Upfront Taxane Could Be Superior to Pegylated Liposomal Doxorubicin (PLD): A Retrospective Real-World Analysis of Treatment Sequence Taxane–PLD versus PLD–Taxane in Patients with Metastatic Breast Cancer
Abstract
:Simple Summary
Abstract
1. Background
2. Material and Methods
2.1. Study Design and Patient Population
2.2. Aims and Endpoints
2.3. Statistical Analysis
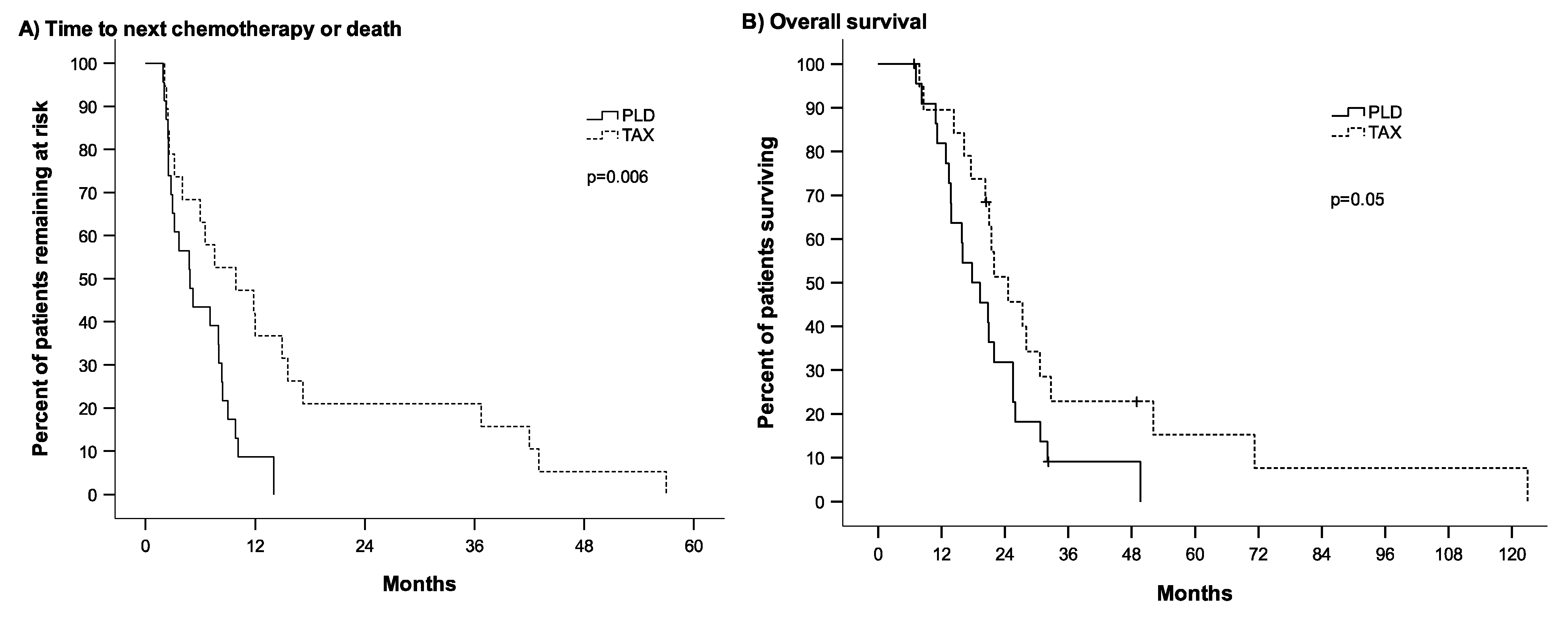
3. Results
3.1. Patients
3.2. Outcome Parameters
3.3. Subsequent Treatments and Crossover
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| CBR | Clinical benefit rate |
| Cdk4/6 | Cyclin-dependent kinase 4/6 |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| ER | Estrogen receptor |
| Her2 | Human epidermal growth factor receptor 2 |
| HR | Hazard ratio |
| MBC | Metastatic breast cancer |
| ORR | Objective response rate |
| OS | Overall Survival |
| PD-L1 | Programmed cell death 1 ligand 1 |
| PFS | Progression-free survival |
| PLD | Pegylated liposomal doxorubicin |
| PPE | Palmar–plantar erythrodysesthesia |
| PR | Progesterone receptor |
| SD | Stable disease |
| SERD | Selective estrogen receptor degrader |
| TNBC | Triple-negative breast cancer |
| TTNC | Time to next chemotherapy |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Voduc, K.D.; Cheang, M.C.U.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast Cancer Subtypes and the Risk of Local and Regional Relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Tolaney, S.M.; Arteaga, C.; Cortes, J.; Sohn, J.; Marmé, F.; Hong, Q.; Delaney, R.J.; Hafeez, A.; et al. TROPiCS-02: A Phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer. Future Oncol. 2020, 16, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar]
- Ditsch, N.; Untch, M.; Kolberg-Liedtke, C.; Jackisch, C.; Krug, D.; Friedrich, M.; Janni, W.; Müller, V.; Albert, U.-S.; Banys-Paluchowski, M.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2020. Breast Care 2020, 15, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.C.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYXTM/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Smorenburg, C.H.; de Groot, S.M.; van Leeuwen-Stok, A.E.; Hamaker, M.E.; Wymenga, A.N.; de Graaf, H.; de Jongh, F.E.; Braun, J.J.; Los, M.; Maartense, E.; et al. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: Results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Ann. Oncol. 2014, 25, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Saupe, S.; Jäger, E.; Schmidt, M.; Kreienberg, R.; Müller, L.; Otremba, B.J.; Waldenmaier, D.; Dorn, J.; Warm, M.; et al. A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: Results of the PELICAN study. Breast Cancer Res. Treat. 2017, 161, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.M.; Mennel, R.G.; Georgoulias, V.A.; Nabholtz, J.-M.; Erazo, A.; Lluch, A.; Vogel, C.L.; Kaufmann, M.; von Minckwitz, G.; Henderson, I.C.; et al. Randomized Phase III Trial of Pegylated Liposomal Doxorubicin versus Vinorelbine or Mitomycin C Plus Vinblastine in Women with Taxane-Refractory Advanced Breast Cancer. J. Clin. Oncol. 2004, 22, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Neuberg, D.; Bernardo, P.; Ingle, J.N.; Martino, S.; Rowinsky, E.K.; Wood, W.C. Phase III Trial of Doxorubicin, Paclitaxel, and the Combination of Doxorubicin and Paclitaxel as Front-Line Chemotherapy for Metastatic Breast Cancer: An Intergroup Trial (E1193). J. Clin. Oncol. 2003, 21, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Xue, L.; Young, L.J.; Burke, P.A.; Cheung, A.T. Paclitaxel (Taxol): An Inhibitor of Angiogenesis in a Highly Vascularized Transgenic Breast Cancer. Cancer Biother. Radiopharm. 1999, 14, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Belotti, D.; Vergani, V.; Drudis, T.; Borsotti, P.; Pitelli, M.R.; Viale, G.; Giavazzi, R.; Taraboletti, G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 1843–1849. [Google Scholar]
- Verras, G.-I.; Tchabashvili, L.; Mulita, F.; Grypari, I.M.; Sourouni, S.; Panagodimou, E.; Argentou, M.-I. Micropapillary Breast Carcinoma: From Molecular Pathogenesis to Prognosis. Breast Cancer Targets Ther. 2022, 14, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Kachur, M.E.; Rechache, K.; Wells, J.M.; Lipkowitz, S. Rare Breast Cancer Subtypes. Curr. Oncol. Rep. 2021, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Güntner, M.; Pauligk, C.; Scholz, M.; Chen, R.; Beiss, B.; Stopatschinskaja, S.; Lerbs, W.; Harbeck, N.; Jäger, E. Anthracycline rechallenge using pegylated liposomal doxorubicin in patients with metastatic breast cancer: A pooled analysis using individual data from four prospective trials. Br. J. Cancer. 2010, 103, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
| O’Brien (2003) [10] | Harbeck (2017) [12] | Smorenburg (2013) [11] | Keller (2004) [13] | |
|---|---|---|---|---|
| Number of patients (PLD) | 254 | 105 | 40 | 150 |
| Control arm | doxorubicin | capecitabine | capecitabine | vinorelbin/MV |
| Treatment line | first line | first line | first | second or later |
| Inclusion criteria | ||||
| - Performance status | ECOG ≤ 2 | ECOG ≤ 2 | ECOG ≤ 2 | Karnofsky ≥ 60% |
| - Age (years) | >18 | >18 | ≥65 | >18 |
| - Type | All | All | All | taxane-refractory |
| Patient criteria | ||||
| - Median age | 59 | 62 | 75 | 56 |
| - ≥3 sites of metastasis (%) | 30 | -- | -- | 26 |
| - Her2-positive (%) | -- | -- | 2 | -- |
| - HR-positive (%) | 35 | -- | 62 | 47 |
| - Visceral disease (%) | 59 | -- | -- | 63 |
| Endocrine therapy (%) | -- | -- | -- | 65 |
| - adjuvant | -- | -- | 48 | 54 |
| - in MBC | -- | -- | 60 | 64 |
| Prior chemotherapy (%) | -- | -- | 12 | 100 |
| - thereof adjuvant | 12 | 78 | ||
| - in MBC | 0 | 96 | ||
| Dosing (mg/m2) | 50, q4w | 50, q4w | 45, q4w | 50, q4w |
| Outcome measure | ||||
| - ORR | 33% | 11% | 18% | 10% |
| - CBR | 58% | -- | 72% | 38% |
| - PFS (months) | 6.9 | 6 | 5.6 | 2.9 |
| - OS (months) | 21 | 23.3 | 13.8 | 10.4 |
| Characteristic | First Line PLD (n = 23) | First Line Taxane (n = 19) | p |
|---|---|---|---|
| Median age at treatment initiation | 61.5 | 59.8 | 0.74 |
| Breast cancer type | |||
| Luminal A (%) | 8 (35) | 6 (32) | 1 |
| Luminal B (%) | 12 (52) | 11 (58) | 0.76 |
| Triple-negative breast cancer (%) | 3 (13) | 2 (11) | 1 |
| Receptor Status | |||
| ER-positive (%) | 20 (87) | 17 (89) | 1 |
| PR-positive (%) | 18 (78) | 15 (79) | 1 |
| M-stage at primary diagnosis | |||
| M0/cMx (%) | 18 (78) | 16 (84) | 0.71 |
| M1 (%) | 5 (22) | 3 (16) | |
| Relevant comorbidity | |||
| Heart/Cardiovascular (%) | 2 (9) | 1 (5) | 1 |
| Pulmonary (%) | 3 (13) | 0 (0) | 0.24 |
| Diabetes (%) | 3 (13) | 2 (11) | 1 |
| Arterial hypertension (%) | 6 (26) | 7 (37) | 0.52 |
| Other (%) | 8 (35) | 3 (16) | 0.29 |
| Metastases at diagnosis of MBC | |||
| Bone (%) | 17 (74) | 10 (53) | 0.11 |
| Lung (%) | 9 (39) | 5 (26) | 0.51 |
| Liver (%) | 5 (22) | 6 (32) | 0.50 |
| Pleural (%) | 2 (9) | 2 (11) | 1 |
| CNS (%) | 1 (4) | 1 (5) | 1 |
| Peritoneal/abdominal (%) | 2 (9) | 2 (11) | 1 |
| Other sites (%) | 11 (48) | 11 (58) | 0.55 |
| Visceral disease (%) | 12 (52) | 10 (53) | 1 |
| 1–2 organs involved (%) | 15 (65) | 12 (63) | 1 |
| ≥3 organs involved (%) | 8 (35) | 7 (37) | -- |
| Primary treatment | |||
| Breast conservative surgery (%) | 12 (52) | 11 (58) | 0.76 |
| Breast ablative surgery (%) | 6 (26) | 7 (37) | 0.52 |
| Sentinel node resection (%) | 10 (43) | 7 (37) | 0.76 |
| Axillar revision (%) | 11 (48) | 10 (53) | 1 |
| Neoadjuvant chemotherapy | |||
| Any (%) | 1 (4) | 3 (16) | 0.31 |
| Neoadjuvant taxanes (%) | 1 (4) | 2 (11) | 0.58 |
| Neoadjuvant anthracycline (%) | 1 (4) | 3 (16) | 0.31 |
| Adjuvant chemotherapy | |||
| Any (%) | 7 (30) | 10 (53) | 0.21 |
| Taxane (%) | 3 (13) | 4 (21) | 0.68 |
| Anthracycline (%) | 6 (26) | 8 (42) | 0.33 |
| Adjuvant endocrine therapy | |||
| Any (%) | 13 (57) | 14 (74) | 0.34 |
| Minimum of 5 years (%) | 7 (30) | 8 (42) | 0.52 |
| Radiotherapy | |||
| Adjuvant RT (%) | 14 (61) | 16 (84) | 0.17 |
| RT for MBC before first line (%) | 14 (61) | 15 (79) | 0.32 |
| Systemic treatment for MBC | |||
| Endocrine therapy before first CT (%) | 17 (74) | 13 (68) | 0.74 |
| cdk4/6 inhibitor before first CT (%) | 9 (39) | 4 (21) | 0.32 |
| Choice of taxane: paclitaxel weekly (%) | 21 (91) | 17 (89) | |
| Choice of taxane: docetaxel q3w (%) | 2 (9) | 2 (11) | 1 |
| Median endocrine lines before first CT | 2 | 1 | 0.08 |
| Median total treatment lines | 5 | 5 | 0.84 |
| Median subsequent treatment lines | 3 | 3 | 0.36 |
| Bisphosphonate/denosumab (%) | 15 (65) | 11 (58) | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallrabenstein, T.; Oseledchyk, A.; Daetwyler, E.; Rochlitz, C.; Vetter, M. Upfront Taxane Could Be Superior to Pegylated Liposomal Doxorubicin (PLD): A Retrospective Real-World Analysis of Treatment Sequence Taxane–PLD versus PLD–Taxane in Patients with Metastatic Breast Cancer. Cancers 2023, 15, 4953. https://doi.org/10.3390/cancers15204953
Wallrabenstein T, Oseledchyk A, Daetwyler E, Rochlitz C, Vetter M. Upfront Taxane Could Be Superior to Pegylated Liposomal Doxorubicin (PLD): A Retrospective Real-World Analysis of Treatment Sequence Taxane–PLD versus PLD–Taxane in Patients with Metastatic Breast Cancer. Cancers. 2023; 15(20):4953. https://doi.org/10.3390/cancers15204953
Chicago/Turabian StyleWallrabenstein, Till, Anton Oseledchyk, Eveline Daetwyler, Christoph Rochlitz, and Marcus Vetter. 2023. "Upfront Taxane Could Be Superior to Pegylated Liposomal Doxorubicin (PLD): A Retrospective Real-World Analysis of Treatment Sequence Taxane–PLD versus PLD–Taxane in Patients with Metastatic Breast Cancer" Cancers 15, no. 20: 4953. https://doi.org/10.3390/cancers15204953
APA StyleWallrabenstein, T., Oseledchyk, A., Daetwyler, E., Rochlitz, C., & Vetter, M. (2023). Upfront Taxane Could Be Superior to Pegylated Liposomal Doxorubicin (PLD): A Retrospective Real-World Analysis of Treatment Sequence Taxane–PLD versus PLD–Taxane in Patients with Metastatic Breast Cancer. Cancers, 15(20), 4953. https://doi.org/10.3390/cancers15204953







