Simple Summary
The microbiome emerges as a crucial participant in gastric cancer (GC), a severe cancer burden worldwide. Current understanding has primarily focused on the impact of H. pylori and Epstein-Barr virus (EBV) on GC. Still, recent evidence suggests that other influences like microbiota and viral and fungal infections also contribute. This review highlights established factors like H. pylori and EBV and other microbes like Lactobacillus, Streptococcus, hepatitis B virus, hepatitis C virus, and Candida albicans. Improved understanding of the gut microbiome may lead to advanced diagnosis and therapies for GC.
Abstract
While previous research has primarily focused on the impact of H. pylori and Epstein-Barr virus (EBV), emerging evidence suggests that other microbial influences, including viral and fungal infections, may also contribute to gastric cancer (GC) development. The intricate interactions between these microbes and the host’s immune response provide a more comprehensive understanding of gastric cancer pathogenesis, diagnosis, and treatment. The review highlights the roles of established players such as H. pylori and EBV and the potential impacts of gut bacteria, mainly Lactobacillus, Streptococcus, hepatitis B virus, hepatitis C virus, and fungi such as Candida albicans. Advanced sequencing technologies offer unprecedented insights into the complexities of the gastric microbiome, from microbial diversity to potential diagnostic applications. Furthermore, the review highlights the potential for advanced GC diagnosis and therapies through a better understanding of the gut microbiome.
1. Introduction
Over the past few decades, gastric cancer (GC) incidence and mortality rates have declined [1]. However, GC remained the fifth most common cancer worldwide and was estimated to cause 769,000 deaths in 2020 [2,3]. GC could be classified into two primary topographical subsites: cardia gastric cancer, which originated near the esophagogastric junction of the stomach, and non-cardia gastric cancer, which emerged from regions farther away from that junction [4].
Significant racial differences have been found in GC incidences. All non-white groups exhibited considerably higher non-cardia GC incidences than non-Hispanic white participants; the incidence was highest in Korean-American men above 50 [5]. Ethnic differences were also present in terms of GC prognosis. Asian patients had significantly longer median survival times than their Caucasian counterparts, especially for early-stage GC, i.e., Stage IA, IB, IA, and IIB [6].
GC is a complex disease that various factors, such as environmental, genetic, and infectious factors, can influence [7]. Previous researchers have extensively studied infective factors such as Helicobacter pylori (H. pylori) infection and Epstein-Barr virus (EBV) for GC.
While H. pylori and EBV have traditionally been the focus of GC research, recent advancements in high-throughput sequencing technology have provided new insights into the gut microbiome in this malignancy.
This literature review aims to give an update on the involvement of the gut microbiota in GC pathogenesis, progression, and treatment response beyond the scope of H. pylori and EBV. By examining recent studies on microbial dysbiosis and two hepatitis viruses associated with GC, this review also highlights the potential for improvement in GC treatment methods by harnessing the power of the diverse and complex gut microbiota.
2. Bacteria
2.1. H. pylori
Three components of the gastric microbiota, namely H. pylori, Lactobacillus, and Streptococcus, have been confirmed to significantly contribute to the progression of gastric cancer through various pathways. These bacteria are not only involved in infections and inflammation that primarily occur in the precancerous stage but also play a role in the immune microenvironment, angiogenesis, and tumor metastasis in cancer. Table 1 provides a comprehensive summary of the detailed effects of gastric microbiome biological processes in gastric tumorigenesis.

Table 1.
Biological processes affected by gastric microbiome.
GC and H. pylori have a well-established and complex relationship. Estimated to have colonized approximately 50% of the world’s population [33], H. pylori infection increases the risk of developing both intestinal-type and diffuse-type GC [34]. It is a Gram-negative bacterium that colonizes gastric mucosa [35,36], classified as a class I carcinogen by the World Health Organization, and responsible for approximately 89% of all GC [37]. H. pylori infection can lead to the development of GC through two main pathways: indirect effects mediated by inflammatory processes and direct effects on the molecular composition of gastric epithelial cells through the toxic action of virulence factors [38]. Infection with H. pylori can lead to chronic inflammation of the gastric mucosa, leading to histological changes, i.e., atrophic gastritis, small intestinal metaplasia, colonic metaplasia, dysplasia, and eventually gastric carcinoma [38,39]. Additionally, H. pylori’s virulence factors, such as cytotoxin-associated gene A (CagA) contained with the Cag pathogenicity island (cagPAI) and vacuolating cytotoxin A (VacA), can directly interact with and influence the molecular makeup of gastric epithelial cells, contributing to the initiation of gastric carcinogenesis [38]. Urease, another virulence factor for GC, could facilitate bacterial colonization and modulate host immune responses via mechanisms such as facilitated apoptosis by binding to the class II major histocompatibility complex (MHC) receptors. A high level of urease might induce histopathological alterations within the gastric mucosa and lead to further gastric carcinogenesis [40].
More than 50% of people worldwide have H. pylori infection, but the projected lifetime risk of GC is only 1–2 percent [41]. However, due to the large number of infected individuals, many H. pylori-infected patients still develop GC. H. pylori eradication, primarily using amoxicillin (AMPC) and clarithromycin (CAM) along with proton pump inhibitors (PPI) to suppress gastric acid secretion [42], and H. pylori treatment has been recognized as a crucial strategy for achieving multiple benefits. These benefits include reducing proliferation rates [43], and lowering the incidence of GC, especially the intestinal type [44], minimizing the risk of its development [45], especially for patients without precancerous lesions [46], and ultimately reducing mortality from GC [47].
2.2. Gastric Microbiota
H. pylori, as previously discussed as a risk factor for GC, can modulate the gastric environment [48], also known as the dysbiosis [49]. H. pylori neutralizes the gastric acidic environment via urease activity [24,25], reducing gastric acid secretion and promoting chronic inflammation, thereby increasing gastric pH. Gastric acid could be a robust barrier against the invasion of orally ingested microorganisms into the gastrointestinal tract. Elevated gastric pH resulting from H. pylori infection might facilitate the overgrowth of non- H. pylori microbes within the gastric ecological niche [50]. In particular, the colonization of H. pylori was linked to significantly reduced alpha and beta diversities in the gastric mucosa [51]. Moreover, the virulent genotypes of H. pylori are linked to variations in the microbial profile, suggesting that the bacterial virulence of H. pylori might influence the composition of the gastric microbiome [52].
There has been insufficient evidence to directly link the non-H. pylori bacterial community in the stomach to gastric carcinogenesis [49]. However, with technological advances, more researchers have found significant differences in the gastric microbiota in patients with GC compared to healthy individuals. Microbiota profiles gradually change from gastritis to a pre-neoplastic lesion to GC [53]. Bacterial load is significantly higher in GC patients, indicating that bacterial overgrowth in the stomach is a possible marker for GC [54]. In addition to richness, GC patients have increased diversity in their gastric microbiota [55]. At the phylum level, Firmicutes are significantly higher in GC patients [56]. In terms of genera, some genera of bacteria are enriched in GC patients, while others show a decrease. Table 2 summarizes recent studies conducted in the past six years, focusing on the difference in genera between healthy and GC patients.

Table 2.
Recent studies (2018–2023) of the gastric microbiota in GC and the non-GC stomach.
As shown in Table 1, several studies support that Lactobacillus, a type of lactic acid-producing bacteria, is significantly more abundant in both progressive histological phases of gastric carcinogenesis and patients diagnosed with GC [68]. An average level of Lactobacillus exerts a probiotic effect on humans, conferring several benefits to the human stomach. These benefits include excluding pathogens, maintaining the gastric barrier function, anti-inflammatory properties, and potential anti-cancer effects [69]. However, the detailed mechanism of how excess Lactobacillus stimulates GC carcinogenesis has remained unclear. Some research has proposed that instead of causing GC development, Lactobacillus overgrowth might change the composition of the stomach microbiome through interactions with other bacteria and potentially cause dysbiosis [14].
On the other hand, as summarized in Figure 1, other studies have suggested that an excessive rise in Lactobacillus could be detrimental because lactic acid, produced by these bacteria, could act as an energy source for tumor cells and promote tumor proliferation and survival [70]. Consequently, it induces glycolytic enzymes, leading to an enhanced supply of ATP. It might also promote inflammation, tumor angiogenesis, and metastasis, further contributing to the complex dynamics of GC development [71,72].
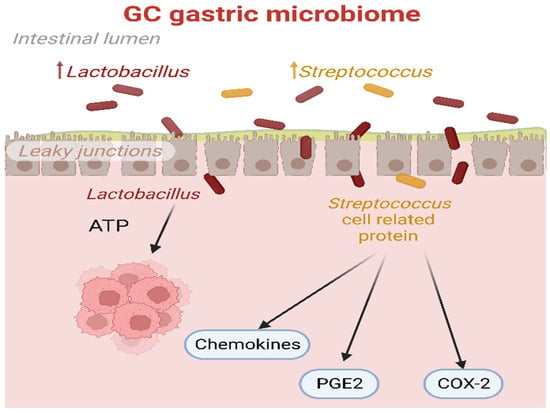
Figure 1.
The biological processes triggered by Lactobacillus and Streptococcus in the GC microbiome. Lactic acid produced by Lactobacillus could serve as a potential energy source for tumor cells. Additionally, proteins associated with Streptococcus cells could induce the release of chemokines, PGE2, and over-expression of COX-2, thereby promoting the angiogenesis process in cancer. COX-2: prostaglandin-endoperoxide synthase 2; PGE2: prostaglandin E2.
The oral microbiota affect the gastric microbiota, and this influence occurs in a bidirectional manner [73]. Due to the weakened immunity of GC patients, GC individuals exhibit a more complex oral bacterial profile compared to the average control population. Oral microbiota might even be a future diagnostic tool [74]. A Korean case-control study revealed that the enriched bacterial genera Streptococcus, Abiotrophia, and Leuconostoc and decreased Prevotella, Haemophilus, and Neisseria increased the risk of cancer [75]. This suggested the potential to make use of oral microbiota for the diagnosis of cancer, including GC. In fact, with a cohort of 293 patients in China, a study has already revealed that the salivary microbiota could accurately identify GC among patients with non-malignant gastric diseases such as superficial gastritis and atrophic gastritis [76]. Another Chinese study of 50 participants revealed a highly sensitive scoring system for oral microbiota screening GC [77]. All of these studies concluded that a strong potential exists for oral microbiota as a diagnostic tool.
However, the heterogeneity of oral bacteria in different places must be considered when formulating a GC diagnosis tool based on oral microbiota. GC patients in Singapore and Malaysia had higher abundances of oral bacteria such as Leptotrichia, Fusobacterium, Haemophilus, Veillonella, and Campylobacter than other regions [78].
Another possible reason behind altered gastric microbiota might be GC treatment methods. Subtotal gastrectomy, a standard surgical treatment for early distal GC, also led to dramatic changes in gastric microbiota. After surgery, the gastric microbiota showed phylum-level shifts, with decreased Proteobacteria and Actinobacteria and increased Firmicutes and Bacteroidetes. Specific genera, such as Ralstonia and Helicobacter, were abundant in GC patients, while Streptococcus and Prevotella dominated after surgery. Notably, predicted gene functions also varied, with denitrification and nitrosation genes prevalent before surgery and increased bile salt hydrolase and NO and N2O reductases after surgery, indicating metabolic changes in response to the surgical intervention [79].
Recently, contrasting findings about the prevalence of various bacteria with or without GC have been shown. A Chinese study discovered no statistical difference in the abundance and prevalence of H. pylori between GC and non-GC individuals [80]. In contrast, two studies from Taiwan and Portugal revealed a decrease in H. pylori for GC patients, as shown in Table 2. Moreover, in the United States, a study using shotgun metagenomic sequencing demonstrated a significant decrease in the abundance of Streptococcus in the gastric microbiota of individuals with GC compared to those with chronic gastritis. In contrast, several other studies employing 16S rRNA gene sequencing reported a noteworthy increase in Lactobacillus and Streptococcus in GC relative to chronic gastritis [81]. Contrasting findings have also been shown in terms of microbial diversity. Indeed, some studies indicate a lack of difference between cancer patients and those with functional dyspepsia or chronic gastritis. However, contrasting reports pointed to increased microbial diversity or decreased diversity in cancer patients compared to the mentioned groups [82]. These varying results highlighted the complexity and ongoing exploration of the relationship between gastric microbiota and GC. A very recent study in 2023 by Nikitina et al. reported significant differences in the relative abundance of the gastric tissue microbiome when comparing 16S rRNA gene transcript and 16S rRNA gene levels in all study groups, including control, tumor, and tumor-adjacent samples. This finding suggested that the molecular analysis method might influence the results and findings. The two methods, 16S rRNA gene transcript analysis and 16S rRNA gene analysis, might capture different aspects of the microbial community, leading to variations in the identified taxa and their relative abundance [83]. Despite the controversial impact of gut microbiota on GC development [84], the gastric microbiome might be a future biomarker for GC diagnosis after more extensive research. The potential mechanism of the gastric microbiome contributing to GC development has remained unclear.
3. Viruses
3.1. EBV
The connection between GC and EBV is well established. EBV, a worldwide oncogenic g-type herpes virus, has been detected in a subset of GC cases (EBVaGC) [85,86], particularly more frequent among tubular adenocarcinomas and medullary carcinomas [87]. It has been suggested that EBV infects both B and epithelial cells [88], causing epithelial cancer, e.g., GC [89]. EBVaGC represents approximately 10% of all GC worldwide [90]. EBV microRNAs are often highly expressed even during the latent infection period. Specifically, the overexpression of EBV-miR-BART5-3p has stimulated the proliferation of GC cells in both laboratory settings and in living organisms [91,92]. BART5-3p targets the tumor suppressor gene TP53 within its 3′-untranslated region, suppressing CDKN1A, BAX, and FAS expression. This accelerates the progression of the cell cycle and inhibited cellular apoptosis. Additionally, BART5-3p contributes to resistance against chemotherapeutic agents and obstructs the increase in p53 induced by ionizing irradiation [92]. This heightened expression of EBV miRNAs generally translates into a threefold increase in the mortality risk associated with GC [93]. While some studies have suggested that EBVaGC patients had a better survival rate than EBV-negative patients [94], another study found that the overall survival of Stage I–III EBVaGC was similar to non-EBVaGC [95]. EBV-related GCs had significant differences in their phenotypic and clinical attributes compared to EBV-negative GC, including the absence of the cyclin-dependent kinase (CDK) inhibitor p16INK4A (encoded by CDKN2A) expression, the presence of unaltered p53, observable driver mutations in the PI3K catalytic subunit-α (PIK3CA) [96], higher PD-L1 [97,98,99] and PD-L2 expression [100], extreme viral and host DNA hypermethylation [101,102,103], and the loss of p16 expression [104]. Transitioning to advanced EBV-associated GC, their histological features differed and had a notably higher rate of aberrant p53 immunostaining patterns than conventional EBV-associated GC [105]. Table 3 summarizes EBV’s mechanism in promoting GC, alongside other viruses.
3.2. Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV)
HBV and HCV, which are both transmitted parenterally [106], have been considered potential risk factors for gastric cancer (GC) [107], not solely for hepatocellular carcinoma [108].
3.2.1. Infection of HBV and HCV
From the World Health Organization (WHO), in 2019, approximately 296 million individuals lived with chronic HBV infection worldwide, with an estimated 1.5 million new infections occurring annually [109]. HBV is primarily transmitted through vertical transmission, sexual contact, and blood [110]. However, the main transmission routes vary depending on geographical area. For instance, vertical transmission plays a significant role in Asia, whereas horizontal transmission from child to child is the most frequent in Africa [111]. With the highly effective vaccine against HBV that has been available since 1982, the prevalence of HBV in developed countries has significantly decreased. However, in Sub-Saharan Africa and East Asia, it was estimated that 5–10% of adults still suffer from chronic HBV infection [112]. HBV has been widely known to play a vital role in developing hepatocellular carcinoma [113,114]. Some recent meta-analyses have shown that HBV infection could also significantly increase people’s risk of developing GC [115,116], but the data on such an association were still limited and not robust. A Kailuan cohort study found a nonsignificant association between HBV infection and GC [117]. Another study further suggested that HBV infection alone was not a strong etiology for GC, but its coexistence with H. pylori would affect the GC progression significantly [118].
Currently, unlike HBV, no HCV vaccine exists. HCV infection remains a significant global health issue [119]. According to the WHO, approximately 58 million individuals worldwide live with chronic HCV infection, and approximately 1.5 million new infections are reported yearly [120]. HCV is typically transmitted through substantial or recurrent contact with blood, such as transfusion [121]. It was found to be primarily associated with liver-related issues like cirrhosis and could also lead to complications beyond the liver, such as diabetes mellitus [122]. As for its association with GC, current research has not established a direct link between HCV infection and the development of GC. However, it is essential to note that chronic HCV, similar to HBV, has been gradually found to be a possible risk factor for GC [108]. The likelihood of developing GC among patients infected with HCV was observed to be 88% higher compared to individuals without HCV infection (p = 0.001) [123].
3.2.2. Molecular Pathogenesis of HBV-Related and HCV-Related GC
The detailed underlying molecular pathogenesis of HBV-related and HCV-related GC remains unknown [124]. However, many previous studies have strongly supported chronic HBV and HCV as an etiology of liver cirrhosis [125,126,127], and liver cirrhosis was recognized as a risk factor of GC [128,129]. This indicates a possible indirect relationship between HBV, HCV, and GC.
Specifically for HBV, as illustrated in Figure 2, upon infection, HBV replicates in the hepatocytes. It does not stay only in hepatocytes but also in extrahepatic tissues [108], including gastric mucosal epithelial cells. HBV DNA has been detected in 12.4% of GC tissues [130]. Based on gastric mucosa epithelial cells also infected with HBV, a study suggests that HBV infection may increase the risk of GC through an analogous mechanism to HBV-related hepatocellular cancer [131]. HBV may cause permanent DNA alteration in the gastric cells by changing the methylation status of cellular DNA, known as a “subthreshold neoplastic state” [132,133]. This, in turn, induces local inflammation in the stomach. Solid evidence supports inflammation as a pivotal factor in etiopathogenesis and ongoing tumor development [134].
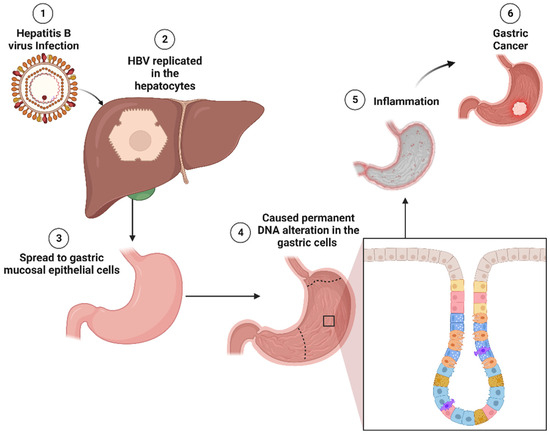
Figure 2.
Molecular pathogenesis of HBV-induced GC. HBV replicates primarily in hepatocytes and can spread to gastric mucosal epithelial cells. In gastric cells, HBV has the potential to induce permanent DNA alterations by modifying the methylation status of cellular DNA. These alterations may trigger inflammation in the gastric tissue, ultimately contributing to gastric tumorigenesis. HBV: hepatitis B virus; DNA: deoxyribonucleic acid.
In addition, the HBV genome encodes seven proteins, including the X protein (HBx) [135]. HBx is widely established to be a critical factor in the development of numerous types of cancer. HBx is significantly expressed in GC cells but not normal cells [136]. The binding of HBx to various cellular proteins, including hepatitis B virus X-interacting protein (HBXIP) [137], could be attributed to the elevated expression of HBXIP in GC tissues compared to paracancerous tissues [138]. HBXIP regulates cell proliferation and migration through activating NF-κB and IL-8 [139], apoptosis, and cell division and is overexpressed in several cancers [140]. NF-κB promotes survival genes within cancer cells and inflammation-promoting genes in tumor microenvironment components [141]. The expression of IL-8 in GC cells indicates the extent of angiogenesis in the tumor [142]. HBXIP promotes cancer progression by causing a regulatory cascade that influences signaling pathways and the miRNA [143]. HBXIP enhances glucose uptake, particularly in GC, stimulating glycolysis and mitochondrial respiration in GC cells [140]. A study reveals that HBXIP also plays an oncogenic role in GC via METTL3-mediated MYC mRNA m6A modification [138]. Moreover, the anti-HBc antibodies, the most sensitive and reliable seromarker for previous HBV exposure, are expressed highly in GC subjects compared to the control [136,144], supporting a possible association between GC and HBV.
However, immunohistochemical findings have implied that the HBx protein and HBcAg (or HBc protein or HBV core protein) expression are restricted to the cytoplasm instead of being found in the nucleolus. Furthermore, the low detection rate of cccDNA suggests that HBV may exhibit diminished replication activity in tissues outside the liver. These observations provide insights into the potential extrahepatic involvement in the molecular pathogenesis of HBV-related GC. Additional functional analyses and research are necessary to elucidate the detailed mechanisms through which HBV infection and protein expression may influence GC development [136].
The gut microbiota is a significant component of a huge human symbiotic community with trillions of microorganisms [145,146]. Such microbiota is essential in many physiological functions, such as maintaining the intestinal mucosal barrier and immune protection [147]. Notably, the gastric microbiome has been suggested to be a risk factor for GC [71]. The microbial diversity decreases constantly throughout GC progression, and cancer patients and non-cancer individuals may have a significantly different microbial composition [66]. Research employing advanced 16S rRNA gene sequencing techniques revealed a reduced bacterial diversity in individuals suffering from HCV infection. These findings demonstrated a decline in the presence of Clostridiales while observing an increase in Streptococcus and Lactobacillus species [148]. Such microbiome dysbiosis resulting from HCV infection may lead to an increased risk for GC.
3.2.3. Host Immunity of HBV-Related and HCV-Related GC
The host immune system prevents HBV and HCV infection with innate and adaptive immunity [149]. Innate immunity acts as a first line of defense and is typically activated quickly upon exposure to a foreign pathogen [150], including HBV and HCV. Both HBV and HCV are known to activate inflammasomes such as the NLRP3 inflammasome [151,152].
One innate pathway against HBV involves toll-like receptors (TLR3, 7, 8, and 9) in endosomal membranes of specialized cell types, e.g., plasmacytoid dendritic cells, which recognize HBV, as shown in Figure 3A. They then recruit proteins such as MYD88 to activate protein kinases, including IKK-related kinases [153]. A previous GC mouse model also supported that lacking the essential TLR signaling adaptor MYD88 could suppress tumor formation [154]. Nuclear factor kappa B subunit 1 (NF-κB) inhibitor (I-B) is then phosphorylated by the IKK complex, and this causes I-B to be targeted for destruction by the ubiquitin-proteasome pathway, allowing NF-κB to enter the nucleus and activate a wide range of genes involved in immunological and inflammatory responses. IRF3 or IRF7 is phosphorylated by TBK1 and IKK, which causes dimerization and nuclear translocation. Interferons are produced and released to attach to their receptors on HBV-infected cells and nearby non-infected cells by the nuclear IRFs, NF-κB, and other transcription factors, forming an enhanceosome complex. Interferon-stimulated genes (ISGs), which prevent HBV replication, are induced when interferon receptors are engaged by activating the activator of transcription (STAT) signaling pathway and the Janus kinase (JAK)-signal transducer [153]. Researchers have validated the ability of TLRs to inhibit HBV replication through intravenous injection of the panel of ligands specific for TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 and analyzing HBV DNA via Southern blot analysis [155].
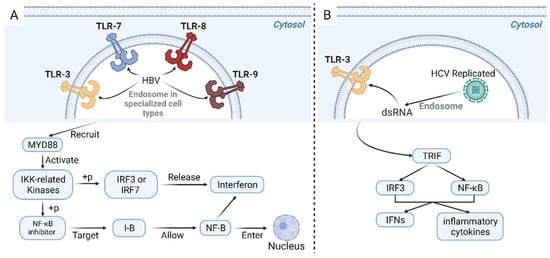
Figure 3.
Innate pathway in response to HBV infection (A). TLR3, 7, 8, and 9 are receptors located on endosomal membranes of specific cell types, responsible for the recognition of the presence of HBV. These receptors recruit proteins such as MYD88, activating protein kinases, including the IKK-related kinases. The IKK complex phosphorylates the NF-κB inhibitor I-B, targeting it for degradation through the ubiquitin-proteasome pathway. This degradation allows NF-κB to enter the nucleus and activate various genes involved in immunological and inflammatory responses. Additionally, phosphorylation of IRF3 or IRF7 by TBK1 and IKK leads to their dimerization and translocation into the nucleus. This event triggers the production and release of interferons, which bind to their receptors on both HBV-infected cells and nearby non-infected cells, forming an enhanceosome complex involving the nuclear IRFs, NF-κB, and other transcription factors. Innate pathway in response to HCV infection (B). The virus replicates and accumulates dsRNA intermediates during HCV infection. These dsRNA intermediates are recognized by TLR3 and subsequently initiate a signaling cascade. This cascade activates the TRIF. Consequently, the transcription factors IRF3 and NF-κB are activated, promoting the synthesis of IFNs and inflammatory cytokines. TLR: toll-like receptors; HBV: hepatitis B virus; MYD88: myeloid differentiation primary response 88; IKK: IκB kinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; IRF: interferon regulatory factor; TBK1: TANK-binding kinase 1; dsRNA: double-stranded RNA viruses; TRIF: TIR-domain-containing adapter-inducing interferon-β; IFNs: interferons.
HCV infection has been primarily detected by cytosolic pattern recognition receptors and endosome membrane receptor toll-like receptor 3, the previously mentioned receptor, which promote the synthesis of interferons (IFNs) and inflammatory cytokines through different signaling pathways [156,157,158]. Illustrated in Figure 3B, HCV replicates and accumulated double-stranded RNA (dsRNA) intermediates, which are identified and then activated TLR3. This process subsequently leads to the activation of the toll/interleukin-1 receptor-domain-containing adapter-inducing interferon-β (TRIF). Consequently, the transcription factors IRF3 and NF-κB are activated, promoting the synthesis of interferons (IFNs) and inflammatory cytokines [157].
However, HBV and HCV affect some innate immunity signaling pathways by interfering with various components of the innate immune response. HBV impacts innate immunity signaling by being invisible to pattern recognition receptors [159]. The type 1 interferon response, an effective anti-viral response, typically activates interferon-stimulated genes, which stimulate the effector function of immune cells (e.g., T cells) for eliminating pathogens [160]. A study found that genes encoding critical factors in the type 1 interferon response, such as IFNB, were absent in early HBV-infected organisms [161]. HCV NS3/4A protease effectively cleave and deactivate the mitochondrial anti-viral signaling protein (MAVS) and the toll-interleukin-1 receptor-domain-containing adaptor-inducing interferon-β (TRIF) involved in the detection pathways that respond to HCV pathogen-associated molecular patterns (PAMPs) to stimulate interferons (IFNs), which aid in escaping from the innate immune response [162].
3.2.4. Diagnosis and Treatment of HBV-Related and HCV-Related GC
The prognosis of HBV-related GC patients tends to be poorer than other GC patients. Through Cox proportional hazards regression, a recent study revealed that chronic HBV infection led to poor prognosis in the GC development [163]. HBV-related GC patients were more likely to suffer liver metastasis after GC surgery and poorer disease-free survival and 5-year overall survival rates than GC patients without HBV. Hence, HBV might be an adverse biomarker in predicting GC patient survival rates [163].
Monoclonal antibodies in immune checkpoint blockade therapy have targeted mainly PD-1/PD-L1 proteins [164]. However, patients with HBV-related GC exhibit a reduced expression of programmed cell death ligand 1 (PD-L1), suggesting a decreased possibility of successful immune checkpoint blockade therapy [165]. Immune checkpoint blockade therapy has become a modern standard of care for advanced GC patients [166] and profoundly transformed the landscape of cancer treatment approaches [167,168]. Also, the high expression of HBXIP in HBV-related GC patients has led to higher drug resistance in tumor cells [143], which might bring primary resistance to the immunotherapy [169], decreasing the effectiveness of immune checkpoint inhibitors for HBXIP+ GC patients [170].
The prognosis of HCV-related GC has yet to be studied extensively. A population-based retrospective cohort study in Taiwan showed the lowest GC incidence and mortality rates in HCV-uninfected individuals. At the same time, treatment against HCV did not lower the GC risk for patients. This indicated that HCV antiviral treatment might not effectively reduce GC incidences [171].

Table 3.
Biological processes affected by viruses.
Table 3.
Biological processes affected by viruses.
| Biological Process | Virus Type | Mechanisms |
|---|---|---|
| Infection and Entry Process | EBV | EBV-infected naive B cells, forming proliferating latently infected lymphoblasts expressing latent proteins. In the germinal center, these cells exhibit a confined protein profile, expressing either EBNA1 or remaining in a latent state. A small subset might undergo lytic reactivation under signals at any time, releasing infectious viruses for spread or reinfection [172] |
| HBV and HCV | Infected hepatocytes specifically [108,173]; HBV first bound to hepatocytes through low-affinity binding to heparin sulfate proteoglycans and high-affinity binding to the NTCP receptor, then entered through endocytosis. HCV interacts with 14 or more host cell factors for efficient infection [174] | |
| Carcinogenic Factor | EBV | The latent membrane protein 2A (LMP2A) participated in cellular biosynthesis and influenced subsequent genes and cellular behaviors by engaging the AKT and AMPK signaling pathways [175] |
| HBV | HBXIP stimulated both cellular growth, migration, and invasion in laboratory settings and living organisms [176] | |
| HCV | HCV nonstructural protein genes contributed to fibrosis development, which might indirectly promote carcinogenesis, by triggering the synthesis of transforming growth factor beta and activating hepatic stellate cells; HCV core protein might play a role in promoting the development of cancer [177] | |
| Inflammation | EBV | Latent EBV-positive B cells might lead inflammation via upregulated cytokines in EBV-transformed B cells such as TNF-α, TNF-β, and G-CSF [178]. Non-resolving inflammation was conducive to forming a tumor microenvironment for GC tumor initiation and development [134] |
| HBV | HBx stimulated toll-like receptor (TLR) and nuclear factor-kappa B (NF-κB) signaling pathways, leading to increased pro-inflammatory cytokine expression; and it triggered NLRP3 inflammasome activation, hastening the release of IL-1β and IL-18 [179] | |
| HCV | Continued HCV replication within hepatocytes resulted in unregulated inflammation and the production of chemokines [180]. Non-resolving inflammation induced by different viruses was conducive to forming a tumor microenvironment for GC tumor initiation and development [134] | |
| DNA Damage | EBV | Increased DNA hypermethylation of PD-L1/2 [181] |
| HBV | Within HBV-infected cell nuclei, the transformation of genomic viral DNA into transcriptionally active episomal DNA (cccDNA) or transcription of viral mRNAs from cccDNA relied on cellular proteins’ enzymatic activities. HBV DNA integration into host chromosomal DNA and the accumulation of mutations in host DNA potentially triggered carcinogenesis [182] | |
| HCV | The chronic inflammation caused by hepatitis C virus (HCV) might trigger oxidative stress, potentially hindering the repair of DNA damage. This could increase the vulnerability of cells to spontaneous or mutagen-induced changes [183] | |
| Immune Evasion and Immune System | EBV | Evaded immune responses by utilizing lytic gene products, such as BGLF5, which diminished the levels of innate immune EBV-sensing TLR2 and the lipid antigen-presenting CD1d molecule [184] |
| HBV | Did not induce significant innate immune activation via pro-inflammatory cytokines and interferons (IFN) [185] | |
| HCV | To evade the initiation of interferon regulatory factor 3 (IRF3) and subsequent interferon (IFN) production triggered by RIG-I/MDA5, the HCV protease NS3/4a has developed a dual role: processing the HCV polyprotein and cleaving the vital adapter protein MAVS, essential for relaying RIG-I/MDA5-initiated signals, as well as TRIF, which mediates downstream signaling from toll-like receptor (TLR)3 that recognizes double-stranded RNA within endosomes. Consequently, this obstructs the expression of type I and III IFN [185] | |
| Angiogenesis | EBV | The components produced by EBV could encourage the development of tumor angiogenesis through the PI3K/AKT signaling pathway [186] |
| HBV | HBx could induce an angiogenic response by directly stimulating angiogenesis alone; and it stimulated angiogenesis through both the transcriptional activation and stabilization of HIF-1α [187] | |
| HCV | Caused higher serum concentrations of the angiogenic proteins placenta growth factor (PlGF) and Ang-2 [187] | |
| Cellular Proliferation | EBV | Expressed viral oncogenes that facilitate cell growth and hinder the apoptotic response, leading to uncontrolled cell proliferation [188] |
| HBV | Induced cellular proliferation in part via HBx-induced miRNA-21 expression [189,190] | |
| HCV | Inhibited cell proliferation via overexpression of HCV E2 [191] | |
| Metastasis | EBV | The elevated expression of Indian Hedgehog gene increased metastatic potential via angiogenesis and Snail protein expression [192] |
| HBV | Hepatitis B surface antigen (HBsAg) was associated with an elevated risk of distant metastasis in cancer patients [193] | |
| HCV | Proteins encoded by HCV could directly trigger cellular functions that promote metastasis [194] |
3.3. Human Papillomavirus (HPV)
HPV is a sexually transmitted virus with a well-established role in cervical cancer [195]. While specific high-risk subtypes, such as HPV type 16 [196], have been extensively studied for their role in cervical cancer, their potential involvement in other cancers, including GC, is gaining attention.
3.3.1. HPV Infection
Repetitive and persistent HPV infection was assumed to lead to precursor lesions like dysplasia or adenocarcinoma in situ, eventually resulting in malignancy [197]. Under such an assumption, it was suggested that HPV may enter the anus and colorectum through transmission from anogenital sites [197]. Alternatively, oral entry is possible, leading to downward infection from the mouth to the esophagus and ultimately to the stomach [197].
3.3.2. HPV Gastric Oncogenesis
HPV has been believed to contribute to neoplastic changes in the gastric mucosa, potentially resulting in the advancement of GC [198]. HPV increases gastrointestinal cancer risk, particularly of squamous cell origin [199]. HPV oncoproteins, specifically E6 and E7, disrupt cellular pathways regarding cell cycle regulation, apoptosis, and cell polarity control networks [200]. E6 affects cell cycle regulation and stimulates excessive cellular proliferation by exerting its inhibitory effect on p53 through different pathways. One mechanism involves the disruption of E6AP, a ubiquitin ligase that facilitates proteasome-dependent degradation. E6 forms a complex with E6AP and p53 in this process to promote p53 degradation [201]. Such an HPV oncogenesis mechanism might lead to GC tumor initiation and progression.
3.3.3. Clinical Significance of HPV in GC
The clinical significance of HPV in gastric cancer remains an active area of investigation. Studies have shown conflicting results regarding HPV positivity in gastric cancer patients. According to a meta-analysis, the overall prevalence of HPV in gastric cancer (GC) was 23.6%. Interestingly, there were significant variations in HPV status between Chinese and non-Chinese studies. Notably, HPV positivity was 1.43-times higher in Chinese GC patients than in non-Chinese patients, indicating a potential association between HPV infection and GC development in the Chinese population [202]. However, another study did not detect HPV DNA in the gastric mucosa tissue of GC patients in China [203]. Further large-scale studies and rigorous analyses are required to determine any distinct clinicopathological features and response patterns to treatment associated with HPV-positive gastric tumors to improve clinical practices for better patient outcomes.
3.4. Other Types of Viruses
Apart from HBV, HCV, HPV, and the well-studied EBV, many other oncoviruses are still suspected to be a risk for GC. A meta-analysis in 2020 revealed that patients with GC exhibited a notably increased viral load of human cytomegalovirus (HCMV) [204], suggesting a possibility that it might also play a role in GC progression. The role of HCMV in oncogenesis has remained a subject of debate since most infected individuals do not develop cancer, and the virus alone is generally not potent enough to initiate tumor formation [205].
4. Fungi
Fungi have played a notable role in gastric carcinogenesis. These eukaryotic microorganisms have been widely distributed globally, with two hundred orders and a dozen phyla. Particularly, a few hundred fungal species that belonged to a few lineages have caused most related infections and deaths [206].
Intriguingly, despite the acidic nature of the stomach, many species of fungi have been identified in the healthy human gut. However, only a limited number of fungi can thrive and establish colonization within the gut [207]. Candida and Phialemonium were the main fungi among them [208]. Among fungal species in a study using endoscopic and mycological examinations, Candida albicans was isolated most frequently in normal subjects at 48%, and Candida tropicalis came second at 29.3% [209]. Many recent studies have shown that fungi infection, especially Candida albicans, a member of the microbiota that could cause infection under certain circumstances, increases the host risk of GC [210,211,212].
Most pathogenic fungi are promptly identified and eliminated by innate immune cells with diverse pattern-recognition receptors. Typically, these fungi remain harmless unless there is a disturbance in the immune equilibrium [213]. Factors for such disturbances include deficiencies and polymorphisms in pattern recognition receptor systems [214], systemic immunosuppression due to conditions like acquired immune deficiency syndrome (AIDS) [215], leukemia [216], diabetes mellitus [217], solid organ transplantation [218], and long-term mechanical ventilation [219]. Significant gastric fungal imbalance was also found in GC. Candida and Alternaria were elevated in GC patients at the genus level, while Saitozyma and Thermomyces (p = 0.009) ere decreased. In particular, Candida albicans were significantly increased in GC [211].
It is crucial to acknowledge the significant morbidity and mortality risks associated with opportunistic Candida infections in GC patients [220]. While fungal microorganism infections in the context of GC are relatively rare, when they do occur, they can have severe consequences. Therefore, it is imperative to recognize the potential impact of fungal microorganisms, such as Candida, and emphasize the importance of studying their role in GC. By understanding their contribution to disease progression, we can advance our knowledge and potentially identify new therapeutic approaches to improve patient outcomes.
5. Conclusion and Future Perspectives
The rapid advancements in understanding the microbiome’s role in GC have opened up new avenues of research and potential therapeutic interventions. While most existing research has been directed towards established factors such as H. pylori and EBV, emerging evidence suggested that a broader spectrum of microbial factors, including viral and fungal infections, could contribute to gastric carcinogenesis. This literature review delved into the intricate interplay between the gut microbiome and GC, focusing on familiar and lesser-explored microbes.
H. pylori has remained a significant player in gastric carcinogenesis. This Gram-negative bacterium was intricately linked with chronic inflammation and molecular alterations that drive oncogenic processes. While extensive research has unveiled its association with DNA damage, oncogene activation, and tumor suppressor inhibition, recent high-throughput sequencing techniques allowed us to delve deeper into its influence on the gastric ecosystem. The modulation of the gastric environment through H. pylori’s activities could pave the way for the overgrowth of non-H. pylori microbes within the stomach, altering the microbial balance and potentially influencing cancer development.
Furthermore, EBV has long been associated with GC, particularly the unique subset known as EBVaGC. This oncovirus exerts its influence through complex molecular mechanisms, including miRNA-initiated methylation pathways. Recent immunotherapy advances have provided avenues for targeted treatment in this specific subtype. However, the relationship between EBV and GC is far from one-dimensional, with differing clinical and phenotypic attributes highlighting the need for nuanced investigations.
Including HBV and HCV in the discussion further broadens our understanding of viral influences on GC. Chronic infections with HBV and HCV have traditionally been associated with hepatocellular carcinoma, but their potential role in gastric carcinogenesis has also garnered attention. While the precise molecular mechanisms linking these viruses to GC remain unclear, the interaction between these viruses and the host immune response could be crucial in shaping disease outcomes.
Interestingly, the less-explored territory of fungal infections in GC is also gaining traction. Candida albicans, a common fungus in the human digestive tract, has been implicated in gastric carcinogenesis. Research has suggested that an overgrowth of Candida albicans in the stomach could potentially impact the development of GC. Various factors could influence this fungal imbalance, including systemic immunosuppression and other underlying health conditions.
Moving forward, several intriguing areas could be explored. The emergence of high-throughput sequencing technologies offers unprecedented opportunities to comprehensively profile the gastric microbiome in health and disease. Understanding the dynamics of microbial diversity, abundance, and interplay could shed light on the intricate relationship between microbes and GC. The potential of the gut microbiome as a diagnostic tool is particularly promising, with studies hinting at its role in distinguishing healthy individuals from those with precancerous lesions or malignancies.
Moreover, therapeutic avenues could emerge as we uncover the molecular intricacies of viral and fungal interactions. Expanding the scope of immunotherapies to encompass a broader spectrum of infections and focusing on modulating the gut microbiome could hold promise for more effective treatment strategies. Additionally, unraveling the role of microbes in influencing treatment responses, such as immune checkpoint blockade therapies, could pave the way for personalized approaches to GC management.
In conclusion, the landscape of microbial influences on GC is evolving rapidly. Beyond the established role of H. pylori and EBV, viruses such as HBV and HCV and fungal species like Candida albicans are emerging as potential contributors to gastric carcinogenesis. However, these viruses’ studies are still in their infancy, and the detailed molecular mechanism remains unclear. More deep mechanism studies on the interaction between these oncoviruses and GC cells are required to shed light on the treatment methods targeting the microbiome.
Author Contributions
W.K. offered directions on this manuscript. W.S.S., F.X. and B.C. reviewed the literature and drafted this manuscript. J.Y., K.W.L., G.M.K.T. and K.F.T. reviewed the manuscript and gave comments. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (NSFC) (2022, No. 82272990), Health and Medical Research Fund (HMRF, 08190586), CUHK direct research grant (2022.001, 2020.004), and Cheng Yue Pui Charity Foundation.
Acknowledgments
We also appreciate the technical support from Core Utilities of Cancer Genomics and Pathobiology of the Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, M.C.; Huang, J.; Chan, P.S.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global incidence and mortality of gastric cancer, 1980–2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.O.; Laversanne, M. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Ning, F.-L.; Lyu, J.; Pei, J.-P.; Gu, W.-J.; Zhang, N.-N.; Cao, S.-Y.; Zeng, Y.-J.; Abe, M.; Nishiyama, K.; Zhang, C.-D. The burden and trend of gastric cancer and possible risk factors in five Asian countries from 1990 to 2019. Sci. Rep. 2022, 12, 5980. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol. Rev./Przegląd Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Shah, S.C.; McKinley, M.; Gupta, S.; Peek, R.M., Jr.; Martinez, M.E.; Gomez, S.L. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology 2020, 159, 1705–1714.e1702. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Bertagnolli, M.M. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: Results from the Surveillance Epidemiology and End Results (SEER) database. Ann. Surg. Oncol. 2015, 22, 2965–2971. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Y.; Xia, S.; Feng, L.; Zhou, W.; Zhang, M.; Dong, R.; Tian, D.; Liu, M.; Liao, J. Epstein-Barr virus is associated with gastric cancer precursor: Atrophic gastritis. Int. J. Med. Sci. 2022, 19, 924. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Corcoran, B.; Stanton, C.; Fitzgerald, G.; Ross, R. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Zi, M.; Zhang, Y.; Hu, C.; Zhang, S.; Chen, J.; Yuan, L.; Cheng, X. A literature review on the potential clinical implications of streptococci in gastric cancer. Front. Microbiol. 2022, 13, 1010465. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef]
- McClain, M.S.; Beckett, A.C.; Cover, T.L. Helicobacter pylori vacuolating toxin and gastric cancer. Toxins 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-P.; Liu, J.-X.; Lu, L.-L.; Wang, L.-L.; Xu, L.; Guo, Z.-H.; Dong, Q.-J. Overgrowth of Lactobacillus in gastric cancer. World J. Gastrointest. Oncol. 2021, 13, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Yang, J.; Jin, M.; Zhou, B.; Shi, L.; Zhao, L.; Zhang, J.; Lin, Z.; Ren, J.; Liu, L. Fecal Streptococcus alteration is associated with gastric cancer occurrence and liver metastasis. MBio 2021, 12, e02994-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022, 10, e635. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Zhou, L. Detection of gastritis-associated pathogens by culturing of gastric juice and mucosa. Int. J. Clin. Exp. Pathol. 2018, 11, 2214. [Google Scholar]
- Shi, Y.; Wang, P.; Guo, Y.; Liang, X.; Li, Y.; Ding, S. Helicobacter pylori-induced DNA damage is a potential driver for human gastric cancer AGS cells. DNA Cell Biol. 2019, 38, 272–280. [Google Scholar] [CrossRef]
- Bernard, J.N.; Chinnaiyan, V.; Almeda, J.; Catala-Valentin, A.; Andl, C.D. Lactobacillus sp. Facilitate the Repair of DNA Damage Caused by Bile-Induced Reactive Oxygen Species in Experimental Models of Gastroesophageal Reflux Disease. Antioxidants 2023, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.; Zhao, H.; Gong, S.-G.; Lévesque, C.M. A DNA-damage inducible gene promotes the formation of antibiotic persisters in response to the quorum sensing signaling peptide in Streptococcus mutans. Genes 2022, 13, 1434. [Google Scholar] [CrossRef]
- Lina, T.T.; Alzahrani, S.; Gonzalez, J.; Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Immune evasion strategies used by Helicobacter pylori. World J. Gastroenterol. WJG 2014, 20, 12753. [Google Scholar] [CrossRef]
- Tomkinson, S.; Triscott, C.; Schenk, E.; Foey, A. The Potential of Probiotics as Ingestible Adjuvants and Immune Modulators for Antiviral Immunity and Management of SARS-CoV-2 Infection and COVID-19. Pathogens 2023, 12, 928. [Google Scholar] [CrossRef]
- Kolar, S.L.; Kyme, P.; Tseng, C.W.; Soliman, A.; Kaplan, A.; Liang, J.; Nizet, V.; Jiang, D.; Murali, R.; Arditi, M. Group B Streptococcus evades host immunity by degrading hyaluronan. Cell Host Microbe 2015, 18, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, N.; Chai, N.; Liu, X.; Jiang, H.; Wu, Q.; Li, Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 2016, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Mager, D. Bacteria and cancer: Cause, coincidence or cure? A review. J. Transl. Med. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Wang, X.; Wang, Y.; Li, R.; Li, G.; He, Y.; Liu, S.; Luo, Y.; Wang, L.; Lei, Z. Helicobacter pylori promotes gastric cancer progression by upregulating semaphorin 5A expression via ERK/MMP9 signaling. Mol. Ther.-Oncolytics 2021, 22, 256–264. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, S.; Shi, J.; Xie, Q.; Li, N.; Guan, J.; Evivie, S.E.; Liu, F.; Li, B.; Huo, G. Effects of Lactobacillus acidophilus KLDS1. 0901 on proliferation and apoptosis of colon cancer cells. Front. Microbiol. 2022, 12, 788040. [Google Scholar] [CrossRef]
- Kumar, R.; Taylor, J.C.; Jain, A.; Jung, S.Y.; Garza, V.; Xu, Y. Modulation of the extracellular matrix by Streptococcus gallolyticus subsp. gallolyticus and importance in cell proliferation. PLoS Pathog. 2022, 18, e1010894. [Google Scholar] [CrossRef]
- Liu, L.-P.; Sheng, X.-P.; Shuai, T.-K.; Zhao, Y.-X.; Li, B.; Li, Y.-M. Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression. World J. Gastroenterol. 2018, 24, 4565. [Google Scholar] [CrossRef]
- Motevaseli, E.; Dianatpour, A.; Ghafouri-Fard, S. The role of probiotics in cancer treatment: Emphasis on their in vivo and in vitro anti-metastatic effects. Int. J. Mol. Cell. Med. 2017, 6, 66. [Google Scholar]
- Yu, L.; Maishi, N.; Akahori, E.; Hasebe, A.; Takeda, R.; Matsuda, A.Y.; Hida, Y.; Nam, J.M.; Onodera, Y.; Kitagawa, Y. The oral bacterium Streptococcus mutans promotes tumor metastasis by inducing vascular inflammation. Cancer Sci. 2022, 113, 3980. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Jang, J.Y.; Chun, H.J. Efficacy of Helicobacter pylori eradication for the prevention of metachronous gastric cancer after endoscopic resection for early gastric cancer. World J. Gastroenterol. WJG 2014, 20, 2760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Lin, J.C.; Tu, S.P. Etiology and prevention of gastric cancer. Gastrointest. Tumors 2016, 3, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.; Lai, W.Y.; Ng, W.K.; Suen, M.M.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.; Wu, J.C. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Ishaq, S.; Nunn, L. Helicobacter pylori and gastric cancer: A state of the art review. Gastroenterol. Hepatol. Bed Bench 2015, 8, S6. [Google Scholar] [PubMed]
- Kumar, S.; Patel, G.K.; Ghoshal, U.C. Helicobacter pylori-induced inflammation: Possible factors modulating the risk of gastric cancer. Pathogens 2021, 10, 1099. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori virulence factors—Mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef]
- Mungazi, S.G.; Chihaka, O.B.; Muguti, G.I. Prevalence of Helicobacter pylori in asymptomatic patients at surgical outpatient department: Harare hospitals. Ann. Med. Surg. 2018, 35, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Kodama, M.; Fukuda, M.; Hirashita, Y.; Tsutsumi, K.; Fukuda, K.; Ogawa, R.; Okamoto, K.; Okimoto, T.; Murakami, K. Comparison of the improvement in gastric mucosal tissue after Helicobacter pylori eradication between young and elderly people. Arab J. Gastroenterol. 2023, 24, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef]
- Takenaka, R.; Okada, H.; Kato, J.; Makidono, C.; Hori, S.; Kawahara, Y.; Miyoshi, M.; Yumoto, E.; Imagawa, A.; Toyokawa, T. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment. Pharmacol. Ther. 2007, 25, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.-J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.-H. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Wong, B.C.-Y.; Lam, S.K.; Wong, W.M.; Chen, J.S.; Zheng, T.T.; Feng, R.E.; Lai, K.C.; Hu, W.H.C.; Yuen, S.T.; Leung, S.Y. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA 2004, 291, 187–194. [Google Scholar] [CrossRef]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.-I.; Kook, M.-C.; Park, B.; Joo, J. Family history of gastric cancer and Helicobacter pylori treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Liu, X.; Ling, Z.; Ji, F. Role of the gastric microbiome in gastric cancer: From carcinogenesis to treatment. Front. Microbiol. 2021, 12, 641322. [Google Scholar] [CrossRef]
- Pereira-Marques, J.; Ferreira, R.M.; Pinto-Ribeiro, I.; Figueiredo, C. Helicobacter pylori infection, the gastric microbiome and gastric cancer. Adv. Exp. Med. Biol. 2019, 1149, 195–210. [Google Scholar]
- Wen, J.; Lau, H.C.-H.; Peppelenbosch, M.P.; Yu, J. Gastric Microbiota beyond H. pylori: An Emerging Critical Character in Gastric Carcinogenesis. Biomedicines 2021, 9, 1680. [Google Scholar] [CrossRef]
- Weng, C.-Y.; Xu, J.-L.; Sun, S.-P.; Wang, K.-J.; Lv, B. Helicobacter pylori eradication: Exploring its impacts on the gastric mucosa. World J. Gastroenterol. 2021, 27, 5152. [Google Scholar] [CrossRef]
- Wang, L.; Xin, Y.; Zhou, J.; Tian, Z.; Liu, C.; Yu, X.; Meng, X.-y.; Jiang, W.; Zhao, S.; Dong, Q. Gastric Mucosa-Associated Microbial Signatures of Early Gastric Cancer. Front. Microbiol. 2020, 11, 1548. [Google Scholar] [CrossRef]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef]
- Chen, X.-H.; Wang, A.; Chu, A.-N.; Gong, Y.-H.; Yuan, Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared With Non-cancer Tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, R.; Zhao, X.; Cao, X.; Wang, Q.; Jiang, Q.; Zhang, Y.; Zheng, W.; Wu, X.; Yang, A. Alterations in Gastric Mucosal Microbiota in Gastric Carcinogenesis: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 754959. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Yen, C.-W.; Xu, H.-W.; Lin, Y.-J.; Deng, Y.-F.; Hsu, W.-T.; Wu, C.-S.; Li, C. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, M.N.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Ahn, Y.; Park, C.; Kim, J. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: A case-control study. Sci. Rep. 2019, 9, 13589. [Google Scholar] [CrossRef]
- Qi, Y.-F.; Sun, J.-N.; Ren, L.-F.; Cao, X.-L.; Dong, J.-H.; Tao, K.; Guan, X.-M.; Cui, Y.-N.; Su, W. Intestinal Microbiota Is Altered in Patients with Gastric Cancer from Shanxi Province, China. Dig. Dis. Sci. 2019, 64, 1193–1203. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, B.; Pan, H.; Wang, D.; Liu, M.; Yang, Y.; Zou, M.; Yang, J.; Xiao, K.; Zhao, R. Detection of microbial 16S rRNA gene in the serum of patients with gastric cancer. Front. Oncol. 2019, 9, 608. [Google Scholar] [CrossRef]
- Liang, W.; Yang, Y.; Wang, H.; Wang, H.; Yu, X.; Lu, Y.; Shen, S.; Teng, L. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine 2019, 98, e16626. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; El Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Uchida, T.; Oyuntsetseg, K.; Bolor, D.; Yamaoka, Y. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment. Pharmacol. Ther. 2020, 51, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, J.; Shi, X.; Du, Y.; Niu, Y.; Jin, G.; Wang, Z.; Lyu, J. Gut microbiome analysis as a predictive marker for the gastric cancer patients. Appl. Microbiol. Biotechnol. 2021, 105, 803–814. [Google Scholar] [PubMed]
- Park, J.Y.; Seo, H.; Kang, C.-S.; Shin, T.-S.; Kim, J.W.; Park, J.-M.; Kim, J.G.; Kim, Y.-K. Dysbiotic change in gastric microbiome and its functional implication in gastric carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [PubMed]
- Ai, B.; Mei, Y.; Liang, D.; Wang, T.; Cai, H.; Yu, D. Uncovering the special microbiota associated with occurrence and progression of gastric cancer by using RNA-sequencing. Sci. Rep. 2023, 13, 5722. [Google Scholar] [CrossRef]
- Bakhti, S.Z.; Latifi-Navid, S. Interplay and cooperation of Helicobacter pylori and gut microbiota in gastric carcinogenesis. BMC Microbiol. 2021, 21, 258. [Google Scholar]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor microenvironment: Lactic acid promotes tumor development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Goh, K.-L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar]
- Vinasco, K.; Mitchell, H.M.; Kaakoush, N.O.; Castano-Rodriguez, N. Microbial carcinogenesis: Lactic acid bacteria in gastric cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1872, 188309. [Google Scholar]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar]
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 953718. [Google Scholar]
- Nouri, Z.; Choi, S.W.; Choi, I.J.; Ryu, K.W.; Woo, S.M.; Park, S.-J.; Lee, W.J.; Choi, W.; Jung, Y.-S.; Myung, S.-K. Exploring Connections between Oral Microbiota, Short-Chain Fatty Acids, and Specific Cancer Types: A Study of Oral Cancer, Head and Neck Cancer, Pancreatic Cancer, and Gastric Cancer. Cancers 2023, 15, 2898. [Google Scholar]
- Huang, K.; Gao, X.; Wu, L.; Yan, B.; Wang, Z.; Zhang, X.; Peng, L.; Yu, J.; Sun, G.; Yang, Y. Salivary microbiota for gastric cancer prediction: An exploratory study. Front. Cell. Infect. Microbiol. 2021, 11, 640309. [Google Scholar]
- Sun, J.H.; Li, X.L.; Yin, J.; Li, Y.H.; Hou, B.X.; Zhang, Z. A screening method for gastric cancer by oral microbiome detection. Oncol. Rep. 2018, 39, 2217–2224. [Google Scholar]
- Bakhti, S.Z.; Latifi-Navid, S. Oral microbiota and Helicobacter pylori in gastric carcinogenesis: What do we know and where next? BMC Microbiol. 2021, 21, 71. [Google Scholar]
- Tseng, C.-H.; Lin, J.-T.; Ho, H.J.; Lai, Z.-L.; Wang, C.-B.; Tang, S.-L.; Wu, C.-Y. Gastric microbiota and predicted gene functions are altered after subtotal gastrectomy in patients with gastric cancer. Sci. Rep. 2016, 6, 20701. [Google Scholar]
- Hu, Y.; Pang, W.; Huang, Y.; Zhang, Y.; Zhang, C. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front. Cell. Infect. Microbiol. 2018, 8, 433. [Google Scholar]
- Wu, F.; Yang, L.; Hao, Y.; Zhou, B.; Hu, J.; Yang, Y.; Bedi, S.; Sanichar, N.G.; Cheng, C.; Perez-Perez, G.I.; et al. Oral and gastric microbiome in relation to gastric intestinal metaplasia. Int. J. Cancer 2022, 150, 928–940. [Google Scholar] [CrossRef]
- Pereira-Marques, J.; Ferreira, R.M.; Machado, J.C.; Figueiredo, C. The influence of the gastric microbiota in gastric cancer development. Best Pract. Res. Clin. Gastroenterol. 2021, 50, 101734. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, D.; Lehr, K.; Vilchez-Vargas, R.; Jonaitis, L.V.; Urba, M.; Kupcinskas, J.; Skieceviciene, J.; Link, A. Comparison of genomic and transcriptional microbiome analysis in gastric cancer patients and healthy individuals. World J. Gastroenterol. 2023, 29, 1202. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Tong, D.; Jiang, C.; Zhu, X.; Wei, Z.; Gong, T.; Jin, C. Relationships among microbiota, gastric cancer, and immunotherapy. Front. Microbiol. 2022, 13, 987763. [Google Scholar] [CrossRef]
- Cho, J.; Kang, M.-S.; Kim, K.-M. Epstein-Barr virus-associated gastric carcinoma and specific features of the accompanying immune response. J. Gastric Cancer 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Zhang, X.; Zhang, Y.; Xiao, H.; Luo, B. LMP2A induces DNA methylation and expression repression of AQP3 in EBV-associated gastric carcinoma. Virology 2019, 534, 87–95. [Google Scholar] [CrossRef]
- Ribeiro, J.; Oliveira, A.; Malta, M.; Oliveira, C.; Silva, F.; Galaghar, A.; Afonso, L.P.; Neves, M.C.; Medeiros, R.; Pimentel-Nunes, P. Clinical and pathological characterization of Epstein-Barr virus-associated gastric carcinomas in Portugal. World J. Gastroenterol. 2017, 23, 7292. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, K.-M. Role of Exosomes and Their Potential as Biomarkers in Epstein-Barr Virus-Associated Gastric Cancer. Cancers 2023, 15, 469. [Google Scholar] [CrossRef]
- De Re, V.; Brisotto, G.; Repetto, O.; De Zorzi, M.; Caggiari, L.; Zanussi, S.; Alessandrini, L.; Canzonieri, V.; Miolo, G.; Puglisi, F. Overview of epstein–barr-virus-associated gastric cancer correlated with prognostic classification and development of therapeutic options. Int. J. Mol. Sci. 2020, 21, 9400. [Google Scholar] [CrossRef]
- de Sousa, H.M.L.; Ribeiro, J.P.C.; Timóteo, M.B. Epstein-Barr virus-associated gastric cancer: Old entity with new relevance. In Epstein-Barr Virus: New Trends; IntechOpen: London, UK, 2021; p. 45. [Google Scholar]
- Wang, J.; Zheng, X.; Qin, Z.; Wei, L.; Lu, Y.; Peng, Q.; Gao, Y.; Zhang, X.; Zhang, X.; Li, Z. Epstein–Barr virus miR-BART3-3p promotes tumorigenesis by regulating the senescence pathway in gastric cancer. J. Biol. Chem. 2019, 294, 4854–4866. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Wei, L.; Peng, Q.; Gao, Y.; Fu, Y.; Lu, Y.; Qin, Z.; Zhang, X.; Lu, J. Epstein-Barr virus microRNA miR-BART5-3p inhibits p53 expression. J. Virol. 2018, 92, e01022-18. [Google Scholar] [CrossRef]
- Abusalah, M.A.H.; Irekeola, A.A.; Hanim Shueb, R.; Jarrar, M.t.; Yean Yean, C. Prognostic Epstein-Barr Virus (EBV) miRNA biomarkers for survival outcome in EBV-associated epithelial malignancies: Systematic review and meta-analysis. PLoS ONE 2022, 17, e0266893. [Google Scholar] [CrossRef]
- Qiu, M.Z.; He, C.Y.; Lu, S.X.; Guan, W.L.; Wang, F.; Wang, X.J.; Jin, Y.; Wang, F.H.; Li, Y.H.; Shao, J.Y. Prospective observation: Clinical utility of plasma Epstein–Barr virus DNA load in EBV-associated gastric carcinoma patients. Int. J. Cancer 2020, 146, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Liu, Y.Y.; Liu, K.H.; Hsu, J.T.; Chen, T.C.; Chiu, C.T.; Yeh, T.S. Comprehensive profiling of virus microRNAs of Epstein–Barr virus-associated gastric carcinoma: Highlighting the interactions of ebv-Bart9 and host tumor cells. J. Gastroenterol. Hepatol. 2017, 32, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein–Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Lima, Á.; Sousa, H.; Medeiros, R.; Nobre, A.; Machado, M. PD-L1 expression in EBV associated gastric cancer: A systematic review and meta-analysis. Discov. Oncol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Nishikawa, J.; Sakai, K.; Iizasa, H.; Yoshiyama, H.; Yanagihara, M.; Shuto, T.; Shimokuri, K.; Kanda, T.; Suehiro, Y. EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer 2019, 22, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Dong, M.; Wang, H.-y.; Zhao, X.-x.; Chen, J.-n.; Zhang, Y.-w.; Huang, Y.; Xue, L.; Li, H.-g.; Du, H.; Wu, X.-y. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus–associated gastric carcinoma. Hum. Pathol. 2016, 53, 25–34. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Zeng, B.; Hu, G.; Gan, R. Epstein–Barr virus-associated gastric cancer: A distinct subtype. Cancer Lett. 2020, 495, 191–199. [Google Scholar] [CrossRef]
- Liu, W.; Luo, B. The impact of EBV on the epigenetics of gastric carcinoma. Future Virol. 2020, 15, 183–193. [Google Scholar] [CrossRef]
- Yang, X.; Lou, J.; Shan, W.; Ding, J.; Jin, Z.; Hu, Y.; Du, Q.; Liao, Q.; Xie, R.; Xu, J. Pathophysiologic Role of Neurotransmitters in Digestive Diseases. Front. Physiol. 2021, 12, 567650. [Google Scholar] [CrossRef] [PubMed]
- Samir, S.; Ahmed, H.O.; Diab, T.M.; Mostafa, A.; Elmeligy, H.A.; Kamel, A.; Khalil, H. Rate of Epstein-Barr Virus in Gastric Adenocarcinoma in Egyptian Patients in View of the WHO Classification and Correlation with p16 Immunoreactivity. Open Access Maced. J. Med. Sci. 2022, 10, 1218–1225. [Google Scholar] [CrossRef]
- Jang, H.; Seo, A.N.; Kim, M. Clinicopathological Characteristics of Advanced Epstein–Barr Virus-associated Gastric Cancer Highlighting Aberrant p53 Expression. Anticancer Res. 2022, 42, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Paul, C.; Banerjee, J.; Kaul, R.; Banerjee, S. Virus Association with Gastric Inflammation and Cancer: An Updated Overview. J. Med. Microbiol. Infect. Dis. 2022, 10, 163–178. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.Z.; Chen, X.L.; Zhang, W.H.; Liu, K.; Wang, Y.J.; Tang, H.R.; Hu, J.K.; the SIGES research group. Associations between hepatitis B virus exposure and the risk of extrahepatic digestive system cancers: A hospital-based, case–control study (SIGES). Cancer Med. 2021, 10, 3741–3755. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Z.; Wu, W.; Ruan, L.; Yu, C.; Xi, Y.; Wang, L.; Wang, K.; Mo, J.; Zhao, S. Chronic hepatitis virus infection are associated with high risk of gastric cancer: A systematic review and cumulative analysis. Front. Oncol. 2021, 11, 703558. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 10 August 2023).
- Sabeena, S.; Ravishankar, N. Horizontal Modes of Transmission of Hepatitis B Virus (HBV): A Systematic Review and Meta-Analysis. Iran. J. Public Health 2022, 51, 2181. [Google Scholar] [CrossRef]
- Lemoine, M.; Nayagam, S.; Thursz, M. Viral hepatitis in resource-limited countries and access to antiviral therapies: Current and future challenges. Future Virol. 2013, 8, 371–380. [Google Scholar] [CrossRef]
- Stasi, C.; Silvestri, C.; Voller, F. Emerging trends in epidemiology of hepatitis B virus infection. J. Clin. Transl. Hepatol. 2017, 5, 272. [Google Scholar] [CrossRef]
- Tian, T.; Song, C.; Jiang, L.; Dai, J.; Lin, Y.; Xu, X.; Yu, C.; Ge, Z.; Ding, Y.; Wen, Y. Hepatitis B virus infection and the risk of cancer among the Chinese population. Int. J. Cancer 2020, 147, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, K.; Sundquist, J.; Ji, J. Risk of hepatocellular carcinoma and cancers at other sites among patients diagnosed with chronic hepatitis B virus infection in Sweden. J. Med. Virol. 2014, 86, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, W.; Charoenngam, N.; Ponvilawan, B.; Rujirachun, P.; Wattanachayakul, P.; Srikulmontri, T.; Hong, N.; Rai, P.; Ungprasert, P. Hepatitis B virus infection and risk of gastric cancer: A systematic review and meta-analysis. Minerva Gastroenterol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Yu, R.; Huang, J.; Peng, H.; Yin, S.; Xie, W.; Ren, S.; Peng, X.-E. Association Between Hepatitis B Virus and Gastric Cancer: A Systematic Review and Meta-Analysis. Infect. Med. 2023, 11, e00201. [Google Scholar] [CrossRef]
- Liu, T.; Song, C.; Zhang, Y.; Siyin, S.T.; Zhang, Q.; Song, M.; Cao, L.; Shi, H. Hepatitis B virus infection and the risk of gastrointestinal cancers among Chinese population: A prospective cohort study. Int. J. Cancer 2022, 150, 1018–1028. [Google Scholar] [CrossRef]
- Niedźwiedzka-Rystwej, P.; Grywalska, E.; Hrynkiewicz, R.; Wołącewicz, M.; Becht, R.; Roliński, J. The double-edged sword role of viruses in gastric cancer. Cancers 2020, 12, 1680. [Google Scholar] [CrossRef]
- Manns, M.P.; Maasoumy, B. Breakthroughs in hepatitis C research: From discovery to cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 10 October 2023).
- Alter, M.J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. WJG 2007, 13, 2436. [Google Scholar] [CrossRef]
- Cheng, J.-S.; Hu, J.-H.; Lin, Y.-s.; Lin, M.-S.; Ku, H.-P.; Chien, R.-N.; Chang, M.-L. Interferon-based therapy did not attenuate hepatitis C-associated rheumatic disorder risk: A nationwide population-based cohort study. Res. Sq. 2020. preprint. [Google Scholar] [CrossRef]
- Sun, D.; Ding, M.; Ruan, M.; Yang, L.; Qi, X. Association between Hepatitis C Virus and Extrahepatic Tumors; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Morozov, S.; Batskikh, S. Commentary: Hepatitis B virus infection: An insight into the clinical connection and molecular interaction between hepatitis B virus and host extrahepatic cancer risk. Front. Immunol. 2023, 14, 1200405. [Google Scholar] [CrossRef]
- Shiffman, M.L. Approach to the patient with chronic hepatitis B and decompensated cirrhosis. Liver Int. 2020, 40, 22–26. [Google Scholar] [CrossRef]
- Huang, X.; Yan, M.; Deng, Z.; Yao, L.; Han, D.; Sun, L. Natural history of decompensated cirrhosis with serum hepatitis B DNA < 2000 IU/mL: A retrospective study. BMC Gastroenterol. 2022, 22, 452. [Google Scholar]
- Toshikuni, N.; Arisawa, T.; Tsutsumi, M. Hepatitis C-related liver cirrhosis-strategies for the prevention of hepatic decompensation, hepatocarcinogenesis, and mortality. World J. Gastroenterol. WJG 2014, 20, 2876. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.-S.; Chang, C.-F.; Sheen, I.-S.; Jeng, C.-J.; Wang, C.-H. Upper Gastrointestinal Cancer and Liver Cirrhosis. Cancers 2022, 14, 2269. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Zhu, C.; Hong, T.; He, X.; Zhu, H.; Jiang, D. Gastrointestinal Cancers and Liver Cirrhosis: Implications on Treatments and Prognosis. Front. Oncol. 2021, 11, 766069. [Google Scholar] [CrossRef]
- Wei, X.l.; Luo, H.y.; Li, C.f.; Jin, Y.; Zeng, Z.l.; Ju, H.q.; Wu, Q.n.; Wang, Y.; Mao, M.j.; Liu, W.l. Hepatitis B virus infection is associated with younger median age at diagnosis and death in cancers. Int. J. Cancer 2017, 141, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qiu, M.; Jin, Y.; Huang, Y.; Wang, R.; Chen, W.; Wang, D.; Wang, F.; Luo, H.; Zhang, D. Hepatitis B virus infection is associated with gastric cancer in China: An endemic area of both diseases. Br. J. Cancer 2015, 112, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Jaroenlapnopparat, A.; Bhatia, K.; Coban, S. Inflammation and Gastric Cancer. Diseases 2022, 10, 35. [Google Scholar] [CrossRef]
- Tian, Y.; Ou, J.-h.J. Genetic and epigenetic alterations in hepatitis B virus-associated hepatocellular carcinoma. Virol. Sin. 2015, 30, 85–91. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Riechelmann, R.P.; Wilson, K.T.; Algood, H.M.S. Resolution of gastric cancer-promoting inflammation: A novel strategy for anti-cancer therapy. Curr. Top Microbiol. Immunol. 2019, 421, 319–359. [Google Scholar]
- Ali, A.; Abdel-Hafiz, H.; Suhail, M.; Al-Mars, A.; Zakaria, M.K.; Fatima, K.; Ahmad, S.; Azhar, E.; Chaudhary, A.; Qadri, I. Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma. World J. Gastroenterol. WJG 2014, 20, 10238. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lv, J.; Liu, Y.; Chen, J.G.; Ge, Z.; Zhu, J.; Dai, J.; Du, L.-B.; Yu, C.; Guo, Y. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw. Open 2019, 2, e195718. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Zhu, C.; Wen, Y.; Marusawa, H.; Bailly-Maitre, B.; Matsuzawa, S.-i.; Zhang, H.; Kim, Y.; Bennett, C.F.; Jiang, W. HBXIP, cellular target of hepatitis B virus oncoprotein, is a regulator of centrosome dynamics and cytokinesis. Cancer Res. 2006, 66, 9099–9107. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, X.; Li, D.; Jiang, X. HBXIP promotes gastric cancer via METTL3-mediated MYC mRNA m6A modification. Aging 2020, 12, 24967. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.; Zhang, S.; Cui, M.; Liu, F.; Sun, B.; Zhang, W.; Zhang, X.; Ye, L. HBXIP up-regulates ACSL1 through activating transcriptional factor Sp1 in breast cancer. Biochem. Biophys. Res. Commun. 2017, 484, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Lu, F.; Zhang, L.; Wang, G.; Geng, R.; Miao, Y. HBXIP regulates gastric cancer glucose metabolism and malignancy through PI3K/AKT and p53 signaling. OncoTargets Ther. 2020, 13, 3359–3374. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hou, Y.; Yao, Z.; Zhan, Y.; Chen, W.; Liu, Y. Expressivity of Interleukin-8 and gastric cancer prognosis susceptibility: A systematic review and meta-analysis. Dose-Response 2021, 19, 15593258211037127. [Google Scholar] [CrossRef]
- Xiu, M.; Zeng, X.; Shan, R.; Wen, W.; Li, J.; Wan, R. The oncogenic role of HBXIP. Biomed. Pharmacother. 2021, 133, 111045. [Google Scholar] [CrossRef]
- Gish, R.G.; Basit, S.A.; Ryan, J.; Dawood, A.; Protzer, U. Hepatitis B core antibody: Role in clinical practice in 2020. Curr. Hepatol. Rep. 2020, 19, 254–265. [Google Scholar] [CrossRef]
- Malczewski, A.B.; Navarro, S.; Coward, J.I.; Ketheesan, N. Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Gu, X.; Zhang, J.; Nomoto, D.; Okadome, K.; Baba, H.; Qiu, P. Gut microbiome in gastrointestinal cancer: A friend or foe? Int. J. Biol. Sci. 2022, 18, 4101–4117. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakayama, J.; Moriya, K.; Kawaratani, H.; Momoda, R.; Ito, K.; Iio, E.; Nojiri, S.; Fujiwara, K.; Yoneda, M. Gut dysbiosis associated with hepatitis C virus infection. Clin. Infect. Dis. 2018, 67, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Garcia-Montero, C.; Fraile-Martinez, O.; Boaru, D.L.; García-Puente, L.; Rios-Parra, A.; Garrido-Gil, M.J.; Casanova-Martín, C.; García-Honduvilla, N.; Bujan, J. AIF1: Function and Connection with Inflammatory Diseases. Biology 2023, 12, 694. [Google Scholar] [CrossRef]
- Negash, A.A.; Ramos, H.J.; Crochet, N.; Lau, D.T.; Doehle, B.; Papic, N.; Delker, D.A.; Jo, J.; Bertoletti, A.; Hagedorn, C.H. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013, 9, e1003330. [Google Scholar] [CrossRef]
- Xie, W.-h.; Ding, J.; Xie, X.-x.; Yang, X.-h.; Wu, X.-F.; Chen, Z.-x.; Guo, Q.-l.; Gao, W.-y.; Wang, X.-z.; Li, D. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm. Res. 2020, 69, 683–696. [Google Scholar] [CrossRef]
- Megahed, F.A.K.; Zhou, X.; Sun, P. The interactions between HBV and the innate immunity of hepatocytes. Viruses 2020, 12, 285. [Google Scholar] [CrossRef]
- Marusawa, H.; Jenkins, B.J. Inflammation and gastrointestinal cancer: An overview. Cancer Lett. 2014, 345, 153–156. [Google Scholar] [CrossRef]
- Isogawa, M.; Robek, M.D.; Furuichi, Y.; Chisari, F.V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 2005, 79, 7269–7272. [Google Scholar] [CrossRef]
- Li, K.; Lemon, S.M. Innate immune responses in hepatitis C virus infection. Proc. Semin. Immunopathol. 2013, 35, 53–72. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Ramos, B.; Nunes, A.; Ribeiro, D. Hepatitis C virus: Evading the intracellular innate immunity. J. Clin. Med. 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, J.; Negash, A.; Savan, R.; Gale, M. Innate immunity in hepatitis C virus infection. Cold Spring Harb. Perspect. Med. 2021, 11, a036988. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Toll-like receptor response to hepatitis B virus infection and potential of TLR agonists as immunomodulators for treating chronic hepatitis B: An overview. Int. J. Mol. Sci. 2021, 22, 10462. [Google Scholar] [CrossRef]
- Murira, A.; Lamarre, A. Type-I interferon responses: From friend to foe in the battle against chronic viral infection. Front. Immunol. 2016, 7, 609. [Google Scholar] [CrossRef]
- Sanada, T.; Tsukiyama-Kohara, K.; Shin-I, T.; Yamamoto, N.; Kayesh, M.E.H.; Yamane, D.; Takano, J.-i.; Shiogama, Y.; Yasutomi, Y.; Ikeo, K. Construction of complete Tupaia belangeri transcriptome database by whole-genome and comprehensive RNA sequencing. Sci. Rep. 2019, 9, 12372. [Google Scholar] [CrossRef]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Luo, H.; Niu, J.; Yan, Y.; Fang, Y.; Ke, L.; Chen, W.; Xu, H.; Li, H. Serological and Molecular Characterization of Hepatitis B Virus Infection in Gastric Cancer. Front. Cell. Infect. Microbiol. 2022, 524, 894836. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Hasan, A.; Pozzoli, G.; Cenciarelli, C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): Potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jin, Y.; Chen, F.; Ni, H.; Hu, C.; Xu, Y.; Xuan, H.; Hu, D.; Deng, W.; Zhang, Y. Clinicopathological evidence of hepatitis B virus infection in the development of gastric adenocarcinoma. J. Med. Virol. 2020, 92, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Högner, A.; Moehler, M. Immunotherapy in gastric cancer. Curr. Oncol. 2022, 29, 1559–1574. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, Y.; Zhang, P.; Chu, Q. Acquired resistance to immune checkpoint blockades: The underlying mechanisms and potential strategies. Front. Immunol. 2021, 12, 693609. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Malouf, G.G.; Jacobs, I.; Chou, J.; Liu, L.; Johnson, M.L. Addressing resistance to immune checkpoint inhibitor therapy: An urgent unmet need. Future Oncol. 2021, 17, 1401–1439. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of cancer resistance to immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune Checkpoint Inhibitors in Cancer Therapy—How to Overcome Drug Resistance? Cancers 2022, 14, 3575. [Google Scholar] [CrossRef]
- Chen, C.-W.; Cheng, J.-S.; Chen, T.-D.; Le, P.-H.; Ku, H.-P.; Chang, M.-L. The irreversible HCV-associated risk of gastric cancer following interferon-based therapy: A joint study of hospital-based cases and nationwide population-based cohorts. Ther. Adv. Gastroenterol. 2019, 12, 1756284819855732. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.A.; Hawkins, J.B.; Tracy, S.I.; Shapiro, M. The pathogenesis of Epstein–Barr virus persistent infection. Curr. Opin. Virol. 2013, 3, 227–232. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Qian, X.-J.; Zhao, P.; Qi, Z.-T. How hepatitis C virus invades hepatocytes: The mystery of viral entry. World J. Gastroenterol. WJG 2014, 20, 3457. [Google Scholar] [CrossRef] [PubMed]
- Herrscher, C.; Roingeard, P.; Blanchard, E. Hepatitis B virus entry into cells. Cells 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xiao, H.; Song, H.; An, S.; Luo, B. Transcriptome sequencing of LMP2A-transfected gastric cancer cells identifies potential biomarkers in EBV-associated gastric cancer. Virus Genes 2022, 58, 515–526. [Google Scholar] [CrossRef]
- Fang, X.; Tan, T.; Gao, B.; Zhao, Y.; Liu, T.; Xia, Q. Germacrone Regulates HBXIP-Mediated Cell Cycle, Apoptosis and Promotes the Formation of Autophagosomes to Inhibit the Proliferation of Gastric Cancer Cells. Front Oncol. 2020, 10, 537322. [Google Scholar] [CrossRef] [PubMed]
- Zamor, P.J.; Delemos, A.S.; Russo, M.W. Viral hepatitis and hepatocellular carcinoma: Etiology and management. J. Gastrointest. Oncol. 2017, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.A.; Bhaduri-McIntosh, S. Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses 2023, 15, 949. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wan, P.; Zhang, Y.; Tan, Q.; Qudus, M.S.; Yue, Z.; Luo, W.; Zhang, W.; Ouyang, J.; Li, Y. Innate immunity, inflammation, and intervention in HBV infection. Viruses 2022, 14, 2275. [Google Scholar] [CrossRef]
- Li, H.; Huang, M.-H.; Jiang, J.-D.; Peng, Z.-G. Hepatitis C: From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J. Gastroenterol. 2018, 24, 5297. [Google Scholar] [CrossRef]
- Naseem, M.; Barzi, A.; Brezden-Masley, C.; Puccini, A.; Berger, M.D.; Tokunaga, R.; Battaglin, F.; Soni, S.; McSkane, M.; Zhang, W. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat. Rev. 2018, 66, 15–22. [Google Scholar] [CrossRef]
- Gómez-Moreno, A.; Garaigorta, U. Hepatitis B virus and DNA damage response: Interactions and consequences for the infection. Viruses 2017, 9, 304. [Google Scholar] [CrossRef]
- Pal, S.; Polyak, S.J.; Bano, N.; Qiu, W.C.; Carithers, R.L.; Shuhart, M.; Gretch, D.R.; Das, A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J. Gastroenterol. Hepatol. 2010, 25, 627–634. [Google Scholar] [CrossRef]
- Yao, Y.; Kong, W.; Yang, L.; Ding, Y.; Cui, H. Immunity and Immune Evasion Mechanisms of Epstein–Barr Virus. Viral Immunol. 2023, 36, 303–317. [Google Scholar] [CrossRef]
- Ortega-Prieto, A.M.; Dorner, M. Immune evasion strategies during chronic hepatitis B and C virus infection. Vaccines 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Y.; Wang, C.; Gan, R. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int. 2021, 21, 93. [Google Scholar] [CrossRef]
- Vrancken, K.; Paeshuyse, J.; Liekens, S. Angiogenic activity of hepatitis B and C viruses. Antivir. Chem. Chemother. 2012, 22, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Dai, J.; Bazot, Q.; Patel, L.; Nikitin, P.A.; Djavadian, R.; Winter, P.S.; Salinas, C.A.; Barry, A.P.; Wood, K.C. Epstein-Barr virus ensures B cell survival by uniquely modulating apoptosis at early and late times after infection. Elife 2017, 6, e22509. [Google Scholar] [CrossRef]
- Madden, C.R.; Slagle, B.L. Stimulation of cellular proliferation by hepatitis B virus X protein. Dis. Markers 2001, 17, 153–157. [Google Scholar] [CrossRef]
- Damania, P.; Sen, B.; Dar, S.B.; Kumar, S.; Kumari, A.; Gupta, E.; Sarin, S.K.; Venugopal, S.K. Hepatitis B virus induces cell proliferation via HBx-induced microRNA-21 in hepatocellular carcinoma by targeting programmed cell death protein4 (PDCD4) and phosphatase and tensin homologue (PTEN). PLoS ONE 2014, 9, e91745. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.-Y.; Chen, S.S.-L. Hepatitis C virus and cellular stress response: Implications to molecular pathogenesis of liver diseases. Viruses 2012, 4, 2251–2290. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.O.; Tang, C.-M.; Yu, J. Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: Disease and treatments. World J. Gastroenterol. WJG 2014, 20, 6448. [Google Scholar] [CrossRef]
- He, B.; Gao, R.; Lv, S.; Chen, A.; Huang, J.; Wang, L.; Feng, Y.; Feng, J.; Liu, B.; Lei, J.; et al. Cancer cell employs a microenvironmental neural signal trans-activating nucleus-mitochondria coordination to acquire stemness. Signal Transduct. Target. Ther. 2023, 8, 275. [Google Scholar] [CrossRef]
- Khera, L.; Paul, C.; Kaul, R. Hepatitis C Virus mediated metastasis in hepatocellular carcinoma as a therapeutic target for cancer management. Curr. Drug Metab. 2018, 19, 224–235. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, A.; Schiffman, M.; Bromley, C.; Wacholder, S.; Herrero, R.; Rodriguez, A.C.; Bratti, M.C.; Sherman, M.E.; Scarpidis, U.; Lin, Q.-Q. Human papillomavirus type 16 variants and risk of cervical cancer. J. Natl. Cancer Inst. 2001, 93, 315–318. [Google Scholar] [CrossRef]
- Zeng, Z.-m.; Luo, F.-f.; Zou, L.-x.; He, R.-q.; Pan, D.-h.; Chen, X.; Xie, T.-t.; Li, Y.-q.; Peng, Z.-g.; Chen, G. Human papillomavirus as a potential risk factor for gastric cancer: A meta-analysis of 1917 cases. OncoTargets Ther. 2016, 9, 7105–7114. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Dudek, I.; Chilimoniuk, Z.; Dobosz, M.; Dobrzyński, M.; Teresiński, G.; Buszewicz, G.; Flieger, J.; Portincasa, P. The Involvement of Human Papilloma Virus in Gastrointestinal Cancers. Cancers 2022, 14, 2607. [Google Scholar] [CrossRef] [PubMed]
- Roesch-Dietlen, F.; Cano-Contreras, A.; Sánchez-Maza, Y.; Espinosa-González, J.; Vázquez-Prieto, M.; Díaz-Roesch, F.; Carrasco-Arroniz, M.; Cruz-Palacios, A.; Grube-Pagola, P.; Sumoza-Toledo, A. Frequency of human papillomavirus infection in patients with gastrointestinal cancer. Rev. Gastroenterol. México 2018, 83, 253–258. [Google Scholar] [CrossRef]
- Tomaić, V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers 2016, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Sofiani, V.H.; Veisi, P.; Rukerd, M.R.Z.; Ghazi, R.; Nakhaie, M. The complexity of human papilloma virus in cancers: A narrative review. Infect. Agents Cancer 2023, 18, 13. [Google Scholar] [CrossRef]
- Xu, Q.; Dong, H.; Wang, Z.; Zhang, P.; Albers, A.E.; Kaufmann, A.M.; Zheng, Z.M.; Qian, X. Integration and viral oncogene expression of human papillomavirus type 16 in oropharyngeal squamous cell carcinoma and gastric cancer. J. Med. Virol. 2023, 95, e28761. [Google Scholar] [CrossRef]
- Yuan, X.-y.; Wang, M.-y.; Wang, X.-y.; Chang, A.-y.; Li, J. Non-detection of Epstein-Barr virus and Human Papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genet. Mol. Biol. 2013, 36, 183–184. [Google Scholar] [CrossRef]
- Firoz, A.; Ali, H.M.; Rehman, S.; Rather, I.A. Gastric Cancer and Viruses: A Fine Line between Friend or Foe. Vaccines 2022, 10, 600. [Google Scholar] [CrossRef]
- Guven-Maiorov, E.; Tsai, C.-J.; Nussinov, R. Oncoviruses can drive cancer by rewiring signaling pathways through interface mimicry. Front. Oncol. 2019, 9, 1236. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O. Non-bacteria microbiome (virus, fungi, and archaea) in gastrointestinal cancer. Microbiome Gastrointest. Cancer 2023, 37, 91–106. [Google Scholar]
- Zwolińska-Wciso, M.; Budak, A.; Trojanowska, D.; Bogda–, J.; Stachura, J. Fungal colonization of the stomach and its clinical relevance: Die Pilzbesiedlung des Magens und ihre klinische Relevanz. Mycoses 1998, 41, 327–334. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Dmitrović, B.; Juzbašić, M.; Matijević, T.; Bekić, S.; Erić, S.; Flam, J.; Belić, D.; Petek Erić, A. A Putative Role of Candida albicans in Promoting Cancer Development: A Current State of Evidence and Proposed Mechanisms. Microorganisms 2023, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Xiong, Y.; Zhao, J.; Gao, Z.; Ma, J.; Wu, Z.; Song, Y.; Hong, X. Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 2021, 11, 4945. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, Z. The research progress in the interaction between Candida albicans and cancers. Front. Microbiol. 2022, 13, 988734. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Gaffen, S.L.; Hise, A.G.; Brown, G.D. Innate defense against fungal pathogens. Cold Spring Harb. Perspect. Med. 2015, 5, a019620. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Leventakos, K.; Lewis, R.E.; Kontoyiannis, D.P. Fungal infections in leukemia patients: How do we prevent and treat them? Clin. Infect. Dis. 2010, 50, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lao, M.; Li, C.; Li, J.; Chen, D.; Ding, M.; Gong, Y. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from Southern China: Clinical features and associated factors. J. Diabetes Investig. 2020, 11, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Marr, K.A. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012, 7, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, A.; Jaiswal, A.; Verma, P.K.; Singhal, R. A prospective study of fungal colonization and invasive fungal disease in long-term mechanically ventilated patients in a respiratory intensive care unit. Indian J. Crit. Care Med. 2018, 22, 597. [Google Scholar]
- Zhao, Y.; Zhang, J.; Cheng, A.S.; Yu, J.; To, K.F.; Kang, W. Gastric cancer: Genome damaged by bugs. Oncogene 2020, 39, 3427–3442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).