Multiple Laparoscopic Liver Resection for Colorectal Liver Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Feasibility of Multiple Concomitant LLR
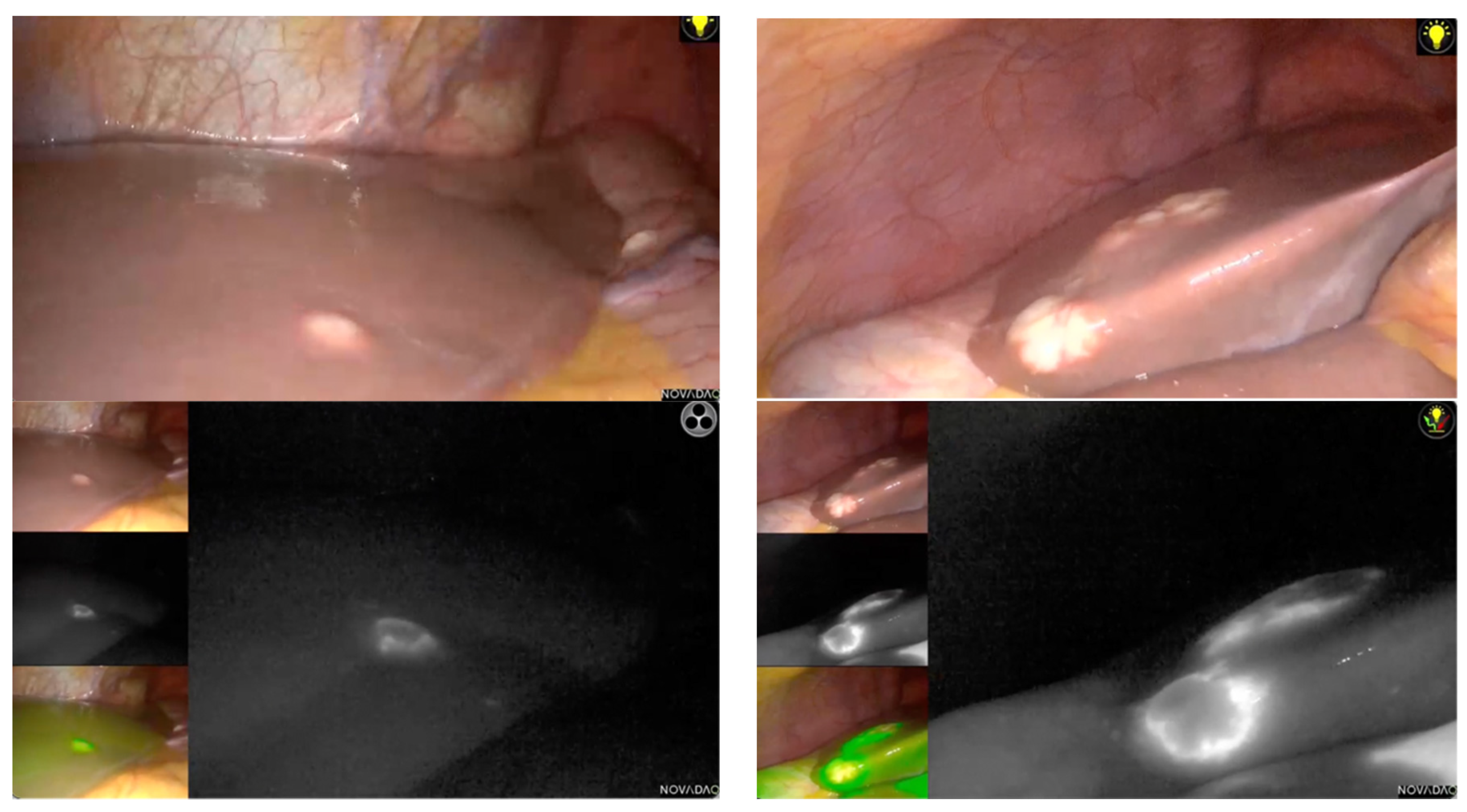
3. Ultrasonography and Other Operative Guidance in Multiple LLR for CRLM
4. Postoperative Short-Term Outcomes after Multiple LLR
5. Oncological Outcomes after Multiple LLR for CRLM
6. Two-Stage Laparoscopic Hepatectomy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abu Hilal, M.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Halls, M.C.; Alseidi, A.; Berardi, G.; Cipriani, F.; Van der Poel, M.; Davila, D.; Ciria, R.; Besselink, M.; D’Hondt, M.; Dagher, I.; et al. A Comparison of the Learning Curves of Laparoscopic Liver Surgeons in Differing Stages of the IDEAL Paradigm of Surgical Innovation: Standing on the Shoulders of Pioneers. Ann. Surg. 2019, 269, 221–228. [Google Scholar] [CrossRef]
- Phelip, J.M.; Tougeron, D.; Léonard, D.; Benhaim, L.; Desolneux, G.; Dupré, A.; Michel, P.; Penna, C.; Tournigand, C.; Louvet, C.; et al. Metastatic Colorectal Cancer (MCRC): French Intergroup Clinical Practice Guidelines for Diagnosis, Treatments and Follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2019, 51, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann. Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef]
- Robles-Campos, R.; Lopez-Lopez, V.; Brusadin, R.; Lopez-Conesa, A.; Gil-Vazquez, P.J.; Navarro-Barrios, Á.; Parrilla, P. Open versus Minimally Invasive Liver Surgery for Colorectal Liver Metastases (LapOpHuva): A Prospective Randomized Controlled Trial. Surg. Endosc. 2019, 33, 3926–3936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Liu, R.-F.; Zhang, D.; Zhang, Y.-S.; Wang, T. Laparoscopic versus Open Liver Resection for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis of Studies with Propensity Score-Based Analysis. Int. J. Surg. 2017, 44, 191–203. [Google Scholar] [CrossRef]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The International Position on Laparoscopic Liver Surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for Laparoscopic Liver Resection: A Report from the Second International Consensus Conference Held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef]
- van der Werf, L.R.; Kok, N.F.M.; Buis, C.I.; Grünhagen, D.J.; Hoogwater, F.J.H.; Swijnenburg, R.J.; den Dulk, M.; Dejong, K.C.H.C.; Klaase, J.M. Dutch Hepato Biliary Audit Group Implementation and First Results of a Mandatory, Nationwide Audit on Liver Surgery. HPB 2019, 21, 1400–1410. [Google Scholar] [CrossRef]
- Alvarez, F.A.; Sanchez Claria, R.; Oggero, S.; de Santibañes, E. Parenchymal-Sparing Liver Surgery in Patients with Colorectal Carcinoma Liver Metastases. World J. Gastrointest. Surg. 2016, 8, 407–423. [Google Scholar] [CrossRef]
- Gold, J.S.; Are, C.; Kornprat, P.; Jarnagin, W.R.; Gönen, M.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; D’Angelica, M. Increased Use of Parenchymal-Sparing Surgery for Bilateral Liver Metastases from Colorectal Cancer Is Associated with Improved Mortality without Change in Oncologic Outcome: Trends in Treatment over Time in 440 Patients. Ann. Surg. 2008, 247, 109–117. [Google Scholar] [CrossRef]
- Torzilli, G.; Viganò, L.; Cimino, M.; Imai, K.; Vibert, E.; Donadon, M.; Mansour, D.; Castaing, D.; Adam, R. Is Enhanced One-Stage Hepatectomy a Safe and Feasible Alternative to the Two-Stage Hepatectomy in the Setting of Multiple Bilobar Colorectal Liver Metastases? A Comparative Analysis between Two Pioneering Centers. Dig. Surg. 2018, 35, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Tabchouri, N.; Leung, U.; Tinguely, P.; Louvet, C.; Beaussier, M.; Gayet, B.; Fuks, D. Laparoscopic Parenchymal-Sparing Hepatectomy for Multiple Colorectal Liver Metastases Improves Outcomes and Salvageability: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2019, 26, 4576–4586. [Google Scholar] [CrossRef] [PubMed]
- Kalil, J.A.; Poirier, J.; Becker, B.; Van Dam, R.; Keutgen, X.; Schadde, E. Laparoscopic Parenchymal-Sparing Hepatectomy: The New Maximally Minimal Invasive Surgery of the Liver-a Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2019, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Montalti, R.; Tomassini, F.; Laurent, S.; Smeets, P.; De Man, M.; Geboes, K.; Libbrecht, L.J.; Troisi, R.I. Impact of Surgical Margins on Overall and Recurrence-Free Survival in Parenchymal-Sparing Laparoscopic Liver Resections of Colorectal Metastases. Surg. Endosc. 2015, 29, 2736–2747. [Google Scholar] [CrossRef]
- Ferrero, A.; Lo Tesoriere, R.; Russolillo, N.; Viganò, L.; Forchino, F.; Capussotti, L. Ultrasound-Guided Laparoscopic Liver Resections. Surg. Endosc. 2015, 29, 1002–1005. [Google Scholar] [CrossRef]
- Torzilli, G.; Procopio, F.; Botea, F.; Marconi, M.; Del Fabbro, D.; Donadon, M.; Palmisano, A.; Spinelli, A.; Montorsi, M. One-Stage Ultrasonographically Guided Hepatectomy for Multiple Bilobar Colorectal Metastases: A Feasible and Effective Alternative to the 2-Stage Approach. Surgery 2009, 146, 60–71. [Google Scholar] [CrossRef]
- Langella, S.; Russolillo, N.; D’Eletto, M.; Forchino, F.; Lo Tesoriere, R.; Ferrero, A. Oncological Safety of Ultrasound-Guided Laparoscopic Liver Resection for Colorectal Metastases: A Case-Control Study. Updates Surg. 2015, 67, 147–155. [Google Scholar] [CrossRef]
- Russolillo, N.; Aldrighetti, L.; Cillo, U.; Guglielmi, A.; Ettorre, G.M.; Giuliante, F.; Mazzaferro, V.; Dalla Valle, R.; De Carlis, L.; Jovine, E.; et al. Risk-Adjusted Benchmarks in Laparoscopic Liver Surgery in a National Cohort. Br. J. Surg. 2020, 107, 845–853. [Google Scholar] [CrossRef]
- Kazaryan, A.M.; Aghayan, D.L.; Barkhatov, L.I.; Fretland, Å.A.; Edwin, B. Laparoscopic Multiple Parenchyma-Sparing Concomitant Liver Resections for Colorectal Liver Metastases. Surg. Laparosc. Endosc. Percutan. Tech. 2019, 29, 187–193. [Google Scholar] [CrossRef]
- D’Hondt, M.; Pironet, Z.; Parmentier, I.; De Meyere, C.; Besselink, M.; Pottel, H.; Vansteenkiste, F.; Verslype, C. One-Stage Laparoscopic Parenchymal Sparing Liver Resection for Bilobar Colorectal Liver Metastases: Safety, Recurrence Patterns and Oncologic Outcomes. Surg. Endosc. 2022, 36, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Tribillon, E.; Marchese, U.; Faermark, N.; Bonnet, S.; Beaussier, M.; Gayet, B.; Fuks, D. Feasibility and Outcomes of Multiple Simultaneous Laparoscopic Liver Resections. Surg. Endosc. 2022, 36, 2466–2472. [Google Scholar] [CrossRef]
- Aghayan, D.L.; Pelanis, E.; Avdem Fretland, Å.; Kazaryan, A.M.; Sahakyan, M.A.; Røsok, B.I.; Barkhatov, L.; Bjørnbeth, B.A.; Jakob Elle, O.; Edwin, B. Laparoscopic Parenchyma-Sparing Liver Resection for Colorectal Metastases. Radiol. Oncol. 2018, 52, 36–41. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Fuks, D.; Kokudo, N.; Gayet, B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann. Surg. 2018, 267, 13–17. [Google Scholar] [CrossRef]
- Viganò, L.; Ferrero, A.; Amisano, M.; Russolillo, N.; Capussotti, L. Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br. J. Surg. 2013, 100, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, T.; Kakisaka, T.; Orimo, T. Current role of intraoperative ultrasonography in hepatectomy. Surg. Today 2021, 51, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Lo Tesoriere, R.; Russolillo, N. Ultrasound Liver Map Technique for Laparoscopic Liver Resections. World J. Surg. 2019, 43, 2607–2611. [Google Scholar] [CrossRef]
- Wang, X.; Teh, C.S.C.; Ishizawa, T.; Aoki, T.; Cavallucci, D.; Lee, S.-Y.; Panganiban, K.M.; Perini, M.V.; Shah, S.R.; Wang, H.; et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann. Surg. 2021, 274, 97–106. [Google Scholar] [CrossRef]
- Lu, H.; Gu, J.; Qian, X.-F.; Dai, X.-Z. Indocyanine Green Fluorescence Navigation in Laparoscopic Hepatectomy: A Retrospective Single-Center Study of 120 Cases. Surg. Today 2021, 51, 695–702. [Google Scholar] [CrossRef]
- Takahashi, H.; Zaidi, N.; Berber, E. An initial report on the intraoperative use of indocyanine green fluorescence imaging in the surgical management of liver tumorss. J. Surg. Oncol. 2016, 114, 625–629. [Google Scholar] [CrossRef]
- Procopio, F.; Cimino, M.; Viganò, L.; Colombo, A.E.; Franchi, E.; Costa, G.; Donadon, M.; Del Fabbro, D.; Torzilli, G. Prediction of Remnant Liver Volume Using 3D Simulation Software in Patients Undergoing R1vasc Parenchyma-Sparing Hepatectomy for Multiple Bilobar Colorectal Liver Metastases: Reliability, Clinical Impact, and Learning Curve. HPB 2021, 23, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Budzyński, A.; Grochowska, A.; Ballard, D.H.; Major, P.; Rubinkiewicz, M.; Złahoda-Huzior, A.; Popiela, T.J.; Wierdak, M.; Pędziwiatr, M. Decision-making based on 3D printed models in laparoscopic liver resections with intraoperative ultrasound: A prospective observational study. Eur. Radiol. 2020, 30, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Sahara, K.; Moris, D.; Mehta, R.; Paredes, A.Z.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; et al. Assessing Textbook Outcomes Following Liver Surgery for Primary Liver Cancer Over a 12-Year Time Period at Major Hepatobiliary Centers. Ann. Surg. Oncol. 2020, 27, 3318–3327. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Nobili, C.; Marzano, E.; Oussoultzoglou, E.; Rosso, E.; Addeo, P.; Bachellier, P.; Jaeck, D.; Pessaux, P. Multivariate Analysis of Risk Factors for Pulmonary Complications after Hepatic Resection. Ann. Surg. 2012, 255, 540–550. [Google Scholar] [CrossRef]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.-M. Epidemiology and Management of Liver Metastases from Colorectal Cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef]
- Aghayan, D.L.; Kazaryan, A.M.; Dagenborg, V.J.; Røsok, B.I.; Fagerland, M.W.; Waaler Bjørnelv, G.M.; Kristiansen, R.; Flatmark, K.; Fretland, Å.A.; Edwin, B.; et al. Long-Term Oncologic Outcomes After Laparoscopic Versus Open Resection for Colorectal Liver Metastases: A Randomized Trial. Ann. Intern. Med. 2021, 174, 175–182. [Google Scholar] [CrossRef]
- Bolton, J.S.; Fuhrman, G.M. Survival after Resection of Multiple Bilobar Hepatic Metastases from Colorectal Carcinoma. Ann. Surg. 2000, 231, 743–751. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Croner, R.; Lorenz, E.; Cacciaguerra, A.B.; Tsai, T.-J.; Starker, L.; Flanagan, J.; Yu, N.J.; Chouillard, E.; Abu Hilal, M. Survival Study: International Multicentric Minimally Invasive Liver Resection for Colorectal Liver Metastases (SIMMILR-2). Cancers 2022, 14, 4190. [Google Scholar] [CrossRef]
- Adam, R.; Laurent, A.; Azoulay, D.; Castaing, D.; Bismuth, H. Two-Stage Hepatectomy: A Planned Strategy to Treat Irresectable Liver Tumors. Ann. Surg. 2000, 232, 777–785. [Google Scholar] [CrossRef]
- Jaeck, D.; Oussoultzoglou, E.; Rosso, E.; Greget, M.; Weber, J.-C.; Bachellier, P. A Two-Stage Hepatectomy Procedure Combined with Portal Vein Embolization to Achieve Curative Resection for Initially Unresectable Multiple and Bilobar Colorectal Liver Metastases. Ann. Surg. 2004, 240, 1037–1049; discussion 1049–1051. [Google Scholar] [CrossRef]
- Wicherts, D.A.; Miller, R.; de Haas, R.J.; Bitsakou, G.; Vibert, E.; Veilhan, L.-A.; Azoulay, D.; Bismuth, H.; Castaing, D.; Adam, R. Long-Term Results of Two-Stage Hepatectomy for Irresectable Colorectal Cancer Liver Metastases. Ann. Surg. 2008, 248, 994–1005. [Google Scholar] [CrossRef]
- Fuks, D.; Nomi, T.; Ogiso, S.; Gelli, M.; Velayutham, V.; Conrad, C.; Louvet, C.; Gayet, B. Laparoscopic Two-Stage Hepatectomy for Bilobar Colorectal Liver Metastases. Br. J. Surg. 2015, 102, 1684–1690. [Google Scholar] [CrossRef]
- Okumura, S.; Goumard, C.; Gayet, B.; Fuks, D.; Scatton, O. Laparoscopic versus Open Two-Stage Hepatectomy for Bilobar Colorectal Liver Metastases: A Bi-Institutional, Propensity Score-Matched Study. Surgery 2019, 166, 959–966. [Google Scholar] [CrossRef]
- Kilburn, D.J.; Chiow, A.K.H.; Lewin, J.; Kienzle, N.; Cavallucci, D.J.; Bryant, R.; O’Rourke, N. Laparoscopic Approach to a Planned Two-Stage Hepatectomy for Bilobar Colorectal Liver Metastases. ANZ J. Surg. 2016, 86, 811–815. [Google Scholar] [CrossRef]
- Taillieu, E.; De Meyere, C.; D’Hondt, M. The Role of the Laparoscopic Approach in Two-Stage Hepatectomy for Colorectal Liver Metastases: A Single-Center Experience. Surg. Endosc. 2022, 36, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Di Fabio, F.; Whistance, R.; Rahman, S.; Primrose, J.N.; Pearce, N.W.; Abu Hilal, M. Exploring the Role of Laparoscopic Surgery in Two-Stage Hepatectomy for Bilobar Colorectal Liver Metastases. J. Laparoendosc. Adv. Surg. Tech. A 2012, 22, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Görgec, B.; Suhool, A.; Al-Jarrah, R.; Fontana, M.; Tehami, N.A.; Modi, S.; Abu Hilal, M. Surgical Technique and Clinical Results of One- or Two-Stage Laparoscopic Right Hemihepatectomy after Portal Vein Embolization in Patients with Initially Unresectable Colorectal Liver Metastases: A Case Series. Int. J. Surg. 2020, 77, 69–75. [Google Scholar] [CrossRef]
- Levi Sandri, G.B.; Santoro, R.; Vennarecci, G.; Lepiane, P.; Colasanti, M.; Ettorre, G.M. Two-Stage Hepatectomy, a 10 Years Experience. Updates Surg. 2015, 67, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, L.; Li, H.; Wang, L.; Huang, Y.; Wu, L.; Zhang, Y. Laparoscopic versus open liver resection for colorectal liver metastases: A systematic review. J. Surg. Res. 2017, 220, 234–246. [Google Scholar] [CrossRef]

| Variable | Number of Patients Reported [20,21,22,23] | ||||
|---|---|---|---|---|---|
| Number of LLR | |||||
| 2 | 178 (65.7%) | ||||
| 3–4 | 69 (25.5%) | ||||
| ≥5 | 24 (8.9%) | ||||
| Variable | Number of Patients Reported | Kazaryan et al. [20] (n = 104) | D’Hondt et al. [21] (n = 36) | Nassar et al. [22] (n = 39) | Aghayan et al. [23] (n = 92) |
| Type of LLR | Not described | ||||
| Left lateral sectionectomy with right atypical | 29 | 20 | 9 | ||
| Left hemihepatectomy with right atypical | 1 | 1 | |||
| Multiple atypical resections | 126 | 83 | 23 | 20 | |
| Left | 13/126 (10.3%) | 6 | 7 | ||
| Right | 36/126 (28.6%) | 22 | 14 | ||
| Bilateral | 77/126 (61.1%) | 54 | 23 | ||
| Right bi-segmentectomy or hemihepatectomy with left atypical | 5 | 1 | 4 |
| Article | Tumor Maximum Size (mm) | p (vs. Single) | Mean Blood Loss (mL Range) | p (vs. Single) | Mean Operative Time (min (Range) | p (vs. Single) | Conversion Rate | p (vs. Single) |
|---|---|---|---|---|---|---|---|---|
| Karazyan et al. [20] (n = 104) | 22 | 0.12 | 300 (50–5000) | 0.75 | 186 (75–390) | 0.26 | 2.9% | 0.41 |
| D’Hondt et al. [21] (n = 36) | Not described | 250 (150–450) | <0.001, higher for multiple | 200 (170–230) | <0.001, longer for multiple | 8.3% | 0.07 | |
| Nassar et al. [22] (n = 39) | 23.9 | 0.69 | 188.9 (0–1000) | 0.39 | 217.3 (90–369) | 0.039, longer for multiple | 0% | 0.88 |
| Technique | Location of Tumors Detected | Tumor Margins | Detection of Missing Tumors from Preoperative CT Scan | Availability in OR | Disadvantages |
|---|---|---|---|---|---|
| Intraoperative ultrasound | Superficial or deep | Location relative to veins | Yes | Available | Technicality |
| Indocyanine green fluorescence | Only superficial | Real-time visualization | Yes, if superficial | Available | No deep lesion visualization |
| 3D models | Superficial and deep | Location relative to anatomical structures | No | Not available | Location of tumor detected only if by preoperative CT-scan |
| Article | 90-Days Morbidity Rate | p (vs. Single) | Major Complication (Clavien III–IV) | p (vs. Single) | 90-Days Mortality Rate (n) | p (vs. Single) | Length of Stay (Days) | p (vs. Single) | 5-Year OS | p (vs. Single) | 5-Year RFS | p (vs. Single) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karazyan et al. [20] (n = 104) | 20 (19.2%) | 0.17 | 14 (13.4%) | Not described | 1 (0.96%) | 3 (1–26) | 0.62 | 42% | 0.62 | 16% | 0.14 | |
| D’Hondt et al. [21] (n = 36) | 2 (5.6%) | 1.0 | 1 (2.8%) | 1.0 | 0 (0%) | 1.0 | 5 (4–7) | 0.015, longer for multiple | 66% | 0.49 | 28% | 0.62 |
| Nassar et al. [22] (n = 39) | 14 (27.5%) | 0.82 | 1 (1.9%) | 0.45 | 0 (0%) | 0.94 | 5.5 | 0.59 | Not described | Not described |
| Article | 1st Stage Laparoscopy | 2nd Stage Laparoscopy | Interval Time (Months) | Mean Number of CRLM | Conversion Rate 1st/2nd | 90-Day Morbidity Rate 1st/2nd |
|---|---|---|---|---|---|---|
| Fuks et al. [42] | 34 | 26 | 3 | 2/4 | ||
| Okumura et al. [43] | 38 | 38 | 2.8 | 6 | 1/4 | 16%/26% |
| Kilburn et al. [44] | 7 | 1 | 3.4 | 5.2 | 0/1 | 0%/0% |
| Taillieu et al. [45] | 23 | 7 | 1.9 | Not described | 1/1 | 0%/14% |
| Di Fabio et al. [46] | 8 | 3 | 2.9 | 4 | 0/1 | 0%/Not described |
| Görgec et al. [47] | Not described | 12 | Not described | 3.6 | -/2 | Not described/17% |
| Levi Sandri et al. [48] | 5 | 0 | 2.2 | 6.6 | 0/- | Not described |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, A.; Tzedakis, S.; Dhote, A.; Strigalev, M.; Coriat, R.; Karoui, M.; Dohan, A.; Gaillard, M.; Marchese, U.; Fuks, D. Multiple Laparoscopic Liver Resection for Colorectal Liver Metastases. Cancers 2023, 15, 435. https://doi.org/10.3390/cancers15020435
Nassar A, Tzedakis S, Dhote A, Strigalev M, Coriat R, Karoui M, Dohan A, Gaillard M, Marchese U, Fuks D. Multiple Laparoscopic Liver Resection for Colorectal Liver Metastases. Cancers. 2023; 15(2):435. https://doi.org/10.3390/cancers15020435
Chicago/Turabian StyleNassar, Alexandra, Stylianos Tzedakis, Alix Dhote, Marie Strigalev, Romain Coriat, Mehdi Karoui, Anthony Dohan, Martin Gaillard, Ugo Marchese, and David Fuks. 2023. "Multiple Laparoscopic Liver Resection for Colorectal Liver Metastases" Cancers 15, no. 2: 435. https://doi.org/10.3390/cancers15020435
APA StyleNassar, A., Tzedakis, S., Dhote, A., Strigalev, M., Coriat, R., Karoui, M., Dohan, A., Gaillard, M., Marchese, U., & Fuks, D. (2023). Multiple Laparoscopic Liver Resection for Colorectal Liver Metastases. Cancers, 15(2), 435. https://doi.org/10.3390/cancers15020435








