Prognostic Relevance of Tumor-Infiltrating Immune Cells in Cervix Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Sample
2.2. Ethical Approval
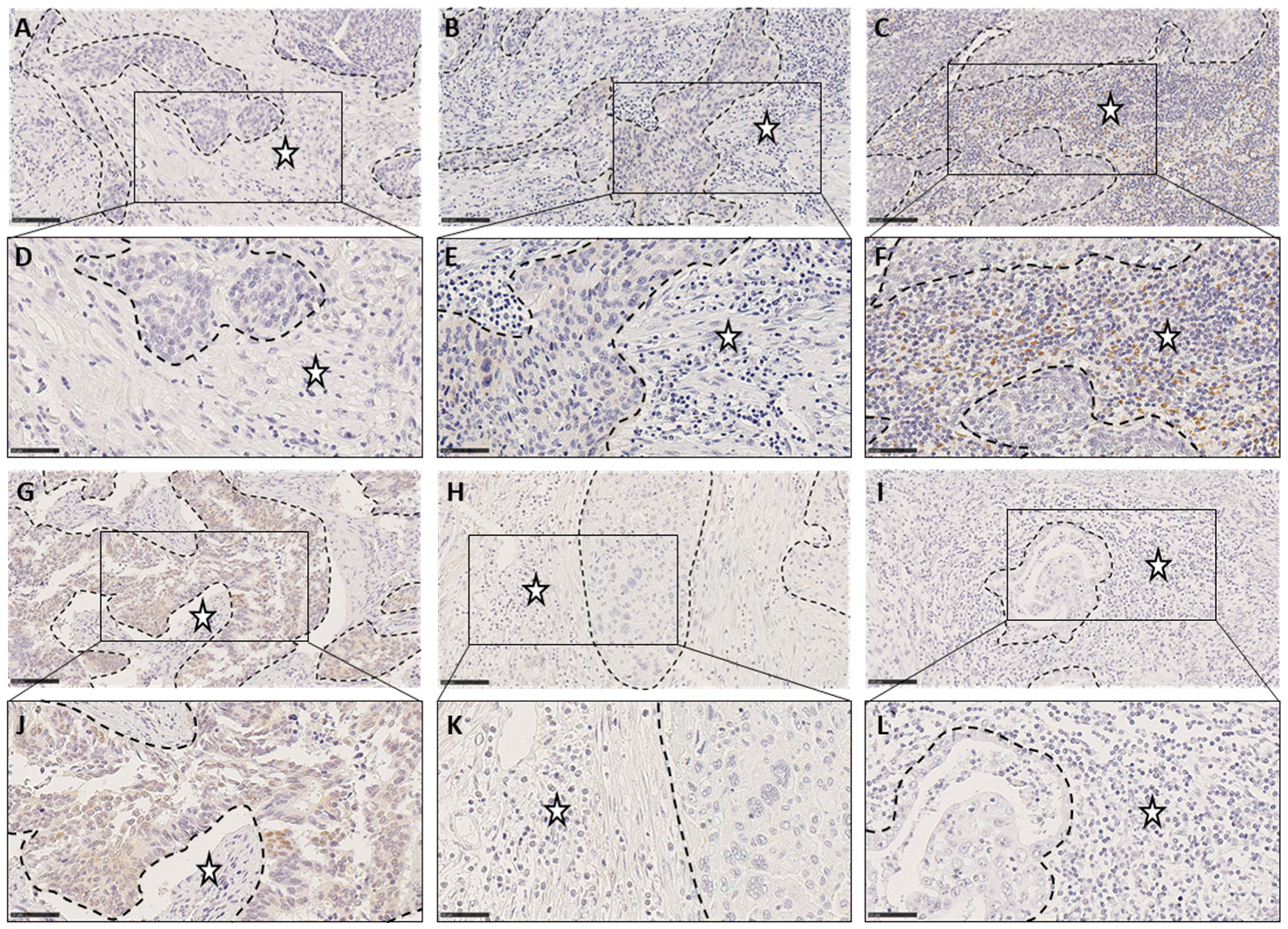
2.3. Quantification of TIICs
2.4. Statistical Analysis
3. Results
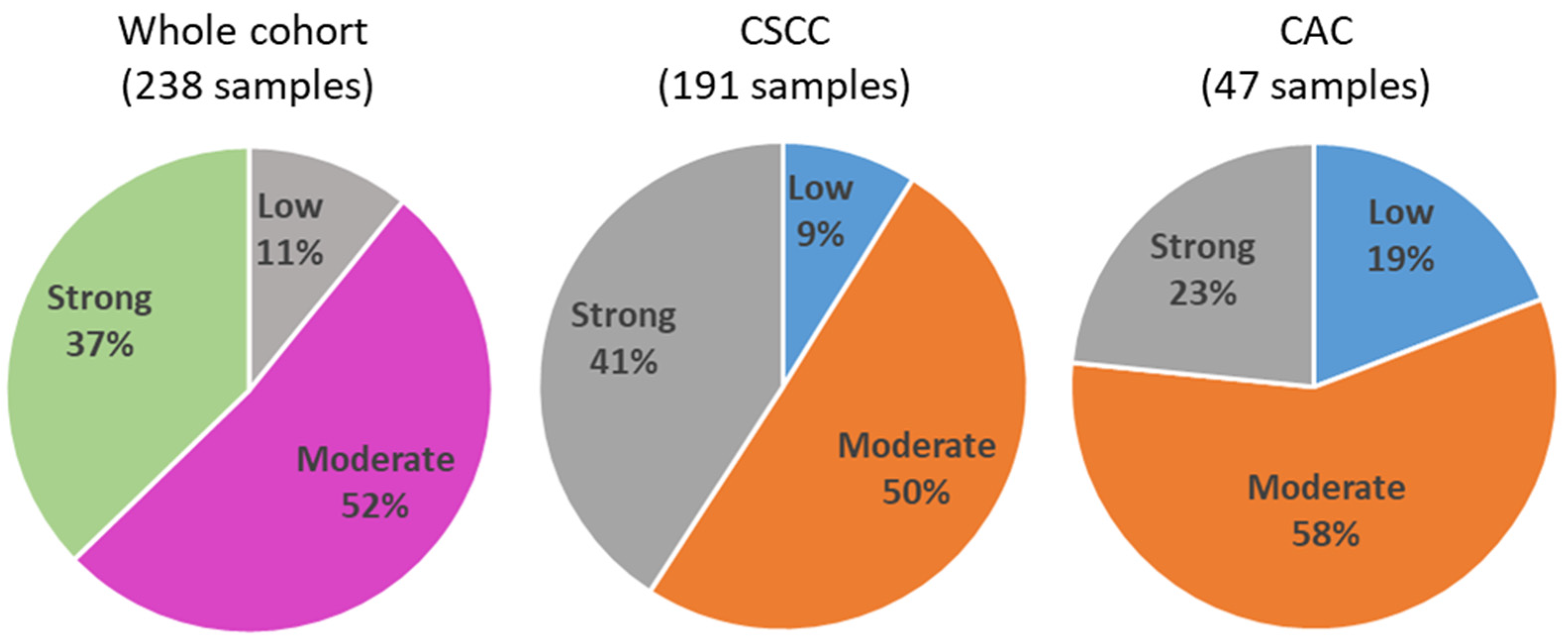
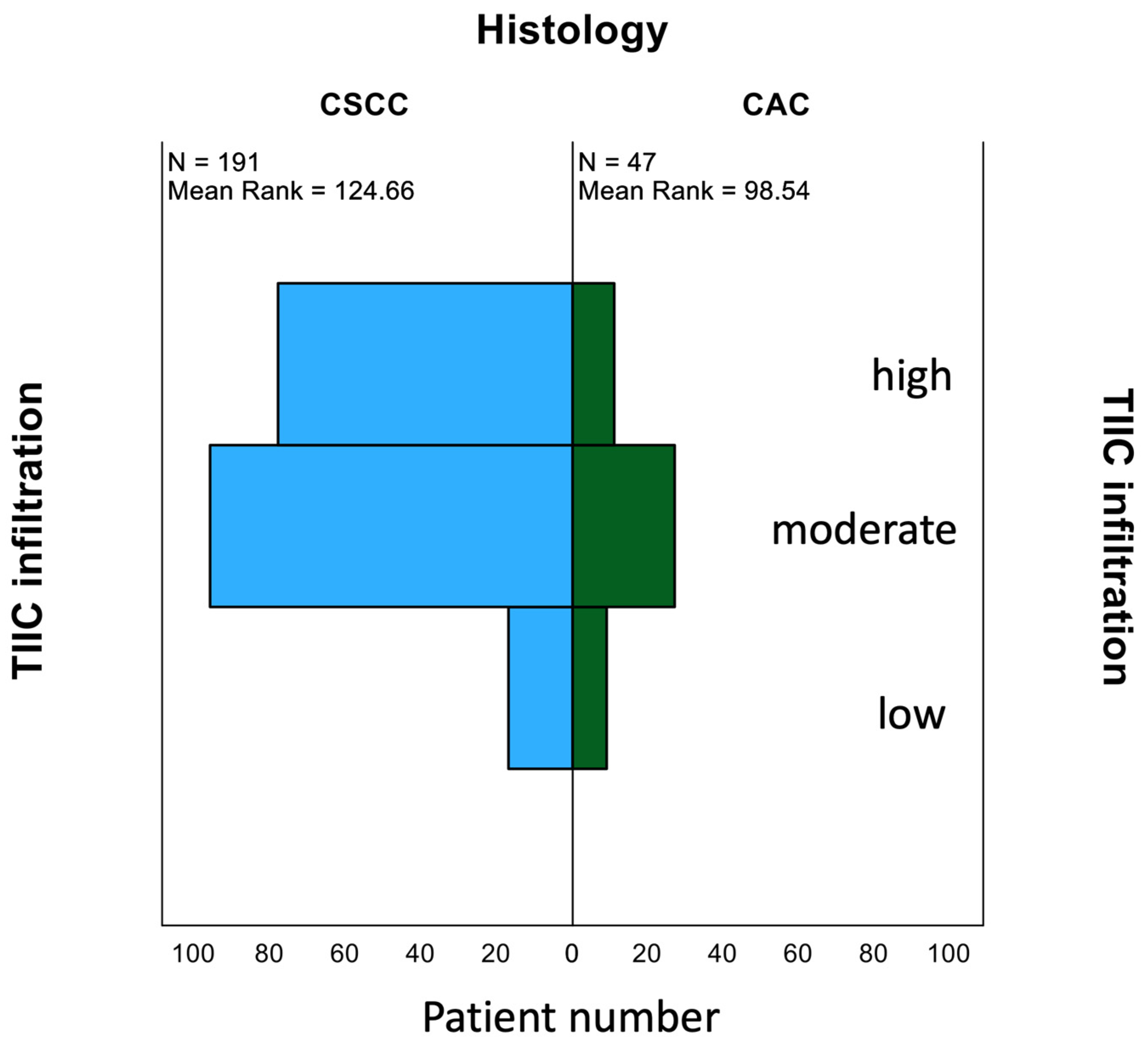
3.1. Quantification of TIICs
3.2. Correlation Analyses of TIIC Levels with Tumor Properties and Protein Markers
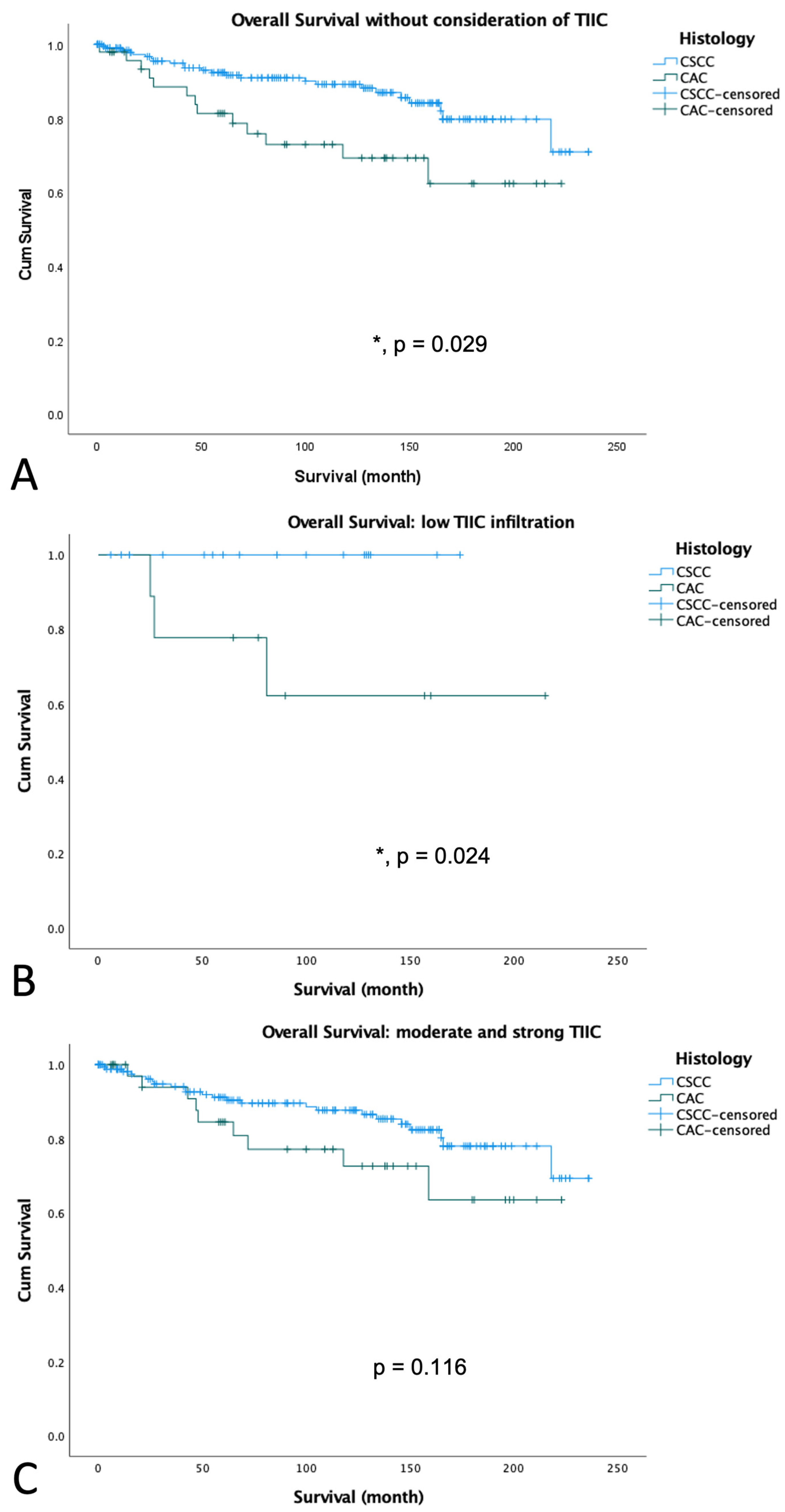
3.3. Survival Analyses According to Histology
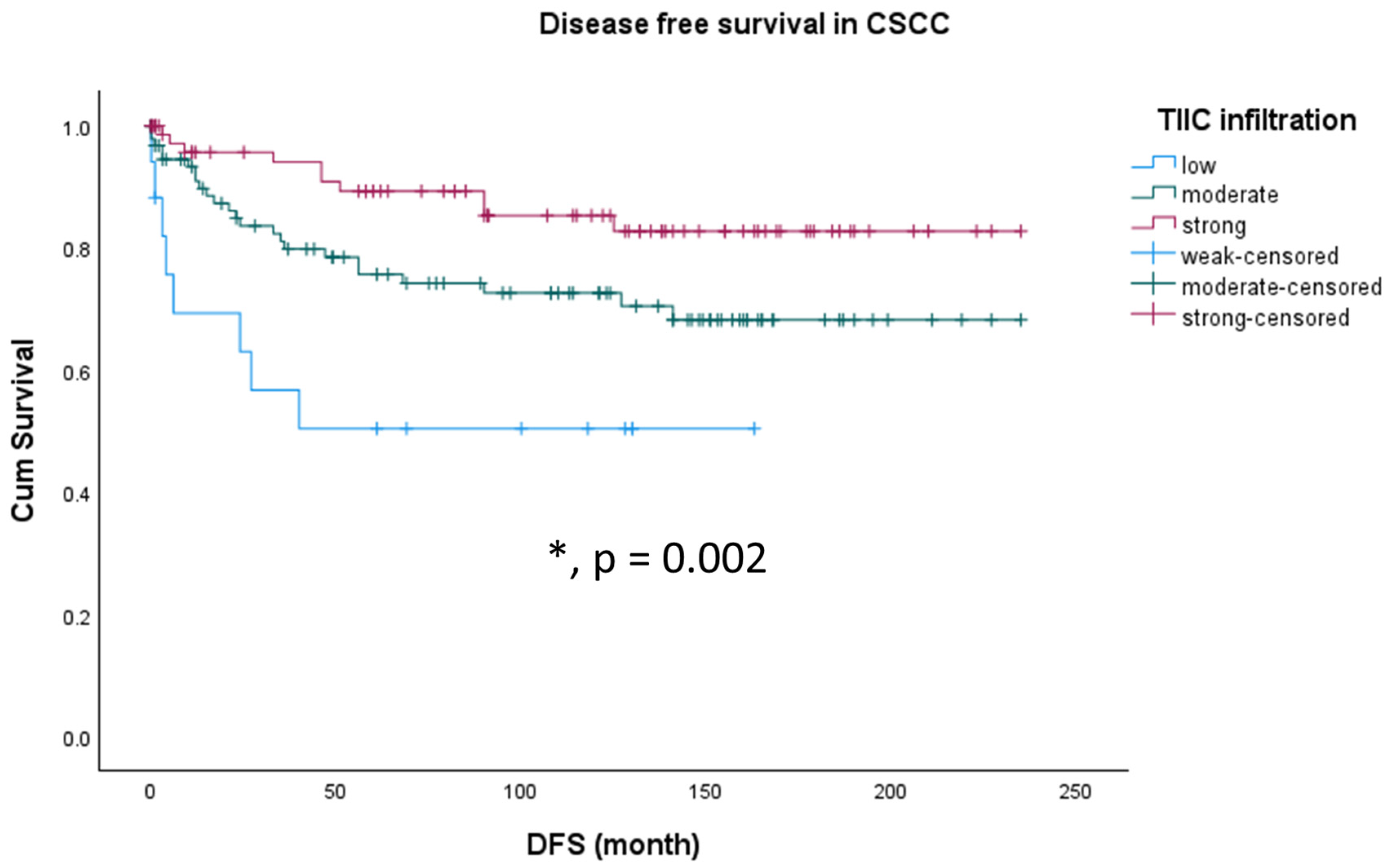
3.4. Survival Analyses According to TIIC in Different Histological Subtypes of Cervical Cancer
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front. Immunol. 2022, 13, 1018962. [Google Scholar] [CrossRef]
- Okada, Y.; Yahata, G.; Takeuchi, S.; Seidoh, T.; Tanaka, K. A correlation between the expression of CD 8 antigen and specific cytotoxicity of tumor-infiltrating lymphocytes. Jpn. J. Cancer Res. 1989, 80, 249–256. [Google Scholar] [CrossRef]
- Hilders, C.G.; Ras, L.; van Eendenburg, J.D.; Nooyen, Y.; Fleuren, G.J. Isolation and characterization of tumor-infiltrating lymphocytes from cervical carcinoma. Int. J. Cancer 1994, 57, 805–813. [Google Scholar] [CrossRef]
- Hohn, H.; Pilch, H.; Gunzel, S.; Neukirch, C.; Freitag, K.; Necker, A.; Maeurer, M.J. Human papillomavirus type 33 E7 peptides presented by HLA-DR*0402 to tumor-infiltrating T cells in cervical cancer. J. Virol. 2000, 74, 6632–6636. [Google Scholar] [CrossRef]
- Santin, A.D.; Ravaggi, A.; Bellone, S.; Pecorelli, S.; Cannon, M.; Parham, G.P.; Hermonat, P.L. Tumor-infiltrating lymphocytes contain higher numbers of type 1 cytokine expressors and DR+ T cells compared with lymphocytes from tumor draining lymph nodes and peripheral blood in patients with cancer of the uterine cervix. Gynecol. Oncol. 2001, 81, 424–432. [Google Scholar] [CrossRef]
- Sheu, B.C.; Chang, W.C.; Lin, H.H.; Chow, S.N.; Huang, S.C. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. J. Obstet. Gynaecol. Res. 2007, 33, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Kuo, T.Y.; Ho, H.N. Tumor-infiltrating lymphocytes contain a higher proportion of FOXP3(+) T lymphocytes in cervical cancer. J. Formos. Med. Assoc. = Taiwan Yi Zhi 2011, 110, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Schmoeckel, E.; Kost, B.P.; Kuhn, C.; Vattai, A.; Vilsmaier, T.; Mahner, S.; Mayr, D.; Jeschke, U.; Heidegger, H.H. Higher CCL22+ Cell Infiltration is Associated with Poor Prognosis in Cervical Cancer Patients. Cancers 2019, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sudan, K.; Schmoeckel, E.; Kost, B.P.; Kuhn, C.; Vattai, A.; Vilsmaier, T.; Mahner, S.; Jeschke, U.; Heidegger, H.H. CCL22-Polarized TAMs to M2a Macrophages in Cervical Cancer In Vitro Model. Cells 2022, 11, 2027. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Wild, C.M.; Jeschke, U.; Dannecker, C.; Mayr, D.; Cavailles, V.; Mahner, S.; Kost, B.; Heidegger, H.H.; Vattai, A. Expression of Progesterone Receptor A as an Independent Negative Prognosticator for Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 2815. [Google Scholar] [CrossRef]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Freier, C.P.; Stiasny, A.; Kuhn, C.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Jeschke, U.; Kost, B. Immunohistochemical Evaluation of the Role of p53 Mutation in Cervical Cancer: Ser-20 p53-Mutant Correlates with Better Prognosis. Anticancer Res. 2016, 36, 3131–3137. [Google Scholar]
- Kost, B.P.; Beyer, S.; Schroder, L.; Zhou, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Mahner, S.; Jeschke, U.; et al. Glucocorticoid receptor in cervical cancer: An immunhistochemical analysis. Arch. Gynecol. Obstet. 2019, 299, 203–209. [Google Scholar] [CrossRef]
- Beyer, S.; Zhu, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Dannecker, C.; Jeschke, U.; Kost, B.P. Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer. Int. J. Mol. Sci. 2017, 18, 477. [Google Scholar] [CrossRef]
- Stiasny, A.; Freier, C.P.; Kuhn, C.; Schulze, S.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Dannecker, C.; Jeschke, U.; et al. The involvement of E6, p53, p16, MDM2 and Gal-3 in the clinical outcome of patients with cervical cancer. Oncol. Lett. 2017, 14, 4467–4476. [Google Scholar] [CrossRef][Green Version]
- Silcocks, P.B.; Thornton-Jones, H.; Murphy, M. Squamous and adenocarcinoma of the uterine cervix: A comparison using routine data. Br. J. Cancer 1987, 55, 321–325. [Google Scholar] [CrossRef]
- Chen, T.H.; Fukuhara, K.; Mandai, M.; Matsumura, N.; Kariya, M.; Takakura, K.; Fujii, S. Increased cyclooxygenase-2 expression is correlated with suppressed antitumor immunity in cervical adenocarcinomas. Int. J. Gynecol. Cancer 2006, 16, 772–779. [Google Scholar] [CrossRef]
- Takahashi, Y.H.; Lee, J.S.; Swanson, S.K.; Saraf, A.; Florens, L.; Washburn, M.P.; Trievel, R.C.; Shilatifard, A. Regulation of H3K4 trimethylation via Cps40 (Spp1) of COMPASS is monoubiquitination independent: Implication for a Phe/Tyr switch by the catalytic domain of Set1. Mol. Cell Biol. 2009, 29, 3478–3486. [Google Scholar] [CrossRef]
- Takahashi, Y.H.; Shilatifard, A. Structural basis for H3K4 trimethylation by yeast Set1/COMPASS. Adv. Enzyme Regul. 2010, 50, 104–110. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Shen, L.; Chen, K.N. Association between H3K4 methylation and cancer prognosis: A meta-analysis. Thorac. Cancer 2018, 9, 794–799. [Google Scholar] [CrossRef]
- Mao, C.; Balasubramanian, A.; Yu, M.; Kiviat, N.; Ridder, R.; Reichert, A.; Herkert, M.; von Knebel Doeberitz, M.; Koutsky, L.A. Evaluation of a new p16(INK4A) ELISA test and a high-risk HPV DNA test for cervical cancer screening: Results from proof-of-concept study. Int. J. Cancer 2007, 120, 2435–2438. [Google Scholar] [CrossRef]
- Melkane, A.E.; Mirghani, H.; Auperin, A.; Saulnier, P.; Lacroix, L.; Vielh, P.; Casiraghi, O.; Griscelli, F.; Temam, S. HPV-related oropharyngeal squamous cell carcinomas: A comparison between three diagnostic approaches. Am. J. Otolaryngol. 2014, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Atipas, K.; Laokulrath, N.; Petsuksiri, J.; Ratanaprasert, N.; Pongsapich, W. CD8+ T Cells and PD-L1 Expression as Prognostic Indicators in a Low Prevalence of HPV-Associated Oropharyngeal Squamous Cell Carcinoma. Curr. Oncol. 2023, 30, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Lin, L.; Huang, Q.; Hu, C.; Zhang, M. HPV16 status might correlate to increasing tumor-infiltrating lymphocytes in hypopharyngeal cancer. Acta Oto-Laryngol. 2023, 143, 543–550. [Google Scholar] [CrossRef]
- Calderon, G.; Castaneda, C.A.; Castillo, M.; Sanchez, J.; Bernabe, L.; Suarez, N.; Tello, K.; Torres, E.; Cotrina, J.M.; Dunstan, J.; et al. Human Papillomavirus, Cytomegalovirus Infection and P16 Staining in Breast Tumors from Peruvian Women. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 1571–1576. [Google Scholar] [CrossRef]
- Ljokjel, B.; Haave, H.; Lybak, S.; Vintermyr, O.K.; Helgeland, L.; Aarstad, H.J. Tumor Infiltration Levels of CD3, Foxp3 (+) Lymphocytes and CD68 Macrophages at Diagnosis Predict 5-Year Disease-Specific Survival in Patients with Oropharynx Squamous Cell Carcinoma. Cancers 2022, 14, 1508. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Sotlar, K. HPV-associated squamous cell carcinogenesis. Pathologe 2011, 32, 391–398. [Google Scholar] [CrossRef]
- Adams, A.K.; Wise-Draper, T.M.; Wells, S.I. Human papillomavirus induced transformation in cervical and head and neck cancers. Cancers 2014, 6, 1793–1820. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Scholtz, L.U.; Gendreizig, S.; Martinez-Ruiz, L.; Florido, J.; Escames, G.; Schurmann, M.; Hain, C.; Hose, L.; Mentz, A.; et al. Primary head and neck cancer cell cultures are susceptible to proliferation of Epstein-Barr virus infected lymphocytes. BMC Cancer 2023, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, J.S.; Patil, S.; Rajarajan, S.; Ce, A.; Nair, M.; Alexander, A.; Ramesh, R.; Bs, S.; Sridhar, T. Triple-negative breast cancers with expression of glucocorticoid receptor in immune cells show better prognosis. Ann. Oncol. 2021, 32, S35. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lin, Y.H.; Chen, C.A.; Huang, S.C.; Chow, S.N.; Hsieh, C.Y. Influence of histologic type and age on survival rates for invasive cervical carcinoma in Taiwan. Gynecol. Oncol. 1999, 73, 184–190. [Google Scholar] [CrossRef]
- Sigurdsson, K.; Hrafnkelsson, J.; Geirsson, G.; Gudmundsson, J.; Salvarsdottir, A. Screening as a prognostic factor in cervical cancer: Analysis of survival and prognostic factors based on Icelandic population data, 1964–1988. Gynecol. Oncol. 1991, 43, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Yamasaki, S.; Shintani, T.; Matsui, K.; Obayashi, F.; Koizumi, K.; Tani, R.; Yanamoto, S.; Okamoto, T. Tumor-Infiltrating CD45RO(+) Memory Cells Are Associated with Favorable Prognosis in Oral Squamous Cell Carcinoma Patients. Cancers 2023, 15, 2221. [Google Scholar] [CrossRef]
- Subramaniam, N.; Nambiar, A.; Dhar, S.; Thankappan, K.; Koyakutty, M.; Balasubramanian, D.; Das, M.; Iyer, S. Low PDL1 Expression in Tumour Infiltrating Lymphocytes Predicts Local Recurrence in Oral Squamous Cell Carcinoma. Indian J. Surg. Oncol. 2021, 12, 408–414. [Google Scholar] [CrossRef] [PubMed]
| Parameter | CSCC (n = 191) | CAC (n = 47) | ||||
|---|---|---|---|---|---|---|
| Mean | Std. dev. | Mean | Std. dev. | p | ||
| Age | 49.69 | 12.89 | 48.94 | 12.31 | 0.718 | |
| Number | Percentage | Number | Percentage | |||
| pT (summarized) | pT1 | 42 | 22.0 | 10 | 21.3 | 0.280 |
| pT2 | 37 | 19.4 | 14 | 29.8 | ||
| pT3 | 112 | 58.6 | 24 | 48.9 | ||
| pN | pN1 | 76 | 39.8 | 14 | 29.8 | 0.205 |
| pN0 | 115 | 60.2 | 33 | 70.2 | ||
| Grading | G1 | 12 | 6.3 | 7 | 14.9 | 0.129 |
| G2 | 112 | 58.6 | 26 | 55.3 | ||
| G3 | 61 | 31.9 | 12 | 25.5 | ||
| Missing | 6 | 3.1 | 2 | 4.3 | ||
| FIGO 2009 | 1–1B2 | 43 | 22.5 | 18 | 38.3 | 0.090 |
| 2–4 | 74 | 38.7 | 16 | 34.0 | ||
| Missing | 74 | 38.7 | 13 | 27.7 | ||
| Age | Correlation Coefficient | −0.177 |
| Sig. (2-tailed) | 0.015 | |
| N | 187 | |
| FIGO | Correlation Coefficient | −0.184 |
| Sig. (2-tailed) | 0.011 | |
| N | 191 | |
| H3K4me3 (nuclear) | Correlation Coefficient | −0.293 |
| Sig. (2-tailed) | <0.001 | |
| N | 191 |
| p16 (cytoplasmic) | Correlation Coefficient | 0.322 |
| Sig. (2-tailed) | 0.031 | |
| N | 45 | |
| MDM2 (nuclear) | Correlation Coefficient | −0.422 |
| Sig. (2-tailed) | 0.003 | |
| N | 47 | |
| Glucocorticoid receptor (nuclear) | Correlation Coefficient | 0.389 |
| Sig. (2-tailed) | 0.007 | |
| N | 47 |
| Histology | Mean Estimate | Std. Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| CSCC | 204.839 | 5.783 | 193.505 | 216.173 |
| CAC | 169.266 | 12.948 | 143.887 | 194.645 |
| Overall | 199.147 | 5.502 | 188.363 | 209.930 |
| Histology | TIIC | Mean Estimate | Std. Error | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| CSCC | Low | 88.798 | 18.835 | 51.882 | 125.714 |
| Moderate | 174.022 | 10.521 | 153.401 | 194.643 | |
| Strong | 204.295 | 8.942 | 186.768 | 221.821 | |
| Overall | 181.431 | 7.160 | 167.398 | 195.465 | |
| Coefficient | Significance | Hazard Ratio | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Immune infiltration | −0.669 | 0.007 | 0.512 | 0.316 | 0.830 |
| pN | 0.255 | 0.477 | 10.291 | 0.638 | 2.611 |
| pM | −0.526 | 0.263 | 0.591 | 0.235 | 1.485 |
| age | 0.008 | 0.520 | 1.008 | 0.983 | 1.034 |
| pT | 0.225 | 0.016 | 1.253 | 1.043 | 1.506 |
| FIGO | −0.018 | 0.632 | 0.982 | 0.910 | 1.059 |
| Grading | 0.597 | 0.056 | 1.816 | 0.984 | 3.351 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wild, C.M.; Garrido, F.; Dannecker, C.; Köpke, M.B.; Chateau, M.-C.; Boissière-Michot, F.; Heidegger, H.H.; Vattai, A.; Kessler, M.; Jeschke, U.; et al. Prognostic Relevance of Tumor-Infiltrating Immune Cells in Cervix Squamous Cell Carcinoma. Cancers 2023, 15, 4952. https://doi.org/10.3390/cancers15204952
Wild CM, Garrido F, Dannecker C, Köpke MB, Chateau M-C, Boissière-Michot F, Heidegger HH, Vattai A, Kessler M, Jeschke U, et al. Prognostic Relevance of Tumor-Infiltrating Immune Cells in Cervix Squamous Cell Carcinoma. Cancers. 2023; 15(20):4952. https://doi.org/10.3390/cancers15204952
Chicago/Turabian StyleWild, Carl Mathis, Fabian Garrido, Christian Dannecker, Melitta B. Köpke, Marie-Christine Chateau, Florence Boissière-Michot, Helene H. Heidegger, Aurelia Vattai, Mirjana Kessler, Udo Jeschke, and et al. 2023. "Prognostic Relevance of Tumor-Infiltrating Immune Cells in Cervix Squamous Cell Carcinoma" Cancers 15, no. 20: 4952. https://doi.org/10.3390/cancers15204952
APA StyleWild, C. M., Garrido, F., Dannecker, C., Köpke, M. B., Chateau, M.-C., Boissière-Michot, F., Heidegger, H. H., Vattai, A., Kessler, M., Jeschke, U., & Cavaillès, V. (2023). Prognostic Relevance of Tumor-Infiltrating Immune Cells in Cervix Squamous Cell Carcinoma. Cancers, 15(20), 4952. https://doi.org/10.3390/cancers15204952









