Locus-Specific Bisulfate NGS Sequencing of GSTP1, RNF219, and KIAA1539 Genes in the Total Pool of Cell-Free and Cell-Surface-Bound DNA in Prostate Cancer: A Novel Approach for Prostate Cancer Diagnostics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Blood Collection
2.2. DNA Extraction, Quantification, and Bisulfate Conversion
2.3. Amplification of Selected Loci
2.4. Preparation of Sequencing Libraries
2.5. NGS Data and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022.
- Ornstein, D.; Pruthi, R. Prostate-specific antigen. Expert Opin. Pharmacother. 2000, 1, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Bill-Axelson, A.; Holmberg, L.; Filen, F.; Ruutu, M.; Garmo, H.; Busch, C.; Nordling, S.; Haggman, M.; Andersson, S.-O.; Bratell, S.; et al. Radical Prostatectomy Versus Watchful Waiting in Localized Prostate Cancer: The Scandinavian Prostate Cancer Group-4 Randomized Trial. JNCI J. Natl. Cancer Inst. 2008, 100, 1144–1154. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2012, 157, 120. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Foj, L.; Milà, M.; Augé, J.M.; Molina, R.; Jiménez, W. PCA3 in the Detection and Management of Early Prostate Cancer. Tumor Biol. 2013, 34, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Haese, A.; de la Taille, A.; van Poppel, H.; Marberger, M.; Stenzl, A.; Mulders, P.F.A.; Huland, H.; Abbou, C.-C.; Remzi, M.; Tinzl, M.; et al. Clinical Utility of the PCA3 Urine Assay in European Men Scheduled for Repeat Biopsy. Eur. Urol. 2008, 54, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Deras, I.L.; Aubin, S.M.J.; Blase, A.; Day, J.R.; Koo, S.; Partin, A.W.; Ellis, W.J.; Marks, L.S.; Fradet, Y.; Rittenhouse, H.; et al. PCA3: A Molecular Urine Assay for Predicting Prostate Biopsy Outcome. J. Urol. 2008, 179, 1587–1592. [Google Scholar] [CrossRef]
- McMahon, K.W.; Karunasena, E.; Ahuja, N. The Roles of DNA Methylation in the Stages of Cancer. Cancer J. 2017, 23, 257–261. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG DNA: A Pathogenic Factor in Systemic Lupus Erythematosus? J. Clin. Immunol. 1995, 15, 284–292. [Google Scholar] [CrossRef]
- Wong, I.H.; Lo, Y.M.; Johnson, P.J. Epigenetic Tumor Markers in Plasma and Serum: Biology and Applications to Molecular Diagnosis and Disease Monitoring. Ann. N. Y. Acad. Sci. 2001, 945, 36–50. [Google Scholar] [CrossRef]
- Ziegler, A.; Zangemeister-Wittke, U.; Stahel, R.A. Circulating DNA: A New Diagnostic Gold Mine? Cancer Treat. Rev. 2002, 28, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.J.; Alemany-Cosme, E.; Goñi, S.; Bandres, E.; Palanca-Ballester, C.; Sandoval, J. Epigenetic Regulation of MicroRNAs in Cancer: Shortening the Distance from Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 7350. [Google Scholar] [CrossRef] [PubMed]
- Flores, B.C.T.; Correia, M.P.; Rodríguez, J.G.; Henrique, R.; Jerónimo, C. Bridging the Gaps between Circulating Tumor Cells and DNA Methylation in Prostate Cancer. Cancers 2021, 13, 4209. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Sanchez-Cespedes, M.; Rosell, R.; Sidransky, D.; Baylin, S.B.; Herman, J.G. Detection of Aberrant Promoter Hypermethylation of Tumor Suppressor Genes in Serum DNA from Non-Small Cell Lung Cancer Patients. Cancer Res. 1999, 59, 67–70. [Google Scholar] [PubMed]
- Epigenomics AG Detecting Cancer in Blood. Available online: www.epigenomics.com (accessed on 13 November 2022).
- Bryzgunova, O.E.; Laktionov, P.P. Current Methods of Extracellular DNA Methylation Analysis. Mol. Biol. 2017, 51, 167–183. [Google Scholar] [CrossRef]
- Affinito, O.; Scala, G.; Palumbo, D.; Florio, E.; Monticelli, A.; Miele, G.; Avvedimento, V.E.; Usiello, A.; Chiariotti, L.; Cocozza, S. Modeling DNA Methylation by Analyzing the Individual Configurations of Single Molecules. Epigenetics 2016, 11, 881–888. [Google Scholar] [CrossRef]
- Mikeska, T.; Candiloro, I.L.; Dobrovic, A. The Implications of Heterogeneous DNA Methylation for the Accurate Quantification of Methylation. Epigenomics 2010, 2, 561–573. [Google Scholar] [CrossRef]
- Korshunova, Y.; Maloney, R.K.; Lakey, N.; Citek, R.W.; Bacher, B.; Budiman, A.; Ordway, J.M.; McCombie, W.R.; Leon, J.; Jeddeloh, J.A.; et al. Massively Parallel Bisulphite Pyrosequencing Reveals the Molecular Complexity of Breast Cancer-Associated Cytosine-Methylation Patterns Obtained from Tissue and Serum DNA. Genome Res. 2008, 18, 19–29. [Google Scholar] [CrossRef]
- Bryzgunova, O.; Bondar, A.; Ruzankin, P.; Laktionov, P.; Tarasenko, A.; Kurilshikov, A.; Epifanov, R.; Zaripov, M.; Kabilov, M.; Laktionov, P. Locus-Specific Methylation of GSTP1, RNF219, and KIAA1539 Genes with Single Molecule Resolution in Cell-Free DNA from Healthy Donors and Prostate Tumor Patients: Application in Diagnostics. Cancers 2021, 13, 6234. [Google Scholar] [CrossRef]
- Gerashchenko, T.S.; Denisov, E.V.; Litviakov, N.V.; Zavyalova, M.V.; Vtorushin, S.V.; Tsyganov, M.M.; Perelmuter, V.M.; Cherdyntseva, N.V. Intratumor Heterogeneity: Nature and Biological Significance. Biochem. Mosc. 2013, 78, 1201–1215. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Linehan, W.M. Intratumoral Heterogeneity in Kidney Cancer. Nat. Genet. 2014, 46, 214–215. [Google Scholar] [CrossRef]
- Cheow, L.F.; Courtois, E.T.; Tan, Y.; Viswanathan, R.; Xing, Q.; Tan, R.Z.; Tan, D.S.W.; Robson, P.; Loh, Y.-H.; Quake, S.R.; et al. Single-Cell Multimodal Profiling Reveals Cellular Epigenetic Heterogeneity. Nat. Methods 2016, 13, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, N.C.; Pierron, G.; Klughammer, J.; Datlinger, P.; Schönegger, A.; Schuster, M.; Hadler, J.; Surdez, D.; Guillemot, D.; Lapouble, E.; et al. DNA Methylation Heterogeneity Defines a Disease Spectrum in Ewing Sarcoma. Nat. Med. 2017, 23, 386–395. [Google Scholar] [CrossRef]
- Tutanov, O.; Tamkovich, S. The Influence of Proteins on Fate and Biological Role of Circulating DNA. Int. J. Mol. Sci. 2022, 23, 7224. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, T.E.; Rykova, E.Y.; Tamkovich, S.N.; Bryzgunova, O.E.; Starikov, A.V.; Kuznetsova, N.P.; Vlassov, V.V.; Laktionov, P.P. Cell-Free and Cell-Bound Circulating DNA in Breast Tumours: DNA Quantification and Analysis of Tumour-Related Gene Methylation. Br. J. Cancer 2006, 94, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.; Laktionov, P. Cell-surface-bound Circulating DNA in the Blood: Biology and Clinical Application. IUBMB Life 2019, 71, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Bryzgunova, O.E.; Morozkin, E.S.; Yarmoschuk, S.V.; Vlassov, V.V.; Laktionov, P.P. Methylation-Specific Sequencing of GSTP1 Gene Promoter in Circulating/Extracellular DNA from Blood and Urine of Healthy Donors and Prostate Cancer Patients. Ann. N. Y. Acad. Sci. 2008, 1137, 222–225. [Google Scholar] [CrossRef]
- Ponomaryova, A.A.; Rykova, E.Y.; Cherdyntseva, N.V.; Skvortsova, T.E.; Dobrodeev, A.Y.; Zav’yalov, A.A.; Tuzikov, S.A.; Vlassov, V.V.; Laktionov, P.P. RARβ2 Gene Methylation Level in the Circulating DNA from Blood of Patients with Lung Cancer. Eur. J. Cancer Prev. 2011, 20, 453–455. [Google Scholar] [CrossRef]
- Gainetdinov, I.V.; Kapitskaya, K.Y.; Rykova, E.Y.; Ponomaryova, A.A.; Cherdyntseva, N.V.; Vlassov, V.V.; Laktionov, P.P.; Azhikina, T.L. Hypomethylation of Human-Specific Family of LINE-1 Retrotransposons in Circulating DNA of Lung Cancer Patients. Lung Cancer 2016, 99, 127–130. [Google Scholar] [CrossRef]
- Laktionov, P.P.; Skvortsova, T.E.; Morozkin, E.S.; Bondar, A.A.; Mileiko, V.V.; Vlasov, V.V. Method for isolating the total fraction of extracellular nucleic acids from blood. RF Patent 2554746, 1 June 2015. [Google Scholar]
- Bryzgunova, O.; Bondar, A.; Morozkin, E.; Mileyko, V.; Vlassov, V.; Laktionov, P. A Reliable Method to Concentrate Circulating DNA. Anal. Biochem. 2011, 408, 354–356. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Yegnasubramanian, S.; De Marzo, A.M.; Nelson, W.G. Prostate Cancer Epigenetics: From Basic Mechanisms to Clinical Implications. Cold Spring Harb. Perspect. Med. 2019, 9, a030445. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.A.; Jiang, P.; Chan, C.W.M.; Sun, K.; Wong, J.; Hui, E.P.; Chan, S.L.; Chan, W.C.; Hui, D.S.C.; Ng, S.S.M.; et al. Noninvasive Detection of Cancer-Associated Genome-Wide Hypomethylation and Copy Number Aberrations by Plasma DNA Bisulfite Sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 18761–18768. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Rigau, M.; Olivan, M.; Garcia, M.; Sequeiros, T.; Montes, M.; Colás, E.; Llauradó, M.; Planas, J.; de Torres, I.; Morote, J.; et al. The Present and Future of Prostate Cancer Urine Biomarkers. Int. J. Mol. Sci. 2013, 14, 12620–12649. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.S.; Girdhani, S.; Hlatky, L. A Quest to Identify Prostate Cancer Circulating Biomarkers with a Bench-to-Bedside Potential. J. Biomark. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Martignano, F.; Gurioli, G.; Salvi, S.; Calistri, D.; Costantini, M.; Gunelli, R.; De Giorgi, U.; Foca, F.; Casadio, V. GSTP1 Methylation and Protein Expression in Prostate Cancer: Diagnostic Implications. Dis. Markers 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Liu, R.; Su, X.; Long, Y.; Zhou, D.; Zhang, X.; Ye, Z.; Ma, J.; Tang, T.; Wang, F.; He, C. A Systematic Review and Quantitative Assessment of Methylation Biomarkers in Fecal DNA and Colorectal Cancer and Its Precursor, Colorectal Adenoma. Mutat. Res. Mutat. Res. 2019, 779, 45–57. [Google Scholar] [CrossRef]
- Cortese, R.; Kwan, A.; Lalonde, E.; Bryzgunova, O.; Bondar, A.; Wu, Y.; Gordevicius, J.; Park, M.; Oh, G.; Kaminsky, Z.; et al. Epigenetic Markers of Prostate Cancer in Plasma Circulating DNA. Hum. Mol. Genet. 2012, 21, 3619–3631. [Google Scholar] [CrossRef]
- Gurioli, G.; Martignano, F.; Salvi, S.; Costantini, M.; Gunelli, R.; Casadio, V. GSTP1 Methylation in Cancer: A Liquid Biopsy Biomarker? Clin. Chem. Lab. Med. CCLM 2018, 56, 702–717. [Google Scholar] [CrossRef]
- Kim, J.H.; Dhanasekaran, S.M.; Prensner, J.R.; Cao, X.; Robinson, D.; Kalyana-Sundaram, S.; Huang, C.; Shankar, S.; Jing, X.; Iyer, M.; et al. Deep Sequencing Reveals Distinct Patterns of DNA Methylation in Prostate Cancer. Genome Res. 2011, 21, 1028–1041. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Bryzgunova, O.E.; Konoshenko, M.Y.; Laktionov, P.P. Concentration of Cell-Free DNA in Different Tumor Types. Expert Rev. Mol. Diagn. 2021, 21, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C. Cell-Free DNA (CfDNA): Clinical Significance and Utility in Cancer Shaped By Emerging Technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Bryzgunova, O.E.; Tamkovich, S.N.; Cherepanova, A.V.; Yarmoshchuk, S.V.; Permyakova, V.I.; Anykeeva, O.Y.; Laktionov, P.P. Redistribution of Free- and Cell-Surface-Bound DNA in Blood of Benign and Malignant Prostate Tumor Patients. Acta Naturae 2015, 7, 115–118. [Google Scholar] [CrossRef]
- Kolesnikova, E.V.; Tamkovich, S.N.; Bryzgunova, O.E.; Shelestyuk, P.I.; Permyakova, V.I.; Vlassov, V.V.; Tuzikov, A.S.; Laktionov, P.P.; Rykova, E.Y. Circulating DNA in the Blood of Gastric Cancer Patients. Ann. N. Y. Acad. Sci. 2008, 1137, 226–231. [Google Scholar] [CrossRef]
- Bryzgunova, O.E.; Laktionov, P.P. Generation of blood circulating DNA: The sources, peculiarities of circulation and structure. Biomeditsinskaya Khimiya 2015, 61, 409–426. [Google Scholar] [CrossRef]
- Kitchen, M.O.; Bryan, R.T.; Emes, R.D.; Luscombe, C.J.; Cheng, K.; Zeegers, M.P.; James, N.D.; Gommersall, L.M.; Fryer, A.A. HumanMethylation450K Array–Identified Biomarkers Predict Tumour Recurrence/Progression at Initial Diagnosis of High-Risk Non-Muscle Invasive Bladder Cancer. Biomark. Cancer 2018, 10, 1179299X1775192. [Google Scholar] [CrossRef]
- Pisanic, T.R.; Athamanolap, P.; Poh, W.; Chen, C.; Hulbert, A.; Brock, M.V.; Herman, J.G.; Wang, T.-H. DREAMing: A Simple and Ultrasensitive Method for Assessing Intratumor Epigenetic Heterogeneity Directly from Liquid Biopsies. Nucleic Acids Res. 2015, 43, e154. [Google Scholar] [CrossRef] [PubMed]
- Vinci, G.; Buffat, C.; Simoncini, S.; Boubred, F.; Ligi, I.; Dumont, F.; Le Bonniec, B.; Fournier, T.; Vaiman, D.; Dignat-George, F.; et al. Gestational Age-Related Patterns of AMOT Methylation Are Revealed in Preterm Infant Endothelial Progenitors. PLoS ONE 2017, 12, e0186321. [Google Scholar] [CrossRef]
- Jeon, J.-P.; Koh, I.-U.; Choi, N.-H.; Kim, B.-J.; Han, B.-G.; Lee, S. Differential DNA Methylation of MSI2 and Its Correlation with Diabetic Traits. PLoS ONE 2017, 12, e0177406. [Google Scholar] [CrossRef] [PubMed]
- Giarraputo, J.; DeLoach, J.; Padbury, J.; Uzun, A.; Marsit, C.; Hawes, K.; Lester, B. Medical Morbidities and DNA Methylation of NR3C1 in Preterm Infants. Pediatr. Res. 2017, 81, 68–74. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Xiao, H.; Lee, M.-T.; Levin, L.; Leung, Y.-K.; Ho, S.-M. Methylation of a Single Intronic CpG Mediates Expression Silencing of the PMP24 Gene in Prostate Cancer: Intronic Single CpG Methylation Regulates PMP24. Prostate 2010, 70, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Fackler, M.J.; Lopez Bujanda, Z.; Umbricht, C.; Teo, W.W.; Cho, S.; Zhang, Z.; Visvanathan, K.; Jeter, S.; Argani, P.; Wang, C.; et al. Novel Methylated Biomarkers and a Robust Assay to Detect Circulating Tumor DNA in Metastatic Breast Cancer. Cancer Res. 2014, 74, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Warr, A.; Robert, C.; Hume, D.; Archibald, A.; Deeb, N.; Watson, M. Exome Sequencing: Current and Future Perspectives. G3 GenesGenomesGenetics 2015, 5, 1543–1550. [Google Scholar] [CrossRef]
- How Kit, A.; Nielsen, H.M.; Tost, J. DNA Methylation Based Biomarkers: Practical Considerations and Applications. Biochimie 2012, 94, 2314–2337. [Google Scholar] [CrossRef]
- Levenson, V.V. DNA Methylation as a Universal Biomarker. Expert Rev. Mol. Diagn. 2010, 10, 481–488. [Google Scholar] [CrossRef]
- Koochekpour, S. Genetic and Epigenetic Changes in Human Prostate Cancer. Iran. Red Crescent Med. J. 2011, 13, 80–98. [Google Scholar] [PubMed]
- Tirado-Magallanes, R.; Rebbani, K.; Lim, R.; Pradhan, S.; Benoukraf, T. Whole Genome DNA Methylation: Beyond Genes Silencing. Oncotarget 2017, 8, 5629–5637. [Google Scholar] [CrossRef]
- Skvortsova, T.E.; Bryzgunova, O.E.; Lebedeva, A.O.; Mak, V.V.; Vlassov, V.V.; Laktionov, P.P. Methylated Cell-Free DNA In Vitro and In Vivo. In Circulating Nucleic Acids in Plasma and Serum; Gahan, P.B., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 185–194. ISBN 978-90-481-9381-3. [Google Scholar]
- Ponomaryova, A.A.; Cherdyntseva, N.V.; Bondar, A.A.; Dobrodeev, A.Y.; Zavyalov, A.A.; Tuzikov, S.A.; Vlassov, V.V.; Choinzonov, E.L.; Laktionov, P.P.; Rykova, E.Y. Dynamics of LINE-1 Retrotransposon Methylation Levels in Circulating DNA from Lung Cancer Patients Undergoing Antitumor Therapy. Mol. Biol. 2017, 51, 622–628. [Google Scholar] [CrossRef]
- Tamkovich, S.; Tupikin, A.; Kozyakov, A.; Laktionov, P. Size and Methylation Index of Cell-Free and Cell-Surface-Bound DNA in Blood of Breast Cancer Patients in the Contest of Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 8919. [Google Scholar] [CrossRef] [PubMed]
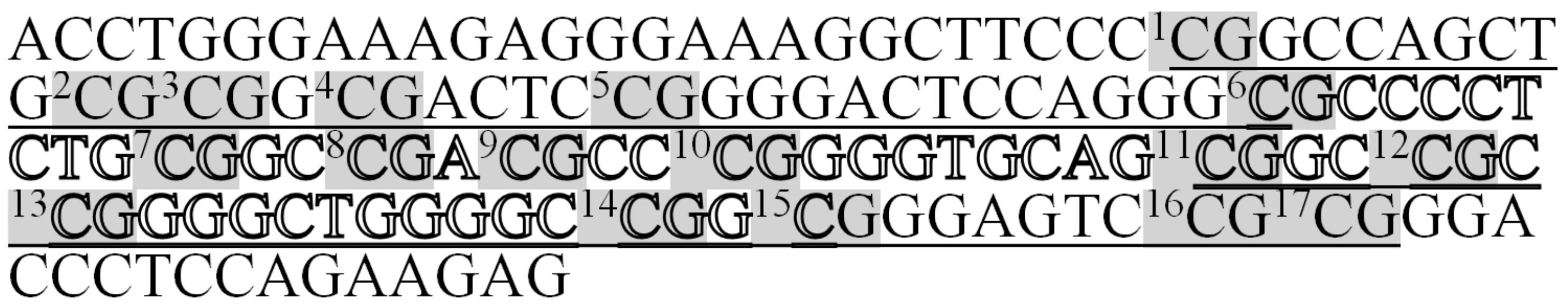
| Characteristic | Groups | |
|---|---|---|
| Prostate Cancer Patients n = 19 | Healthy Donors n = 18 | |
| Age | ||
| Mean ± SD | 67.7 ± 7.0 | 61.6 ± 6.6 |
| Range | 55–77 | 53–74 |
| Total PSA, ng/mL | ||
| Mean ± SD | 17.3 ± 12.9 | 1.2 ± 0.7 |
| Range | 4.8–48.7 | 0.2–2.3 |
| Tumor stage | ||
| T2bNxMx | 6 | N/A |
| T2bNxMx | 5 | |
| T3aNxMx | 4 | |
| T3bNxMx | 3 | |
| Gleason scale | ||
| Unknown | 1 | N/A |
| 4–5 | 1 | |
| 5 | 2 | |
| 5–6 | 3 | |
| 6 | 6 | |
| 7 | 4 | |
| 8 | 2 | |
| Target’s Name | Primer’s Sequence (without Barcodes) | Primers/Probe Concentration, nM | Length of PCR Product, b.p. | Length of PCR Product without Barcodes, b.p. | CG Number | 1× Buffer Composition | PCR Conditions |
|---|---|---|---|---|---|---|---|
| LINE1-For LINE1-Rev LINE1-Probe | 5′-AATGGAAGATGAAATGAATGAAATGA-3′ | 600/300 | - | 155 | - | BioMaster qPCR Mix from Biolabmix (Novosibirsk, Russia) | 95 °C-3 min, (95 °C-15 s, 60 °C-60 s) ×40 |
| 5′-TTCCATTCTCCCCATCACTTTCA-3′ | |||||||
| 5′-FAM-GAGAAGGGAAGTTTAGAGAAAAAAGAAT-FQ-3′ | |||||||
| RNF219-For RNF219-Rev | 5′-(Y1-12)GTGATTGTGGGTATAGTTATAAAA-3′ | 600 | 177 | 161 | 17 | Hotstart PCR buffer with additional MgCl2 (final concentration 5 mM), 1 mM dNTPs and 0.65 units of Hotstart Taq polymerase | 95 °C-15 min (95 °C-60 s, 58 °C-45 s, 72 °C-60 s) ×50 |
| 5′-(X1-8)ACTACCCCCATCTCCCAAAA-3′ | |||||||
| KIAA1539-For KIAA1539-Rev | 5′-(X1-8)AGGAAGGAGGAGATAAAGTGAT-3′ | 600 | 105 | 89 | 5 | ||
| 5′-(Y1-12)CCCCTCTAAACTTATCATCACA-3′ | |||||||
| GSTP1-For GSTP1-Rev | 5′-(Y1-12)ATTTGGGAAAGAGGGAAAGGTT-3′ | 600 | 158 | 142 | 17 | ||
| 5′-(X1-8)CTCTTCTAAAAAATCC-3′ |
| BARCODES | With Forward or Reverse Primer the Exact Barcode Was Used for Each Target | ||
|---|---|---|---|
| RNF219 | KIAA1539 | GSTP1 | |
| X1: TAGATCGC, X2: CTCTCTAT, X3: TATCCTCT, X4: AGAGTAGA, X5: ACTGCATA, X6: AAGGAGTA, X7: CTAAGCCT, X8: CCTCTCTG | Reverse | Forward | Reverse |
| Y1: TCGCCTTA, Y2: CTAGTACG, Y3: TTCTGCCT, Y4: GCTCAGGA, Y5: AGGAGTCC, Y6: CATGCCTA, Y7: GTAGAGAG, Y8: CCTCTCTG, Y9: AGCGTAGC, Y10: CAGCCTCG, Y11: TGCCTCTT, Y12: TCCTCTAC | Forward | Reverse | Forward |
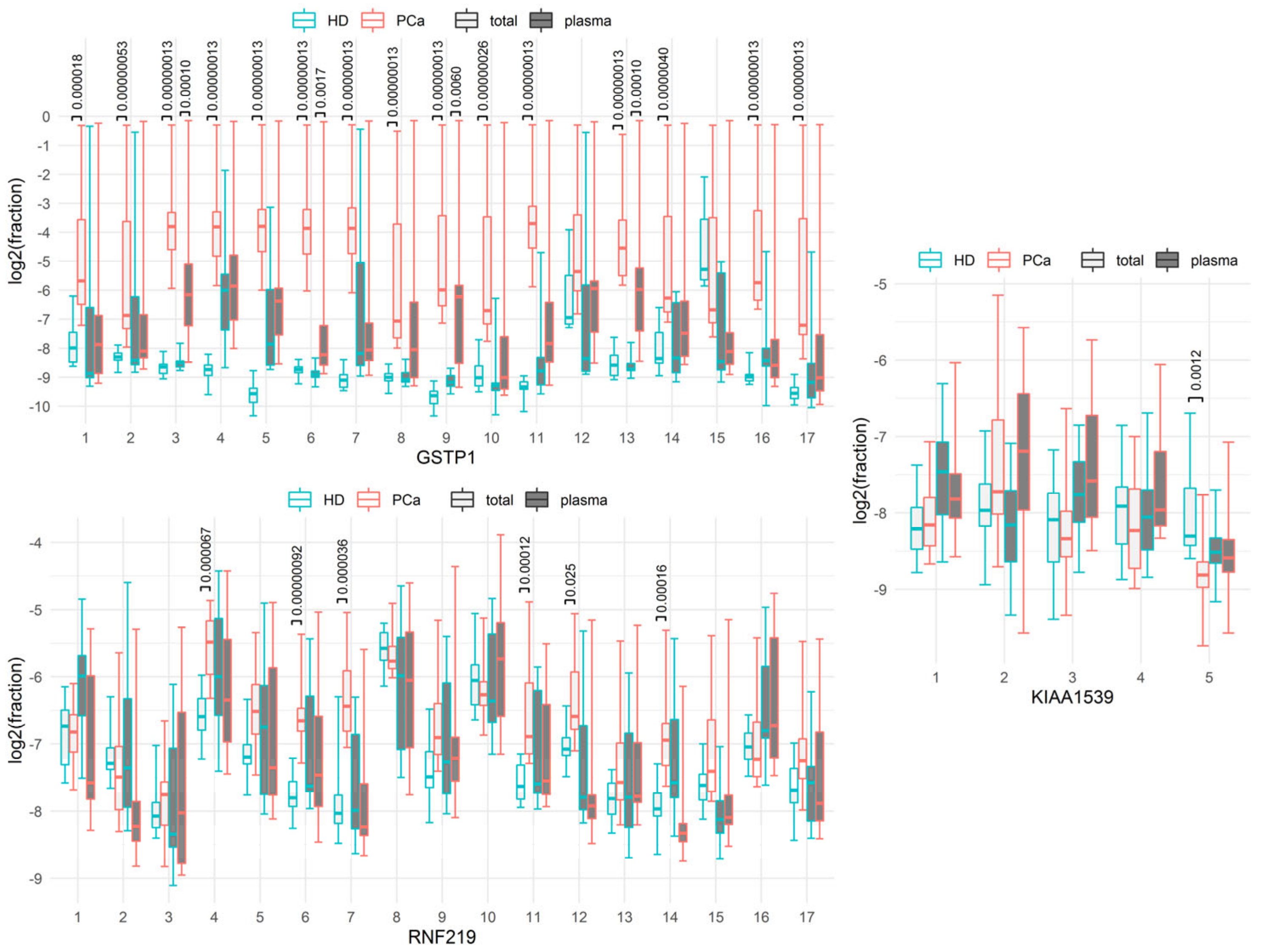
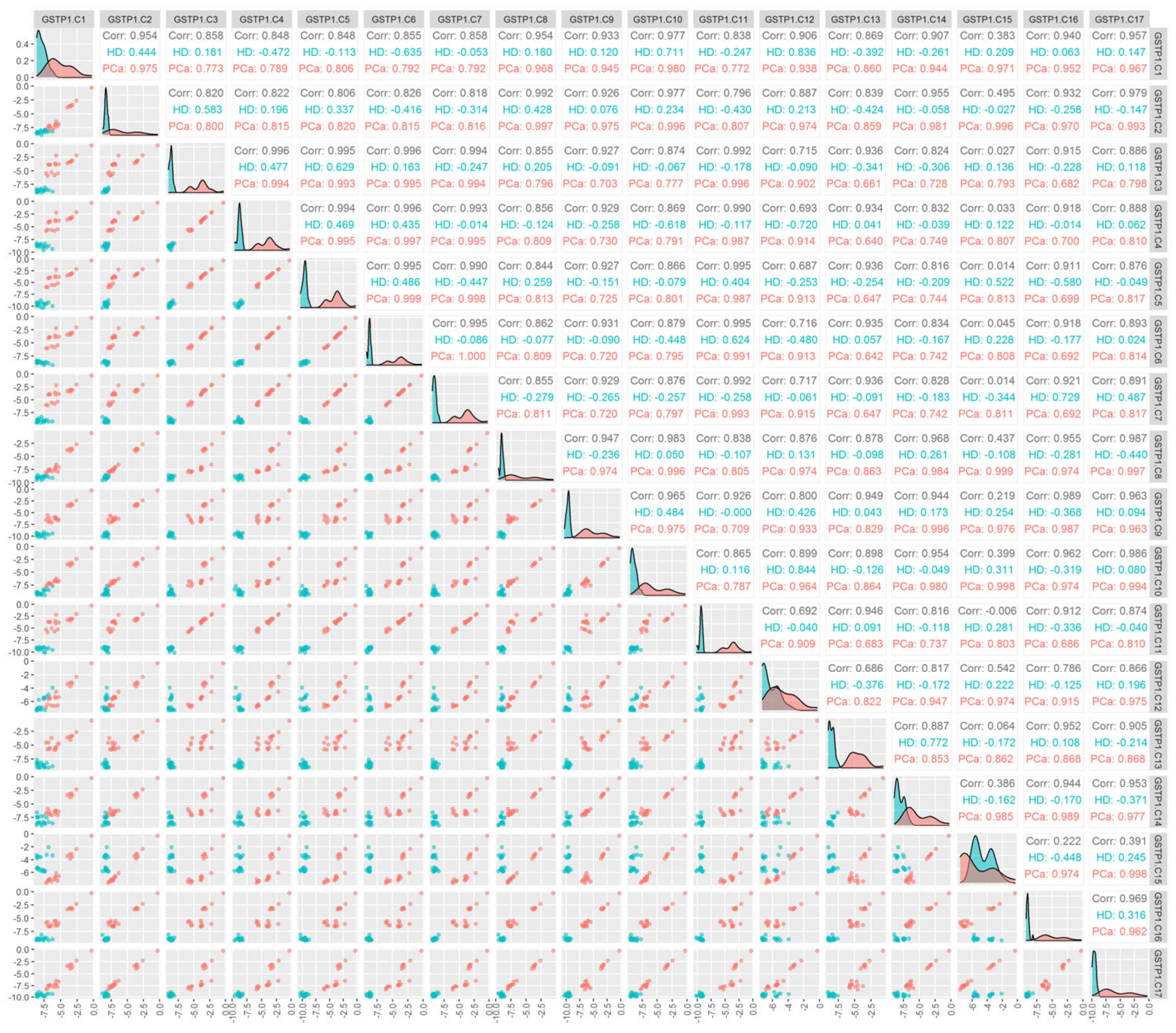
| p-Value × 1167 | Means, % (PCa/HD) | Sensitivity for 100% Specificity, % | Specificity for 100% Sensitivity, % | CV Accuracy, % | CV Sensitivity, % | CV Specificity, % | |
|---|---|---|---|---|---|---|---|
| GSTP1.C1, total | 0.000018 | 8.36/0.483 | 68.4 | 83.3 | 86.5 | 84.2 | 88.9 |
| GSTP1.C1, plasma | >1 | 5.83/5.73 | 5.6 | 12.5 | 0 | 0 | 0 |
| GSTP1.C2, total | 0.00000053 | 7.72/0.321 | 94.7 | 88.9 | 94.6 | 94.7 | 94.4 |
| GSTP1.C2, plasma | >1 | 5.42/5.11 | 5.6 | 6.2 | 11.8 | 0 | 25.0 |
| GSTP1.C3, total | 0.00000013 | 12.0/0.247 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C3, plasma | 0.00010 | 7.74/0.293 | 88.9 | 68.8 | 85.3 | 83.3 | 87.5 |
| GSTP1.C4, total | 0.00000013 | 11.8/0.235 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C4, plasma | >1 | 9.67/4.16 | 11.1 | 18.8 | 50.0 | 22.2 | 81.2 |
| GSTP1.C5, total | 0.00000013 | 12.1/0.133 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C5, plasma | >1 | 8.46/1.41 | 16.7 | 37.5 | 55.9 | 22.2 | 93.8 |
| GSTP1.C6, total | 0.00000013 | 11.9/0.234 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C6, plasma | 0.0017 | 5.36/0.213 | 55.6 | 56.2 | 82.4 | 83.3 | 81.2 |
| GSTP1.C7, total | 0.00000013 | 12.0/0.187 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C7, plasma | > 1 | 5.48/5.89 | 5.6 | 6.2 | 50.0 | 94.4 | 0 |
| GSTP1.C8, total | 0.00000013 | 6.95/0.196 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C8, plasma | >1 | 5.77/0.198 | 61.1 | 12.5 | 76.5 | 61.1 | 93.8 |
| GSTP1.C9, total | 0.00000013 | 8.62/0.124 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C9, plasma | 0.0060 | 6.04/0.179 | 77.8 | 25.0 | 85.3 | 77.8 | 93.8 |
| GSTP1.C10, total | 0.00000026 | 8.23/0.217 | 94.7 | 94.4 | 94.6 | 94.7 | 94.4 |
| GSTP1.C10, plasma | >1 | 5.20/0.222 | 22.2 | 6.2 | 64.7 | 38.9 | 93.8 |
| GSTP1.C11, total | 0.00000013 | 12.6/0.155 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C11, plasma | >1 | 5.73/0.497 | 11.1 | 18.8 | 55.9 | 27.8 | 87.5 |
| GSTP1.C12, total | 0.63 | 9.10/1.61 | 36.8 | 61.1 | 64.9 | 42.1 | 88.9 |
| GSTP1.C12, plasma | >1 | 9.30/5.07 | 5.6 | 43.8 | 47.1 | 5.6 | 93.8 |
| GSTP1.C13, total | 0.00000013 | 8.42/0.274 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C13, plasma | 0.00010 | 11.0/0.258 | 77.8 | 81.2 | 85.3 | 77.8 | 93.8 |
| GSTP1.C14, total | 0.0000040 | 8.50/0.420 | 68.4 | 94.4 | 91.9 | 89.5 | 94.4 |
| GSTP1.C14, plasma | >1 | 5.55/0.645 | 22.2 | 37.5 | 44.1 | 27.8 | 62.5 |
| GSTP1.C15, total | >1 | 8.27/5.92 | 63.2 | 0 | 40.5 | 26.3 | 55.6 |
| GSTP1.C15, plasma | >1 | 5.54/1.03 | 5.6 | 18.8 | 32.4 | 0 | 68.8 |
| GSTP1.C16, total | 0.00000013 | 9.12/0.204 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C16, plasma | >1 | 5.00/0.569 | 5.6 | 6.2 | 44.1 | 0 | 93.8 |
| GSTP1.C17, total | 0.00000013 | 8.11/0.136 | 100 | 100 | 100 | 100 | 100 |
| GSTP1.C17, plasma | >1 | 4.96/0.413 | 5.6 | 6.2 | 52.9 | 16.7 | 93.8 |
| Option Number | Gene, Position, Status (C or T after Conversion) | p-Value × 1167 | Means, % (PCa/HD) | Sensitivity for 100% Specificity, % | Specificity for 100% Sensitivity, % | Cut Off, % (Ratio) | CV Accuracy, % | CV Sensitivity, % | CV Specificity, % | CV AUC,% (DeLong’s CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GSTP1.C3 | 0.00000013 | 12.0/0.247 | 100 | 100 | 0.768 (4.53) | 100 | 100 | 100 | 100 |
| 2 | GSTP1.C11 | 0.00000013 | 12.6/0.155 | 100 | 100 | 0.586 (8.42) | 100 | 100 | 100 | 100 |
| 3 | GSTP1.T3.T5 | 0.00000013 | 87.5/99.7 | 100 | 100 | 98.8 (1.02) | 100 | 100 | 100 | 100 |
| 4 | GSTP1.T1.C6 | 0.00000013 | 4.69/0.232 | 100 | 100 | 0.607 (4.13) | 100 | 100 | 100 | 100 |
| 5 | GSTP1.C2.C3 | 0.00000013 | 7.51/0.0478 | 100 | 100 | 0.174 (1.99) | 100 | 100 | 100 | 100 |
| · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · · | ||||||||||
| 474 | RNF219.C2.C4 | 0.00000013 | 0.301/0.00174 | 100 | 100 | 0.0226 (6.29) | 100 | 100 | 100 | 100 |
| 475 | RNF219.C3.C12 | 0.00000013 | 0.157/0.00132 | 100 | 100 | 0.0199 (3.30) | 100 | 100 | 100 | 100 |
| 476 | RNF219.C10.C14 | 0.00000013 | 0.483/0.00485 | 100 | 100 | 0.0574 (4.08) | 100 | 100 | 100 | 100 |
| Average in HD, % | Average in PCa, % | Cutoff, % (Ratio) | |
|---|---|---|---|
| Forward primer area | |||
| GSTP1.C5.C6 | 0.002 | 11.448 | 0.0909 (180.63) |
| GSTP1.C4.C5.C6 | 0.002 | 11.361 | 0.0896 (175.54) |
| GSTP1.C1.C2.C3 | 0.001 | 7.237 | 0.0299 (43.88) |
| GSTP1.C1.C2.C3.C4 | 0.001 | 7.221 | 0.0295 (42.85) |
| GSTP1.C1.C2.C3.C4.C5 | 0.001 | 7.211 | 0.0288 (40.65) |
| TaqMan-probe area | |||
| GSTP1.C6.C7 | 0.002 | 11.608 | 0.104 (145.99) |
| GSTP1.C7.C8.C9.C10 | 0.001 | 6.724 | 0.0293 (50.48) |
| GSTP1.C8.C9.C10.C11.C12 | 0.001 | 6.718 | 0.0293 (50.48) |
| GSTP1.C7.C8.C9.C10.C11 | 0.001 | 6.698 | 0.0289 (49.27) |
| GSTP1.C7.C8.C9.C10.C11.C12 | 0.001 | 6.683 | 0.0289 (49.27) |
| GSTP1.C6.C7.C8 | 0.001 | 6.608 | 0.0282 (46.87) |
| GSTP1.C6.C7.C8.C9 | 0.001 | 6.592 | 0.0282 (46.87) |
| GSTP1.C6.C7.C8.C9.C10 | 0.001 | 6.579 | 0.0282 (46.87) |
| Reverse primer area | |||
| GSTP1.C14.C15.C16 | 0.001 | 7.881 | 0.0289 (49.27) |
| GSTP1.C15.C16.C17 | 0.001 | 7.878 | 0.0289 (49.27) |
| GSTP1.C14.C15.C16.C17 | 0.001 | 7.870 | 0.0289 (49.27) |
| Average in HD, % | Average in PCa,% | Cutoff, % (Ratio) | |
|---|---|---|---|
| GSTP1.C4.C5.C6.C7.C8.C9.C10.C11.C15.C16.C17 | 0.001 | 6.231 | 0.024 (35.5) |
| GSTP1.C4.C5.C6.C7.C8.C9.C10.C11.C12.C15.C16.C17 | 0.001 | 6.227 | 0.024 (35.5) |
| GSTP1.C4.C5.C6.C7.C8.C9.C10.C11.C14.C15.C16.C17 | 0.001 | 6.227 | 0.024 (35.5) |
| GSTP1.C4.C5.C6.C7.C8.C9.C10.C11.C12.C14.C15.C16.C17 | 0.001 | 6.223 | 0.024 (35.5) |
| Average in HD, % | Average in PCa, % | |
|---|---|---|
| Potential area of forward primer | ||
| GSTP1.C4.T5.C6 | 0.000630 | 0.0100 |
| Potential area of TaqMan-probe | ||
| GSTP1.C7.C8.T9.C10.C11 | 0 | 0.00281 |
| GSTP1.C7.C8.C9.T10.C11 | 0 | 0.00657 |
| GSTP1.C7.C8.T9.T10.C11 | 0 | 0.0118 |
| GSTP1.C7.C8.T9.C10.C11.C12 | 0 | 0.00281 |
| GSTP1.C7.C8.C9.T10.C11.C12 | 0 | 0.00594 |
| GSTP1.C7.C8.T9.T10.C11.C12 | 0 | 0 |
| Potential missmatch molecules in tcfDNA | ||
| GSTP1.C4.T5.C6.C7.C8.T9.C10.C11.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.C9.T10.C11.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.T10.C11.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.C10.C11.C12.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.C9.T10.C11.C12.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.T10.C11.C12.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.C10.C11.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.C9.T10.C11.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.T10.C11.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.C10.C11.C12.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.C9.T10.C11.C12.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.T5.C6.C7.C8.T9.T10.C11.C12.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.C5.C6.C7.C8.T9.C10.C11.C15.C16.C17 | 0 | 0.00281 |
| GSTP1.C4.C5.C6.C7.C8.C9.T10.C11.C15.C16.C17 | 0 | 0.00575 |
| GSTP1.C4.C5.C6.C7.C8.T9.T10.C11.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.C5.C6.C7.C8.T9.C10.C11.C12.C15.C16.C17 | 0 | 0.00281 |
| GSTP1.C4.C5.C6.C7.C8.C9.T10.C11.C12.C15.C16.C17 | 0 | 0.00575 |
| GSTP1.C4.C5.C6.C7.C8.T9.T10.C11.C12.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.C5.C6.C7.C8.T9.C10.C11.C14.C15.C16.C17 | 0 | 0.00281 |
| GSTP1.C4.C5.C6.C7.C8.C9.T10.C11.C14.C15.C16.C17 | 0 | 0.00575 |
| GSTP1.C4.C5.C6.C7.C8.T9.T10.C11.C14.C15.C16.C17 | 0 | 0 |
| GSTP1.C4.C5.C6.C7.C8.T9.C10.C11.C12.C14.C15.C16.C17 | 0 | 0.00281 |
| GSTP1.C4.C5.C6.C7.C8.C9.T10.C11.C12.C14.C15.C16.C17 | 0 | 0.00575 |
| GSTP1.C4.C5.C6.C7.C8.T9.T10.C11.C12.C14.C15.C16.C17 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryzgunova, O.; Bondar, A.; Ruzankin, P.; Tarasenko, A.; Zaripov, M.; Kabilov, M.; Laktionov, P. Locus-Specific Bisulfate NGS Sequencing of GSTP1, RNF219, and KIAA1539 Genes in the Total Pool of Cell-Free and Cell-Surface-Bound DNA in Prostate Cancer: A Novel Approach for Prostate Cancer Diagnostics. Cancers 2023, 15, 431. https://doi.org/10.3390/cancers15020431
Bryzgunova O, Bondar A, Ruzankin P, Tarasenko A, Zaripov M, Kabilov M, Laktionov P. Locus-Specific Bisulfate NGS Sequencing of GSTP1, RNF219, and KIAA1539 Genes in the Total Pool of Cell-Free and Cell-Surface-Bound DNA in Prostate Cancer: A Novel Approach for Prostate Cancer Diagnostics. Cancers. 2023; 15(2):431. https://doi.org/10.3390/cancers15020431
Chicago/Turabian StyleBryzgunova, Olga, Anna Bondar, Pavel Ruzankin, Anton Tarasenko, Marat Zaripov, Marsel Kabilov, and Pavel Laktionov. 2023. "Locus-Specific Bisulfate NGS Sequencing of GSTP1, RNF219, and KIAA1539 Genes in the Total Pool of Cell-Free and Cell-Surface-Bound DNA in Prostate Cancer: A Novel Approach for Prostate Cancer Diagnostics" Cancers 15, no. 2: 431. https://doi.org/10.3390/cancers15020431
APA StyleBryzgunova, O., Bondar, A., Ruzankin, P., Tarasenko, A., Zaripov, M., Kabilov, M., & Laktionov, P. (2023). Locus-Specific Bisulfate NGS Sequencing of GSTP1, RNF219, and KIAA1539 Genes in the Total Pool of Cell-Free and Cell-Surface-Bound DNA in Prostate Cancer: A Novel Approach for Prostate Cancer Diagnostics. Cancers, 15(2), 431. https://doi.org/10.3390/cancers15020431








