Endoscopic Ultrasound Guided Biliary Drainage in Malignant Distal Biliary Obstruction
Abstract
Simple Summary
Abstract
1. Introduction
2. Endoscopic Retrograde Cholangiopancreatography (ERCP), Percutaneous Transhepatic Biliary Drainage (PTBD) and EUS-Guided Biliary Drainage (EUS-BD) in MBO
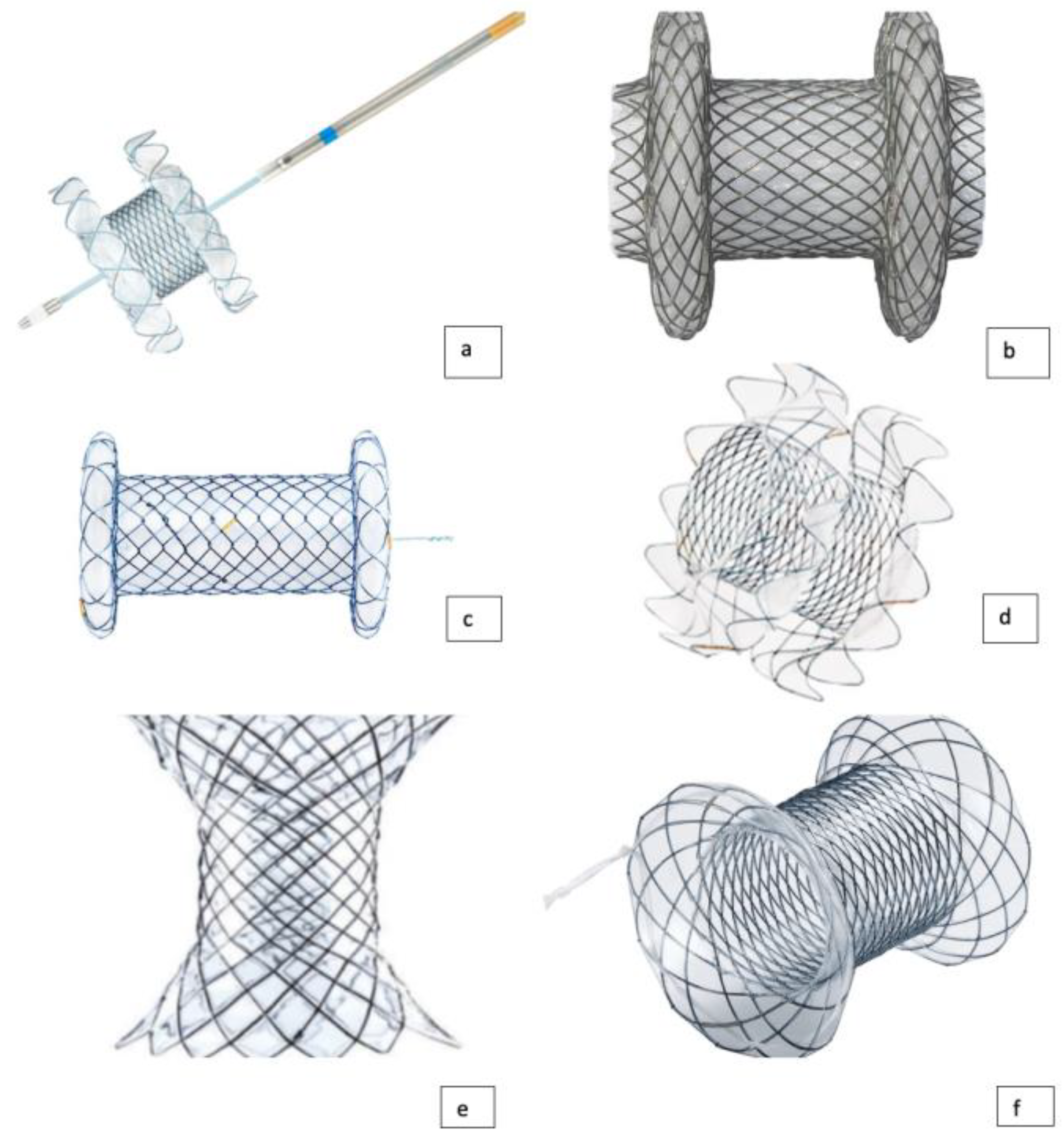
3. Types of LAMS Currently Available
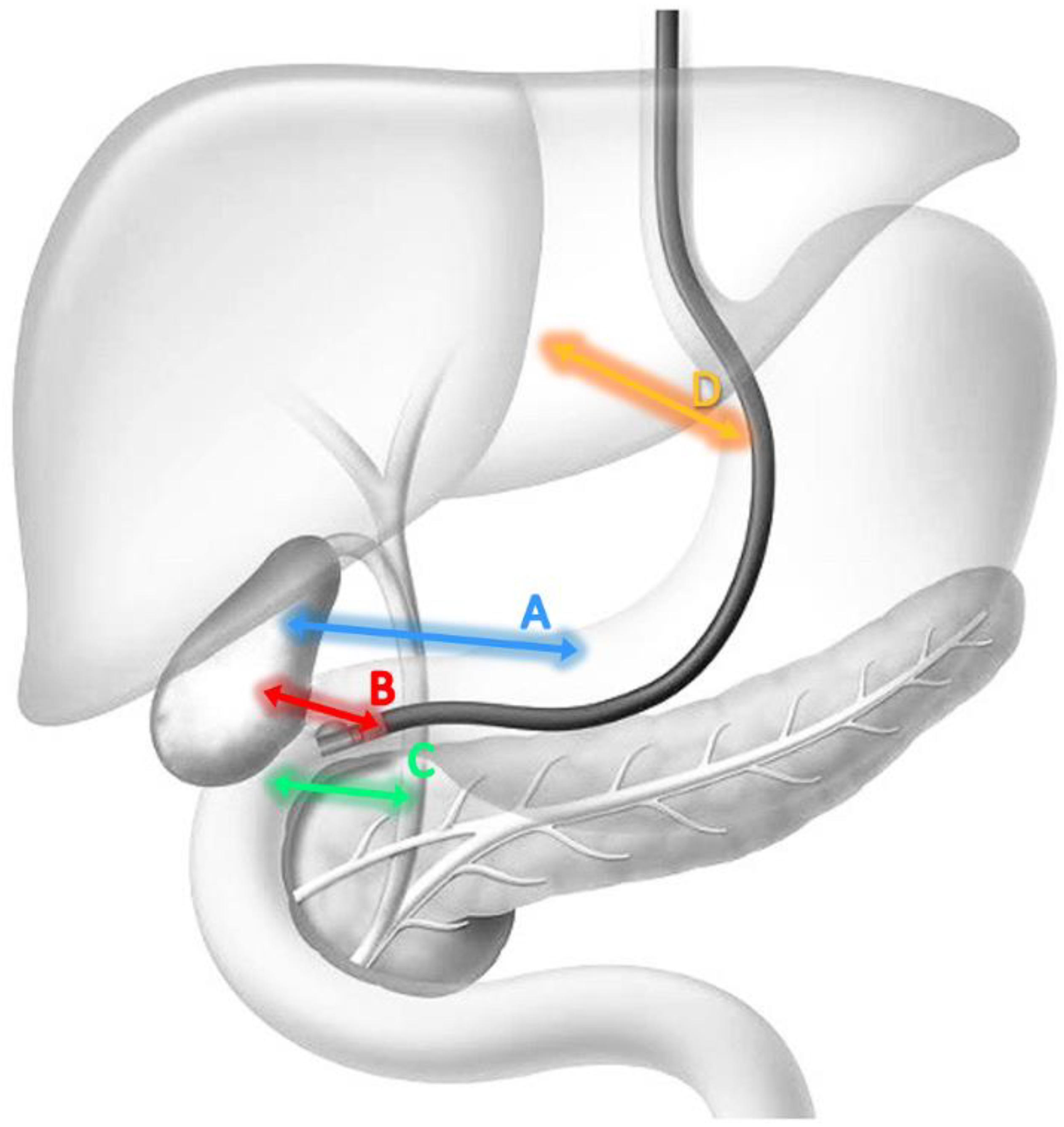
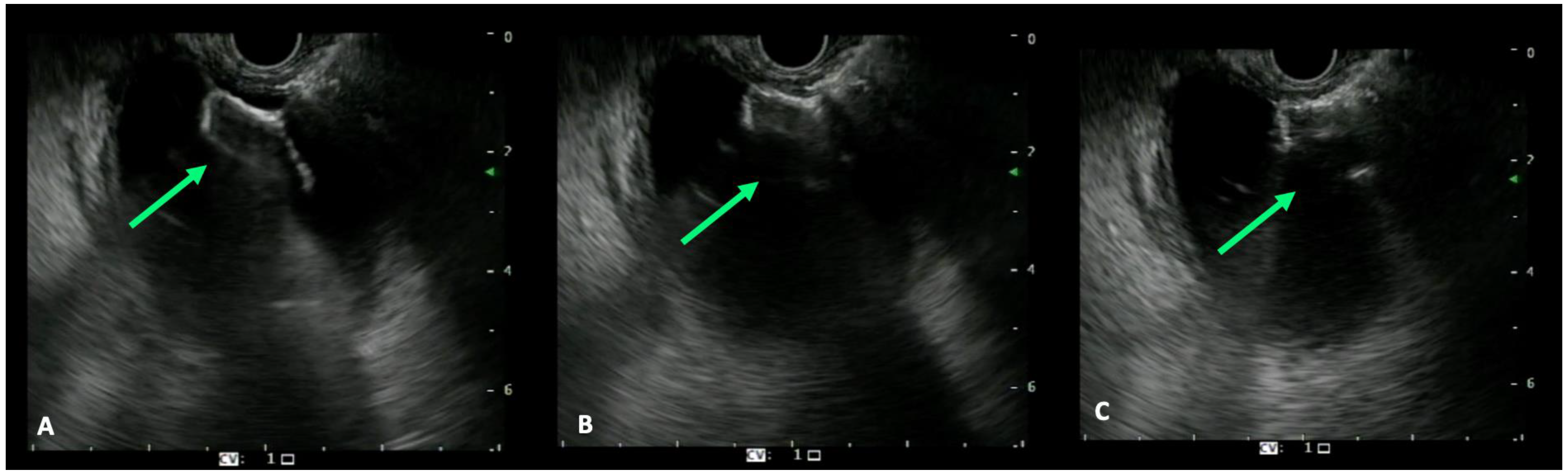
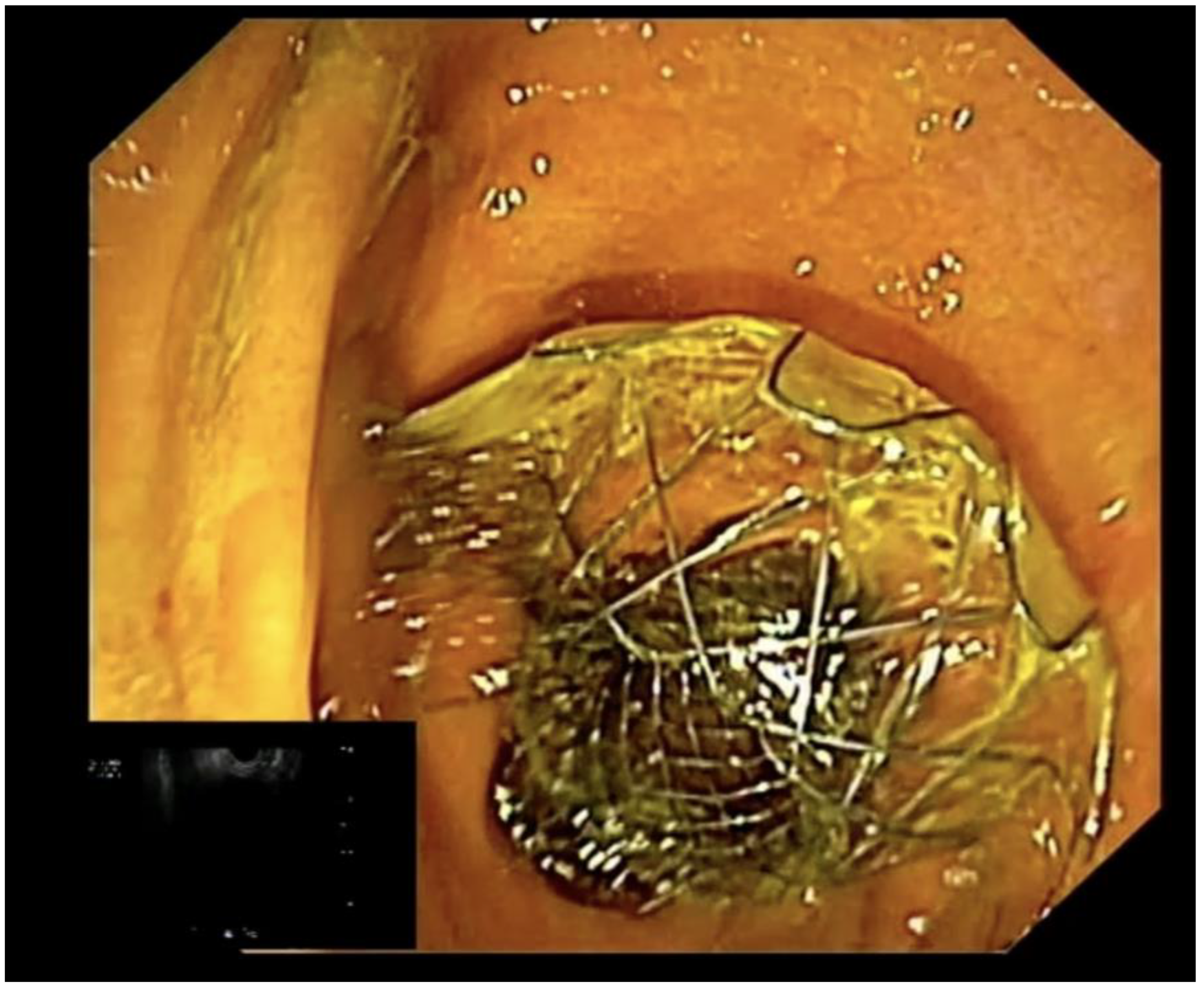
4. EUS-Guided Biliary Drainage Techniques
- -
- access the target organ by using a 19 G EUS FNA needle;
- -
- aspiration of 2–3 mL of bile to confirm the position of the needle tip;
- -
- contrast injection from the 19 G FNA needle (2–3 mL) for the anatomy study;
- -
- 0.035”, 0.025” or 0.032” guidewire insertion;
- -
- FNA needle exchange with a 6 Fr cystotome or a 4 mm balloon to dilate the tract;
- -
- stent placement.
5. Adverse Events
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pu, L.Z.; Singh, R.; Loong, C.K.; de Moura, E.G. Malignant Biliary Obstruction: Evidence for Best Practice. Gastroenterol. Res. Pract. 2016, 2016, 3296801. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.; Do, N.D.T.; Kongkam, P. Efficacy and safety of EUS biliary drainage in malignant distal and hilar biliary obstruction: A comprehensive review of literature and algorithm. Endosc. Ultrasound 2020, 9, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Aadam, A.A.; Liu, K. Endoscopic palliation of biliary obstruction. J. Surg. Oncol. 2019, 120, 57–64. [Google Scholar] [CrossRef]
- Boulay, B.R.; Birg, A. Malignant biliary obstruction: From palliation to treatment. World J. Gastrointest. Oncol. 2016, 8, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Mangiavillano, B.; Papanikolaou, I.S.; Arvanitakis, M.; Auriemma, F.; Bianchetti, M.; Tarantino, I.; Traina, M.; Deviere, J.; Repici, A. Endoscopic drainage in patients with malignant extrahepatic biliary obstruction: When and how. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1279–1283. [Google Scholar] [CrossRef]
- Inamdar, S.; Slattery, E.; Bhalla, R.; Sejpal, D.V.; Trindade, A.J. Comparison of Adverse Events for Endoscopic vs Percutaneous Biliary Drainage in the Treatment of Malignant Biliary Tract Obstruction in an Inpatient National Cohort. JAMA Oncol. 2016, 2, 112–117. [Google Scholar] [CrossRef]
- Mangiavillano, B.; Sosa-Valencia, L.; Deprez, P.; Eisendrath, P.; Robles-Medranda, C.; Eusebi, L.H.; Di Leo, M.; Auriemma, F.; Bianchetti, M.; Anderloni, A.; et al. Tissue acquisition and pancreatic masses: Which needle and which acquisition technique should be used? Endosc. Int. Open 2020, 8, E1315–E1320. [Google Scholar] [CrossRef]
- Di Mitri, R.; Amata, M.; Mocciaro, F.; Conte, E.; Bonaccorso, A.; Scrivo, B.; Scimeca, D. EUS-guided biliary drainage with LAMS for distal malignant biliary obstruction when ERCP fails: Single-center retrospective study and maldeployment management. Surg. Endosc. 2022, 36, 4553–4569. [Google Scholar] [CrossRef]
- Conio, M.; Mangiavillano, B.; Caruso, A.; Filiberti, R.A.; Baron, T.H.; De Luca, L.; Signorelli, S.; Crespi, M.; Marini, M.; Ravelli, P.; et al. Covered versus uncovered self-expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: A randomized multicenter study. Gastrointest. Endosc. 2018, 88, 283–291.e3. [Google Scholar] [CrossRef]
- van Wanrooij, R.L.J.; Bronswijk, M.; Kunda, R.; Everett, S.M.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Badaoui, A.; Law, R.; Arcidiacono, P.G.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2022, 54, 310–332. [Google Scholar] [CrossRef]
- Smith, A.C.; Dowsett, J.F.; Russell, R.C.; Hatfield, A.R.; Cotton, P.B. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet 1994, 344, 1655–1660. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline- Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Nabi, Z.; Talukdar, R.; Reddy, D.N. Primary EUS-guided drainage for malignant distal biliary obstruction: Not yet prime time! Gastrointest. Endosc. 2018, 88, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Baron, T.H.; Davila, R.E.; Egan, J.; Hirota, W.K.; Leighton, J.A.; Qureshi, W.; Rajan, E.; Zuckerman, M.J.; Fanelli, R.; et al. ASGE guideline: The role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest. Endosc. 2005, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fung, B.M.; Pitea, T.C.; Tabibian, J.H. Difficult Biliary Cannulation in Endoscopic Retrograde Cholangiopancreatography: An Overview of Advanced Techniques. Eur. Med. J. Hepatol. 2021, 9, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Nennstiel, S.; Weber, A.; Frick, G.; Haller, B.; Meining, A.; Schmid, R.M.; Neu, B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J. Clin. Gastroenterol. 2015, 49, 764–770. [Google Scholar] [CrossRef]
- Leng, J.J.; Zhang, N.; Dong, J.H. Percutaneous transhepatic and endoscopic biliary drainage for malignant biliary tract obstruction: A meta-analysis. World J. Surg. Oncol. 2014, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Efthymiou, M.; Kaffes, A.; Chen, J.W.; Kwan, V.; Murray, M.; Williams, D.; Nguyen, N.Q.; Tam, W.; Welch, C.; et al. Management of pancreatic collections with a novel endoscopically placed fully covered self-expandable metal stent: A national experience (with videos). Gastrointest. Endosc. 2015, 81, 127–135. [Google Scholar] [CrossRef]
- Anderloni, A.; Buda, A.; Vieceli, F.; Khashab, M.A.; Hassan, C.; Repici, A. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: A systematic review and pooled analysis. Surg. Endosc. 2016, 30, 5200–5208. [Google Scholar] [CrossRef]
- Khashab, M.A.; Kumbhari, V.; Grimm, I.S.; Ngamruengphong, S.; Aguila, G.; El Zein, M.; Kalloo, A.N.; Baron, T.H. EUS-guided gastroenterostomy: The first U.S. clinical experience (with video). Gastrointest. Endosc. 2015, 82, 932–938. [Google Scholar] [CrossRef]
- Tyberg, A.; Perez-Miranda, M.; Sanchez-Ocaña, R.; Peñas, I.; de la Serna, C.; Shah, J.; Binmoeller, K.; Gaidhane, M.; Grimm, I.; Baron, T.; et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: A multicenter, international experience. Endosc. Int. Open 2016, 4, E276–E281. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Davies, S.E.C.; Mezzina, N.; Ardizzone, S. Comprehensive review on EUS-guided biliary drainage. World J. Gastrointest. Endosc. 2019, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- El Chafic, A.H.; Shah, J.N.; Hamerski, C.; Binmoeller, K.F.; Irani, S.; James, T.W.; Baron, T.H.; Nieto, J.; Romero, R.V.; Evans, J.A.; et al. EUS-Guided Choledochoduodenostomy for Distal Malignant Biliary Obstruction Using Electrocautery-Enhanced Lumen-Apposing Metal Stents: First US, Multicenter Experience. Dig. Dis. Sci. 2019, 64, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Moole, H.; Bechtold, M.L.; Forcione, D.; Puli, S.R. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine 2017, 96, e5154. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Ávila, F.I.; Herrera-Mora, D.; Duarte-Medrano, G.; Lopez-Arce, G.; Lindoro-Barraza, D.; Casanova, I.; Elizondo-Rivera, J.; Ramírez-Luna, M.; Valdovinos-Andraca, F. Biliary Drainage in Patients with Failed ERCP: Percutaneous Versus EUS-guided Drainage. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 183–187. [Google Scholar] [CrossRef]
- Jo, S.J.; Moon, J.H.; Lee, Y.N.; Park, J.K.; Lee, T.H.; Park, S.H.; Park, S.I.; Jeong, S.; Lee, D.H. A novel bipolar electrocautery-enhanced delivery system with a lumen-apposing metal stent for EUS-guided drainage: A porcine study. J. Hepato-Biliary-Pancreat. Sci. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997, Erratum in Am. J. Gastroenterol. 2018, 113, 1566. [Google Scholar] [CrossRef]
- Bang, J.Y.; Varadarajulu, S. Lumen-apposing metal stents for endoscopic ultrasonography-guided interventions. Dig. Endosc. 2019, 31, 619–626. [Google Scholar] [CrossRef]
- Kunda, R.; Pérez-Miranda, M.; Will, U.; Ullrich, S.; Brenke, D.; Dollhopf, M.; Meier, M.; Larghi, A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg. Endosc. 2016, 30, 5002–5008. [Google Scholar] [CrossRef]
- Jain, D.; Shah, M.; Patel, U.; Sharma, A.; Singhal, S. Endoscopic Ultrasound Guided Choledocho-Enterostomy by Using Lumen Apposing Metal Stent in Patients with Failed Endoscopic Retrograde Cholangiopancreatography: A Literature Review. Digestion. 2018, 98, 1–10. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mangiavillano, B.; Paduano, D.; Binda, C.; Crinò, S.F.; Gkolfakis, P.; Ramai, D.; Fugazza, A.; Tarantino, I.; Lisotti, A.; et al. Methods for Drainage of Distal Malignant Biliary Obstruction after ERCP Failure: A Systematic Review and Network Meta-Analysis. Cancers 2022, 14, 3291. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Tombazzi, C.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef]
- Lee, T.H.; Choi, J.H.; Park, D.H.; Song, T.J.; Kim, D.U.; Paik, W.H.; Hwangbo, Y.; Lee, S.S.; Seo, D.W.; Lee, S.K.; et al. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin. Gastroenterol. Hepatol. 2016, 14, 1011–1019.e3. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.; Binmoeller, K.F.; Lau, J.Y. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest. Endosc. 2014, 80, 1171. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Kunda, R.; Dollhopf, M.; Sanchez-Yague, A.; Will, U.; Tarantino, I.; Gornals Soler, J.; Ullrich, S.; Meining, A.; Esteban, J.M.; et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: A large retrospective study (with video). Gastrointest. Endosc. 2015, 82, 1039–1046. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Adler, D.G.; Nieto, J.; Shah, J.N.; Binmoeller, K.F.; Kane, S.; Yan, L.; Laique, S.N.; Kowalski, T.; Loren, D.E.; et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: A large retrospective, multicenter U.S. experience (with videos). Gastrointest. Endosc. 2016, 83, 699–707. [Google Scholar] [CrossRef]
- Tharian, B.; Varadarajulu, S.; Hawes, R. Drainage of obstructed gallbladder with use of lumen-apposing metal stent. Gastrointest. Endosc. 2016, 83, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Luigiano, C.; Marsico, M.; Cennamo, V. A rare adverse event resulting from the use of a lumen-apposing metal stent for drainage of a pancreatic fluid collection: “the buried sten”. Gastrointest. Endosc. 2015, 82, 585–587. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Dhir, V.; Kida, M.; Yasuda, I.; Jin, Z.D.; Seo, D.W.; Almadi, M.; Ang, T.L.; Hara, K.; Hilmi, I.; et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut 2018, 67, 1209–1228. [Google Scholar] [CrossRef]
- Anderloni, A.; Attili, F.; Carrara, S.; Galasso, D.; Di Leo, M.; Costamagna, G.; Repici, A.; Kunda, R.; Larghi, A. Intra-channel stent release technique for fluoroless endoscopic ultrasound-guided lumen-apposing metal stent placement: Changing the paradigm. Endosc. Int. Open 2017, 5, E25–E29. [Google Scholar] [CrossRef]
- Bhenswala, P.; Lakhana, M.; Gress, F.G.; Andalib, I. Novel Uses of Lumen-apposing Metal Stents: A Review of the Literature. J. Clin. Gastroenterol. 2021, 55, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Mangiavillano, B.; Moon, J.H.; Crinò, S.F.; Larghi, A.; Pham, K.D.; Teoh, A.Y.B.; Paduano, D.; Lee, Y.N.; Yoo, H.W.; Shin, I.S.; et al. Safety and efficacy of a novel electrocautery-enhanced lumen-apposing metal stent in interventional EUS procedures (with video). Gastrointest. Endosc. 2022, 95, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.W.; Waxman, I. Lumen-Apposing Metal Stents: Which One and Why? Gastrointest. Endosc. Clin. N. Am. 2018, 28, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Itoi, T. Technical tips and recent development of endoscopic ultrasound-guided choledochoduodenostomy. DEN Open 2021, 1, e8. [Google Scholar] [CrossRef]
- Dhir, V.; Isayama, H.; Itoi, T.; Almadi, M.; Siripun, A.; Teoh, A.Y.B.; Ho, K.Y. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig. Endosc. 2017, 29, 472–485. [Google Scholar] [CrossRef]
- James, T.W.; Baron, T.H. EUS-guided gallbladder drainage: A review of current practices and procedures. Endosc. Ultrasound 2019, 8 (Suppl. 1), S28–S34. [Google Scholar] [CrossRef]
- Bories, E.; Pesenti, C.; Caillol, F.; Lopes, C.; Giovannini, M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007, 39, 287–291. [Google Scholar] [CrossRef]
- Ogura, T.; Higuchi, K. Technical tips of endoscopic ultrasound-guided choledochoduodenostomy. World J. Gastroenterol. 2015, 21, 820–828. [Google Scholar] [CrossRef]
- Anderloni, A.; Fugazza, A.; Troncone, E.; Auriemma, F.; Carrara, S.; Semeraro, R.; Maselli, R.; Di Leo, M.; D’Amico, F.; Sethi, A.; et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest. Endosc. 2019, 89, 69–76. [Google Scholar] [CrossRef]
- Ogura, T.; Higuchi, K. Endoscopic ultrasound-guided gallbladder drainage: Current status and future prospects. Dig. Endosc. 2019, 31 (Suppl. 1), 55–64. [Google Scholar] [CrossRef]
- Rana, S.S. Endoscopic ultrasound-guided gallbladder drainage: A technical review. Ann. Gastroenterol. 2021, 34, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Park, D.H. Outcomes and limitations: EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8 (Suppl. 1), S44–S49. [Google Scholar] [CrossRef] [PubMed]
- De Bie, C.; Bronswijk, M.; Vanella, G.; Pérez-Cuadrado-Robles, E.; van Malenstein, H.; Laleman, W.; Van der Merwe, S. EUS-guided hepaticogastrostomy for patients with afferent loop syndrome: A comparison with EUS-guided gastroenterostomy or percutaneous drainage. Surg. Endosc. 2022, 36, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Napoléon, B.; Schoch, A.; Lisotti, A.; Walter, T.; Fumex, F.; Leblanc, S.; Artru, P.; Desramé, J.; Brighi, N.; Marsot, J.; et al. Efficacy of EUS-guided hepaticogastrostomy in prolonging survival of patients with perihilar cholangiocarcinoma. Endosc. Ultrasound 2022, 11, 487–494. [Google Scholar] [CrossRef]
- Jagielski, M.; Zieliński, M.; Piątkowski, J.; Jackowski, M. Outcomes and limitations of endoscopic ultrasound-guided hepaticogastrostomy in malignant biliary obstruction. BMC Gastroenterol. 2021, 21, 202. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, S.O.; So, H.; Shin, E.; Kim, D.U.; Park, D.H. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16551. [Google Scholar] [CrossRef]
- Yang, D.; Perbtani, Y.B.; Mramba, L.K.; Kerdsirichairat, T.; Prabhu, A.; Manvar, A.; Ho, S.; Pannu, D.; Keswani, R.N.; Strand, D.S.; et al. Safety and rate of delayed adverse events with lumen-apposing metal stents (LAMS) for pancreatic fluid collections: A multicenter study. Endosc. Int. Open 2018, 6, E1267–E1275. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Teoh, A.Y.B.; Itoi, T.; Yamao, K.; Hara, K.; Nakai, Y.; Isayama, H.; Kitano, M. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: A prospective multicenter study. Gastrointest. Endosc. 2018, 87, 1138–1146. [Google Scholar] [CrossRef]
- Garcia-Alonso, F.J.; Sanchez-Ocana, R.; Peñas-Herrero, I.; Law, R.; Sevilla-Ribota, S.; Torres-Yuste, R.; Gil-Simón, P.; de la Serna Higuera, C.; Perez-Miranda, M. Cumulative risks of stent migration and gastrointestinal bleeding in patients with lumen-apposing metal stents. Endoscopy 2018, 50, 386–395. [Google Scholar] [CrossRef]
- Walter, D.; Teoh, A.Y.; Itoi, T.; Pérez-Miranda, M.; Larghi, A.; Sanchez-Yague, A.; Siersema, P.D.; Vleggaar, F.P. EUS-guided gall bladder drainage with a lumen-apposing metal stent: A prospective long-term evaluation. Gut 2016, 65, 6–8. [Google Scholar] [CrossRef]
- DeSimone, M.L.; Asombang, A.W.; Berzin, T.M. Lumen apposing metal stents for pancreatic fluid collections: Recognition and management of complications. World J. Gastrointest. Endosc. 2017, 9, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Paduano, D.; Auriemma, F.; Spatola, F.; Lamonaca, L.; Repici, A.; Mangiavillano, B. Endoscopic ultrasound-guided choledochoduodenostomy with pyloric occlusion by proximal flange of electrocautery-enhanced lumen-apposing metal stent: Solving a rare adverse event. Endoscopy 2022, 54, E918–E919. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Liu, Q.; He, S.; Zhong, L.; Wu, S.H.; Guo, X.D.; Ning, B. Current uses of electro-cautery lumen apposing metal stents in endoscopic ultrasound guided interventions. Front. Med. 2022, 9, 1002031. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, B.; Aasen, T.; Goenka, P.; Gayam, V. Massive Upper Gastrointestinal Bleeding Following LAMS (Lumen-Apposing Metal Stent) Placement. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620965800. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut 2017, 66, 2054–2056. [Google Scholar] [CrossRef]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Mangiavillano, B.; Auriemma, F.; Paduano, D.; Lamonaca, L.; Spatola, F.; Repici, A. How to solve misplacement of a lumen-apposing metal stent during cholecystogastrostomy: Immediately perform a second one! Endoscopy 2022, 54, E421–E422, Erratum in Endoscopy 2022, 54, C11. [Google Scholar] [CrossRef]
| Procedure | LAMS: Inner Diameter | Endoscope Position | Indications | Recommendations | Supplemental Comments | |
|---|---|---|---|---|---|---|
| Stomach | Cholecystogastrostomy | 8–10 mm | Long or short. Gallbladder visualized in EUS along the posterior wall/greater curvature of the stomach. | Malignant distal biliary obstruction with retro dilatation of common bile duct | Distended gallbladder with diameter ≥ 20 mm. | After locating the target organ, the distance between the tip of the endoscope and the organ itself should be measured. This distance must be ≤10 mm. Subsequently a Doppler examination must be performed to exclude the presence of vessels or other intervening structures. |
| Duodenum | Cholecystoduodenostomy | 6–8 mm | Long from the duodenal bulb. | Malignant distal biliary obstruction with retro dilatation of common bile duct and sparing of the duodenal bulb. | Distended gallbladder with diameter ≥ 20 mm. | |
| Choledochoduodenostomy | 6–8 mm | Distended CBD with diameter ≥ 15 mm. |
| Adverse Events | |
|---|---|
| Early (periprocedural) |
|
| Late |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduano, D.; Facciorusso, A.; De Marco, A.; Ofosu, A.; Auriemma, F.; Calabrese, F.; Tarantino, I.; Franchellucci, G.; Lisotti, A.; Fusaroli, P.; et al. Endoscopic Ultrasound Guided Biliary Drainage in Malignant Distal Biliary Obstruction. Cancers 2023, 15, 490. https://doi.org/10.3390/cancers15020490
Paduano D, Facciorusso A, De Marco A, Ofosu A, Auriemma F, Calabrese F, Tarantino I, Franchellucci G, Lisotti A, Fusaroli P, et al. Endoscopic Ultrasound Guided Biliary Drainage in Malignant Distal Biliary Obstruction. Cancers. 2023; 15(2):490. https://doi.org/10.3390/cancers15020490
Chicago/Turabian StylePaduano, Danilo, Antonio Facciorusso, Alessandro De Marco, Andrew Ofosu, Francesco Auriemma, Federica Calabrese, Ilaria Tarantino, Gianluca Franchellucci, Andrea Lisotti, Pietro Fusaroli, and et al. 2023. "Endoscopic Ultrasound Guided Biliary Drainage in Malignant Distal Biliary Obstruction" Cancers 15, no. 2: 490. https://doi.org/10.3390/cancers15020490
APA StylePaduano, D., Facciorusso, A., De Marco, A., Ofosu, A., Auriemma, F., Calabrese, F., Tarantino, I., Franchellucci, G., Lisotti, A., Fusaroli, P., Repici, A., & Mangiavillano, B. (2023). Endoscopic Ultrasound Guided Biliary Drainage in Malignant Distal Biliary Obstruction. Cancers, 15(2), 490. https://doi.org/10.3390/cancers15020490







