valcavi, R.; Toulis, K.A. Incidental Thyroid Cancer in Toxic and Nontoxic Goiter: Is TSH Associated with Malignany Rate? Results of A Meta-Analysis. Endocr. Pract. 2013, 19, 212–218. [Google Scholar] [CrossRef]
- Wang, G.; Ren, N.; Wang, S.; Zhang, X.; Li, Y.; Sun, N.; Liu, Q.; Zhang, J.; Song, W.; Ni, X. Serum TSH is not a risk factor for malignancy of pediatric thyroid nodules. Endocr. Relat. Cancer 2021, 28, 247–255. [Google Scholar] [CrossRef]
- Hoffmann, S.; Hofbauer, L.C.; Scharrenbach, V.; Wunderlich, A.; Hassan, I.; Lingelbach, S.; Zielke, A. Thyrotropin (TSH)-induced production of vascular endothelial growth factor in thyroid cancer cells in vitro: Evaluation of TSH signal transduction and of angiogenesis-stimulating growth factors. J. Clin. Endocrinol. Metab. 2004, 89, 6139–6145. [Google Scholar] [CrossRef]
- Mao, A.; An, N.; Wang, J.; Wu, Y.; Wang, T.; Wang, Z.; Guan, H. Association between preoperative serum TSH and tumor status in patients with papillary thyroid microcarcinoma. Endocrine 2021, 73, 617–624. [Google Scholar] [CrossRef]
- Gerschpacher, M.; Göbl, C.; Anderwald, C.; Gessl, A.; Krebs, M. Thyrotropin Serum Concentrations in Patients with Papillary Thyroid Microcancers. Thyroid 2010, 20, 389–392. [Google Scholar] [CrossRef]
- McLeod, D.S.; Cooper, D.S.; Ladenson, P.W.; Ain, K.B.; Brierley, J.D.; Fein, H.G.; Haugen, B.R.; Jonklaas, J.; Magner, J.; Ross, D.S.; et al. Prognosis of Differentiated Thyroid Cancer in Relation to Serum Thyrotropin and Thyroglobulin Antibody Status at Time of Diagnosis. Thyroid 2014, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qu, N.; Zhang, L.; Chen, J.-Y.; Ji, Q.-H. Preoperative ultrasonography and serum thyroid-stimulating hormone on predicting central lymph node metastasis in thyroid nodules as or suspicious for papillary thyroid microcarcinoma. Tumor Biol. 2015, 37, 7453–7459. [Google Scholar] [CrossRef] [PubMed]
- Danilovic, D.L.S.; Ferraz-De-Souza, B.; Fabri, A.W.; Santana, N.O.; Kulcsar, M.A.; Cernea, C.R.; Marui, S.; Hoff, A.O. 25-Hydroxyvitamin D and TSH as Risk Factors or Prognostic Markers in Thyroid Carcinoma. PLoS ONE 2016, 11, e0164550. [Google Scholar] [CrossRef]
- Tam, A.A.; Ozdemir, D.; Aydın, C.; Bestepe, N.; Ulusoy, S.; Sungu, N.; Ersoy, R.; Cakir, B. Association between preoperative thyrotrophin and clinicopathological and aggressive features of papillary thyroid cancer. Endocrine 2018, 59, 565–572. [Google Scholar] [CrossRef]
- Lee, M.C.; Kim, M.J.; Choi, H.S.; Cho, S.W.; Lee, G.H.; Park, Y.J.; Park, D.J. Postoperative Thyroid-Stimulating Hormone Levels Did Not Affect Recurrence after Thyroid Lobectomy in Patients with Papillary Thyroid Cancer. Endocrinol. Metab. 2019, 34, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Paparodis, R.D.; Bantouna, D.; Karvounis, E.; Imam, S.; Jaume, J.C. Higher TSH Is Not Associated With Thyroid Cancer Risk in the Presence of Thyroid Autoimmunity. J. Clin. Endocrinol. Metab. 2020, 105, e2389–e2397. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Karyadi, D.M.; Stewart, C.; Bogdanova, T.I.; Dawson, E.T.; Steinberg, M.K.; Dai, J.; Hartley, S.W.; Schonfeld, S.J.; Sampson, J.N.; et al. Radiation-related genomic profile of papillary thyroid carcinoma after the Chernobyl accident. Science 2021, 372, eabg2538. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF Mutation in Papillary Thyroid Cancer: Pathogenic Role, Molecular Bases, and Clinical Implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef]
- Shen, C.-T.; Zhang, X.-Y.; Qiu, Z.-L.; Sun, Z.-K.; Wei, W.-J.; Song, H.-J.; Luo, Q.-Y. Thyroid autoimmune antibodies in patients with papillary thyroid carcinoma: A double-edged sword? Endocrine 2017, 58, 176–183. [Google Scholar] [CrossRef]
- Wu, X.; Lun, Y.; Jiang, H.; Gang, Q.; Xin, S.; Duan, Z.; Zhang, J. Coexistence of thyroglobulin antibodies and thyroid peroxidase antibodies correlates with elevated thyroid-stimulating hormone level and advanced tumor stage of papillary thyroid cancer. Endocrine 2014, 46, 554–560. [Google Scholar] [CrossRef]
- Yazici, P.; Mihmanli, M.; Bozkurt, E.; Ozturk, F.Y.; Uludag, M. Which is the best predictor of thyroid cancer: Thyrotropin, thyroglobulin or their ratio? Hormones 2016, 15, 256–263. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, C.; Liang, L.; Wang, S.; Zheng, X.; Zhang, Q.; Jiang, C.; Zhong, Q.; Huang, F. Fasting serum glucose, thyroid-stimulating hormone, and thyroid hormones and risk of papillary thyroid cancer: A case-control study. Head Neck 2019, 41, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Danielsen, M.; Wang, H. A Pilot Study of Serum Selenium, Vitamin D, and Thyrotropin Concentrations in Patients with Thyroid Cancer. Thyroid 2013, 23, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, F.; Dedhia, P.H.; Ringel, M.D. Thyroid cancer, recent advances in diagnosis and therapy. Int. J. Cancer 2021, 149, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Farrell, S.G.; Grossmann, M. Thyroid nodules: Diagnosis and management. Med. J. Aust. 2018, 209, 92–98. [Google Scholar] [CrossRef]
- Kim, M.K.; Yun, K.-J.; Kim, M.-H.; Lim, D.-J.; Kwon, H.-S.; Song, K.-H.; Kang, M.-I.; Baek, K.H. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone 2015, 71, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, L.; Haymart, M.R. Optimal Thyrotropin Following Lobectomy for Papillary Thyroid Cancer: Does It Exist? Thyroid 2022, 32, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Han, M.; Jeon, M.J.; Kwon, H.; Kim, M.; Sung, T.-Y.; Kim, T.Y.; Hong, S.J.; Shong, Y.K.; Song, E.; et al. Thyrotropin Suppressive Therapy for Low-Risk Small Thyroid Cancer: A Propensity Score–Matched Cohort Study. Thyroid 2017, 27, 1164–1170. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Auh, S.; Gershengorn, M.; Daley, B.; Bikas, A.; Burman, K.; Wartofsky, L.; Urken, M.; Dewey, E.; Smallridge, R.; et al. Association of Thyrotropin Suppression With Survival Outcomes in Patients With Intermediate- and High-Risk Differentiated Thyroid Cancer. JAMA Netw. Open 2019, 2, e187754. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Hercbergs, A.; Luidens, M.K.; Lin, H.-Y. Recurrence of Differentiated Thyroid Carcinoma During Full TSH Suppression: Is the Tumor Now Thyroid Hormone Dependent? Horm. Cancer 2014, 6, 7–12. [Google Scholar] [CrossRef]
- Biondi, B.; Cooper, D.S. Thyroid Hormone Suppression Therapy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Ramundo, V.; Verrienti, A.; Sponziello, M.; Durante, C. Thyroid hormone therapy in differentiated thyroid cancer. Endocrine 2019, 66, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.J.; Yoo, W.S.; Lee, E.K.; Ahn, H.Y.; Woo, S.H.; Hong, J.H.; Chung, H.K.; Park, J.-W. Effect of TSH Suppression Therapy on Bone Mineral Density in Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021, 106, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Song, B.-S.; Kang, Y.M.; Kim, Y.-R.; Kang, Y.E.; Lee, J.H.; Shong, M.; Yi, H.-S. Effect of Thyroid-Stimulating Hormone Suppression on Muscle Function After Total Thyroidectomy in Patients With Thyroid Cancer. Front. Endocrinol. 2021, 12, 769074. [Google Scholar] [CrossRef] [PubMed]
- Papaleontiou, M.; Chen, D.W.; Banerjee, M.; Reyes-Gastelum, D.; Hamilton, A.S.; Ward, K.C.; Haymart, M.R. Thyrotropin Suppression for Papillary Thyroid Cancer: A Physician Survey Study. Thyroid 2021, 31, 1383–1390. [Google Scholar] [CrossRef]
- Hershman, J.M.; Beck-Peccoz, P. Discoveries Around the Hypothalamic–Pituitary–Thyroid Axis. Thyroid 2023, 33, 785–790. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, L.; Chen, L.; Lian, X.; Liu, C.; Shi, B.; Shi, L.; Tong, N.; Wang, S.; Weng, J.; et al. An Age-Specific Serum Thyrotropin Reference Range for the Diagnosis of Thyroid Diseases in Older Adults: A Cross-Sectional Survey in China. Thyroid 2018, 28, 1571–1579. [Google Scholar] [CrossRef]
- Moulana, F.I.; Priyani, A.A.H.; de Silva, M.V.C.; Dassanayake, R.S. BRAF-Oncogene-Induced Senescence and the Role of Thyroid-Stimulating Hormone Signaling in the Progression of Papillary Thyroid Carcinoma. Horm. Cancer 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Mitsumori, K.O.H.; Takahashi, M.; Shimo, T.; Yasuhara, K.; Kitaura, K.; Takahashi, M.; Hayashi, Y. Effect of thyroid stimulating hormone on the development and progression of rat thyroid follicular cell tumors. Cancer Lett. 1995, 92, 193–202. [Google Scholar] [CrossRef]
- Chaimoff, M.; Raiter, A.; Avidan, S.; Shpitzer, T.; Feinmesser, R.; Hardy, B. Effect of exogenous thyroid-stimulating hormone on thyroid papillary carcinoma cells in tissue culture. Head Neck 2001, 23, 479–483. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Sigurdsson, A.; Bergthorsson, J.T.; He, H.; Blondal, T.; Geller, F.; Jakobsdottir, M.; et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 2009, 41, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.; Lu, M.; Schultz, A.; Thomas, D.; Lin, R.Y. CD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropin. PLoS ONE 2009, 4, e5395. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, L.; Ying, H.; Willingham, M.C.; Cheng, S.Y. Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma. Endocrinology 2010, 151, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.W.; Han, J.H.; Lee, J.; Soh, E.Y.; Park, S.H.; Kim, J.-H.; Park, T.J. TSH signaling overcomes B-RafV600E-induced senescence in papillary thyroid carcinogenesis through regulation of DUSP6. Neoplasia 2014, 16, 1107–1120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kopp, P. Human Genome and Diseases: Review The TSH receptor and its role in thyroid disease. Cell. Mol. Life Sci. 2001, 58, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Endo, T.; Ohta, K.; Gunji, K.; Onaya, T. Point mutations in the thyrotropin receptor in human thyroid tumors. Thyroid 1995, 5, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Usadel, H.; Cohen, Y.; Tokumaru, Y.; Guo, Z.; Westra, W.B.; Tong, B.C.; Tallini, G.; Udelsman, R.; Califano, J.A.; et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: A marker of malignancy and a cause of gene silencing. Cancer Res. 2003, 63, 2316–2321. [Google Scholar]
- Tanaka, K.; Sonoo, H.; Yamamoto, Y.; Udagawa, K.; Kunisue, H.; Arime, I.; Yamamoto, S.; Kurebayashi, J.; Shimozuma, K. Changes of expression level of the differentiation markers in papillary thyroid carcinoma under thyrotropin suppression therapy in vivo immunohistochemical detection of thyroglobulin, thyroid peroxidase, and thyrotropin receptor. J. Surg. Oncol. 2000, 75, 108–116. [Google Scholar] [CrossRef]
- Wu, Z.; Xi, Z.; Xiao, Y.; Zhao, X.; Li, J.; Feng, N.; Hu, L.; Zheng, R.; Zhang, N.; Wang, S.; et al. TSH-TSHR axis promotes tumor immune evasion. J. Immunother. Cancer 2022, 10, e004049. [Google Scholar] [CrossRef]
- Zou, M.; Baitei, E.Y.; A Al-Rijjal, R.; Parhar, R.S.; A Al-Mohanna, F.; Kimura, S.; Pritchard, C.; BinEssa, H.; A Alanazi, A.; Alzahrani, A.S.; et al. KRAS(G12D)-mediated oncogenic transformation of thyroid follicular cells requires long-term TSH stimulation and is regulated by SPRY1. Lab. Investig. 2015, 95, 1269–1277. [Google Scholar] [CrossRef]
- Scheffel, R.S.; de Cristo, A.P.; Romitti, M.; Vargas, C.V.F.; Ceolin, L.; Zanella, A.B.; Dora, J.M.; Maia, A.L. The BRAF(V600E) mutation analysis and risk stratification in papillary thyroid carcinoma. Arch. Endocrinol. Metab. 2021, 64, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.T.; Malaguarnera, R.; Refetoff, S.; Liao, X.-H.; Lundsmith, E.; Kimura, S.; Pritchard, C.; Marais, R.; Davies, T.F.; Weinstein, L.S.; et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 1615–1620. [Google Scholar] [CrossRef]
- Zou, M.; Baitei, E.Y.; A Al-Rijjal, R.; Parhar, R.S.; A Al-Mohanna, F.; Kimura, S.; Pritchard, C.; A Binessa, H.; Alzahrani, A.S.; Al-Khalaf, H.H.; et al. TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene 2016, 35, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shi, X.; Guan, H.; Guo, Q.; Fan, C.; Dong, W.; Wang, G.; Li, F.; Shan, Z.; Cao, L.; et al. P21-activated kinase 4 involves TSH induced papillary thyroid cancer cell proliferation. Oncotarget 2017, 8, 24882–24891. [Google Scholar] [CrossRef] [PubMed]
- Zaballos, M.A.; Santisteban, P. FOXO1 Controls Thyroid Cell Proliferation in Response to TSH and IGF-I and Is Involved in Thyroid Tumorigenesis. Mol. Endocrinol. 2013, 27, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Kim, M.J.; Sun, H.J.; Kim, H.H.; Shin, H.S.; Kim, Y.A.; Oh, B.-C.; Cho, S.W.; Park, Y.J. Aberrant Thyroid-Stimulating Hormone Receptor Signaling Increases VEGF-A and CXCL8 Secretion of Thyroid Cancer Cells, Contributing to Angiogenesis and Tumor Growth. Clin. Cancer Res. 2019, 25, 414–425. [Google Scholar] [CrossRef] [PubMed]
| No. | First Author | Publication Year | Location | Main Findings |
|---|---|---|---|---|
| 1 | Jonklaas et al. [23] | 2008 | American | Compared to patients with TSH concentrations in the lowest 1/4, patients with TSH concentrations in the upper 3/4 have a higher risk of TC (OR = 8.7). |
| 2 | Fiore et al. [25] | 2009 | Italy | The TSH levels of PTC are significantly higher than those of benign thyroid diseases and are highest among patients with serum TSH at the upper limit of the normal range. (TSH > 3.4 mU/L, OR = 3.5). |
| 3 | Kim et al. [26] | 2010 | Korea | Benign nodules: 2.1 ± 2.0 mU/L; TC: 2.5 ± 2.8 mU/L Upper 1/4 of TSH levels: OR = 1.72; above normal range of TSH levels: OR = 1.98 |
| 4 | Zafon et al. [27] | 2011 | Spain | Benign nodules: 1.36 ± 1.62 mU/L; TC: 2.08 ± 2.1 mU/L PTMC: 1.71 ± 1.52 mU/L; TCLS: 2.42 ± 2.5 mU/L |
| 5 | Ye et al. [29] | 2013 | China | The risk for malignancy significantly increased with serum TSH 1.97–4.94 mIU/L, compared with TSH less than 0.35 mIU/L (OR = 1.951) |
| 6 | Mussa et al. [30] | 2013 | Italy | Benign nodules: 1.64 ± 0.99 mU/L; TC: 3.23 ± 1.95 mU/L |
| 7 | Zeng et al. [31] | 2014 | China | Benign tumors: 1.94 ± 1.01 mU/L; PTC: 1.16 ± 0.85 mU/L |
| 8 | Rinaldi et al. [43] | 2014 | France | TC risk was negatively associated with TSH level (OR = 0.56) |
| 9 | Huang et al. [42] | 2017 | American | TSH levels below the normal range were associated with an elevated risk of PTC among women (OR = 3.74); TSH levels above the normal range were associated with an increased risk of PTC among men (OR = 1.96). |
| 10 | Golbert et al. [40] | 2017 | Brazil | Patients with TSH levels ≥ 2.26 mU/L have a risk of developing malignant tumors approximately three times higher than those with low TSH levels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tian, L.; Teng, D.; Teng, W. The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma. Cancers 2023, 15, 5017. https://doi.org/10.3390/cancers15205017
Zhang X, Tian L, Teng D, Teng W. The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma. Cancers. 2023; 15(20):5017. https://doi.org/10.3390/cancers15205017
Chicago/Turabian StyleZhang, Xueqi, Lijun Tian, Di Teng, and Weiping Teng. 2023. "The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma" Cancers 15, no. 20: 5017. https://doi.org/10.3390/cancers15205017
APA StyleZhang, X., Tian, L., Teng, D., & Teng, W. (2023). The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma. Cancers, 15(20), 5017. https://doi.org/10.3390/cancers15205017






