PTEN, PTENP1, microRNAs, and ceRNA Networks: Precision Targeting in Cancer Therapeutics
Abstract
Simple Summary
Abstract
1. Introduction
2. PTEN and Cancer: From Mutations to a Continuum Model of Tumorigenesis
PTEN Loss, Tumour Immune Evasion, and Therapy Resistance
3. Post-Transcriptional Regulation of PTEN by microRNAs and Pseudogene lncRNAs
3.1. microRNAs Regulate PTEN Expression at the Post-Transcriptional Level
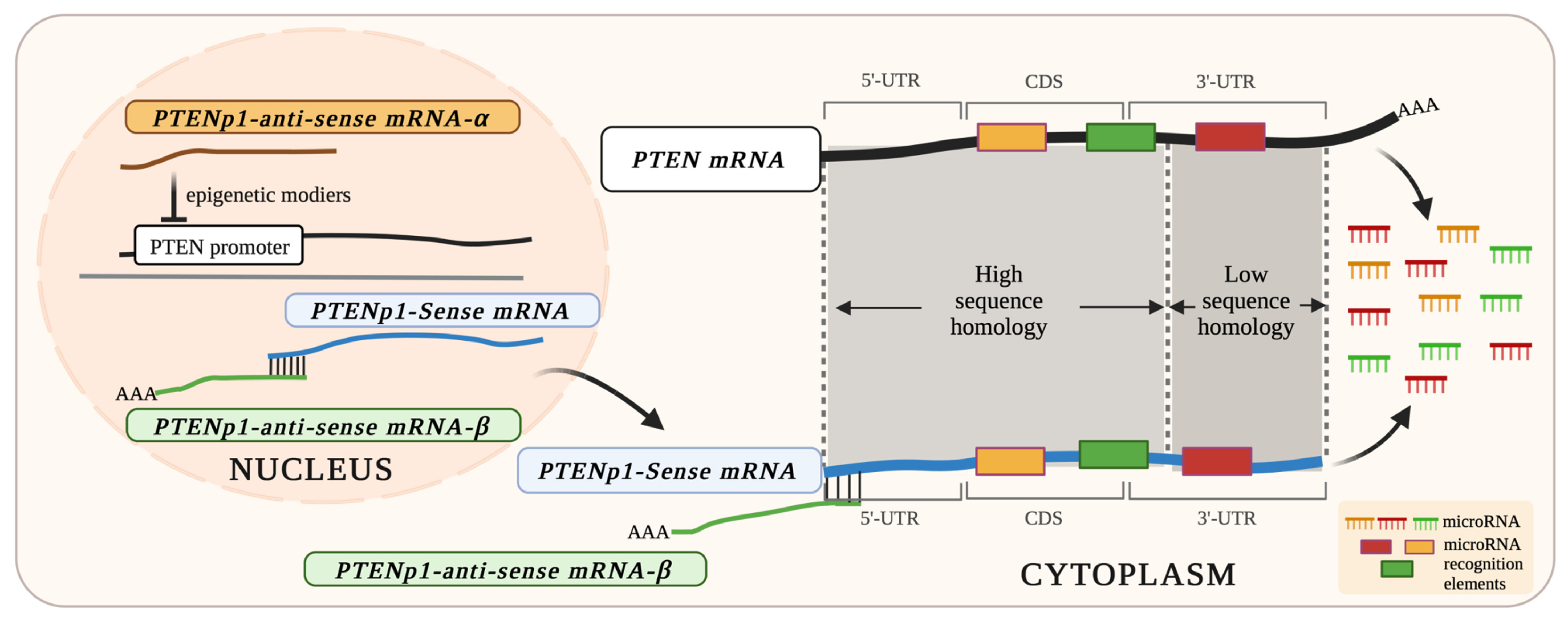
3.2. PTENP1: A Processed Pseudogene of PTEN Produces Bidirectional Transcripts
4. PTEN, miRNA, PTENP1, and the Endogenous Competitive RNA (ceRNA) Binding Hypothesis
Other ceRNAs Regulating PTEN Expression in Cancer
5. Expression of PTEN and PTENP1 in Cancer
5.1. PTENP1 also Functions Independently of the PTEN ceRNA Network
5.2. Evolution of PTENP1 and cross Regulation of PTENP1 by PTEN
6. Overexpression of PTENP1 or Its 3’-UTR: Prelude to Cancer Therapy?
7. Manipulating PTEN, PTENP1, and miRNA Levels as Potential Cancer Therapies
7.1. Increasing PTEN Levels Directly
7.2. Increasing PTEN Levels Indirectly via PTENP1 as an miRNA Competitor
7.3. Altering Levels of miRNAs Targeting PTEN and PTENP1
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Steck, P.A.; Pershouse, M.A.; Jasser, S.A.; Yung, W.K.; Lin, H.; Ligon, A.H.; Langford, L.A.; Baumgard, M.L.; Hattier, T.; Davis, T.; et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997, 15, 356–362. [Google Scholar] [CrossRef]
- Li, D.M.; Sun, H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997, 57, 2124–2129. [Google Scholar]
- Hollander, M.C.; Blumenthal, G.M.; Dennis, P.A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 2011, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C.; Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef]
- Nassif, N.T.; Lobo, G.P.; Wu, X.; Henderson, C.J.; Morrison, C.D.; Eng, C.; Jalaludin, B.; Segelov, E. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene 2004, 23, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef] [PubMed]
- Stambolic, V.; Suzuki, A.; de la Pompa, J.L.; Brothers, G.M.; Mirtsos, C.; Sasaki, T.; Ruland, J.; Penninger, J.M.; Siderovski, D.P.; Mak, T.W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998, 95, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, A.; Carracedo, A.; Clohessy, J.G.; Trotman, L.C.; Nardella, C.; Egia, A.; Salmena, L.; Sampieri, K.; Haveman, W.J.; Brogi, E.; et al. Subtle variations in Pten dose determine cancer susceptibility. Nat. Genet. 2010, 42, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.H.; Knudson, A.G.; Pandolfi, P.P. A continuum model for tumour suppression. Nature 2011, 476, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef]
- Bubien, V.; Bonnet, F.; Brouste, V.; Hoppe, S.; Barouk-Simonet, E.; David, A.; Edery, P.; Bottani, A.; Layet, V.; Caron, O.; et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J. Med. Genet. 2013, 50, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Risinger, J.I.; Hayes, K.; Maxwell, G.L.; Carney, M.E.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 1998, 4, 3005–3010. [Google Scholar]
- Celebi, J.T.; Shendrik, I.; Silvers, D.N.; Peacocke, M. Identification of PTEN mutations in metastatic melanoma specimens. J. Med. Genet. 2000, 37, 653–657. [Google Scholar] [CrossRef]
- Dahia, P.L.; FitzGerald, M.G.; Zhang, X.; Marsh, D.J.; Zheng, Z.; Pietsch, T.; von Deimling, A.; Haluska, F.G.; Haber, D.A.; Eng, C. A highly conserved processed PTEN pseudogene is located on chromosome band 9p21. Oncogene 1998, 16, 2403–2406. [Google Scholar] [CrossRef]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef]
- Whang, Y.E.; Wu, X.; Sawyers, C.L. Identification of a pseudogene that can masquerade as a mutant allele of the PTEN/MMAC1 tumor suppressor gene. J. Natl. Cancer Inst. 1998, 90, 859–861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujii, G.H.; Morimoto, A.M.; Berson, A.E.; Bolen, J.B. Transcriptional analysis of the PTEN/MMAC1 pseudogene, psiPTEN. Oncogene 1999, 18, 1765–1769. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Taheri, M.; Akbari Dilmaghani, N. A review on the role of PTENP1 in human disorders with an especial focus on tumor suppressor role of this lncRNA. Cancer Cell Int. 2022, 22, 207. [Google Scholar] [CrossRef]
- Marsh, D.J.; Coulon, V.; Lunetta, K.L.; Rocca-Serra, P.; Dahia, P.L.; Zheng, Z.; Liaw, D.; Caron, S.; Duboue, B.; Lin, A.Y.; et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum. Mol. Genet. 1998, 7, 507–515. [Google Scholar] [CrossRef]
- Marsh, D.J.; Dahia, P.L.; Zheng, Z.; Liaw, D.; Parsons, R.; Gorlin, R.J.; Eng, C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat. Genet. 1997, 16, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Zigman, A.F.; Lavine, J.E.; Jones, M.C.; Boland, C.R.; Carethers, J.M. Localization of the Bannayan-Riley-Ruvalcaba syndrome gene to chromosome 10q23. Gastroenterology 1997, 113, 1433–1437. [Google Scholar] [CrossRef]
- Yehia, L.; Eng, C. 65 YEARS OF THE DOUBLE HELIX: One gene, many endocrine and metabolic syndromes: PTEN-opathies and precision medicine. Endocr. Relat. Cancer 2018, 25, T121–T140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.P.; Waite, K.A.; Pilarski, R.; Hampel, H.; Fernandez, M.J.; Bos, C.; Dasouki, M.; Feldman, G.L.; Greenberg, L.A.; Ivanovich, J.; et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am. J. Hum. Genet. 2003, 73, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef]
- Marsh, D.J.; Kum, J.B.; Lunetta, K.L.; Bennett, M.J.; Gorlin, R.J.; Ahmed, S.F.; Bodurtha, J.; Crowe, C.; Curtis, M.A.; Dasouki, M.; et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum. Mol. Genet. 1999, 8, 1461–1472. [Google Scholar] [CrossRef]
- Zhou, X.; Hampel, H.; Thiele, H.; Gorlin, R.J.; Hennekam, R.C.; Parisi, M.; Winter, R.M.; Eng, C. Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromes. Lancet 2001, 358, 210–211. [Google Scholar] [CrossRef]
- Butler, M.G.; Dasouki, M.J.; Zhou, X.P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Cairns, P.; Okami, K.; Halachmi, S.; Halachmi, N.; Esteller, M.; Herman, J.G.; Jen, J.; Isaacs, W.B.; Bova, G.S.; Sidransky, D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997, 57, 4997–5000. [Google Scholar] [PubMed]
- Mutter, G.L.; Lin, M.C.; Fitzgerald, J.T.; Kum, J.B.; Baak, J.P.; Lees, J.A.; Weng, L.P.; Eng, C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J. Natl. Cancer Inst. 2000, 92, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Zhou, X.P.; Cummings, M.C.; Pavey, S.; Hayward, N.K.; Eng, C. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int. J. Cancer 2002, 99, 63–67. [Google Scholar] [CrossRef]
- Guldberg, P.; thor Straten, P.; Birck, A.; Ahrenkiel, V.; Kirkin, A.F.; Zeuthen, J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997, 57, 3660–3663. [Google Scholar] [PubMed]
- Cancer Genome Atlas Research, N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Heo, M.H.; Kim, J.H.; Cho, B.C.; Cho, E.K.; Min, Y.J.; Lee, K.H.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; et al. Molecular Screening of Small Biopsy Samples Using Next-Generation Sequencing in Korean Patients with Advanced Non-small Cell Lung Cancer: Korean Lung Cancer Consortium (KLCC-13-01). J. Pathol. Transl. Med. 2018, 52, 148–156. [Google Scholar] [CrossRef]
- Shuch, B.; Ricketts, C.J.; Vocke, C.D.; Komiya, T.; Middelton, L.A.; Kauffman, E.C.; Merino, M.J.; Metwalli, A.R.; Dennis, P.; Linehan, W.M. Germline PTEN mutation Cowden syndrome: An underappreciated form of hereditary kidney cancer. J. Urol. 2013, 190, 1990–1998. [Google Scholar] [CrossRef]
- Carbognin, L.; Miglietta, F.; Paris, I.; Dieci, M.V. Prognostic and Predictive Implications of PTEN in Breast Cancer: Unfulfilled Promises but Intriguing Perspectives. Cancers 2019, 11, 1401. [Google Scholar] [CrossRef]
- Wang, S.I.; Puc, J.; Li, J.; Bruce, J.N.; Cairns, P.; Sidransky, D.; Parsons, R. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997, 57, 4183–4186. [Google Scholar] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lin, B.; Ye, Z.; Chen, S.; Yu, H.; Chen, C.; Zhang, X.; Zhou, K.; Zeng, J. Triple-high expression of phosphatase and tensin homolog (PTEN), estrogen receptor (ER) and progesterone receptor (PR) may predict favorable prognosis for patients with Type I endometrial carcinoma. J. Cancer 2020, 11, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Xiang, H.; Ding, P.; Wu, T.; Ji, G. The biochemical and clinical implications of phosphatase and tensin homolog deleted on chromosome ten in different cancers. Am. J. Cancer Res. 2021, 11, 5833–5855. [Google Scholar] [PubMed]
- Denning, G.; Jean-Joseph, B.; Prince, C.; Durden, D.L.; Vogt, P.K. A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene 2007, 26, 3930–3940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, S.M.; Leslie, N.R.; Perera, N.M.; Batty, I.H.; Downes, C.P. The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem. J. 2004, 379, 301–307. [Google Scholar] [CrossRef]
- Yang, J.M.; Schiapparelli, P.; Nguyen, H.N.; Igarashi, A.; Zhang, Q.; Abbadi, S.; Amzel, L.M.; Sesaki, H.; Quinones-Hinojosa, A.; Iijima, M. Characterization of PTEN mutations in brain cancer reveals that pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene 2017, 36, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Ruano, Y.; Ribalta, T.; de Lope, A.R.; Campos-Martin, Y.; Fiano, C.; Perez-Magan, E.; Hernandez-Moneo, J.L.; Mollejo, M.; Melendez, B. Worse outcome in primary glioblastoma multiforme with concurrent epidermal growth factor receptor and p53 alteration. Am. J. Clin. Pathol. 2009, 131, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Wiencke, J.K.; Zheng, S.; Jelluma, N.; Tihan, T.; Vandenberg, S.; Tamguney, T.; Baumber, R.; Parsons, R.; Lamborn, K.R.; Berger, M.S.; et al. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro Oncol. 2007, 9, 271–279. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.Z.; LaPushin, R.; Cuevas, B.; Fang, X.; Yu, S.X.; Davies, M.A.; Khan, H.; Furui, T.; Mao, M.; et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene 1999, 18, 7034–7045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Liang, F.; Jia, Z.L.; Song, S.T.; Jiang, Z.F. PTEN mutation, methylation and expression in breast cancer patients. Oncol. Lett. 2013, 6, 161–168. [Google Scholar] [CrossRef]
- Coughlin, C.M.; Johnston, D.S.; Strahs, A.; Burczynski, M.E.; Bacus, S.; Hill, J.; Feingold, J.M.; Zacharchuk, C.; Berkenblit, A. Approaches and limitations of phosphatidylinositol-3-kinase pathway activation status as a predictive biomarker in the clinical development of targeted therapy. Breast Cancer Res. Treat. 2010, 124, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, C.; Cui, J.; Liu, J.; Li, J.; Jiang, H. The prognostic value and potential drug target of phosphatase and tensin homolog in breast cancer patients: A meta-analysis. Medicine 2017, 96, e8000. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, J.; Mo, X. The association of PTEN hypermethylation and breast cancer: A meta-analysis. OncoTargets Ther. 2016, 9, 5643–5650. [Google Scholar] [CrossRef] [PubMed]
- Gray, I.C.; Phillips, S.M.; Lee, S.J.; Neoptolemos, J.P.; Weissenbach, J.; Spurr, N.K. Loss of the chromosomal region 10q23-25 in prostate cancer. Cancer Res. 1995, 55, 4800–4803. [Google Scholar] [PubMed]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Leinonen, K.A.; Saramaki, O.R.; Furusato, B.; Kimura, T.; Takahashi, H.; Egawa, S.; Suzuki, H.; Keiger, K.; Ho Hahm, S.; Isaacs, W.B.; et al. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Ludkovski, O.; DeGrace, D.; Williams, J.L.; Evans, A.; Sircar, K.; Bismar, T.A.; Nuin, P.; Squire, J.A. PTEN genomic deletions that characterize aggressive prostate cancer originate close to segmental duplications. Genes Chromosomes Cancer 2012, 51, 149–160. [Google Scholar] [CrossRef]
- Leslie, N.R.; Foti, M. Non-genomic loss of PTEN function in cancer: Not in my genes. Trends Pharmacol. Sci. 2011, 32, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Whang, Y.E.; Wu, X.; Suzuki, H.; Reiter, R.E.; Tran, C.; Vessella, R.L.; Said, J.W.; Isaacs, W.B.; Sawyers, C.L. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl. Acad. Sci. USA 1998, 95, 5246–5250. [Google Scholar] [CrossRef]
- Gravina, G.L.; Biordi, L.; Martella, F.; Flati, V.; Ricevuto, E.; Ficorella, C.; Tombolini, V.; Festuccia, C. Epigenetic modulation of PTEN expression during antiandrogenic therapies in human prostate cancer. Int. J. Oncol. 2009, 35, 1133–1139. [Google Scholar] [CrossRef][Green Version]
- Cancer Genome Atlas, N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Serebriiskii, I.G.; Pavlov, V.; Tricarico, R.; Andrianov, G.; Nicolas, E.; Parker, M.I.; Newberg, J.; Frampton, G.; Meyer, J.E.; Golemis, E.A. Comprehensive characterization of PTEN mutational profile in a series of 34,129 colorectal cancers. Nat. Commun. 2022, 13, 1618. [Google Scholar] [CrossRef]
- Berg, M.; Danielsen, S.A.; Ahlquist, T.; Merok, M.A.; Agesen, T.H.; Vatn, M.H.; Mala, T.; Sjo, O.H.; Bakka, A.; Moberg, I.; et al. DNA sequence profiles of the colorectal cancer critical gene set KRAS-BRAF-PIK3CA-PTEN-TP53 related to age at disease onset. PLoS ONE 2010, 5, e13978. [Google Scholar] [CrossRef]
- Bohn, B.A.; Mina, S.; Krohn, A.; Simon, R.; Kluth, M.; Harasimowicz, S.; Quaas, A.; Bockhorn, M.; Izbicki, J.R.; Sauter, G.; et al. Altered PTEN function caused by deletion or gene disruption is associated with poor prognosis in rectal but not in colon cancer. Hum. Pathol. 2013, 44, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Jauhri, M.; Bhatnagar, A.; Gupta, S.; Shokeen, Y.; Minhas, S.; Aggarwal, S. Targeted molecular profiling of rare genetic alterations in colorectal cancer using next-generation sequencing. Med. Oncol. 2016, 33, 106. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, J.K.; Lin, H.H.; Lan, Y.T.; Lin, C.C.; Yang, S.H.; Chen, W.S.; Liang, W.Y.; Jiang, J.K.; Chang, S.C. A comprehensive analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss in colorectal cancer. World J. Surg. Oncol. 2015, 13, 186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goel, A.; Arnold, C.N.; Niedzwiecki, D.; Carethers, J.M.; Dowell, J.M.; Wasserman, L.; Compton, C.; Mayer, R.J.; Bertagnolli, M.M.; Boland, C.R. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004, 64, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J.; Zheng, S.; Aldape, K.; Hinds, P.W.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. PTEN expression in non-small-cell lung cancer: Evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 2005, 36, 768–776. [Google Scholar] [CrossRef]
- Di Cristofano, A.; Ellenson, L.H. Endometrial carcinoma. Annu. Rev. Pathol. 2007, 2, 57–85. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, H.B.; MacDonald, N.; Ryan, A.; Jacobs, I.J.; Lynch, E.D.; Akslen, L.A.; Das, S. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int. J. Cancer 2001, 91, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian Cancers: Genetic Abnormalities, Tumor Heterogeneity and Progression, Clonal Evolution and Cancer Stem Cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef]
- Steelman, L.S.; Bertrand, F.E.; McCubrey, J.A. The complexity of PTEN: Mutation, marker and potential target for therapeutic intervention. Expert Opin. Ther. Targets 2004, 8, 537–550. [Google Scholar] [CrossRef]
- McConechy, M.K.; Ding, J.; Senz, J.; Yang, W.; Melnyk, N.; Tone, A.A.; Prentice, L.M.; Wiegand, K.C.; McAlpine, J.N.; Shah, S.P.; et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod. Pathol. 2014, 27, 128–134. [Google Scholar] [CrossRef]
- Kolasa, I.K.; Rembiszewska, A.; Janiec-Jankowska, A.; Dansonka-Mieszkowska, A.; Lewandowska, A.M.; Konopka, B.; Kupryjanczyk, J. PTEN mutation, expression and LOH at its locus in ovarian carcinomas. Relation to TP53, K-RAS and BRCA1 mutations. Gynecol. Oncol. 2006, 103, 692–697. [Google Scholar] [CrossRef]
- Merritt, M.A.; Cramer, D.W. Molecular pathogenesis of endometrial and ovarian cancer. Cancer Biomark. 2010, 9, 287–305. [Google Scholar] [CrossRef]
- Schondorf, T.; Ebert, M.P.; Hoffmann, J.; Becker, M.; Moser, N.; Pur, S.; Gohring, U.J.; Weisshaar, M.P. Hypermethylation of the PTEN gene in ovarian cancer cell lines. Cancer Lett. 2004, 207, 215–220. [Google Scholar] [CrossRef]
- Alimonti, A. PTEN breast cancer susceptibility: A matter of dose. Ecancermedicalscience 2010, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.T.; Yang, Z.; Lu, N.H. Roles of PTEN (Phosphatase and Tensin Homolog) in gastric cancer development and progression. Asian Pac. J. Cancer Prev. 2014, 15, 17–24. [Google Scholar] [CrossRef]
- Vidotto, T.; Melo, C.M.; Lautert-Dutra, W.; Chaves, L.P.; Reis, R.B.; Squire, J.A. Pan-cancer genomic analysis shows hemizygous PTEN loss tumors are associated with immune evasion and poor outcome. Sci. Rep. 2023, 13, 5049. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, L.; Li, S.L.; Gu, J.; Cui, X.; Zhou, Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer 2021, 21, 429. [Google Scholar] [CrossRef]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Ciuffreda, L.; Ferretti, G.; Vari, S.; Ferraresi, V.; Cognetti, F.; Milella, M. PTEN Function at the Interface between Cancer and Tumor Microenvironment: Implications for Response to Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5337. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Lan, K.H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; Dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef]
- Centomo, M.L.; Vitiello, M.; Poliseno, L.; Pandolfi, P.P. An Immunocompetent Environment Unravels the Proto-Oncogenic Role of miR-22. Cancers 2022, 14, 6255. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Chekulaeva, M.; Filipowicz, W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 2009, 21, 452–460. [Google Scholar] [CrossRef]
- Egawa, H.; Jingushi, K.; Hirono, T.; Ueda, Y.; Kitae, K.; Nakata, W.; Fujita, K.; Uemura, M.; Nonomura, N.; Tsujikawa, K. The miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating PTEN. Sci. Rep. 2016, 6, 20574. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cui, R.; Bahr, J.; Zanesi, N.; Luo, Z.; Meng, W.; Liang, G.; Croce, C.M. miR-130a Deregulates PTEN and Stimulates Tumor Growth. Cancer Res. 2017, 77, 6168–6178. [Google Scholar] [CrossRef] [PubMed]
- Sekino, Y.; Sakamoto, N.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. miR-130b Promotes Sunitinib Resistance through Regulation of PTEN in Renal Cell Carcinoma. Oncology 2019, 97, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, S.; Xu, Y.; Shu, R.; Wang, F.; Chen, C.; Zeng, Y.; Luo, H. Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN. Cancer Res. Treat. 2018, 50, 1396–1417. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Fan, K.C.; Zhang, J.H.; Chen, H.J.; Wang, S.S. Suppression of microRNA-130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int. J. Mol. Med. 2018, 41, 284–292. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.; Sun, L.; Yan, Q.; Zhang, Y.; Zhang, Z.; Su, Y.; Wang, C. MicroRNA-130b targets PTEN to induce resistance to cisplatin in lung cancer cells by activating Wnt/beta-catenin pathway. Cell Biochem. Funct. 2018, 36, 194–202. [Google Scholar] [CrossRef]
- Liu, H.L.; Bao, H.G.; Zheng, C.L.; Teng, C.; Bai, M.H. MiR-130a regulating the biological function of colon cancer by targeting inhibition of PTEN. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, Y.; Nie, L.; Qian, S.; Xu, M. MiR-130 exerts tumor suppressive function on the tumorigenesis of human non-small cell lung cancer by targeting PTEN. Am. J. Transl. Res. 2017, 9, 1856–1865. [Google Scholar] [PubMed]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Xiang, Y.; Tian, Q.; Guan, L.; Niu, S.S. The Dual Role of miR-186 in Cancers: Oncomir Battling with Tumor Suppressor miRNA. Front. Oncol. 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef]
- Johnsson, P.; Ackley, A.; Vidarsdottir, L.; Lui, W.O.; Corcoran, M.; Grander, D.; Morris, K.V. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat. Struct. Mol. Biol. 2013, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Hesson, L.B.; Ward, R.L. The importance of distinguishing pseudogenes from parental genes. Clin. Epigenet. 2014, 6, 90. [Google Scholar] [CrossRef][Green Version]
- Cao, L.Q.; Yang, X.W.; Chen, Y.B.; Zhang, D.W.; Jiang, X.F.; Xue, P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol. Cancer 2019, 18, 148. [Google Scholar] [CrossRef]
- Yu, G.; Yao, W.; Gumireddy, K.; Li, A.; Wang, J.; Xiao, W.; Chen, K.; Xiao, H.; Li, H.; Tang, K.; et al. Pseudogene PTENP1 functions as a competing endogenous RNA to suppress clear-cell renal cell carcinoma progression. Mol. Cancer Ther. 2014, 13, 3086–3097. [Google Scholar] [CrossRef]
- Gao, L.; Ren, W.; Zhang, L.; Li, S.; Kong, X.; Zhang, H.; Dong, J.; Cai, G.; Jin, C.; Zheng, D.; et al. PTENp1, a natural sponge of miR-21, mediates PTEN expression to inhibit the proliferation of oral squamous cell carcinoma. Mol. Carcinog. 2017, 56, 1322–1334. [Google Scholar] [CrossRef]
- Chen, C.L.; Tseng, Y.W.; Wu, J.C.; Chen, G.Y.; Lin, K.C.; Hwang, S.M.; Hu, Y.C. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials 2015, 44, 71–81. [Google Scholar] [CrossRef]
- Qian, Y.Y.; Li, K.; Liu, Q.Y.; Liu, Z.S. Long non-coding RNA PTENP1 interacts with miR-193a-3p to suppress cell migration and invasion through the PTEN pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 107859–107869. [Google Scholar] [CrossRef]
- Li, R.K.; Gao, J.; Guo, L.H.; Huang, G.Q.; Luo, W.H. PTENP1 acts as a ceRNA to regulate PTEN by sponging miR-19b and explores the biological role of PTENP1 in breast cancer. Cancer Gene Ther. 2017, 24, 309–315. [Google Scholar] [CrossRef]
- Shi, X.; Tang, X.; Su, L. Overexpression of Long Noncoding RNA PTENP1 Inhibits Cell Proliferation and Migration via Suppression of miR-19b in Breast Cancer Cells. Oncol. Res. 2018, 26, 869–878. [Google Scholar] [CrossRef]
- Gao, X.; Qin, T.; Mao, J.; Zhang, J.; Fan, S.; Lu, Y.; Sun, Z.; Zhang, Q.; Song, B.; Li, L. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J. Exp. Clin. Cancer Res. 2019, 38, 256. [Google Scholar] [CrossRef]
- Yu, G.; Ou, Z.Y.; Tao, Q.Y.; Wan, G.Y.; Lu, Z.H.; Lang, B. Role of lncRNA PTENP1 in tumorigenesis and progression of bladder cancer and the molecular mechanism. J. South. Med. Univ. 2017, 37, 1494–1500. [Google Scholar] [CrossRef]
- Hao, S.C.; Ma, H.; Niu, Z.F.; Sun, S.Y.; Zou, Y.R.; Xia, H.C. hUC-MSCs secreted exosomes inhibit the glioma cell progression through PTENP1/miR-10a-5p/PTEN pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10013–10023. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, M.; Liu, W.; Chen, H.; Cai, T.; Xiong, H.; Sheng, X.; Liu, S.; Peng, J.; Wang, F.; et al. Estrogen affects the negative feedback loop of PTENP1-miR200c to inhibit PTEN expression in the development of endometrioid endometrial carcinoma. Cell Death Dis. 2018, 10, 4. [Google Scholar] [CrossRef]
- Fan, Y.; Sheng, W.; Meng, Y.; Cao, Y.; Li, R. LncRNA PTENP1 inhibits cervical cancer progression by suppressing miR-106b. Artif. Cells Nanomed. Biotechnol. 2020, 48, 393–407. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, Y.; Ma, Z.; Ma, G.; Xue, Q.; Li, F.; Liu, L. Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget 2017, 8, 26079–26089. [Google Scholar] [CrossRef]
- Lister, N.C.; Johnsson, P.; Waters, P.D.; Morris, K.V. Pseudogenes: A Novel Source of Trans-Acting Antisense RNAs. Methods Mol. Biol. 2021, 2324, 219–236. [Google Scholar] [CrossRef]
- Lister, N.; Shevchenko, G.; Walshe, J.L.; Groen, J.; Johnsson, P.; Vidarsdottir, L.; Grander, D.; Ataide, S.F.; Morris, K.V. The molecular dynamics of long noncoding RNA control of transcription in PTEN and its pseudogene. Proc. Natl. Acad. Sci. USA 2017, 114, 9942–9947. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Tay, Y.; Kats, L.; Salmena, L.; Weiss, D.; Tan, S.M.; Ala, U.; Karreth, F.; Poliseno, L.; Provero, P.; Di Cunto, F.; et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 2011, 147, 344–357. [Google Scholar] [CrossRef]
- Wu, C.; Wang, F.; Tan, L. Role and the molecular mechanism of lncRNA PTENP1 in regulating the proliferation and invasion of cervical cancer cells. Gene Ther. 2020, 29, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Deng, L.; Deng, K.; Wang, H.; Shan, T.; Zhou, H.; Liang, Z.; Xia, J.; Li, C. Pseudogene PTENP1 Suppresses Gastric Cancer Progression by Modulating PTEN. Anticancer Agents Med. Chem. 2016, 16, 456–464. [Google Scholar] [CrossRef]
- Khan, I.; Kerwin, J.; Owen, K.; Griner, E.; Reproducibility Project: Cancer Biology. Registered report: A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Elife 2015, 4, e08245. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.; Khan, I.; Reproducibility Project: Cancer Biology. Replication Study: A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Elife 2020, 9, e51019. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, M.; Evangelista, M.; Zhang, Y.; Salmena, L.; Pandolfi, P.P.; Poliseno, L. PTENP1 is a ceRNA for PTEN: It’s CRISPR clear. J. Hematol. Oncol. 2020, 13, 73. [Google Scholar] [CrossRef]
- Xia, T.; Chen, S.; Jiang, Z.; Shao, Y.; Jiang, X.; Li, P.; Xiao, B.; Guo, J. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci. Rep. 2015, 5, 13445. [Google Scholar] [CrossRef]
- Fei, D.; Zhang, X.; Liu, J.; Tan, L.; Xing, J.; Zhao, D.; Zhang, Y. Long Noncoding RNA FER1L4 Suppresses Tumorigenesis by Regulating the Expression of PTEN Targeting miR-18a-5p in Osteosarcoma. Cell. Physiol. Biochem. 2018, 51, 1364–1375. [Google Scholar] [CrossRef]
- Qin, N.; Tong, G.F.; Sun, L.W.; Xu, X.L. Long Noncoding RNA MEG3 Suppresses Glioma Cell Proliferation, Migration, and Invasion by Acting as a Competing Endogenous RNA of miR-19a. Oncol. Res. 2017, 25, 1471–1478. [Google Scholar] [CrossRef]
- Li, D.; Feng, J.; Wu, T.; Wang, Y.; Sun, Y.; Ren, J.; Liu, M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am. J. Pathol. 2013, 182, 64–70. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Sui, J.; Yang, X.; Qi, W.; Guo, K.; Gao, Z.; Wang, L.; Sun, D. Long Non-Coding RNA Linc-USP16 Functions as a Tumour Suppressor in Hepatocellular Carcinoma by Regulating PTEN Expression. Cell. Physiol. Biochem. 2017, 44, 1188–1198. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zou, W.; Hu, C.; Li, G.; Zhou, S.; He, Y.; Ma, F.; Deng, C.; Sun, L. Modulation of CASC2/miR-21/PTEN pathway sensitizes cervical cancer to cisplatin. Arch. Biochem. Biophys. 2017, 623–624, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, X.Y.; Jin, Z.L. Linc00702 inhibits cell growth and metastasis through regulating PTEN in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Lin, S.; Zheng, M.; Cai, Q.; Tu, Y. Long noncoding RNA NEAT1 promotes the growth of cervical cancer cells via sponging miR-9-5p. Biochem. Cell Biol. 2019, 97, 100–108. [Google Scholar] [CrossRef]
- Chi, H.; Yang, R.; Zheng, X.; Zhang, L.; Jiang, R.; Chen, J. LncRNA RP11-79H23.3 Functions as a Competing Endogenous RNA to Regulate PTEN Expression through Sponging hsa-miR-107 in the Development of Bladder Cancer. Int. J. Mol. Sci. 2018, 19, 2531. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, L.; Zheng, M.; Xu, J.; Wang, W. lncRNA TP73-AS1 Regulates miR-21/PTEN Axis to Affect Cell Proliferation in Acute Myeloid Leukemia. Cancer Biother. Radiopharm. 2021, 36, 268–272. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Gong, R.; Gao, M.; Feng, C.; Liu, T.; Sun, Y.; Jin, M.; Wang, D.; Yuan, Y.; et al. The Long Non-coding RNA-ORLNC1 Regulates Bone Mass by Directing Mesenchymal Stem Cell Fate. Mol. Ther. 2019, 27, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Sumazin, P.; Yang, X.; Chiu, H.S.; Chung, W.J.; Iyer, A.; Llobet-Navas, D.; Rajbhandari, P.; Bansal, M.; Guarnieri, P.; Silva, J.; et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 2011, 147, 370–381. [Google Scholar] [CrossRef]
- Zarringhalam, K.; Tay, Y.; Kulkarni, P.; Bester, A.C.; Pandolfi, P.P.; Kulkarni, R.V. Identification of competing endogenous RNAs of the tumor suppressor gene PTEN: A probabilistic approach. Sci. Rep. 2017, 7, 7755. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.S.; Martinez, M.R.; Komissarova, E.V.; Llobet-Navas, D.; Bansal, M.; Paull, E.O.; Silva, J.; Yang, X.; Sumazin, P.; Califano, A. The number of titrated microRNA species dictates ceRNA regulation. Nucleic Acids Res. 2018, 46, 4354–4369. [Google Scholar] [CrossRef]
- Kovalenko, T.F.; Morozova, K.V.; Pavlyukov, M.S.; Anufrieva, K.S.; Bobrov, M.Y.; Gamisoniya, A.M.; Ozolinya, L.A.; Dobrokhotova, Y.E.; Shakhparonov, M.I.; Patrushev, L.I. Methylation of the PTENP1 pseudogene as potential epigenetic marker of age-related changes in human endometrium. PLoS ONE 2021, 16, e0243093. [Google Scholar] [CrossRef] [PubMed]
- Hesson, L.B.; Packham, D.; Pontzer, E.; Funchain, P.; Eng, C.; Ward, R.L. A reinvestigation of somatic hypermethylation at the PTEN CpG island in cancer cell lines. Biol. Proced. Online 2012, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, S.; Austreid, E.; Skaftnesmo, K.O.; Lonning, P.E.; Eikesdal, H.P. Divergent Activity of the Pseudogene PTENP1 in ER-Positive and Negative Breast Cancer. Mol. Cancer Res. 2018, 16, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, T.F.; Sorokina, A.V.; Ozolinia, L.A.; Patrushev, L.I. Pseudogene PTENP1 5’-region methylation in endometrial cancer and hyperplasias. Russ. J. Bioorg. Chem. 2013, 39, 397–405. [Google Scholar] [CrossRef]
- Kovalenko, T.F.; Morozova, K.V.; Ozolinya, L.A.; Lapina, I.A.; Patrushev, L.I. The PTENP1 Pseudogene, Unlike the PTEN Gene, Is Methylated in Normal Endometrium, As Well As in Endometrial Hyperplasias and Carcinomas in Middle-Aged and Elderly Females. Acta Naturae 2018, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ioffe, Y.J.; Chiappinelli, K.B.; Mutch, D.G.; Zighelboim, I.; Goodfellow, P.J. Phosphatase and tensin homolog (PTEN) pseudogene expression in endometrial cancer: A conserved regulatory mechanism important in tumorigenesis? Gynecol. Oncol. 2012, 124, 340–346. [Google Scholar] [CrossRef]
- Ulger, C.; Toruner, G.A.; Alkan, M.; Mohammed, M.; Damani, S.; Kang, J.; Galante, A.; Aviv, H.; Soteropoulos, P.; Tolias, P.P.; et al. Comprehensive genome-wide comparison of DNA and RNA level scan using microarray technology for identification of candidate cancer-related genes in the HL-60 cell line. Cancer Genet. Cytogenet. 2003, 147, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xing, Y.; Xu, L.; Chen, W.; Cao, W.; Zhang, C. Decreased expression of pseudogene PTENP1 promotes malignant behaviours and is associated with the poor survival of patients with HNSCC. Sci. Rep. 2017, 7, 41179. [Google Scholar] [CrossRef]
- Dai, C.; Sheng, X.; Wang, J.; Dai, Y.; Kuang, Y.; Xu, Z.; Guo, Y. Prognostic Value of PTENP1 Expression in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin. Lab. 2022, 68, 131–140. [Google Scholar] [CrossRef]
- Vidarsdottir, L.; Azimi, A.; Das, I.; Sigvaldadottir, I.; Suryo Rahmanto, A.; Petri, A.; Kauppinen, S.; Ingvar, C.; Jonsson, G.; Olsson, H.; et al. PTENP1-AS contributes to BRAF inhibitor resistance and is associated with adverse clinical outcome in stage III melanoma. Sci. Rep. 2021, 11, 11023. [Google Scholar] [CrossRef]
- Yan, B.; Wubuli, A.; Liu, Y.; Wang, X. Long non-coding RNA phosphatase and tensin homolog pseudogene 1 suppresses osteosarcoma cell growth via the phosphoinositide 3-kinase/protein kinase B signaling pathway. Exp. Ther. Med. 2018, 15, 4829–4837. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Li, L.; Luo, D.; Zhao, H.; Li, D.; Peng, B. Overexpression of lncRNA PTENP1 suppresses glioma cell proliferation and metastasis in vitro. OncoTargets Ther. 2019, 12, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Du, M.; Wang, X.; Xu, W.; Liang, J.; Wang, W.; Lv, Q.; Qin, C.; Chu, H.; Wang, M.; et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer 2018, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, S.; Austreid, E.; Knappskog, S.; Chrisanthar, R.; Lilleng, P.K.; Lonning, P.E.; Eikesdal, H.P. High PTEN gene expression is a negative prognostic marker in human primary breast cancers with preserved p53 function. Breast Cancer Res. Treat. 2017, 163, 177–190. [Google Scholar] [CrossRef]
- Poliseno, L.; Haimovic, A.; Christos, P.J.; Vega, Y.S.d.M.E.C.; Shapiro, R.; Pavlick, A.; Berman, R.S.; Darvishian, F.; Osman, I. Deletion of PTENP1 pseudogene in human melanoma. J. Investig. Dermatol. 2011, 131, 2497–2500. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Zhang, J.H.; Xia, Q.J.; Sun, Q.; Li, Z.K.; Zhang, J.G.; Tang, M.S.; Dong, M.S. Long non-coding RNA PTENP1 inhibits proliferation and migration of breast cancer cells via AKT and MAPK signaling pathways. Oncol. Lett. 2017, 14, 4659–4662. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, J.; Zhong, L.; He, X.; Si, X.; Sun, Y.; Chen, Y.; Zhong, J.; Hu, Y.; Li, B.; et al. The pseudogene PTENP1 regulates smooth muscle cells as a competing endogenous RNA. Clin. Sci. 2019, 133, 1439–1455. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Han, X.; Qu, P.; Wang, W. Long noncoding RNA PTENP1 affects the recovery of spinal cord injury by regulating the expression of miR-19b and miR-21. J. Cell Physiol. 2020, 235, 3634–3645. [Google Scholar] [CrossRef]
- Takamura, M.; Zhou, W.; Rombauts, L.; Dimitriadis, E. The long noncoding RNA PTENP1 regulates human endometrial epithelial adhesive capacity in vitro: Implications in infertility. Biol. Reprod. 2020, 102, 53–62. [Google Scholar] [CrossRef]
- Tang, J.; Ning, R.; Zeng, B.; Li, Y. Molecular Evolution of PTEN Pseudogenes in Mammals. PLoS ONE 2016, 11, e0167851. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, T.F.; Yadav, B.; Anufrieva, K.S.; Larionova, T.D.; Aksinina, T.E.; Latyshev, Y.A.; Bastola, S.; Shakhparonov, M.I.; Pandey, A.K.; Pavlyukov, M.S. PTEN regulates expression of its pseudogene in glioblastoma cells in DNA methylation-dependent manner. Biochimie 2023, in press. [Google Scholar] [CrossRef]
- Gong, T.; Zheng, S.; Huang, S.; Fu, S.; Zhang, X.; Pan, S.; Yang, T.; Sun, Y.; Wang, Y.; Hui, B.; et al. PTENP1 inhibits the growth of esophageal squamous cell carcinoma by regulating SOCS6 expression and correlates with disease prognosis. Mol. Carcinog. 2017, 56, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Wang, Y.; Ding, J.; Jiang, A.; Wang, J.; Yu, M.; Blake, S.; Liu, S.; Bieberich, C.J.; Farokhzad, O.C.; et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 2021, 13, eaba9772. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Li, J.; Su, Q.; Ma, H.; Wei, Q.; Li, H.; Gao, G. rAAV-delivered PTEN therapeutics for prostate cancer. Mol. Ther. Nucleic Acids 2022, 27, 122–132. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Xin, W.; Zhao, S.; Han, X.; Zhao, P.; Yu, H.; Gao, X.; Li, P.; Wu, Q.; Ding, J.; Hua, K. lncRNA LA16c-313D11.11 modulates the development of endometrial cancer by binding to and inhibiting microRNA-205-5p function and indirectly increasing PTEN activity. Int. J. Oncol. 2020, 57, 355–363. [Google Scholar] [CrossRef]
- Chen, L.; Ren, P.; Zhang, Y.; Gong, B.; Yu, D.; Sun, X. Long non-coding RNA GAS5 increases the radiosensitivity of A549 cells through interaction with the miR-21/PTEN/Akt axis. Oncol. Rep. 2020, 43, 897–907. [Google Scholar] [CrossRef]
- Ouyang, L.; Yang, M.; Wang, X.; Fan, J.; Liu, X.; Zhang, Y.; Shu, Y. Long non-coding RNA FER1L4 inhibits cell proliferation and promotes cell apoptosis via the PTEN/AKT/p53 signaling pathway in lung cancer. Oncol. Rep. 2021, 45, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Klicka, K.; Grzywa, T.M.; Mielniczuk, A.; Klinke, A.; Wlodarski, P.K. The role of miR-200 family in the regulation of hallmarks of cancer. Front. Oncol. 2022, 12, 965231. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lu, R.L.; Li, J.X.; Rong, L.J. MiR-200a and miR-200b target PTEN to regulate the endometrial cancer cell growth in vitro. Asian Pac. J. Trop. Med. 2017, 10, 498–502. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Li, L.; Deng, H.; Xian, L. MicroRNA-200a promotes esophageal squamous cell carcinoma cell proliferation, migration and invasion through extensive target genes. Mol. Med. Rep. 2020, 21, 2073–2084. [Google Scholar] [CrossRef]
- Chen, P.; Guo, X.; Zhang, L.; Zhang, W.; Zhou, Q.; Tian, Z.; Zheng, Y.; Liao, Q.; Wang, H.; Li, G.; et al. MiR-200c is a cMyc-activated miRNA that promotes nasopharyngeal carcinoma by downregulating PTEN. Oncotarget 2017, 8, 5206–5218. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Xu, S.; Ma, J.; Wu, J.; Jin, S.; Cao, S.; Yu, Y. MicroRNA-429 induces tumorigenesis of human non-small cell lung cancer cells and targets multiple tumor suppressor genes. Biochem. Biophys. Res. Commun. 2014, 450, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, S.; Hansen, J.B.; Snir, O. Optimized workflow to modify microRNA expression in primary human intravascular cells. BMC Immunol. 2023, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]

| Cancer Type | Mutation | Deletion | Loss of Protein | Promoter Methylation | ||||
|---|---|---|---|---|---|---|---|---|
| Glioblastoma | 30% | [2,3,42,44,45,46] | 78% | [44,45,46,47] | 65% | [48] | 6% | [49] |
| Breast | 3% | [42,50,51] | 27% | [38,52] | 40% | [42] | 35% | [53,54] |
| Prostate | 13% | [55,56,57,58] | 51% | [56,57,58] | 54% | [55,56,57,58] | <5% | [42,59,60,61] |
| Colorectal | 7% | [6,42,62,63,64,65,66] | 8.7% | [42,62,63] | 40% | [67] | 17% | [68] |
| Lung | 8% | [42] | 34% | [42] | 56% | [42] | 38% | [69] |
| Endometrial | 41% | [14,42,70] | 48% | [14,42,70] | 45% | [14,41] | 19% | [42,71] |
| Ovarian | 16% | [42,43,72,73,74,75,76] | 48% | [42,43,72,73,74,75,76] | 44% | [42,43,72,73,74,75,76] | 10% | [42,77] |
| Disease | microRNAs (miR) * | References |
|---|---|---|
| Prostate cancer | miR-17-5p miR-19-3p miR-21-5p miR-26a-5p miR-214-3p | [17] [17] [17] [17] [17] |
| Hepatocellular carcinoma | miR-17-5p miR-19b-3p miR-20a-5p miR-193a-3p miR-21 | [107] [107] [107] [108] [104] |
| Clear cell renal carcinoma | miR-21 | [105] |
| Breast cancer | miR-19b miR-20a | [109,110] [111] |
| Bladder cancer | miR-17 | [112] |
| Glioma | miR-10-5p | [113] |
| Endometrial cancer | miR-200c | [114] |
| Cervical cancer | miR-106b | [115] |
| Gastric cancer | miR-106b miR-93 | [116] [116] |
| Oral squamous cell carcinomas | miR-21-5p | [106] |
| Cancer Tissue Type/Cancer Cells | PTENP1 Promoter Methylation Status | Reference(s) |
|---|---|---|
| Breast cancer | Hypermethylated | [142] |
| MDA-MB-231 breast cancer cells | Hypermethylated | [144] |
| MCF-7 breast cancer cells | Unmethylated | [144] |
| Cervical cancer | Hypermethylated | [142] |
| Ovarian cancer | Hypermethylated | [142] |
| Hepatocellular carcinoma cell lines | Hypermethylated | [142] |
| Lymphoma | Hypermethylated | [143] |
| Colorectal cancer | Hypermethylated | [143] |
| Clear cell renal carcinoma cells | Hypermethylated | [105,143] |
| Endometrial cancer and hyperplasia | Hypermethylated | [142,146] |
| PTEN:PTENP1 Relative Expression Ratio * | Cell Line or Tissue Type | References |
|---|---|---|
| ↑PTEN:PTENP1-S | Osteosarcoma cell lines | [152] |
| Melanoma cell lines | [151] | |
| Breast cancer cell lines and tissue samples | [102,109,110,111] | |
| Bladder cancer tissue | [112] | |
| Gastric cancer cells and tissues | [116] | |
| Oral squamous cell carcinoma cells | [106] | |
| Hepatocellular carcinoma cell lines and tissues | [107,108] | |
| Head and neck squamous cell carcinoma cells | [149] | |
| Glioma tissue | [112] | |
| Prostate cell lines | [17] | |
| Cervical cancer cells | [102,121] | |
| Endometrioid endometrial carcinoma cells | [114] | |
| Melanoma | [151] | |
| ↓PTEN:PTENP1-S | Some prostate cancer tissue samples, gastric cancer cell line, AGS, endometrioid endometrial carcinoma cell lines, RL-952, and JEC | [17] [116] [114] |
| ↓PTENP1-S:PTENP1-AS | Kidney, HEK-293T, breast, MCF-7, cervix, HeLa, bone, U-2OS | [102] [102] [102] [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travis, G.; McGowan, E.M.; Simpson, A.M.; Marsh, D.J.; Nassif, N.T. PTEN, PTENP1, microRNAs, and ceRNA Networks: Precision Targeting in Cancer Therapeutics. Cancers 2023, 15, 4954. https://doi.org/10.3390/cancers15204954
Travis G, McGowan EM, Simpson AM, Marsh DJ, Nassif NT. PTEN, PTENP1, microRNAs, and ceRNA Networks: Precision Targeting in Cancer Therapeutics. Cancers. 2023; 15(20):4954. https://doi.org/10.3390/cancers15204954
Chicago/Turabian StyleTravis, Glena, Eileen M. McGowan, Ann M. Simpson, Deborah J. Marsh, and Najah T. Nassif. 2023. "PTEN, PTENP1, microRNAs, and ceRNA Networks: Precision Targeting in Cancer Therapeutics" Cancers 15, no. 20: 4954. https://doi.org/10.3390/cancers15204954
APA StyleTravis, G., McGowan, E. M., Simpson, A. M., Marsh, D. J., & Nassif, N. T. (2023). PTEN, PTENP1, microRNAs, and ceRNA Networks: Precision Targeting in Cancer Therapeutics. Cancers, 15(20), 4954. https://doi.org/10.3390/cancers15204954









