Niosomal Delivery of Celecoxib and Metformin for Targeted Breast Cancer Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
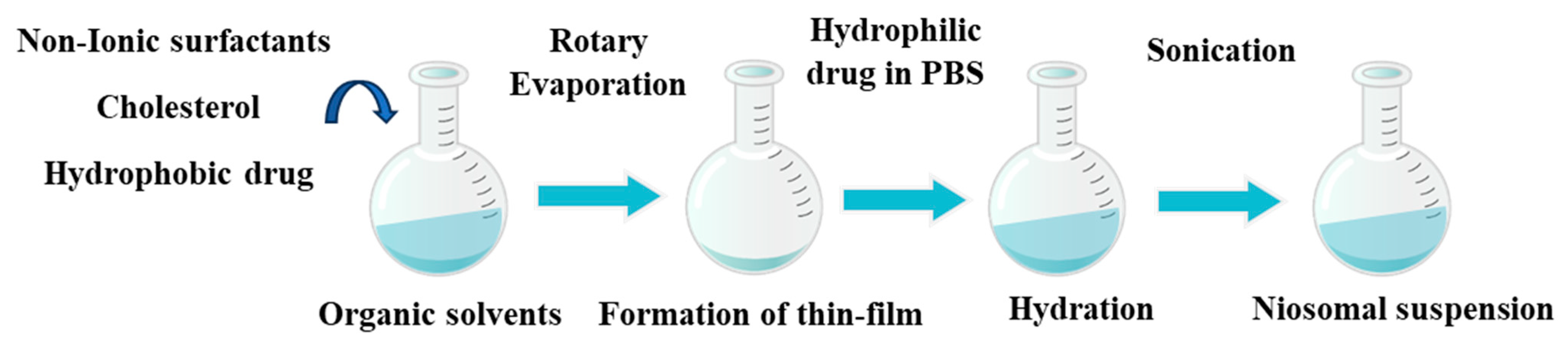
2.2.1. Preparation of Niosomes
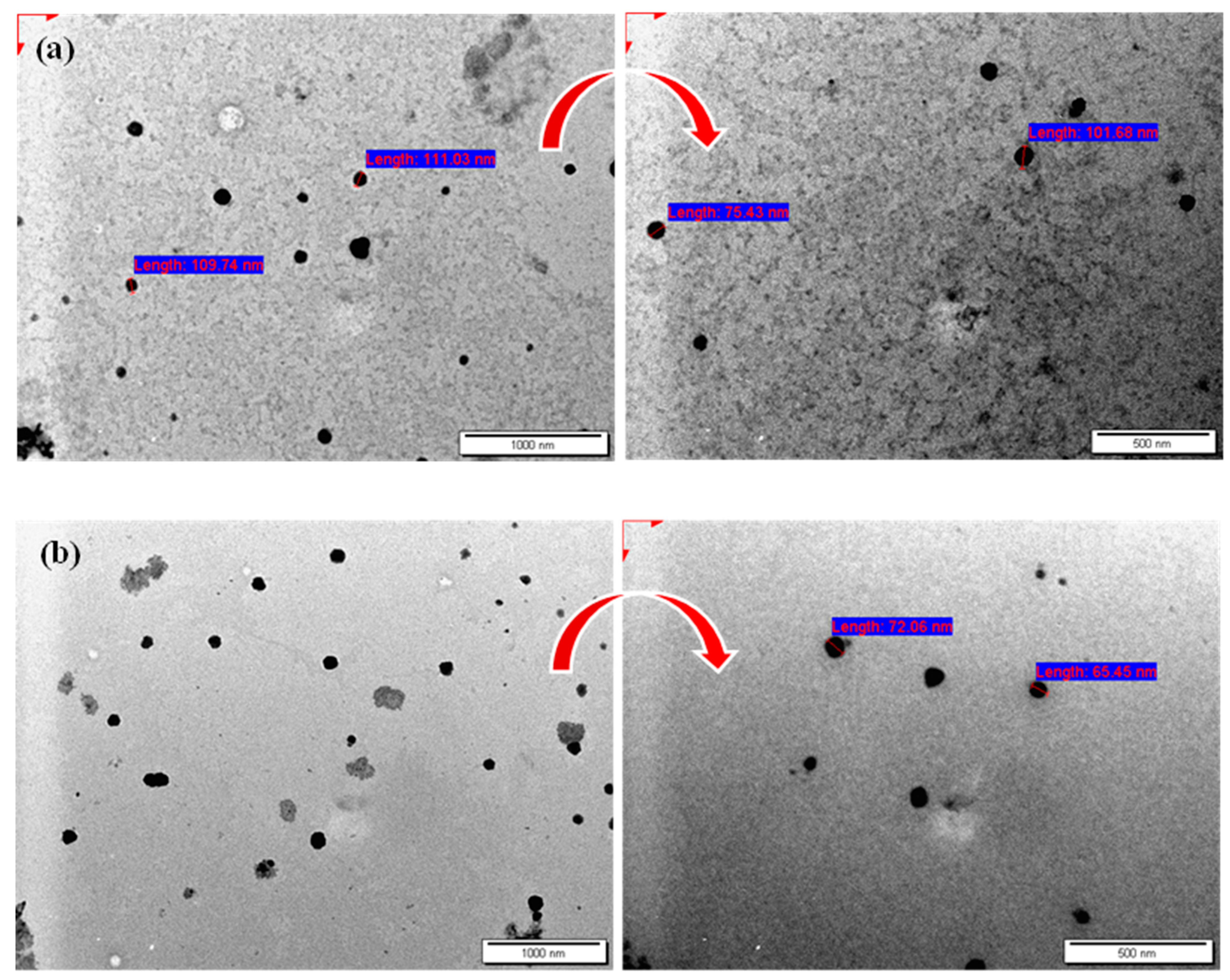
2.2.2. Characterization of Niosomes Using Transmission Electron Microscope (TEM)
2.2.3. Measurement of Particle Size (PS), Zeta Potential (ZP), and Polydispersity Index (PDI)
2.2.4. Purification of Niosomes
2.2.5. Drug Entrapment Efficiency
2.2.6. Attenuated Total Reflectance—Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.2.7. In Vitro Drug Release Study
2.2.8. Stability Study
2.2.9. Chromatographic Method of Analysis of MET NP and CXB NP
2.2.10. Cell Culture
2.2.11. MTT Cell Viability Assay
2.2.12. Spheroid Formation and Viability Assay
2.2.13. Spheroids Penetration Assay
2.2.14. Wound Healing Assay
2.2.15. Statistical Analysis
3. Results
3.1. Characterization of MET- and CXB-Loaded Niosomes
3.2. Purification of Niosomes
3.3. Drug Entrapment Efficiency
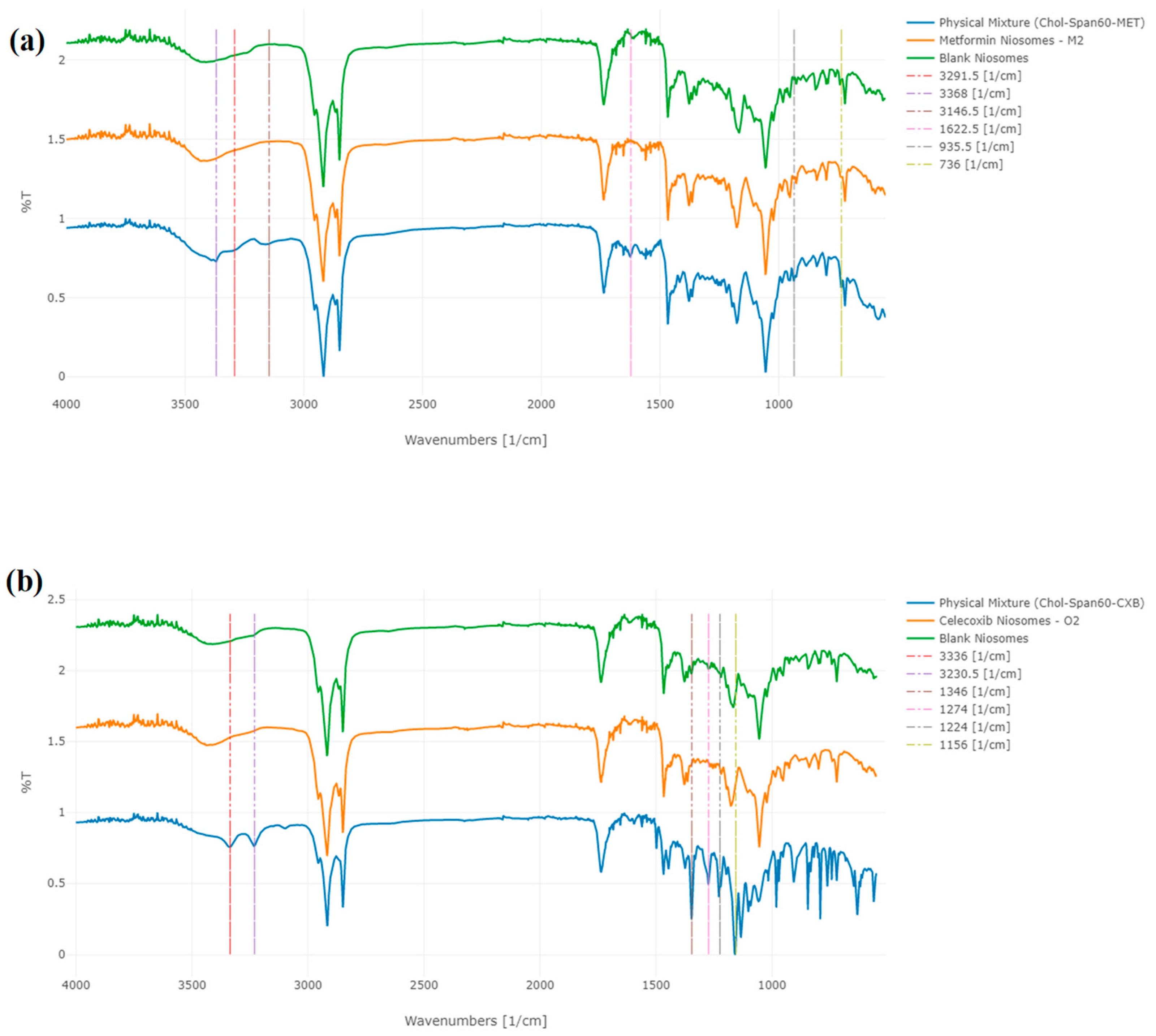
3.4. Attenuated Total Reflectance—Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
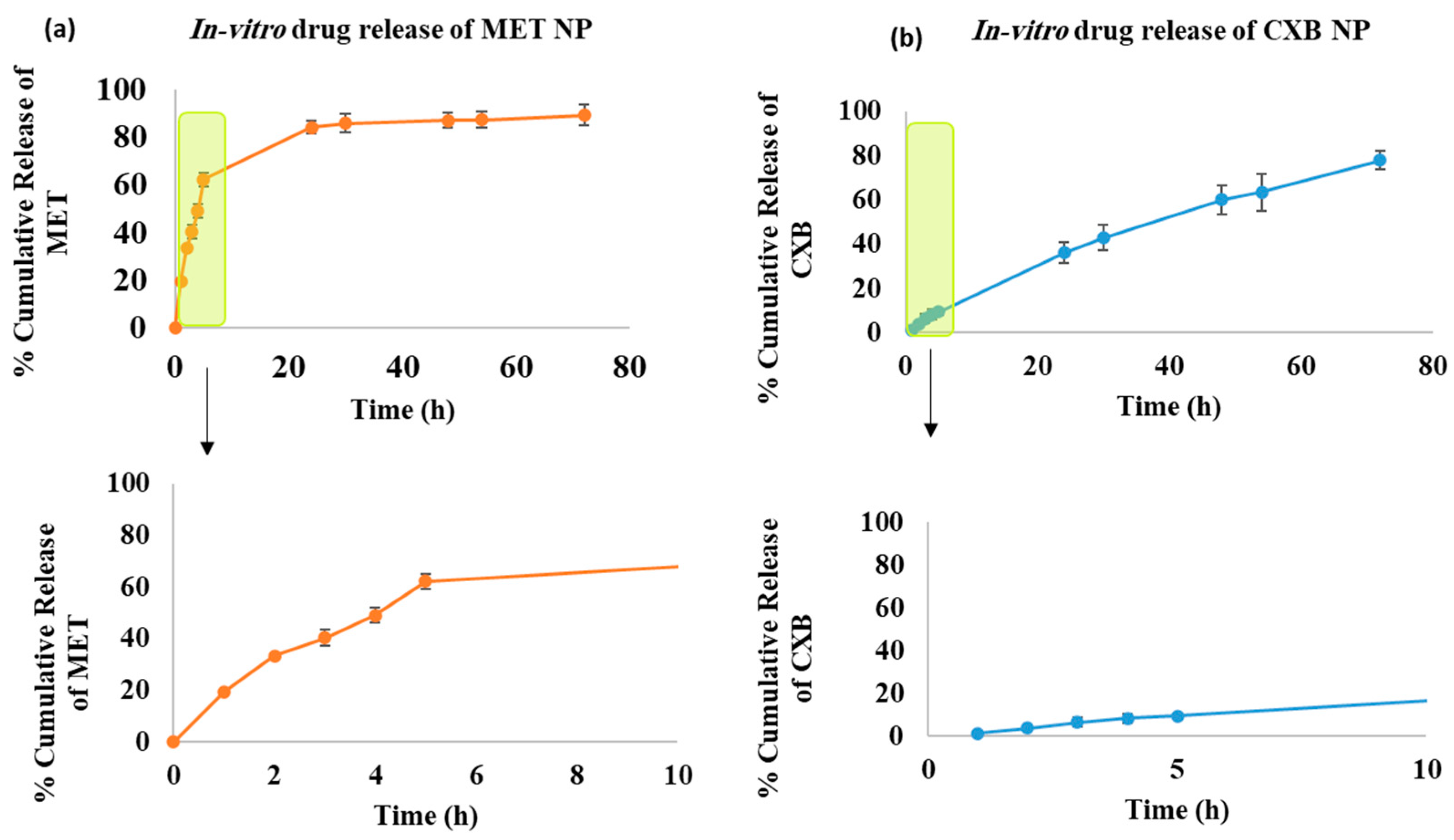
3.5. In Vitro Drug Release Study
3.6. Stability Study
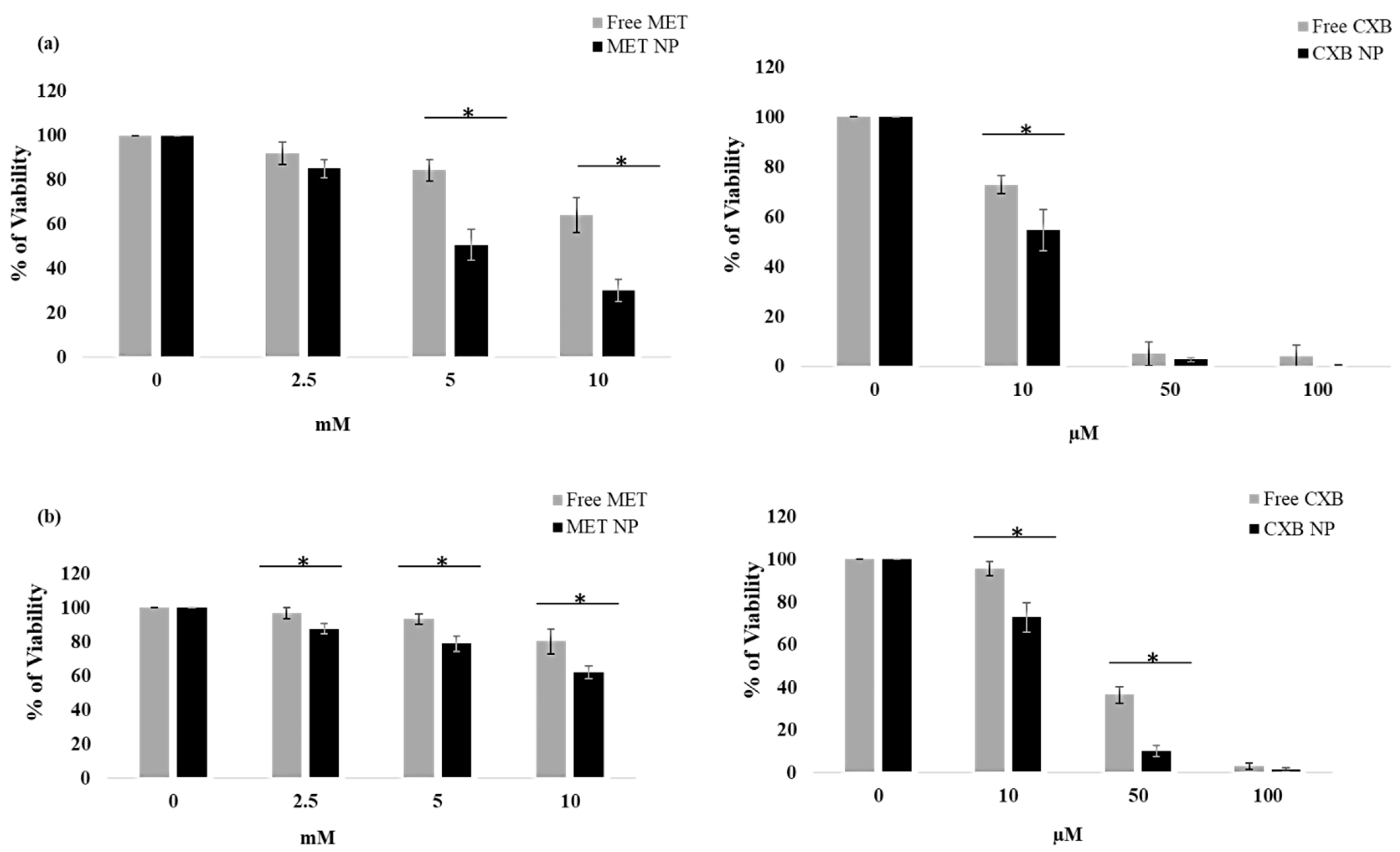
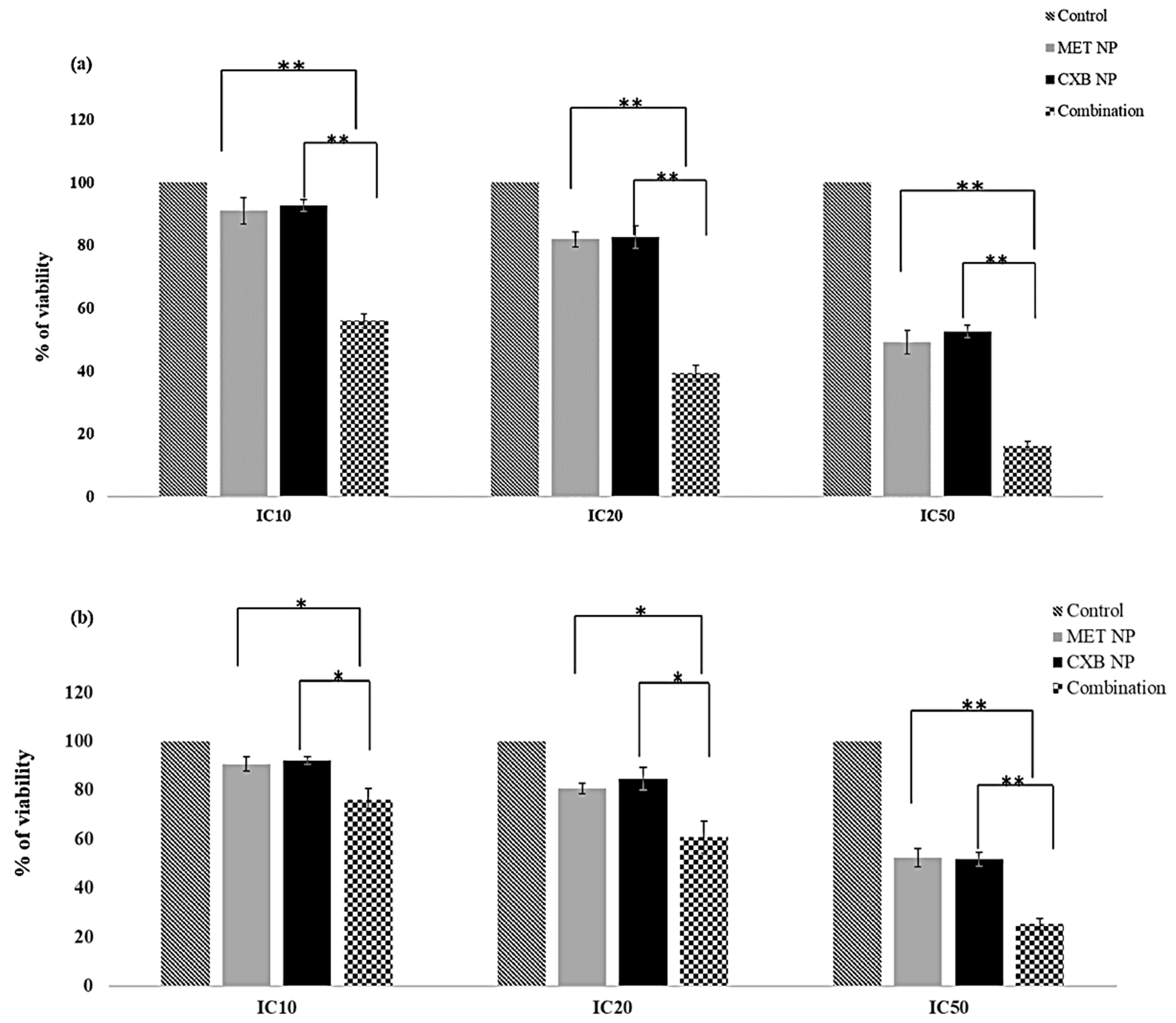
3.7. Effect of the Combination of MET NP and CXB NP on Cell Viability in a Monolayered Cell Culture
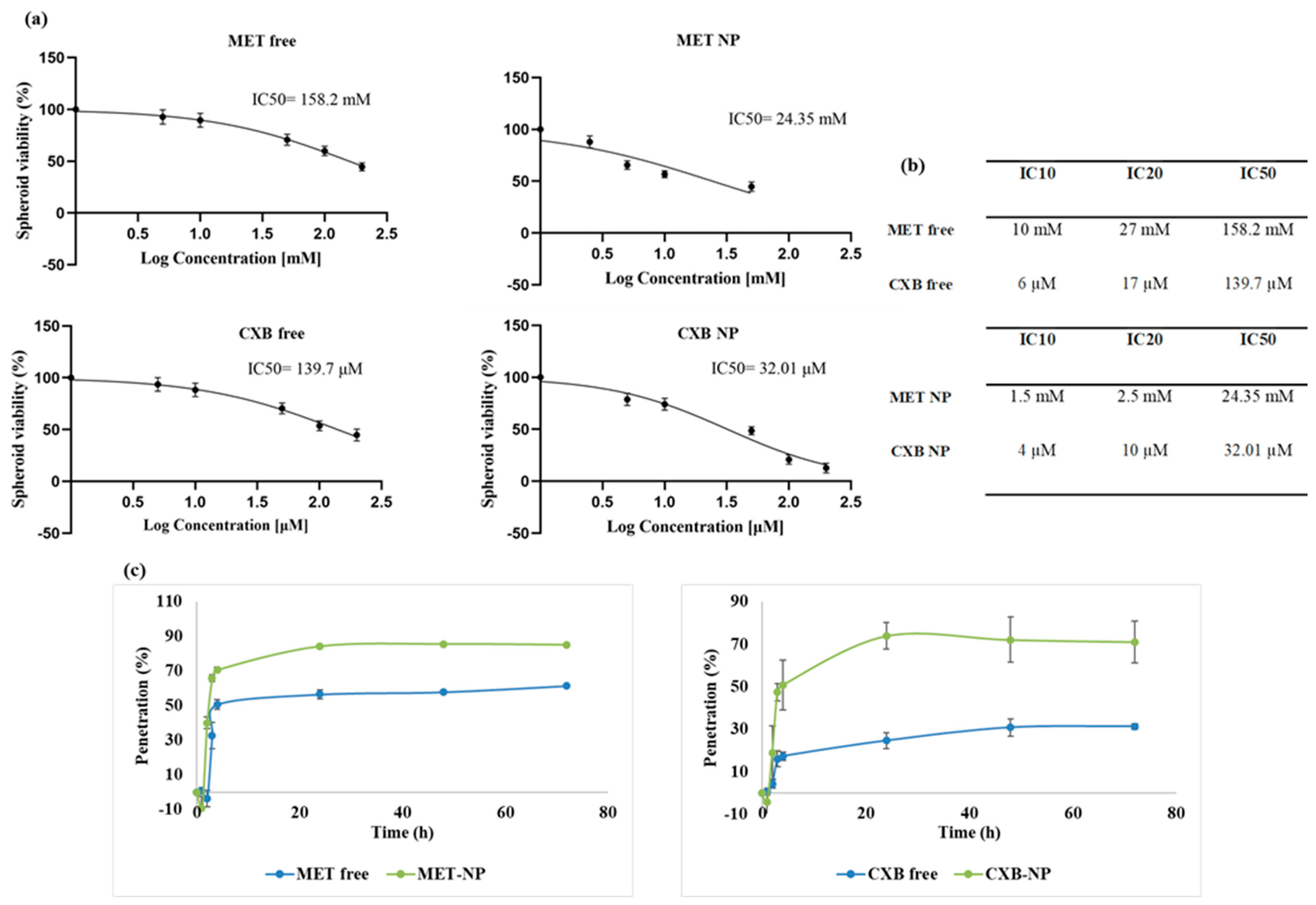
3.8. Effect of the Combination of MET NP and CXB NP on Cell Viability in 3D Spheroids
3.9. Penetration of MET, MET NP, CXB, and CXB NP
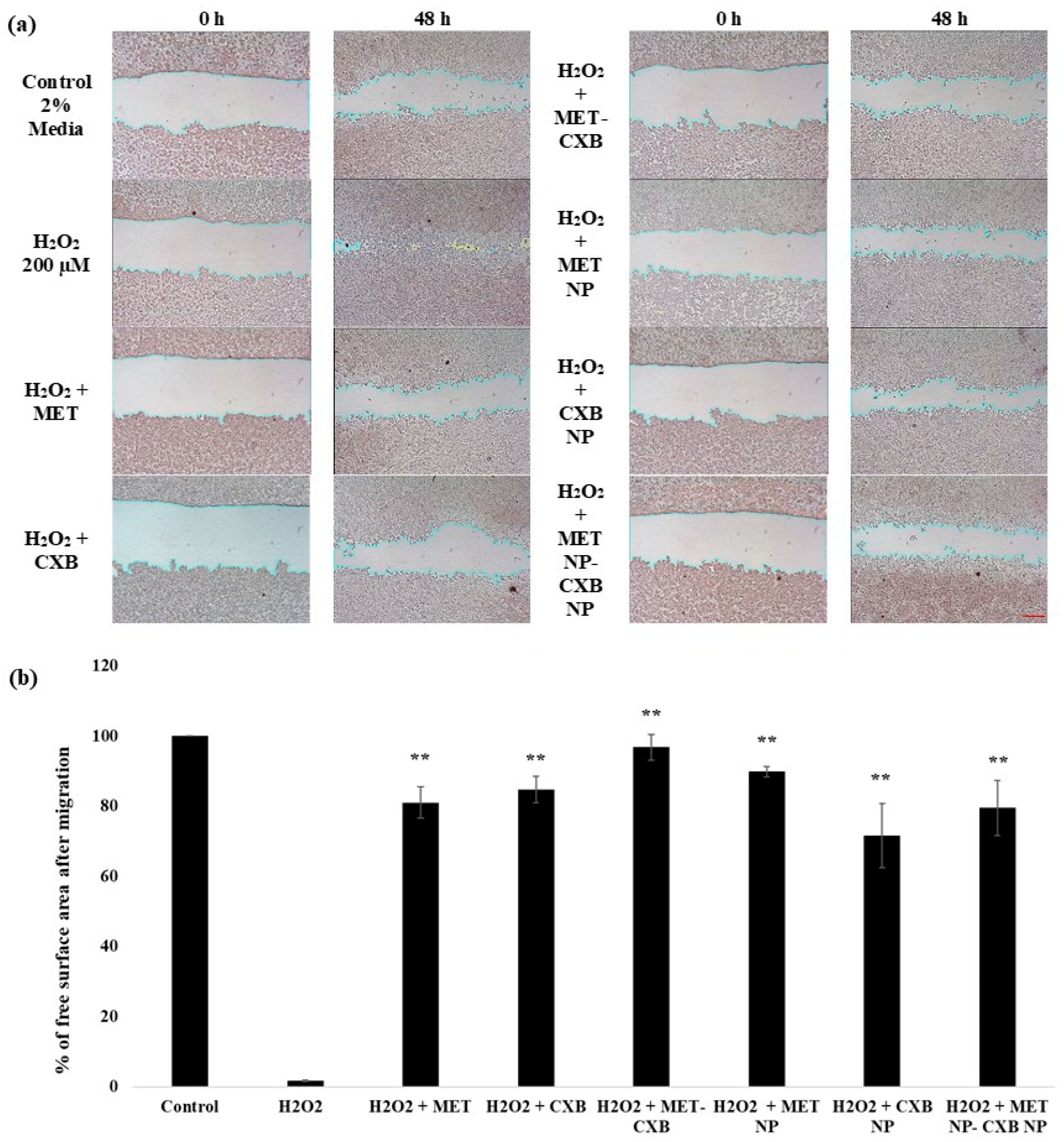
3.10. Effect of the Combination Treatment of MET NP and CXB NP on Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of breast cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar] [PubMed]
- Tang, Y.; Wang, Y.; Kiani, M.F.; Wang, B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin. Breast. Cancer 2016, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Minden, A. Current Molecular Combination Therapies Used for the Treatment of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 11046. [Google Scholar] [CrossRef] [PubMed]
- Hamy, A.S.; Tury, S.; Wang, X.; Gao, J.; Pierga, J.Y.; Giacchetti, S.; Brain, E.; Pistilli, B.; Marty, M.; Espie, M.; et al. Celecoxib with Neoadjuvant Chemotherapy for Breast Cancer Might Worsen Outcomes Differentially by COX-2 Expression and ER Status: Exploratory Analysis of the REMAGUS02 Trial. J. Clin. Oncol. 2019, 37, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Dasgupta, A.; Jiralerspong, S. Metformin in breast cancer—An evolving mystery. Breast Cancer Res. 2015, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Abo-Zour, H.; Basheer, H.A.; Abu-Zour, H.; Donnelly, R.F. Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs. Polymers 2023, 15, 1962. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Hamed, R.; Abdo, H.; Swellmeen, L.; Basheer, H.A.; Wahdan, W.; Abu Kwiak, A.D. Formulation and Evaluation of Azithromycin-Loaded Niosomal Gel: Optimization, In Vitro Studies, Rheological Characterization, and Cytotoxicity Study. ACS Omega 2022, 7, 39782–39793. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Musleh, B.; Hamed, R.; Swellmeen, L.; Basheer, H.A. Preparation and Characterization of Patch Loaded with Clarithromycin Nanovesicles for Transdermal Drug Delivery. J. Funct. Biomater. 2023, 14, 57. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Cejuela, M.; Martin-Castillo, B.; Menendez, J.A.; Pernas, S. Metformin and Breast Cancer: Where Are We Now? Int. J. Mol. Sci. 2022, 23, 2705. [Google Scholar] [CrossRef] [PubMed]
- Toloczko-Iwaniuk, N.; Dziemianczyk-Pakiela, D.; Nowaszewska, B.K.; Celinska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention—Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, e1702858. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, J.; Zhang, L.; Xiao, X.; Li, W. Curcumin inhibits H2O2-induced invasion and migration of human pancreatic cancer via suppression of the ERK/NF-kappaB pathway. Oncol. Rep. 2016, 36, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.; Fu, J.Y.; Htar, T.T.; Muniyandy, S.; Kasbollah, A.; Wan Kamal, W.H.B.; Chuah, L.H. Characterization, optimization, and in vitro evaluation of Technetium-99m-labeled niosomes. Int. J. Nanomed. 2019, 14, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid. Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Jagdale, S.; Patil, S.; Kuchekar, B.; Chabukswar, A. Preparation and Characterization of Metformin Hydrochloride-Compritol 888 ATO Solid Dispersion. J. Young Pharm. 2011, 3, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Samed, N.; Sharma, V.; Sundaramurthy, A. Hydrogen bonded niosomes for encapsulation and release of hydrophilic and hydrophobic anti-diabetic drugs: An efficient system for oral anti-diabetic formulation. Appl. Surf. Sci. 2018, 449, 567–573. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Nnamani, D.O.; Duhu, J.C.; Nmesirionye, B.U.; Momoh, M.A.; Akpa, P.A.; Attama, A.A. Potential enhancement of metformin hydrochloride in solidified reverse micellar solution-based PEGylated lipid nanoparticles targeting therapeutic efficacy in diabetes treatment. Heliyon 2022, 8, e09099. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.; Reddy, M.P.; Kaza, R. Design and characterization of microcrystals for enhanced dissolution rate of celecoxib. Curr. Drug Discov. Technol. 2013, 10, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Kannappan, V.; Sathyanarayanamoorthi, V. DFT analysis and spectral characteristics of Celecoxib a potent COX-2 inhibitor. J. Mol. Struct. 2016, 1121, 16–25. [Google Scholar] [CrossRef]
- Farmoudeh, A.; Akbari, J.; Saeedi, M.; Ghasemi, M.; Asemi, N.; Nokhodchi, A. Methylene blue-loaded niosome: Preparation, physicochemical characterization, and in vivo wound healing assessment. Drug Deliv. Transl. Res. 2020, 10, 1428–1441. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rasul, A.; Khan, M.I.; Hanif, M.; Aamir, M.N.; Waqas, M.K.; Hameed, M.; Akram, M.R. Development of niosomal formulations loaded with cyclosporine A and evaluation of its compatibility. Trop. J. Pharm. Res. 2018, 17, 1457–1464. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Dhavale, R.P.; Bhatia, M.S.; Jadhav, S.U.; Dhanavade, M.J.; Barale, S.S.; Pathak, S.; Parale, V.G.; Sonawane, K.D. Exploring anticancer potential of nintedanib conjugated magnetic nanoparticles: In-vitro and in-silico studies. J. Drug Deliv. Sci. Technol. 2023, 81, 104213. [Google Scholar] [CrossRef]
- Namdeo, A.; Jain, N.K. Niosomal delivery of 5-fluorouracil. J. Microencapsul. 1999, 16, 731–740. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Mirzaie, A.; Zabet, N.; Moammeri, A.; Mansoori-Kermani, A.; Akbarzadeh, I.; Eshrati Yeganeh, F.; Chitgarzadeh, A.; Bagheri Kashtali, A.; Ren, Q. Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation. Nanomaterials 2021, 11, 1573. [Google Scholar] [CrossRef]
- Hasan, A.A.; Madkor, H.; Wageh, S. Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system. Drug Deliv. 2013, 20, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.B.; El-Shanawany, S.M.; Hamad, M.A.; Elsabahy, M. Niosomes: A Strategy toward Prevention of Clinically Significant Drug Incompatibilities. Sci. Rep. 2017, 7, 6340. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A controlled and novel drug delivery system. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M. In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. Aaps Pharmscitech 2010, 11, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, E.; Abou-Taleb, H.A.; Mohamad, S.A.; Elrehany, M.; Gaber, S.S.; Mansour, H.F. Topical Nano-Vesicular Spanlastics of Celecoxib: Enhanced Anti-Inflammatory Effect and Down-Regulation of TNF-alpha, NF-small ka, CyrillicB and COX-2 in Complete Freund’s Adjuvant-Induced Arthritis Model in Rats. Int. J. Nanomed. 2021, 16, 133–145. [Google Scholar] [CrossRef]
- Cui, D. Nanomaterials for Theranostics of Gastric Cancer. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–349. [Google Scholar] [CrossRef]
- Yaghoobian, M.; Haeri, A.; Bolourchian, N.; Shahhosseni, S.; Dadashzadeh, S. The Impact of Surfactant Composition and Surface Charge of Niosomes on the Oral Absorption of Repaglinide as a BCS II Model Drug. Int. J. Nanomed. 2020, 15, 8767–8781. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, Z.; Wei, Z.; Tian, L. Hydrophobic Interaction: A Promising Driving Force for the Biomedical Applications of Nucleic Acids. Adv. Sci. 2020, 7, 2001048. [Google Scholar] [CrossRef]
- Esmaeili Rad, M.; Egil, A.C.; Ozaydin Ince, G.; Yuce, M.; Zarrabi, A. Optimization of curcumin loaded niosomes for drug delivery applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129921. [Google Scholar] [CrossRef]
- Hnin, H.M.; Stefansson, E.; Loftsson, T.; Asasutjarit, R.; Charnvanich, D.; Jansook, P. Physicochemical and Stability Evaluation of Topical Niosomal Encapsulating Fosinopril/gamma-Cyclodextrin Complex for Ocular Delivery. Pharmaceutics 2022, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zaki, R.M.; Alfadhel, M.M.; Alshahrani, S.M.; Alsaqr, A.; Al-Kharashi, L.A.; Anwer, M.K. Formulation of Chitosan-Coated Brigatinib Nanospanlastics: Optimization, Characterization, Stability Assessment and In-Vitro Cytotoxicity Activity against H-1975 Cell Lines. Pharmaceuticals 2022, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, T.; Zhao, M.; Wang, W.; Sun, C.; Liu, L.; Li, Q.; Zhang, F.; Zhao, D.; Li, X. Size and charge dual-transformable mesoporous nanoassemblies for enhanced drug delivery and tumor penetration. Chem. Sci. 2020, 11, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, D.; Li, L.; Sun, K. Charge reversal nano-systems for tumor therapy. J. Nanobiotechnol. 2022, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Kashani-Asadi-Jafari, F.; Aftab, A.; Ghaemmaghami, S. A machine learning framework for predicting entrapment efficiency in niosomal particles. Int. J. Pharm. 2022, 627, 122203. [Google Scholar] [CrossRef] [PubMed]
- Sankhyan, A.; Pawar, P.K. Metformin loaded non-ionic surfactant vesicles: Optimization of formulation, effect of process variables and characterization. Daru 2013, 21, 7. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Continuous supercritical CO2 assisted process for the production of nano-niosomes loaded with a second-generation antibiotic for ocular therapy. J. Supercrit. Fluids 2022, 188, 105673. [Google Scholar] [CrossRef]
- Auda, S.H.; Fathalla, D.; Fetih, G.; El-Badry, M.; Shakeel, F. Niosomes as transdermal drug delivery system for celecoxib: In vitro and in vivo studies. Polym. Bull. 2015, 73, 1229–1245. [Google Scholar] [CrossRef]
- Aguilar-Jimenez, Z.; Gonzalez-Ballesteros, M.; Davila-Manzanilla, S.G.; Espinoza-Guillen, A.; Ruiz-Azuara, L. Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design. Int. J. Mol. Sci. 2022, 23, 12756. [Google Scholar] [CrossRef]
- Cervello, M.; Bachvarov, D.; Lampiasi, N.; Cusimano, A.; Azzolina, A.; McCubrey, J.A.; Montalto, G. Novel combination of sorafenib and celecoxib provides synergistic anti-proliferative and pro-apoptotic effects in human liver cancer cells. PLoS ONE 2013, 8, e65569. [Google Scholar] [CrossRef]
- Gao, J.; Jia, W.D.; Li, J.S.; Wang, W.; Xu, G.L.; Ma, J.L.; Ge, Y.S.; Yu, J.H.; Ren, W.H.; Liu, W.B.; et al. Combined inhibitory effects of celecoxib and fluvastatin on the growth of human hepatocellular carcinoma xenografts in nude mice. J. Int. Med. Res. 2010, 38, 1413–1427. [Google Scholar] [CrossRef]
- Ko, J.C.; Chiu, H.C.; Wo, T.Y.; Huang, Y.J.; Tseng, S.C.; Huang, Y.C.; Chen, H.J.; Syu, J.J.; Chen, C.Y.; Jian, Y.T.; et al. Inhibition of p38 MAPK-dependent MutS homologue-2 (MSH2) expression by metformin enhances gefitinib-induced cytotoxicity in human squamous lung cancer cells. Lung Cancer 2013, 82, 397–406. [Google Scholar] [CrossRef]
- Liu, J.; Hou, M.; Yuan, T.; Yi, G.; Zhang, S.; Shao, X.; Chen, J.; Jia, X.; He, Z. Enhanced cytotoxic effect of low doses of metformin combined with ionizing radiation on hepatoma cells via ATP deprivation and inhibition of DNA repair. Oncol. Rep. 2012, 28, 1406–1412. [Google Scholar] [CrossRef]
- Cao, N.; Lu, Y.; Liu, J.; Cai, F.; Xu, H.; Chen, J.; Zhang, X.; Hua, Z.C.; Zhuang, H. Metformin Synergistically Enhanced the Antitumor Activity of Celecoxib in Human Non-Small Cell Lung Cancer Cells. Front. Pharmacol. 2020, 11, 1094. [Google Scholar] [CrossRef]
- Hu, J.W.; Chen, B.; Zhang, J.; Qi, Y.P.; Liang, J.H.; Zhong, J.H.; Xiang, B.D. Novel combination of celecoxib and metformin improves the antitumor effect by inhibiting the growth of Hepatocellular Carcinoma. J. Cancer 2020, 11, 6437–6444. [Google Scholar] [CrossRef] [PubMed]




| Formulation Code | MET (mg) | CXB (mg) | HLB * | Cholesterol (mM) | Span 60 (mM) | Tween 80 (mM) | Hydration volume (mL) |
|---|---|---|---|---|---|---|---|
| M1 | 100 | - | 4.7 | 75 | 75 | - | 15 |
| M2 | 100 | - | 4.7 | 100 | 100 | - | 15 |
| M3 | 100 | - | 6.4 | 100 | 66.6 | 33.3 | 15 |
| O1 | - | 10 | 4.7 | 75 | 75 | - | 15 |
| O2 | - | 10 | 4.7 | 100 | 100 | - | 15 |
| O3 | - | 10 | 6.4 | 100 | 66.6 | 33.3 | 15 |
| Formulation Code | PS (nm) ± SD | PDI ± SD | ZP (mV) ± SD |
|---|---|---|---|
| M1 | 120.0 ± 1.400 | 0.180 ± 0.003 | −42.15 ± 3.000 |
| M2 | 110.6 ± 0.600 | 0.139 ± 0.017 | −44.42 ± 1.990 |
| M3 | 129.5 ± 3.100 | 0.163 ± 0.007 | −56.18 ± 1.890 |
| O1 | 103.0 ± 1.300 | 0.316 ± 0.014 | −53.93 ± 1.550 |
| O2 | 96.7 ± 0.700 | 0.278 ± 0.003 | −53.89 ± 5.680 |
| O3 | 159.1 ± 1.700 | 0.120 ± 0.020 | −50.43 ± 0.785 |
| Formulation Code | EE ± SD (%) |
|---|---|
| M1 | 64.57 ± 2.02 |
| M2 | 68.94 ± 1.28 |
| M3 | 56.69 ± 3.22 |
| O1 | 72.19 ± 4.97 |
| O2 | 94.44 ± 2.09 |
| O3 | 88.05 ± 3.13 |
| Characteristic Peak (cm−1) of MET | Group Assign to Peak | Characteristic Peak (cm−1) of CXB | Group Assign to Peak | Characteristic Peak (cm−1) of Span 60 | Group Assign to Peak | Characteristic Peak (cm−1) of Cholesterol | Group Assign to Peak |
|---|---|---|---|---|---|---|---|
| 3291.5 | N–H primary stretching | 3336 | –NH2 stretching | 3400 | –OH stretching | 3430.5 | O-H stretching |
| 3368 | N–H primary stretching | 3230.5 | –NH2 stretching | 2917 | –CH stretching | 2931 | C-H stretching |
| 3146.5 | N–H secondary stretching | 1346 | S=O stretching | 1736 | strong C=O ester bond | 2867 | C-O bending vibrations |
| 1622.5 | C–N stretching | 1274.5 | -CF3 | 1174 | C–O and C-C stretching vibration | 1055 | C-O bending vibrations |
| 935.5 | N–H out of plane bending | 1229 | -CF3 | 721 | C–C connections | 958.5 | aromatic substitutions |
| 736 | N–H wagging | 1156 | S=O stretching | 840.5 | aromatic substitutions |
| Formula | Time Interval | PS PS (nm) ± SD | PDI | (%) EE |
|---|---|---|---|---|
| M2 | Freshly prepared | 110.6 ± 0.62 | 0.139 ± 0.01 | 68.94 ± 1.28 |
| One month | 123.9 ± 1.25 | 0.157 ± 0.01 | 66.85 ± 2.06 | |
| Two months | 141.4 ± 0.53 | 0.156 ± 0.02 | 61.42 ± 1.36 | |
| Three months | 149.2 ± 0.70 | 0.204 ± 0.01 | 57.12 ± 0.95 | |
| O2 | Freshly prepared | 96.7 ± 0.71 | 0.278 ± 0.01 | 94.54 ± 2.09 |
| One month | 102.3 ± 0.47 | 0.209 ± 0.01 | 93.81 ± 1.14 | |
| Two months | 113.7 ± 0.14 | 0.189 ± 0.02 | 91.44 ± 1.67 | |
| Three months | 109.7 ± 0.91 | 0.254 ± 0.08 | 90.73 ± 3.26 |
| MET | CXB | |||||
| IC10 | IC20 | IC50 | IC10 | IC20 | IC50 | |
| MCF-7 | 0.39 mM | 1.28 mM | 17.14 mM | 1.39 µM | 5.00 µM | 22.30 µM |
| MDA-MB-231 | 5.00 mM | 8.00 mM | 24.20 mM | 12.59 µM | 18.17 µM | 28.10 µM |
| MET NP | CXB NP | |||||
| IC10 | IC20 | IC50 | IC10 | IC20 | IC50 | |
| MCF-7 | 0.21 mM | 0.60 mM | 5.75 mM | 0.66 µM | 1.42 µM | 10.82 µM |
| MDA-MB-231 | 2.89 mM | 5.00 mM | 16.10 mM | 5.05 µM | 9.00 µM | 16.34 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basheer, H.A.; Alhusban, M.A.; Zaid Alkilani, A.; Alshishani, A.; Elsalem, L.; Afarinkia, K. Niosomal Delivery of Celecoxib and Metformin for Targeted Breast Cancer Treatment. Cancers 2023, 15, 5004. https://doi.org/10.3390/cancers15205004
Basheer HA, Alhusban MA, Zaid Alkilani A, Alshishani A, Elsalem L, Afarinkia K. Niosomal Delivery of Celecoxib and Metformin for Targeted Breast Cancer Treatment. Cancers. 2023; 15(20):5004. https://doi.org/10.3390/cancers15205004
Chicago/Turabian StyleBasheer, Haneen A., Maram A. Alhusban, Ahlam Zaid Alkilani, Anas Alshishani, Lina Elsalem, and Kamyar Afarinkia. 2023. "Niosomal Delivery of Celecoxib and Metformin for Targeted Breast Cancer Treatment" Cancers 15, no. 20: 5004. https://doi.org/10.3390/cancers15205004
APA StyleBasheer, H. A., Alhusban, M. A., Zaid Alkilani, A., Alshishani, A., Elsalem, L., & Afarinkia, K. (2023). Niosomal Delivery of Celecoxib and Metformin for Targeted Breast Cancer Treatment. Cancers, 15(20), 5004. https://doi.org/10.3390/cancers15205004






