Distinct Clinicopathological Features and Prognostic Values of High-, Low-, or Non-Expressing HER2 Status in Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analysis of HER2 Protein Expression by IHC
2.3. Mismatch Repair (MMR) Status Determination
2.4. Gene Mutation Detection
2.5. Treatment and Follow-Up
2.6. Propensity Score Matching
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
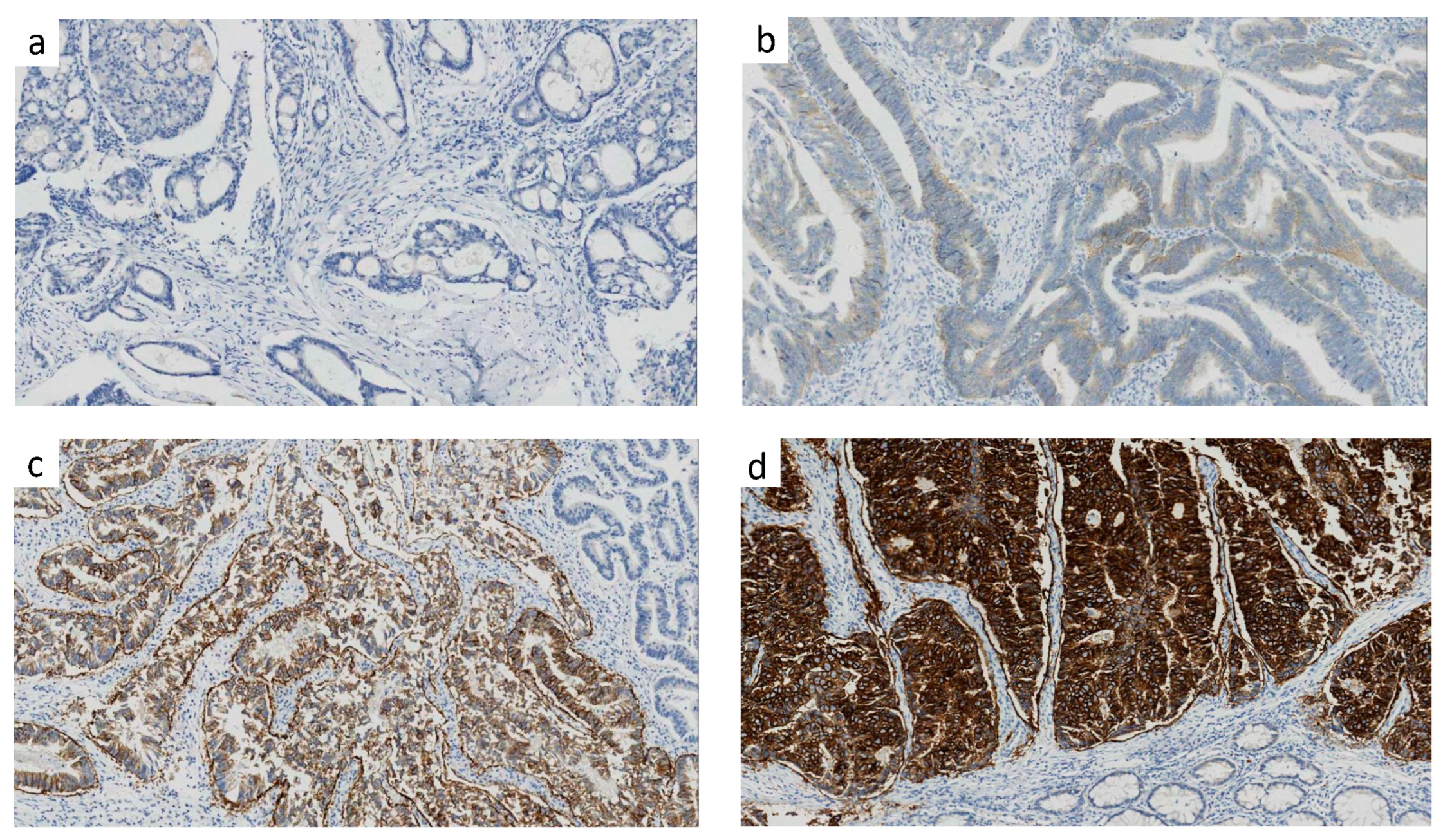
3.2. Distribution of HER2 Expression of Score 0, 1, 2, 3
3.3. Distinct Clinical Features of HER2-Zero, Low, High Colorectal Cancer
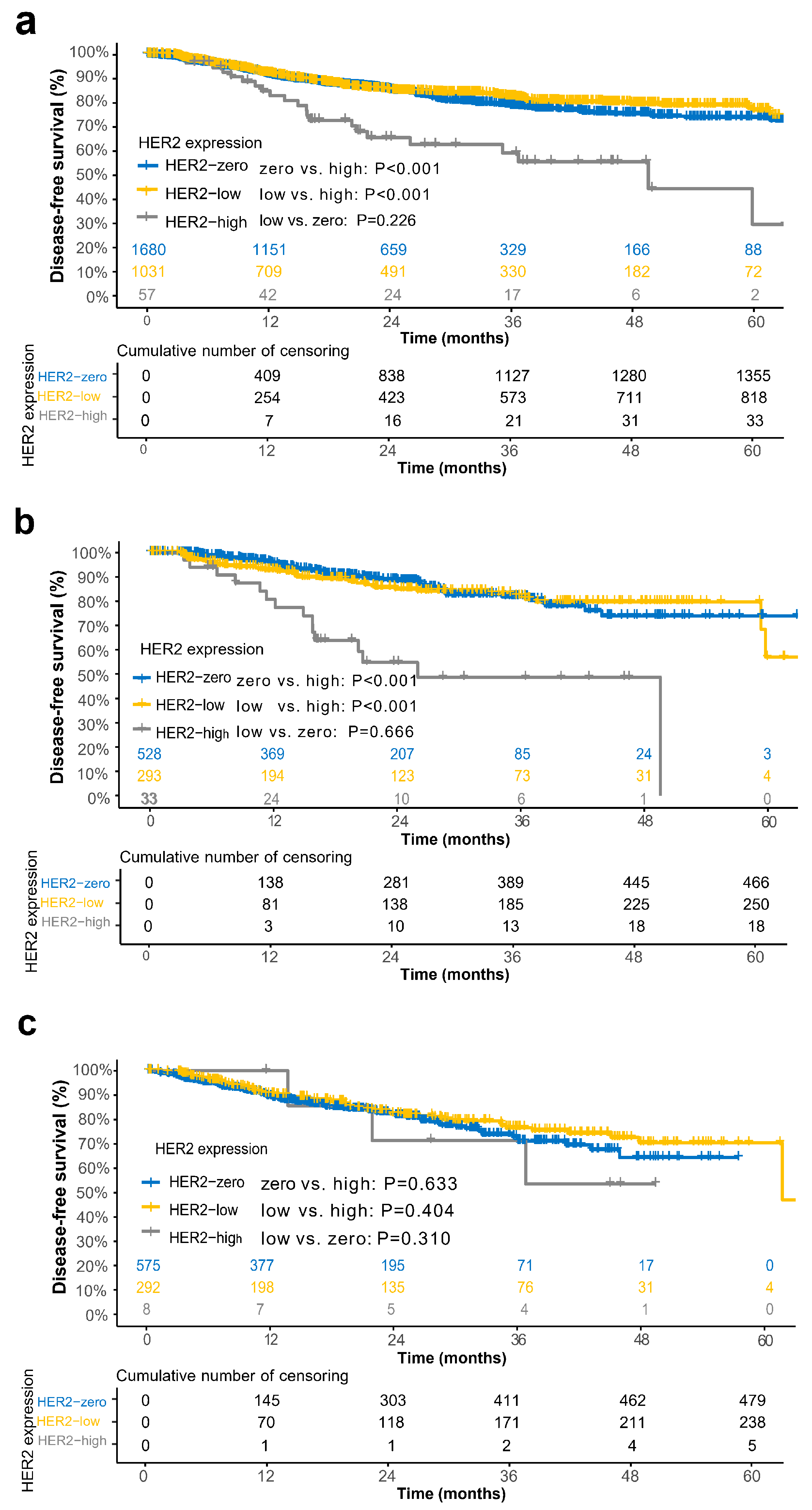
3.4. Prognostic Relevance of HER2 Groups in the Overall Cohort and Subgroups
3.5. Propensity Score Matching
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer (MyPathway): An Updated Report from a Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after Trastuzumab-Based Adjuvant Therapy in Patients with HER2-Positive Breast Cancer (ExteNET): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer,V.1.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 25 February 2022).
- Kavanagh, D.O.; Chambers, G.; O’Grady, L.; Barry, K.M.; Waldron, R.P.; Bennani, F.; Eustace, P.W.; Tobbia, I. Is Overexpression of HER-2 a Predictor of Prognosis in Colorectal Cancer? BMC Cancer 2009, 9, 1. [Google Scholar] [CrossRef]
- Marx, A.H.; Burandt, E.C.; Choschzick, M.; Simon, R.; Yekebas, E.; Kaifi, J.T.; Mirlacher, M.; Atanackovic, D.; Bokemeyer, C.; Fiedler, W.; et al. Heterogenous High-Level HER-2 Amplification in a Small Subset of Colorectal Cancers. Hum. Pathol. 2010, 41, 1577–1585. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zheng, Z.-X.; Sun, Y.; Bai, Y.-H.; Shi, Y.-F.; Zhou, L.-X.; Yao, Y.-F.; Wu, A.-W.; Cao, D.-F. Significance of HER2 Protein Expression and HER2 Gene Amplification in Colorectal Adenocarcinomas. World J. Gastrointest. Oncol. 2019, 11, 335–347. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Li, J.; Chen, P. Clinicopathological and Prognostic Significance of HER-2/Neu and VEGF Expression in Colon Carcinomas. BMC Cancer 2011, 11, 277. [Google Scholar] [CrossRef]
- Nathanson, D.R.; Culliford, A.T.; Shia, J.; Chen, B.; D’Alessio, M.; Zeng, Z.-S.; Nash, G.M.; Gerald, W.; Barany, F.; Paty, P.B. HER 2/Neu Expression and Gene Amplification in Colon Cancer. Int. J. Cancer 2003, 105, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Kang, M.S.; Oh, S.J.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Han, W.K.; Kim, H.; et al. HER-2/Neu Overexpression Is an Independent Prognostic Factor in Colorectal Cancer. Int. J. Colorectal. Dis. 2007, 22, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, Y.; Chang, W.; Ren, L.; Tang, W.; Zheng, P.; Wu, Q.; Liu, T.; Liu, Y.; Wei, Y.; et al. HER2 Positivity as a Biomarker for Poor Prognosis and Unresponsiveness to Anti-EGFR Therapy in Colorectal Cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.-S.; Tice, D.A.; Soria, J.-C. Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.-U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and Molecular Characteristics of HER2-Low-Positive Breast Cancer: Pooled Analysis of Individual Patient Data from Four Prospective, Neoadjuvant Clinical Trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 Scoring System for Colorectal Cancer: Results from a Validation Study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite Instability in Cancer of the Proximal Colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef]
- Austin, P.C. Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity-Score Matched Samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Richman, S.D.; Southward, K.; Chambers, P.; Cross, D.; Barrett, J.; Hemmings, G.; Taylor, M.; Wood, H.; Hutchins, G.; Foster, J.M.; et al. HER2 Overexpression and Amplification as a Potential Therapeutic Target in Colorectal Cancer: Analysis of 3256 Patients Enrolled in the QUASAR, FOCUS and PICCOLO Colorectal Cancer Trials. J. Pathol. 2016, 238, 562–570. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Amatu, A.; Porcu, L.; Ghezzi, S.; Lonardi, S.; Leone, F.; Bergamo, F.; Fenocchio, E.; Martinelli, E.; Borelli, B.; et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist 2019, 24, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Yagisawa, M.; Sawada, K.; Nakamura, Y.; Fujii, S.; Yuki, S.; Komatsu, Y.; Yoshino, T.; Sakamoto, N.; Taniguchi, H. Prognostic Value and Molecular Landscape of HER2 Low-Expressing Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2021, 20, 113–120.e1. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, C.; Li, C.; Wang, Y.; Chen, B.; Wen, L.; Jia, M.; Li, K.; Mok, H.; Cao, L.; et al. Distinct Clinical and Somatic Mutational Features of Breast Tumors with High-, Low-, or Non-Expressing Human Epidermal Growth Factor Receptor 2 Status. BMC Med. 2022, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yu, Y.; Luo, Z.; Guan, H.; Zhu, F.; He, Y.; Chen, Q.; Liu, C.; Nie, B.; Liu, H. Clinical, Pathological Complete Response, and Prognosis Characteristics of HER2-Low Breast Cancer in the Neoadjuvant Chemotherapy Setting: A Retrospective Analysis. Ann. Surg. Oncol. 2022, 29, 8026–8034. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Vitiello, P.P.; Marsoni, S.; Siena, S.; Tabernero, J.; Trusolino, L.; Bernards, R.; Bardelli, A. Precision Oncology in Metastatic Colorectal Cancer—From Biology to Medicine. Nat. Rev. Clin. Oncol. 2021, 18, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the Human Epidermal Growth Factor Receptor 2 (HER2) Oncogene in Colorectal Cancer. Ann. Oncol. 2018, 29, 1108–1119. [Google Scholar] [CrossRef]
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving Concepts in HER2 Evaluation in Breast Cancer: Heterogeneity, HER2-Low Carcinomas and Beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab Deruxtecan (DS-8201) in Patients with HER2-Expressing Metastatic Colorectal Cancer (DESTINY-CRC01): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | All the Population, n = 2768 | HER2-Zero Group, n = 1680 | HER2-Low Group, n = 1031 | HER2 High Group, n = 57 | p |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Age, years | |||||
| <60 | 1325 (47.9%) | 804 (47.9%) | 490 (47.5%) | 31 (54.4%) | ns |
| ≥60 | 1443 (52.1%) | 876 (52.1%) | 541 (52.5%) | 26 (45.6%) | |
| Gender | |||||
| Female | 1125 (40.6%) | 695 (41.4%) | 406 (39.4%) | 24 (42.1%) | ns |
| Male | 1643 (59.4%) | 985 (58.6%) | 625 (60.6%) | 33 (57.9%) | |
| Grade of differentiation | zero vs. low, p < 0.001 | ||||
| Well- or moderately | 2373 (85.7%) | 1395 (83.0%) | 925 (89.7%) | 53 (93.0%) | zero vs. high, ns |
| Poorly | 395 (14.3%) | 285 (17.0%) | 106 (10.3%) | 4 (7.0%) | low vs. high, ns |
| Primary tumor site | |||||
| Left (splenic flexure, descending colon, sigmoid colon, and rectum) | 1685 (60.9%) | 1010 (60.1%) | 638 (61.9%) | 37 (64.9%) | ns |
| Right (cecum, ascending colon, hepatic flexure, and transverse colon) | 1083 (39.1%) | 670 (39.9%) | 393 (38.1%) | 20 (35.1%) | |
| Rectal cancer | |||||
| No | 2719 (98.2%) | 1652 (98.3%) | 1012 (98.2%) | 55 (96.5%) | ns |
| Yes | 49 (1.8%) | 28 (1.7%) | 19 (1.8%) | 2 (3.5%) | |
| Initial bowel obstruction | zero vs. low, p < 0.001 | ||||
| No | 2631 (95.1%) | 1578 (93.9%) | 1000 (97.0%) | 53 (93.0%) | zero vs. high, ns |
| Yes | 137 (4.9%) | 102 (6.1%) | 31 (3.0%) | 4 (7.0%) | low vs. high, ns |
| Vascular invasion and/or lymphatic infiltration | zero vs. low, p = 0.016 | ||||
| No | 2471 (89.3%) | 1483 (88.3%) | 941 (91.3%) | 47 (82.5%) | zero vs. high, ns |
| Yes | 297 (10.7%) | 197 (11.7%) | 90 (8.7%) | 10 (17.5%) | low vs. high, p = 0.045 |
| Perineural invasion | zero vs. low, ns | ||||
| No | 2375 (85.8%) | 1440 (85.7%) | 896 (86.9%) | 39 (68.4%) | zero vs. high, p = 0.001 |
| Yes | 393 (14.2%) | 240 (14.3%) | 135 (13.1%) | 18 (31.6%) | low vs. high, p < 0.001 |
| No. of lymph nodes excised | |||||
| <12 | 284 (10.3%) | 160 (9.5%) | 118 (11.4%) | 6 (10.5%) | ns |
| ≥12 | 2484 (89.7%) | 1520 (90.5%) | 913 (88.6%) | 51 (89.5%) | |
| Pathologic T stage | zero vs. low, p < 0.001 | ||||
| T1–T3 | 2349 (84.9%) | 1467 (87.3%) | 835 (81.0%) | 47 (82.5%) | zero vs. high, ns |
| T4 | 419 (15.1%) | 213 (12.7%) | 196 (19.0%) | 10 (17.5%) | low vs. high, ns |
| Lymph node metastasis | zero vs. low, ns | ||||
| No | 1770 (63.9%) | 1064 (63.3%) | 678 (65.8%) | 28 (49.1%) | zero vs. high, p = 0.041 |
| Yes | 998 (36.1%) | 616 (36.7%) | 353 (34.2%) | 29 (50.9%) | low vs. high, p = 0.016 |
| Tumor deposit | zero vs. low, ns | ||||
| No | 2283 (82.5%) | 1394 (83.0%) | 848 (82.3%) | 41 (71.9%) | zero vs. high, p = 0.047 |
| Yes | 485 (17.5%) | 286 (17.0%) | 183 (17.7%) | 16 (28.1%) | low vs. high, ns |
| Pathologic N stage | zero vs. low, ns | ||||
| N0 | 1622 (58.6%) | 977 (58.2%) | 620 (60.1%) | 25 (43.9%) | zero vs. high, p = 0.044 |
| N1–2 | 1146 (41.4%) | 703 (41.8%) | 411 (39.9%) | 32 (56.1%) | low vs. high, p = 0.022 |
| Mismatch repair status | |||||
| Proficient | 2429 (87.8%) | 1472 (87.6%) | 902 (87.5%) | 55 (96.5%) | ns |
| Deficient | 339 (12.2%) | 208 (12.4%) | 129 (12.5%) | 2 (3.5%) | |
| RAS/BRAF mutation | zero vs. low, ns | ||||
| No | 854 (49.4%) | 528 (47.9%) | 293 (50.1%) | 33 (80.5%) | zero vs. high, p < 0.001 |
| Yes | 875 (50.6%) | 575 (52.1%) | 292 (49.9%) | 8 (19.5%) | low vs. high, p < 0.001 |
| Missing values | |||||
| Neoadjuvant therapy | |||||
| No | 2765 (99.9%) | 1677 (99.8%) | 1031 (100%) | 57 (100%) | ns |
| Yes | 3 (0.1%) | 3 (0.2%) | 0 (0%) | 0 (0%) | |
| Adjuvant therapy | |||||
| No | 1377 (49.7%) | 828 (49.3%) | 526 (51.0%) | 23 (40.4%) | ns |
| Yes | 1391 (50.3%) | 852 (50.7%) | 505 (49.0%) | 34 (59.6%) |
| Variable | No. Patients | No. Events | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |||
| Total | 2768 | 405 | ||||
| Age, years | ||||||
| <60 | 1325 | 188 | 1 | 0.02 | 1 | 0.033 |
| ≥60 | 1443 | 217 | 1.26 (1.04–1.53) | 1.24 (1.02–1.52) | ||
| Gender | ||||||
| Female | 1125 | 166 | 1 | 0.734 | ||
| Male | 1643 | 239 | 1.03 (0.85–1.26) | |||
| Grade of differentiation | ||||||
| Well- or moderately | 2373 | 333 | 1 | 0.017 | 1 | 0.041 |
| Poorly | 395 | 72 | 1.36 (1.06–1.76) | 1.33 (1.01–1.73) | ||
| Pathologic T stage | ||||||
| T1–T3 | 2349 | 289 | 1 | <0.001 | 1 | <0.001 |
| T4 | 419 | 116 | 2.27 (1.83–2.82) | 1.9 (1.52–2.37) | ||
| Vascular invasion and/or lymphatic infiltration | ||||||
| No | 2471 | 331 | 1 | <0.001 | 1 | 0.022 |
| Yes | 297 | 74 | 2.04 (1.59–2.63) | 1.38 (1.05–1.81) | ||
| Perineural invasion | ||||||
| No | 2375 | 286 | 1 | <0.001 | 1 | |
| Yes | 393 | 119 | 2.8 (2.26–3.46) | 1.98 (1.58–2.5) | <0.001 | |
| No. of lymph nodes excised | ||||||
| ≥12 | 284 | 57 | 1 | 0.007 | 1 | 0.121 |
| <12 | 2484 | 348 | 1.47 (1.11–1.95) | 1.26 (0.94–1.67) | ||
| Initial bowel obstruction | ||||||
| No | 2631 | 368 | 1 | 1 | <0.001 | |
| Yes | 137 | 37 | 2.77 (1.97–3.89) | <0.001 | 2.04 (1.44–2.89) | |
| Lymph node metastasis | ||||||
| No | 1770 | 183 | 1 | <0.001 | 1 | 0.005 |
| Yes | 998 | 222 | 1.87 (1.54–2.28) | 1.36 (1.1–1.68) | ||
| Tumor deposit | ||||||
| No | 2283 | 283 | 1 | <0.001 | 1 | 0.058 |
| Yes | 485 | 122 | 1.83 (1.48–2.27) | 1.25 (0.99–1.57) | ||
| Primary tumor site | ||||||
| Left (splenic flexure, descending colon, sigmoid colon, and rectum) | 1685 | 267 | 1 | 0.063 | ||
| Right (cecum, ascending colon, hepatic flexure, and transverse colon) | 1083 | 138 | 0.82 (0.67–1.01) | |||
| Rectal cancer | ||||||
| No | 2719 | 390 | 1 | 0.016 | 1 | 0.031 |
| Yes | 49 | 15 | 1.88 (1.12–3.15) | 1.78 (1.06–3.01) | ||
| Mismatch repair status | ||||||
| Proficient | 2429 | 384 | 1 | <0.001 | 1 | 0.005 |
| Deficient | 339 | 21 | 0.39 (0.25–0.6) | 0.52 (0.33–0.83) | ||
| Adjuvant therapy | ||||||
| No | 1377 | 141 | 1 | <0.001 | ||
| Yes | 1391 | 264 | 1.54 (1.25–1.89) | |||
| HER2 status | ||||||
| Zero and low | 2711 | 383 | 1 | <0.001 | 1 | 0.001 |
| High | 57 | 22 | 2.58 (1.68–3.96) | 2.05 (1.33–3.16) | ||
| Variable | HER2-Zero vs. HER2-High | HER2-Low vs. HER2-High | HER2-Zero vs. HER2-Low | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Total | ||||||
| Age, years | ||||||
| <60 | 1 | 0.045 | 1 | 0.191 | 1 | 0.126 |
| ≥60 | 1.62 (1.01–2.61) | 1.41 (0.84–2.37) | 1.20 (0.95–1.51) | |||
| Rectal cancer | ||||||
| No | 1 | 0.047 | 1 | 0.526 | 1 | 0.055 |
| Yes | 2.62 (1.01–6.76) | 1.40 (0.49–4.00) | 1.86 (0.99–3.52) | |||
| Initial bowel obstruction | ||||||
| No | 1 | 0.434 | 1 | 0.007 | 1 | <0.001 |
| Yes | 1.40 (0.60–3.23) | 2.71 (1.31–5.58) | 2.46 (1.53–3.96) | |||
| Grade of differentiation | ||||||
| Well- or moderately | 1 | 0.684 | 1 | 0.933 | 1 | 0.509 |
| Poorly | 0.79 (0.26–2.40) | 1.04 (0.39–2.78) | 1.14 (0.78–1.66) | |||
| Pathologic T stage | ||||||
| T1–T3 | 1 | 0.010 | 1 | 0.122 | 1 | <0.001 |
| T4 | 2.11 (1.19–3.73) | 1.78 (0.86–3.69) | 1.81 (1.40–2.33) | |||
| Vascular invasion and/or lymphatic infiltration | ||||||
| No | 1 | 0.008 | 1 | 0.989 | 1 | 0.290 |
| Yes | 2.27 (1.24–4.15) | 1.01 (0.46–2.18) | 1.21 (0.85–1.72) | |||
| Perineural invasion | ||||||
| No | 1 | 0.002 | 1 | 0.991 | 1 | <0.001 |
| Yes | 2.24 (1.34–3.74) | 1.00 (0.57–1.74) | 2.26 (1.73–2.95) | |||
| Lymph node metastasis | ||||||
| No | 1 | 0.631 | 1 | 0.228 | 1 | 0.024 |
| Yes | 1.14 (0.67–1.95) | 1.43 (0.80–2.54) | 1.32 (1.04–1.69) | |||
| Tumor deposit | ||||||
| No | 1 | 0.682 | 1 | 0.188 | 1 | 0.056 |
| Yes | 0.89 (0.52–1.54) | 1.47 (0.83–2.61) | 1.3 (0.99–1.70) | |||
| No. of lymph nodes excised | ||||||
| ≥12 | 1 | 0.736 | 1 | 0.988 | 1 | 0.141 |
| <12 | 1.14 (0.53–2.44) | 0.99 (0.47–2.09) | 1.27 (0.92–1.75) | |||
| Mismatch repair status | ||||||
| Proficient | 1 | 0.277 | 1 | 0.996 | 1 | 0.037 |
| Deficient | 0.32 (0.04–2.48) | 0 (0-Inf) | 0.58 (0.35–0.97) | |||
| HER2 status | ||||||
| Zero | 1 | 0.035 | 1 | 0.140 | ||
| Low | 1 | 0.002 | 0.84 (0.67–1.06) | |||
| High | 1.72 (1.04–2.84) | 2.30 (1.36–3.91) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Cheng, Y.; Wang, H.; Liu, D.; Qi, X.; Wang, C.; Zhang, Y.; Zhang, Y.; Cai, R.; Huo, H.; et al. Distinct Clinicopathological Features and Prognostic Values of High-, Low-, or Non-Expressing HER2 Status in Colorectal Cancer. Cancers 2023, 15, 554. https://doi.org/10.3390/cancers15020554
Wu Z, Cheng Y, Wang H, Liu D, Qi X, Wang C, Zhang Y, Zhang Y, Cai R, Huo H, et al. Distinct Clinicopathological Features and Prognostic Values of High-, Low-, or Non-Expressing HER2 Status in Colorectal Cancer. Cancers. 2023; 15(2):554. https://doi.org/10.3390/cancers15020554
Chicago/Turabian StyleWu, Zehua, Yi Cheng, Huaiming Wang, Dian Liu, Xiaoxing Qi, Chao Wang, Yuanzhe Zhang, Yuting Zhang, Runkai Cai, Hong Huo, and et al. 2023. "Distinct Clinicopathological Features and Prognostic Values of High-, Low-, or Non-Expressing HER2 Status in Colorectal Cancer" Cancers 15, no. 2: 554. https://doi.org/10.3390/cancers15020554
APA StyleWu, Z., Cheng, Y., Wang, H., Liu, D., Qi, X., Wang, C., Zhang, Y., Zhang, Y., Cai, R., Huo, H., Zhang, J., Cai, Y., Li, W., Hu, H., & Deng, Y. (2023). Distinct Clinicopathological Features and Prognostic Values of High-, Low-, or Non-Expressing HER2 Status in Colorectal Cancer. Cancers, 15(2), 554. https://doi.org/10.3390/cancers15020554









