Positioning of Minimally Invasive Liver Surgery for Hepatocellular Carcinoma: From Laparoscopic to Robot-Assisted Liver Resection
Abstract
Simple Summary
Abstract
1. Introduction
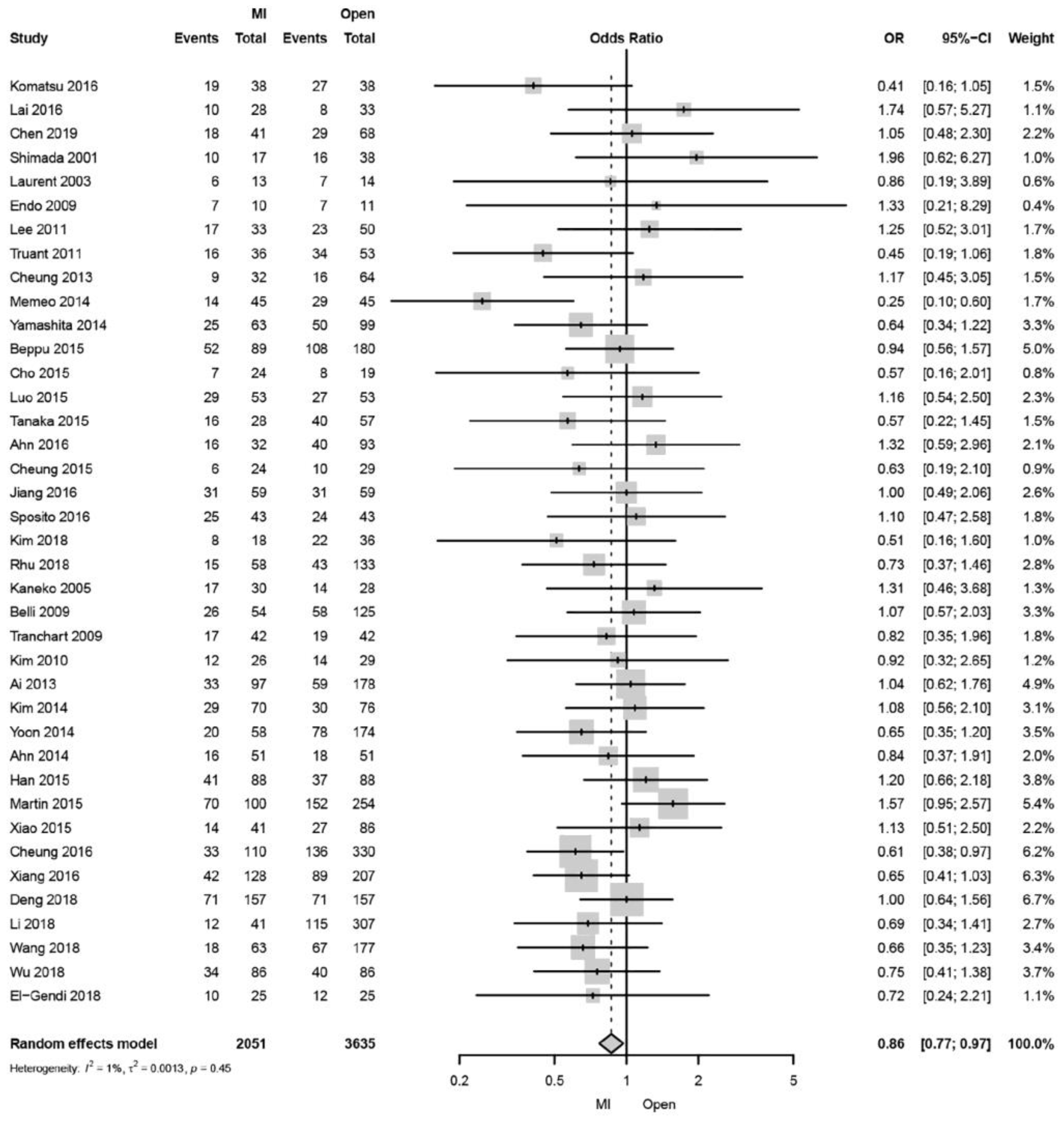
2. Liver Cirrhosis
3. Laparoscopic Repeat Liver Resection (LRLR) for Recurrent HCC
4. Elderly
5. Obesity
6. Robot-Assisted Liver Resection (RALR)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Takenaka, K.; Yanaga, K.; Ohta, M.; Kajiyama, K.; Shirabe, K.; Itasaka, H.; Nishizaki, T.; Sugimachi, K. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg. Endosc. 1995, 9, 1289–1291. [Google Scholar] [CrossRef]
- Laurent, A. Laparoscopic Liver Resection for Subcapsular Hepatocellular Carcinoma Complicating Chronic Liver Disease. Arch. Surg. 2003, 138, 763. [Google Scholar] [CrossRef]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef]
- Cheung, T.T.; Han, H.-S.; She, W.H.; Chen, K.-H.; Chow, P.; Yoong, B.K.; Lee, K.F.; Kubo, S.; Tang, C.N.; Wakabayashi, G. The Asia Pacific Consensus Statement on Laparoscopic Liver Resection for Hepatocellular Carcinoma: A Report from the 7th Asia-Pacific Primary Liver Cancer Expert Meeting Held in Hong Kong. Liver Cancer 2017, 7, 28–39. [Google Scholar] [CrossRef]
- Abu Hilal, M.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Wakabayashi, G.; Nitta, H.; Takahara, T.; Katagiri, H.; Umemura, A.; Makabe, K.; Sasaki, A. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg. Endosc. 2017, 31, 5356–5363. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Han, H.-S.; Kaneko, H.; Buell, J.F. Laparoscopic hepatectomy is theoretically better than open hepatectomy: Preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 723–731. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Fuks, D.; Kokudo, N.; Gayet, B. Difficulty of Laparoscopic Liver Resection. Ann. Surg. 2018, 267, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.; Tanabe, M.; Kumamaru, H.; Nitta, H.; Otsuka, Y.; Miyata, H.; Kakeji, Y.; Kitagawa, Y.; Kaneko, H.; Wakabayashi, G.; et al. Safe Dissemination of Laparoscopic Liver Resection in 27,146 Cases Between 2011 and 2017 From the National Clinical Database of Japan. Ann. Surg. 2020, 274, 1043–1050. [Google Scholar] [CrossRef]
- Han, H.-S.; Shehta, A.; Ahn, S.; Yoon, Y.-S.; Cho, J.Y.; Choi, Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. J. Hepatol. 2015, 63, 643–650. [Google Scholar] [CrossRef]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef]
- Takahara, T.; Wakabayashi, G.; Konno, H.; Gotoh, M.; Yamaue, H.; Yanaga, K.; Fujimoto, J.; Kaneko, H.; Unno, M.; Endo, I.; et al. Comparison of laparoscopic major hepatectomy with propensity score matched open cases from the National Clinical Database in Japan. J. Hepato-Biliary-Pancreat. Sci. 2016, 23, 721–734. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawaguchi, Y.; Kubo, S.; Kanazawa, A.; Takeda, Y.; Hirokawa, F.; Nitta, H.; Nakajima, T.; Kaizu, T.; Kaibori, M.; et al. Validation of index-based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery 2019, 165, 731–740. [Google Scholar] [CrossRef]
- Tanaka, S.; Kubo, S.; Kanazawa, A.; Takeda, Y.; Hirokawa, F.; Nitta, H.; Nakajima, T.; Kaizu, T.; Kaneko, H.; Wakabayashi, G. Validation of a Difficulty Scoring System for Laparoscopic Liver Resection: A Multicenter Analysis by the Endoscopic Liver Surgery Study Group in Japan. J. Am. Coll. Surg. 2017, 225, 249–258e1. [Google Scholar] [CrossRef]
- Li, W.; Han, J.; Xie, G.; Xiao, Y.; Sun, K.; Yuan, K.; Wu, H. Laparoscopic versus open mesohepatectomy for patients with centrally located hepatocellular carcinoma: A propensity score matched analysis. Surg. Endosc. 2018, 33, 2916–2926. [Google Scholar] [CrossRef]
- Meguro, M.; Mizuguchi, T.; Kawamoto, M.; Ota, S.; Ishii, M.; Nishidate, T.; Okita, K.; Kimura, Y.; Hirata, K. Clinical comparison of laparoscopic and open liver resection after propensity matching selection. Surgery 2015, 158, 573–587. [Google Scholar] [CrossRef]
- Xiang, L.; Li, J.; Chen, J.; Wang, X.; Guo, P.; Fan, Y.; Zheng, S. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 1895–1901. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Gujjuri, R.R.; Hilal, M.A.; Manas, D.M.; White, S.A. Does minimally invasive liver resection improve long-term survival compared to open resection for hepatocellular carcinoma? A systematic review and meta-analysis. Scand. J. Surg. 2021, 111, 14574969211042455. [Google Scholar] [CrossRef]
- Tanaka, S.; Takemura, S.; Shinkawa, H.; Nishioka, T.; Hamano, G.; Kinoshita, M.; Ito, T.; Kubo, S. Outcomes of Pure Laparoscopic versus Open Hepatic Resection for Hepatocellular Carcinoma in Cirrhotic Patients: A Case-Control Study with Propensity Score Matching. Eur. Surg. Res. 2015, 55, 291–301. [Google Scholar] [CrossRef]
- Komatsu, S.; Brustia, R.; Goumard, C.; Perdigao, F.; Soubrane, O.; Scatton, O. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: A matched pair analysis. Surg. Endosc. 2015, 30, 1965–1974. [Google Scholar] [CrossRef]
- Lai, C.; Jin, R.-A.; Liang, X.; Cai, X.-J. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J. Zhejiang Univ. B 2016, 17, 236–246. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Wang, Y.-F.; Zheng, X.-Y.; Liang, X.; Yu, H.; Cai, X.-J. Laparoscopic Right Hepatectomy for Hepatocellular Carcinoma: A Propensity Score Matching Analysis of Outcomes Compared with Conventional Open Surgery. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 503–512. [Google Scholar] [CrossRef]
- Shimada, M.; Hashizume, M.; Maehara, S.; Tsujita, E.; Rikimaru, T.; Yamashita, Y.; Tanaka, S.; Adachi, E.; Sugimachi, K. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg. Endosc. 2001, 15, 541–544. [Google Scholar] [CrossRef]
- Endo, Y.; Ohta, M.; Sasaki, A.; Kai, S.; Eguchi, H.; Iwaki, K.; Shibata, K.; Kitano, S. A Comparative Study of the Long-term Outcomes After Laparoscopy-assisted and Open Left Lateral Hepatectomy for Hepatocellular Carcinoma. Surg. Laparosc. Endosc. Percutaneous Tech. 2009, 19, e171–e174. [Google Scholar] [CrossRef]
- Lee, K.F.; Chong, C.N.; Wong, J.; Cheung, Y.S.; Wong, J.; Lai, P. Long-Term Results of Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma: A Case-Matched Analysis. World J. Surg. 2011, 35, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Bouras, A.F.; Hebbar, M.; Boleslawski, E.; Fromont, G.; Dharancy, S.; Leteurtre, E.; Zerbib, P.; Pruvot, F.R. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: A case-matched study. Surg. Endosc. 2011, 25, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.T.; Poon, R.T.P.; Yuen, W.K.; Chok, K.S.H.; Jenkins, C.R.; Chan, S.C.; Fan, S.T.; Lo, C.M. Long-Term Survival Analysis of Pure Laparoscopic Versus Open Hepatectomy for Hepatocellular Carcinoma in Patients with Cirrhosis. Ann. Surg. 2013, 257, 506–511. [Google Scholar] [CrossRef]
- Memeo, R.; De’Angelis, N.; Compagnon, P.; Salloum, C.; Cherqui, D.; Laurent, A.; Azoulay, D. Laparoscopic vs. Open Liver Resection for Hepatocellular Carcinoma of Cirrhotic Liver: A Case–Control Study. World J. Surg. 2014, 38, 2919–2926. [Google Scholar] [CrossRef]
- Yamashita, Y.-I.; Ikeda, T.; Kurihara, T.; Yoshida, Y.; Takeishi, K.; Itoh, S.; Harimoto, N.; Kawanaka, H.; Shirabe, K.; Maehara, Y. Long-Term Favorable Surgical Results of Laparoscopic Hepatic Resection for Hepatocellular Carcinoma in Patients with Cirrhosis: A Single-Center Experience over a 10-Year Period. J. Am. Coll. Surg. 2014, 219, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Wakabayashi, G.; Hasegawa, K.; Gotohda, N.; Mizuguchi, T.; Takahashi, Y.; Hirokawa, F.; Taniai, N.; Watanabe, M.; Katou, M.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 711–720. [Google Scholar] [CrossRef]
- Cho, J.Y.; Han, H.-S.; Yoon, Y.-S.; Choi, Y.; Lee, W. Outcomes of laparoscopic right posterior sectionectomy in patients with hepatocellular carcinoma in the era of laparoscopic surgery. Surgery 2015, 158, 135–141. [Google Scholar] [CrossRef]
- Luo, L.; Zou, H.; Yao, Y.; Huang, X. Laparoscopic versus open hepatectomy for hepatocellular carcinoma: Short- and long-term outcomes comparison. Int. J. Clin. Exp. Med. 2015, 8, 18772–18778. [Google Scholar]
- Ahn, S.; Cho, A.; Kim, E.K.; Paik, K.Y. Favorable Long-Term Oncologic Outcomes of Hepatocellular Carcinoma Following Laparoscopic Liver Resection. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 447–452. [Google Scholar] [CrossRef]
- Cheung, T.T.; Poon, R.T.P.; Dai, W.C.; Chok, K.S.H.; Chan, S.C.; Lo, C.M. Pure Laparoscopic Versus Open Left Lateral Sectionectomy for Hepatocellular Carcinoma: A Single-Center Experience. World J. Surg. 2015, 40, 198–205. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, L.; Zhang, Q.; Jiang, Y.; Huang, J.; Zhou, H.; Zeng, L. Laparoscopic versus open hepatectomy for hepatocellular carcinoma: Long-term outcomes. J. BUON 2016, 21, 135–141. [Google Scholar] [PubMed]
- Sposito, C.; Battiston, C.; Facciorusso, A.; Mazzola, M.; Muscarà, C.; Scotti, M.; Romito, R.; Mariani, L.; Mazzaferro, V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Kim, K.-H.; Kim, S.-H.; Kang, W.-H.; Lee, S.-G. Laparoscopic Versus Open Liver Resection for Centrally Located Hepatocellular Carcinoma in Patients with Cirrhosis: A Propensity Score-matching Analysis. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Rhu, J.; Kim, S.J.; Choi, G.S.; Kim, J.M.; Joh, J.-W.; Kwon, C.H.D. Laparoscopic Versus Open Right Posterior Sectionectomy for Hepatocellular Carcinoma in a High-Volume Center: A Propensity Score Matched Analysis. World J. Surg. 2018, 42, 2930–2937. [Google Scholar] [CrossRef]
- Kaneko, H.; Takagi, S.; Otsuka, Y.; Tsuchiya, M.; Tamura, A.; Katagiri, T.; Maeda, T.; Shiba, T. Laparoscopic liver resection of hepatocellular carcinoma. Am. J. Surg. 2005, 189, 190–194. [Google Scholar] [CrossRef]
- Belli, G.; Limongelli, P.; Fantini, C.; D’Agostino, A.; Cioffi, L.; Belli, A.; Russo, G. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br. J. Surg. 2009, 96, 1041–1048. [Google Scholar] [CrossRef]
- Tranchart, H.; Di Giuro, G.; Lainas, P.; Roudie, J.; Agostini, H.; Franco, D.; Dagher, I. Laparoscopic resection for hepatocellular carcinoma: A matched-pair comparative study. Surg. Endosc. 2009, 24, 1170–1176. [Google Scholar] [CrossRef]
- Kim, H.-H.; Park, E.K.; Seoung, J.S.; Hur, Y.H.; Koh, Y.S.; Kim, J.C.; Cho, C.K.; Kim, H.J. Liver resection for hepatocellular carcinoma: Case-matched analysis of laparoscopic versus open resection. J. Korean Surg. Soc. 2011, 80, 412–419. [Google Scholar] [CrossRef]
- Ai, J.-H.; Li, J.-W.; Chen, J.; Bie, P.; Wang, S.-G.; Zheng, S.-G. Feasibility and Safety of Laparoscopic Liver Resection for Hepatocellular Carcinoma with a Tumor Size of 5–10 cm. PLoS ONE 2013, 8, e72328. [Google Scholar] [CrossRef]
- Kim, S.-J.; Jung, H.-K.; Lee, D.-S.; Yun, S.-S.; Kim, H.-J. The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann. Surg. Treat. Res. 2014, 86, 61–67. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Kim, K.-H.; Jung, D.-H.; Yu, A.; Lee, S.-G. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: Case-matched analysis. Surg. Endosc. 2014, 29, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Kang, K.J.; Kim, Y.H.; Kim, T.-S.; Lim, T.J. A Propensity Score-Matched Case-Control Comparative Study of Laparoscopic and Open Liver Resection for Hepatocellular Carcinoma. J. Laparoendosc. Adv. Surg. Tech. 2014, 24, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G.; Mbah, N.A.; Hill, R.S.; Kooby, D.; Weber, S.; Scoggins, C.R.; Maithel, S.K. Laparoscopic Versus Open Hepatic Resection for Hepatocellular Carcinoma: Improvement in Outcomes and Similar Cost. World J. Surg. 2015, 39, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiang, L.-J.; Li, J.-W.; Chen, J.; Fan, Y.-D.; Zheng, S.-G. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg. Endosc. 2015, 29, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-C.; Jiang, W.-Z.; Tang, X.-D.; Liu, S.-H.; Qin, L.; Qian, H.-X. Laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 157 patients: A case controlled study with propensity score matching at two Chinese centres. Int. J. Surg. 2018, 56, 203–207. [Google Scholar] [CrossRef]
- Wang, W.-H.; Kuo, K.-K.; Wang, S.-N.; Lee, K.-T. Oncological and surgical result of hepatoma after robot surgery. Surg. Endosc. 2018, 32, 3918–3924. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Lau, W.Y.; Li, W.; Lin, P.; Zhang, L.; Chen, Y. Perioperative and long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with well-preserved liver function and cirrhotic background: A propensity score matching study. Surg. Endosc. 2018, 33, 206–215. [Google Scholar] [CrossRef]
- El-Gendi, A.; El-Shafei, M.; El-Gendi, S.; Shawky, A. Laparoscopic Versus Open Hepatic Resection for Solitary Hepatocellular Carcinoma Less Than 5 cm in Cirrhotic Patients: A Randomized Controlled Study. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 302–310. [Google Scholar] [CrossRef]
- Twaij, A. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: Systematic review and meta-analysis. World J. Gastroenterol. 2014, 20, 8274–8281. [Google Scholar] [CrossRef]
- Chen, J.; Bai, T.; Zhang, Y.; Xie, Z.-B.; Wang, X.-B.; Wu, F.-X.; Li, L.-Q. The safety and efficacy of laparoscopic and open hepatectomy in hepatocellular carcinoma patients with liver cirrhosis: A systematic review. Int. J. Clin. Exp. Med. 2015, 8, 20679–20689. [Google Scholar]
- Goh, E.L.; Chidambaram, S.; Ma, S. Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A meta-analysis of the long-term survival outcomes. Int. J. Surg. 2018, 50, 35–42. [Google Scholar] [CrossRef]
- Pan, Y.; Xia, S.; Cai, J.; Chen, K.; Cai, X. Efficacy of Laparoscopic Hepatectomy versus Open Surgery for Hepatocellular Carcinoma With Cirrhosis: A Meta-analysis of Case-Matched Studies. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Kusano, T.; Sasaki, A.; Kai, S.; Endo, Y.; Iwaki, K.; Shibata, K.; Ohta, M.; Kitano, S. Predictors and prognostic significance of operative complications in patients with hepatocellular carcinoma who underwent hepatic resection. Eur. J. Surg. Oncol. (EJSO) 2009, 35, 1179–1185. [Google Scholar] [CrossRef]
- Giuliante, F.; Ardito, F.; Pinna, A.D.; Sarno, G.; Giulini, S.M.; Ercolani, G.; Portolani, N.; Torzilli, G.; Donadon, M.; Aldrighetti, L.; et al. Liver Resection for Hepatocellular Carcinoma ≤3 cm: Results of an Italian Multicenter Study on 588 Patients. J. Am. Coll. Surg. 2012, 215, 244–254. [Google Scholar] [CrossRef]
- Kabir, T.; Syn, N.L.; Tan, Z.Z.; Tan, H.-J.; Yen, C.; Koh, Y.-X.; Kam, J.H.; Teo, J.-Y.; Lee, S.-Y.; Cheow, P.-C.; et al. Predictors of post-operative complications after surgical resection of hepatocellular carcinoma and their prognostic effects on outcome and survival: A propensity-score matched and structural equation modelling study. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 1756–1765. [Google Scholar] [CrossRef]
- Koh, Y.X.; Tan, H.J.; Liew, Y.X.; Syn, N.; Teo, J.Y.; Lee, S.Y.; Goh, B.K.; Goh, G.B.; Chan, C.Y. Liver Resection for Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. J. Am. Coll. Surg. 2019, 229, 467–478e1. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hsieh, P.-M.; Lin, H.-Y.; Hung, C.-M.; Lo, G.-H.; Hsu, Y.-C.; Lu, I.-C.; Lee, C.-Y.; Wu, T.-C.; Yeh, J.-H.; et al. Surgical resection significantly promotes the overall survival of patients with hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2021, 21, 220. [Google Scholar] [CrossRef]
- Brytska, N.; Han, H.-S.; Shehta, A.; Yoon, Y.-S.; Cho, J.Y.; Choi, Y. Laparoscopic liver resection for hepatitis B and C virus-related hepatocellular carcinoma in patients with Child B or C cirrhosis. HepatoBiliary Surg. Nutr. 2015, 4, 373–378. [Google Scholar] [CrossRef]
- Cai, X.; Liang, X.; Yu, T.; Liang, Y.; Jing, R.; Jiang, W.; Li, J.; Ying, H. Liver cirrhosis grading Child-Pugh class B: A Goliath to challenge in laparoscopic liver resection?—Prior experience and matched comparisons. HepatoBiliary Surg. Nutr. 2015, 4, 391–397. [Google Scholar] [CrossRef]
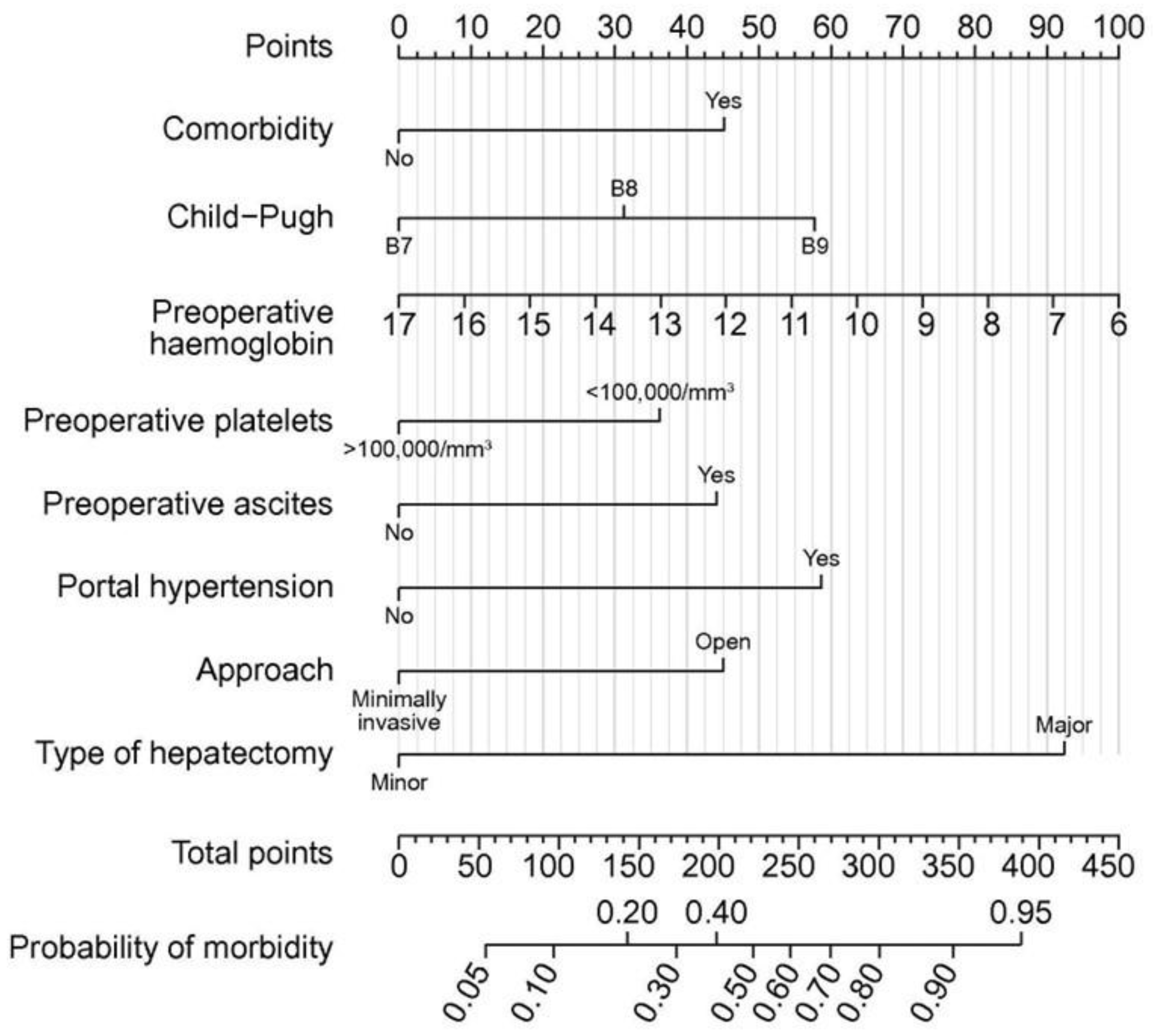
- Berardi, G.; Morise, Z.; Sposito, C.; Igarashi, K.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.; Kubo, S.; Tanaka, S.; et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J. Hepatol. 2019, 72, 75–84. [Google Scholar] [CrossRef]
- Kubo, S.; Tsukamoto, T.; Hirohashi, K.; Tanaka, H.; Shuto, T.; Takemura, S.; Yamamoto, T.; Uenishi, T.; Ogawa, M.; Kinoshita, H. Correlation Between Preoperative Serum Concentration of Type IV Collagen 7s Domain and Hepatic Failure Following Resection of Hepatocellular Carcinoma. Ann. Surg. 2004, 239, 186–193. [Google Scholar] [CrossRef]
- Kanazawa, A.; Tsukamoto, T.; Shimizu, S.; Kodai, S.; Yamazoe, S.; Yamamoto, S.; Kubo, S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg. Endosc. 2013, 27, 2592–2597. [Google Scholar] [CrossRef]
- Cheung, T.T.; Dai, W.C.; Tsang, S.H.Y.; Chan, A.C.Y.; Chok, K.S.H.; Chan, S.C.; Lo, C.M. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients with Liver Cirrhosis. Ann. Surg. 2016, 264, 612–620. [Google Scholar] [CrossRef]
- Koda, M.; Tanaka, S.; Takemura, S.; Shinkawa, H.; Kinoshita, M.; Hamano, G.; Ito, T.; Kawada, N.; Shibata, T.; Kubo, S. Long-Term Prognostic Factors after Hepatic Resection for Hepatitis C Virus-Related Hepatocellular Carcinoma, with a Special Reference to Viral Status. Liver Cancer 2018, 7, 261–276. [Google Scholar] [CrossRef]
- Tanaka, S.; Iimuro, Y.; Hirano, T.; Hai, S.; Suzumura, K.; Fujimoto, J. Outcomes of Hepatic Resection for Large Hepatocellular Carcinoma: Special Reference to Postoperative Recurrence. Am. Surg. 2015, 81, 64–73. [Google Scholar] [CrossRef]
- Tanaka, S.; Shinkawa, H.; Tamori, A.; Takemura, S.; Takahashi, S.; Amano, R.; Kimura, K.; Ohira, G.; Kawada, N.; Kubo, S. Surgical outcomes for hepatocellular carcinoma detected after hepatitis C virus eradiation by direct-acting antivirals. J. Surg. Oncol. 2020, 122, 1543–1552. [Google Scholar] [CrossRef]
- Tanaka, S.; Shinkawa, H.; Tamori, A.; Takemura, S.; Uchida-Kobayashi, S.; Amano, R.; Kimura, K.; Ohira, G.; Nishio, K.; Tauchi, J.; et al. Postoperative direct-acting antiviral treatment after liver resection in patients with hepatitis C virus-related hepatocellular carcinoma. Hepatol. Res. 2021, 51, 1102–1114. [Google Scholar] [CrossRef]
- Kinoshita, M.; Tanaka, S.; Kodai, S.; Takemura, S.; Shinkawa, H.; Ohira, G.; Nishio, K.; Tauchi, J.; Kanazawa, A.; Kubo, S. Increasing incidence and severity of post-hepatectomy adhesion around the liver may be influenced by the hepatectomy-related operative procedures. Asian J. Surg. 2023, 46, 228–235. [Google Scholar] [CrossRef]
- Morise, Z. Status and perspective of laparoscopic repeat liver resection. World J. Hepatol. 2018, 10, 479–484. [Google Scholar] [CrossRef]
- Szomstein, S.; Menzo, E.L.; Simpfendorfer, C.; Zundel, N.; Rosenthal, R.J. Laparoscopic Lysis of Adhesions. World J. Surg. 2006, 30, 535–540. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, G.; Xu, D.; Liu, R. Laparoscopic Repeat Resection of Recurrent Hepatocellular Carcinoma. World J. Surg. 2010, 35, 648–655. [Google Scholar] [CrossRef]
- Belli, G.; Cioffi, L.; Fantini, C.; D’Agostino, A.; Russo, G.; Limongelli, P.; Belli, A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: Feasibility, safety, and results. Surg. Endosc. 2009, 23, 1807–1811. [Google Scholar] [CrossRef]
- Goh, B.K.P.; Teo, J.; Chan, C.; Lee, S.; Cheow, P.; Chung, A.Y.F. Laparoscopic repeat liver resection for recurrent hepatocellular carcinoma. ANZ J. Surg. 2016, 87, E143–E146. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Otsuka, Y.; Maeda, T.; Ishii, J.; Tamura, A.; Kaneko, H. Efficacy of Laparoscopic Surgery for Recurrent Hepatocellular Carcinoma. Hepatogastroenterology 2012, 59, 1333–1337. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Z.; Xiao, Y.; Zhang, W.; Tang, D.; Cheng, B.; Li, Q. Comparison of clinical outcomes of laparoscopic versus open surgery for recurrent hepatocellular carcinoma: A meta-analysis. Surg. Endosc. 2019, 33, 3550–3557. [Google Scholar] [CrossRef]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Belli, A.; Cherqui, D.; Tanabe, M.; Wakabayashi, G.; Cheung, T.T.; Lo, C.M.; et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020, 107, 889–895. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kanazawa, A.; Kodai, S.; Shimizu, S.; Murata, A.; Nishio, K.; Hamano, G.; Shinkawa, H.; Tanaka, S.; Takemura, S.; et al. Difficulty classifications of laparoscopic repeated liver resection in patients with recurrent hepatocellular carcinoma. Asian J. Endosc. Surg. 2019, 13, 366–374. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kanazawa, A.; Tanaka, S.; Takemura, S.; Amano, R.; Kimura, K.; Shinkawa, H.; Ohira, G.; Nishio, K.; Kubo, S. Indications of Laparoscopic Repeat Liver Resection for Recurrent Hepatocellular Carcinoma. Ann. Gastroenterol. Surg. 2021, 6, 119–126. [Google Scholar] [CrossRef]
- Nanashima, A.; Abo, T.; Nonaka, T.; Fukuoka, H.; Hidaka, S.; Takeshita, H.; Ichikawa, T.; Sawai, T.; Yasutake, T.; Nakao, K.; et al. Prognosis of patients with hepatocellular carcinoma after hepatic resection: Are elderly patients suitable for surgery? J. Surg. Oncol. 2011, 104, 284–291. [Google Scholar] [CrossRef]
- Nozawa, A.; Kubo, S.; Takemura, S.; Sakata, C.; Urata, Y.; Nishioka, T.; Kinoshita, M.; Hamano, G.; Uenishi, T.; Suehiro, S. Hepatic resection for hepatocellular carcinoma in super-elderly patients aged 80 years and older in the first decade of the 21st century. Surg. Today 2014, 45, 851–857. [Google Scholar] [CrossRef]
- Wang, W.-L.; Zhu, Y.; Cheng, J.-W.; Li, M.-X.; Xia, J.-M.; Hao, J.; Yu, L.; Lv, Y.; Wu, Z.; Wang, B. Major hepatectomy is safe for hepatocellular carcinoma in elderly patients with cirrhosis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.J.; Welsh, F.K.S.; Chandrakumaran, K.; John, T.G.; Rees, M. Resection of colorectal liver metastases in the elderly: Does age matter? Color. Dis. 2012, 14, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Kishida, N.; Hibi, T.; Itano, O.; Okabayashi, K.; Shinoda, M.; Kitago, M.; Abe, Y.; Yagi, H.; Kitagawa, Y. Validation of Hepatectomy for Elderly Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2015, 22, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Okinaga, H.; Yasunaga, H.; Hasegawa, K.; Fushimi, K.; Kokudo, N. Short-Term Outcomes following Hepatectomy in Elderly Patients with Hepatocellular Carcinoma: An Analysis of 10,805 Septuagenarians and 2,381 Octo- and Nonagenarians in Japan. Liver Cancer 2017, 7, 55–64. [Google Scholar] [CrossRef]
- Nomi, T.; Hirokawa, F.; Kaibori, M.; Ueno, M.; Tanaka, S.; Hokuto, D.; Noda, T.; Nakai, T.; Ikoma, H.; Iida, H.; et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A multi-centre propensity score-based analysis. Surg. Endosc. 2019, 34, 658–666. [Google Scholar] [CrossRef]
- Goh, B.K.P.; Chua, D.; Syn, N.; Teo, J.-Y.; Chan, C.-Y.; Lee, S.-Y.; Jeyaraj, P.R.; Cheow, P.-C.; Chow, P.K.H.; Ooi, L.L.P.J.; et al. Perioperative Outcomes of Laparoscopic Minor Hepatectomy for Hepatocellular Carcinoma in the Elderly. World J. Surg. 2018, 42, 4063–4069. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.; Rhu, J.; Choi, G.-S.; Kwon, C.H.D.; Joh, J.-W. Elderly Hepatocellular Carcinoma Patients: Open or Laparoscopic Approach? Cancers 2020, 12, 2281. [Google Scholar] [CrossRef]
- Mohamedahmed, A.Y.Y.; Zaman, S.; Albendary, M.; Wright, J.; Abdalla, H.; Patel, K.; Mankotia, R.; Sillah, A.K. Laparoscopic versus open hepatectomy for malignant liver tumours in the elderly: Systematic review and meta-analysis. Updat. Surg. 2021, 73, 1623–1641. [Google Scholar] [CrossRef]
- Cho, S.W.; Steel, J.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Gamblin, T.C. Safety of Liver Resection in the Elderly: How Important Is Age? Ann. Surg. Oncol. 2010, 18, 1088–1095. [Google Scholar] [CrossRef]
- Tanaka, S.; Ueno, M.; Iida, H.; Kaibori, M.; Nomi, T.; Hirokawa, F.; Ikoma, H.; Nakai, T.; Eguchi, H.; Kubo, S. Preoperative assessment of frailty predicts age-related events after hepatic resection: A prospective multicenter study. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 377–387. [Google Scholar] [CrossRef]
- Tanaka, S.; Iida, H.; Ueno, M.; Hirokawa, F.; Nomi, T.; Nakai, T.; Kaibori, M.; Ikoma, H.; Eguchi, H.; Shinkawa, H.; et al. Preoperative Risk Assessment for Loss of Independence Following Hepatic Resection in Elderly Patients. Ann. Surg. 2019, 274, e253–e261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Iida, H.; Ueno, M.; Hirokawa, F.; Yoshida, H.; Ishii, H.; Nomi, T.; Nakai, T.; Kaibori, M.; Ikoma, H.; et al. Postoperative loss of independence 1 year after liver resection: Prospective multicentre study. Br. J. Surg. 2022, 109, e54–e55. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 October 2022).
- McCurry, J. Japan battles with obesity. Lancet 2007, 369, 451–452. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare of Japan. Report of national health and nutrition 2018. Available online: https://www.mhlw.go.jp/content/10900000/000688863.pdf (accessed on 1 March 2020).
- Berentzen, T.L.; Gamborg, M.; Holst, C.; Sørensen, T.I.; Baker, J.L. Body mass index in childhood and adult risk of primary liver cancer. J. Hepatol. 2014, 60, 325–330. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Overweight, obesity and risk of liver cancer: A meta-analysis of cohort studies. Br. J. Cancer 2007, 97, 1005–1008. [Google Scholar] [CrossRef]
- Mullen, J.T.; Davenport, D.L.; Hutter, M.M.; Hosokawa, P.W.; Henderson, W.G.; Khuri, S.F.; Moorman, D.W. Impact of Body Mass Index on Perioperative Outcomes in Patients Undergoing Major Intra-abdominal Cancer Surgery. Ann. Surg. Oncol. 2008, 15, 2164–2172. [Google Scholar] [CrossRef]
- Dindo, D.; Muller, M.K.; Weber, M.; Clavien, P.-A. Obesity in general elective surgery. Lancet 2003, 361, 2032–2035. [Google Scholar] [CrossRef]
- Yu, X.; Yu, H.; Fang, X. The impact of body mass index on short-term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiol. 2015, 16, 29. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Ishihara, A.; Tanaka, S.; Shinkawa, H.; Yoshida, H.; Takemura, S.; Amano, R.; Kimura, K.; Ohira, G.; Nishio, K.; Kubo, S. Superiority of laparoscopic liver resection to open liver resection in obese individuals with hepatocellular carcinoma: A retrospective study. Ann. Gastroenterol. Surg. 2021, 6, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Cescon, M.; Ercolani, G.; Di Gioia, P.; Peri, E.; Pinna, A.D. Safety of hepatic resection in overweight and obese patients with cirrhosis. Br. J. Surg. 2011, 98, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Iimuro, Y.; Hirano, T.; Hai, S.; Suzumura, K.; Nakamura, I.; Kondo, Y.; Fujimoto, J. Safety of hepatic resection for hepatocellular carcinoma in obese patients with cirrhosis. Surg. Today 2013, 43, 1290–1297. [Google Scholar] [CrossRef]
- Gedaly, R.; McHugh, P.P.; Johnston, T.D.; Jeon, H.; Ranjan, D.; Davenport, D.L. Obesity, Diabetes, and Smoking are Important Determinants of Resource Utilization in Liver Resection: A Multicenter Analysis of 1029 Patients. Ann. Surg. 2009, 249, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Nagarajan, G.; Farges, O.; Galleano, C.Z.; Dokmak, S.; Paugam-Burtz, C.; Belghiti, J. Safety of Liver Resections in Obese and Overweight Patients. World J. Surg. 2010, 34, 2960–2968. [Google Scholar] [CrossRef]
- Uchida, H.; Iwashita, Y.; Saga, K.; Takayama, H.; Watanabe, K.; Endo, Y.; Yada, K.; Ohta, M.; Inomata, M. Benefit of laparoscopic liver resection in high body mass index patients. World J. Gastroenterol. 2016, 22, 3015–3022. [Google Scholar] [CrossRef]
- Soubrane, O.; Schwarz, L.; Cauchy, F.; Perotto, L.O.; Brustia, R.; Bernard, D.; Scatton, O. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles. Ann. Surg. 2015, 261, 1226–1231. [Google Scholar] [CrossRef]
- Tomishige, H.; Morise, Z.; Kawabe, N.; Nagata, H.; Ohshima, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J. Gastrointest. Surg. 2013, 5, 173–177. [Google Scholar] [CrossRef]
- Kwan, B.; Waters, P.S.; Keogh, C.; Cavallucci, D.J.; O’Rourke, N.; Bryant, R.D. Body mass index and surgical outcomes in laparoscopic liver resections: A systematic review. ANZ J. Surg. 2021, 91, 2296–2307. [Google Scholar] [CrossRef]
- Nomi, T.; Fuks, D.; Ferraz, J.-M.; Kawaguchi, Y.; Nakajima, Y.; Gayet, B. Influence of body mass index on postoperative outcomes after laparoscopic liver resection. Surg. Endosc. 2015, 29, 3647–3654. [Google Scholar] [CrossRef]
- Ome, Y.; Hashida, K.; Yokota, M.; Nagahisa, Y.; Okabe, M.; Kawamoto, K. The safety and efficacy of laparoscopic hepatectomy in obese patients. Asian J. Surg. 2019, 42, 180–188. [Google Scholar] [CrossRef]
- Toriguchi, K.; Hatano, E.; Sakurai, T.; Seo, S.; Taura, K.; Uemoto, S. Laparoscopic Liver Resection in Obese Patients. World J. Surg. 2015, 39, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C. Robotics in General Surgery. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Patriti, A.; Ceccarelli, G.; Bartoli, A.; Spaziani, A.; Lapalorcia, L.M.; Casciola, L. Laparoscopic and robot-assisted one-stage resection of colorectal cancer with synchronous liver metastases: A pilot study. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wakabayashi, G.; Kim, H.-J.; Choi, G.-H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; Troisi, R.I.; Efanov, M.; et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, R.I.; Azimuddin, A.; Cao, H.S.T. Robot-assisted liver resection: The real benefit so far. Langenbeck’s Arch. Surg. 2022, 1–9. [Google Scholar] [CrossRef]
- Troisi, R.I.; Pegoraro, F.; Giglio, M.C.; Rompianesi, G.; Berardi, G.; Tomassini, F.; De Simone, G.; Aprea, G.; Montalti, R.; De Palma, G.D. Robotic approach to the liver: Open surgery in a closed abdomen or laparoscopic surgery with technical constraints? Surg. Oncol. 2019, 33, 239–248. [Google Scholar] [CrossRef]
- Vigano, L.; Laurent, A.; Tayar, C.; Tomatis, M.; Ponti, A.; Cherqui, D. The Learning Curve in Laparoscopic Liver Resection. Ann. Surg. 2009, 250, 772–782. [Google Scholar] [CrossRef]
- Nomi, T.; Fuks, D.; Kawaguchi, Y.; Mal, F.; Nakajima, Y.; Gayet, B. Learning curve for laparoscopic major hepatectomy. Br. J. Surg. 2015, 102, 796–804. [Google Scholar] [CrossRef]
- Lee, W.; Woo, J.-W.; Lee, J.-K.; Park, J.-H.; Kim, J.-Y.; Kwag, S.-J.; Park, T.; Jeong, S.-H.; Ju, Y.-T.; Jeong, E.-J.; et al. Comparison of Learning Curves for Major and Minor Laparoscopic Liver Resection. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 457–464. [Google Scholar] [CrossRef]
- Navarro, J.G.; Kang, I.; Rho, S.Y.; Choi, G.H.; Han, D.H.; Kim, K.S.; Choi, J.S. Major Laparoscopic Versus Open Resection for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis Based on Surgeons’ Learning Curve. Ann. Surg. Oncol. 2020, 28, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, A.R.; Castellanos, A.E.; Desai, J.P.; Meyers, W.C. Robotic Surgery. Ann. Surg. 2004, 239, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Wilson, M.; Waine, E.; Masters, R.S.W.; McGrath, J.S.; Vine, S.J. Robotic technology results in faster and more robust surgical skill acquisition than traditional laparoscopy. J. Robot. Surg. 2014, 9, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Fong, A.; Payyavula, G.; DiMaio, S.; Lafaro, K.; Tallmon, K.; Wren, S.; Sorger, J.; Fong, Y. Study on augmented reality for robotic surgery bedside assistants. J. Robot. Surg. 2021, 1–8. [Google Scholar] [CrossRef]
- Chen, P.-D.; Wu, C.-Y.; Hu, R.-H.; Chen, C.-N.; Yuan, R.-H.; Liang, J.-T.; Lai, H.-S.; Wu, Y.-M. Robotic major hepatectomy: Is there a learning curve? Surgery 2017, 161, 642–649. [Google Scholar] [CrossRef]
- Efanov, M.; Alikhanov, R.; Tsvirkun, V.; Kazakov, I.; Melekhina, O.; Kim, P.; Vankovich, A.; Grendal, K.; Berelavichus, S.; Khatkov, I. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB 2017, 19, 818–824. [Google Scholar] [CrossRef]
- Zhu, P.; Liao, W.; Ding, Z.-Y.; Chen, L.; Zhang, W.-G.; Zhang, B.-X.; Chen, X.-P. Learning Curve in Robot-Assisted Laparoscopic Liver Resection. J. Gastrointest. Surg. 2018, 23, 1778–1787. [Google Scholar] [CrossRef]
- Chong, C.C.; Fuks, D.; Lee, K.-F.; Zhao, J.J.; Choi, G.H.; Sucandy, I.; Chiow, A.K.H.; Marino, M.V.; Gastaca, M.; Wang, X.; et al. Propensity Score–Matched Analysis Comparing Robotic and Laparoscopic Right and Extended Right Hepatectomy. JAMA Surg. 2022, 157, 436. [Google Scholar] [CrossRef]
- Chong, C.C.N.; Lok, H.T.; Fung, A.K.Y.; Fong, A.K.W.; Cheung, Y.S.; Wong, J.; Lee, K.F.; Lai, P.B.S. Robotic versus laparoscopic hepatectomy: Application of the difficulty scoring system. Surg. Endosc. 2019, 34, 2000–2006. [Google Scholar] [CrossRef]
- Lorenz, E.; Arend, J.; Franz, M.; Rahimli, M.; Perrakis, A.; Negrini, V.; Gumbs, A.A.; Croner, R.S. Robotic and laparoscopic liver resection—Comparative experiences at a high-volume German academic center. Langenbeck’s Arch. Surg. 2021, 406, 753–761. [Google Scholar] [CrossRef]
- Jiang, B.; Yan, X.-F.; Zhang, J.-H. Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol. Res. 2018, 48, 635–663. [Google Scholar] [CrossRef] [PubMed]
- Machairas, N.; Papaconstantinou, D.; Tsilimigras, D.I.; Moris, D.; Prodromidou, A.; Paspala, A.; Spartalis, E.; Kostakis, I.D. Comparison between robotic and open liver resection: A systematic review and meta-analysis of short-term outcomes. Updat. Surg. 2019, 71, 39–48. [Google Scholar] [CrossRef]
- Wong, D.J.; Wong, M.J.; Choi, G.H.; Wu, Y.M.; Lai, P.B.; Goh, B.K.P. Systematic review and meta-analysis of robotic versus open hepatectomy. ANZ J. Surg. 2018, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bundred, J.; Manas, D.; Jiao, L.R.; Abu Hilal, M.; White, S.A. Robotic versus conventional laparoscopic liver resections: A systematic review and meta-analysis. Scand. J. Surg. 2020, 110, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Coletta, D.; Sandri, G.B.L.; Giuliani, G.; Guerra, F. Robot-assisted versus conventional laparoscopic major hepatectomies: Systematic review with meta-analysis. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 17, e2218. [Google Scholar] [CrossRef]
- Zhu, P.; Liao, W.; Zhang, W.-G.; Chen, L.; Shu, C.; Zhang, Z.-W.; Huang, Z.-Y.; Chen, Y.-F.; Lau, W.Y.; Zhang, B.-X.M.; et al. A Prospective Study Using Propensity Score Matching to Compare Long-term Survival Outcomes After Robotic-assisted, Laparoscopic, or Open Liver Resection for Patients with BCLC Stage 0-A Hepatocellular Carcinoma. Ann. Surg. 2022, 277, e103–e111. [Google Scholar] [CrossRef]
- Lee, K.-F.; Chong, C.; Cheung, S.; Wong, J.; Fung, A.; Lok, H.-T.; Lo, E.; Lai, P. Robotic versus open hemihepatectomy: A propensity score-matched study. Surg. Endosc. 2020, 35, 2316–2323. [Google Scholar] [CrossRef]
- Magistri, P.; Tarantino, G.; Guidetti, C.; Assirati, G.; Olivieri, T.; Ballarin, R.; Coratti, A.; Di Benedetto, F. Laparoscopic versus robotic surgery for hepatocellular carcinoma: The first 46 consecutive cases. J. Surg. Res. 2017, 217, 92–99. [Google Scholar] [CrossRef]
- Schmelzle, M.; Feldbrügge, L.; Galindo, S.A.O.; Moosburner, S.; Kästner, A.; Krenzien, F.; Benzing, C.; Biebl, M.; Öllinger, R.; Malinka, T.; et al. Robotic vs. laparoscopic liver surgery: A single-center analysis of 600 consecutive patients in 6 years. Surg. Endosc. 2022, 36, 5854–5862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, S.; Kubo, S.; Ishizawa, T. Positioning of Minimally Invasive Liver Surgery for Hepatocellular Carcinoma: From Laparoscopic to Robot-Assisted Liver Resection. Cancers 2023, 15, 488. https://doi.org/10.3390/cancers15020488
Tanaka S, Kubo S, Ishizawa T. Positioning of Minimally Invasive Liver Surgery for Hepatocellular Carcinoma: From Laparoscopic to Robot-Assisted Liver Resection. Cancers. 2023; 15(2):488. https://doi.org/10.3390/cancers15020488
Chicago/Turabian StyleTanaka, Shogo, Shoji Kubo, and Takeaki Ishizawa. 2023. "Positioning of Minimally Invasive Liver Surgery for Hepatocellular Carcinoma: From Laparoscopic to Robot-Assisted Liver Resection" Cancers 15, no. 2: 488. https://doi.org/10.3390/cancers15020488
APA StyleTanaka, S., Kubo, S., & Ishizawa, T. (2023). Positioning of Minimally Invasive Liver Surgery for Hepatocellular Carcinoma: From Laparoscopic to Robot-Assisted Liver Resection. Cancers, 15(2), 488. https://doi.org/10.3390/cancers15020488




