Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes
2.3. Procedures
2.3.1. Stem Cell Mobilization and HDCT Regimens
2.3.2. Supportive Therapy
2.3.3. Blood Samples Collection and Analysis
2.4. Response Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Multiple Myeloma Treatment before Stem Cell Mobilization
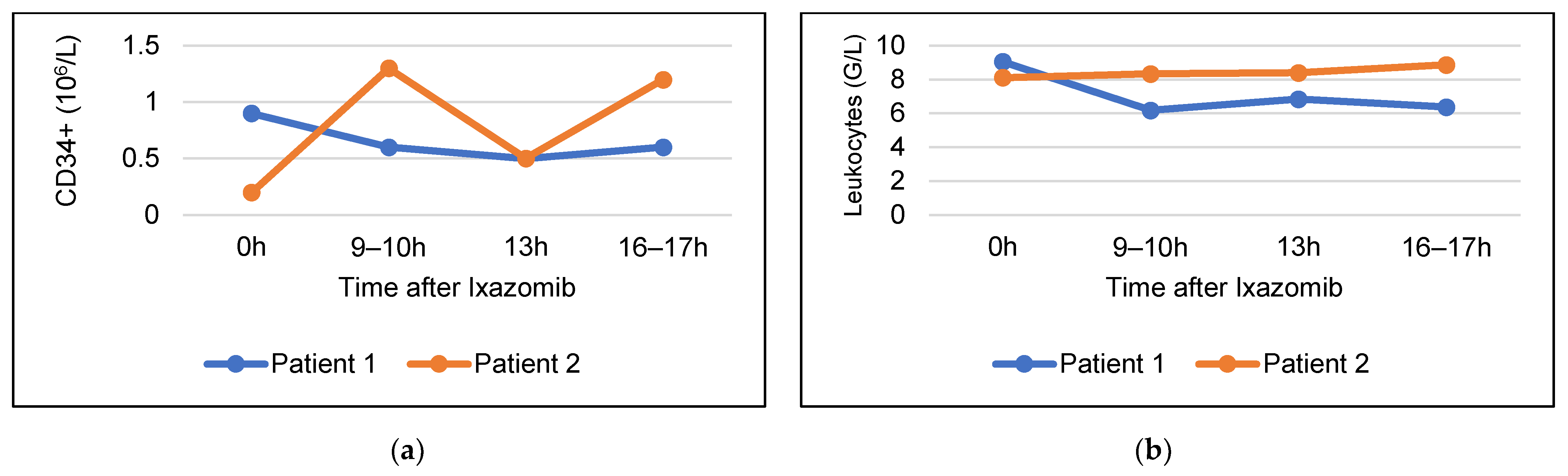
3.3. Stem Cell Mobilizing Efficacy of Ixazomib without G-CSF
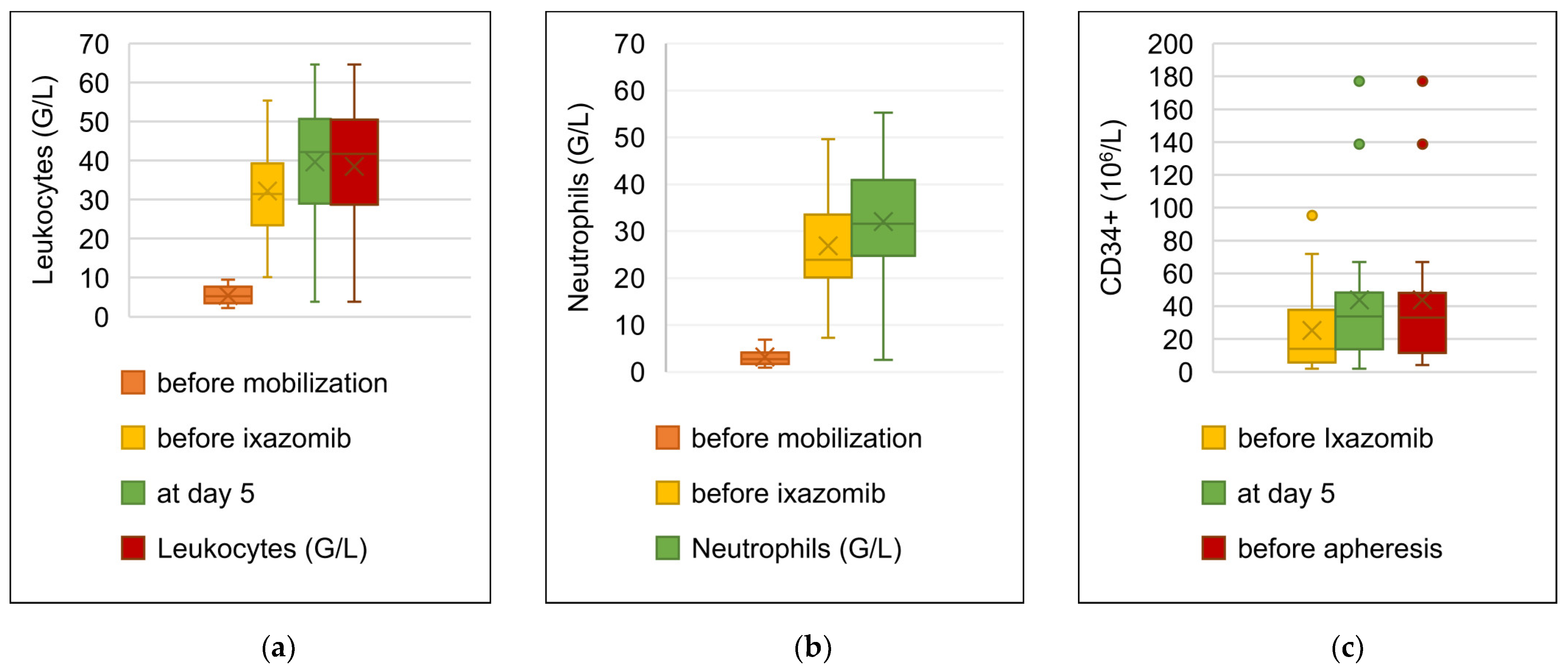
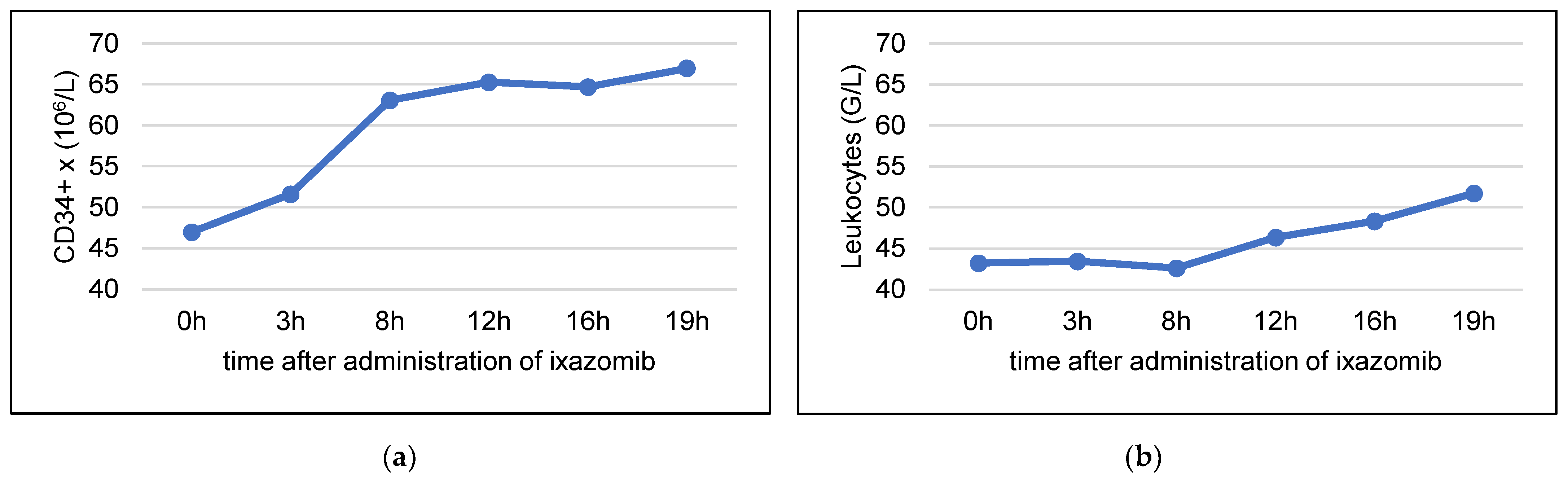
3.4. Stem Cell Mobilization with Ixazomib and G-CSF
3.5. Results of Stem Cell Apheresis
3.6. Safety of the Mobilization with Ixazomib and G-CSF
3.7. High-Dose Chemotherapy and Autologous Stem Cell Transplantation
3.8. Hematologic Recovery after HDCT-ASCT and Infectious Complications
3.9. Remission Status after HDCT and ASCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Group, T.I.M.W. Criteria for the Classification of Monoclonal Gammopathies, Multiple Myeloma and Related Disorders: A Report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef]
- Kehrer, M.; Koob, S.; Strauss, A.; Wirtz, D.C.; Schmolders, J. Multiple Myeloma-Current Status in Diagnostic Testing and Therapy. Z. Orthopädie Unf. 2017, 155, 575–586. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.-L.; Mohty, M. Current Status of Autologous Stem Cell Transplantation for Multiple Myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Ricciuti, G.; Falcone, A.; Cascavilla, N.; Martinelli, G.; Cerchione, C. Autologous Stem Cell Transplantation in Multiple Myeloma. Panminerva Med. 2020, 62, 220–224. [Google Scholar] [CrossRef]
- Mueller, B.U.; Keller, S.; Seipel, K.; Mansouri Taleghani, B.; Rauch, D.; Betticher, D.; Egger, T.; Pabst, T. Stem Cell Mobilization Chemotherapy with Gemcitabine Is Effective and Safe in Myeloma Patients with Bortezomib-Induced Neurotoxicity. Leuk. Lymphoma 2016, 57, 1122–1129. [Google Scholar] [CrossRef]
- Samaras, P.; Bargetzi, M.; Betticher, D.C.; Driessen, C.; Duchosal, M.A.; Heim, D.; Ketterer, N.; Lerch, E.; Matthes, T.; Mey, U.; et al. Updated Recommendations for Diagnosis and Treatment of Plasma Cell Myeloma in Switzerland. Swiss Med. Wkly. 2019, 149, w20031. [Google Scholar] [CrossRef]
- Stettler, J.; Novak, U.; Baerlocher, G.M.; Seipel, K.; Mansouri Taleghani, B.; Pabst, T. Autologous Stem Cell Transplantation in Elderly Patients with Multiple Myeloma: Evaluation of Its Safety and Efficacy. Leuk. Lymphoma 2017, 58, 1076–1083. [Google Scholar] [CrossRef]
- Straka, C.; Dietzfelbinger, H. Multiples Myelom, 5th ed.; W Zuckschwerdt Verlag: München, Deutschland, 2017. [Google Scholar]
- Elice, F.; Raimondi, R.; Tosetto, A.; D’Emilio, A.; Di Bona, E.; Piccin, A.; Rodeghiero, F. Prolonged Overall Survival with Second On-Demand Autologous Transplant in Multiple Myeloma. Am. J. Hematol. 2006, 81, 426–431. [Google Scholar] [CrossRef]
- Arora, S.; Majhail, N.S.; Liu, H. Hematopoietic Progenitor Cell Mobilization for Autologous Stem Cell Transplantation in Multiple Myeloma in Contemporary Era. Clin. Lymphoma Myeloma Leuk. 2019, 19, 200–205. [Google Scholar] [CrossRef]
- Pusic, I.; DiPersio, J.F. The Use of Growth Factors in Hematopoietic Stem Cell Transplantation. Curr. Pharm. Des. 2008, 14, 1950–1961. [Google Scholar] [CrossRef]
- Sahin, U.; Demirer, T. Current Strategies for the Management of Autologous Peripheral Blood Stem Cell Mobilization Failures in Patients with Multiple Myeloma. J. Clin. Apher. 2018, 33, 357–370. [Google Scholar] [CrossRef]
- Giralt, S.; Costa, L.; Schriber, J.; DiPersio, J.; Maziarz, R.; McCarty, J.; Shaughnessy, P.; Snyder, E.; Bensinger, W.; Copelan, E.; et al. Optimizing Autologous Stem Cell Mobilization Strategies to Improve Patient Outcomes: Consensus Guidelines and Recommendations. Biol. Blood Marrow Transplant. 2014, 20, 295–308. [Google Scholar] [CrossRef]
- Micallef, I.N.; Stiff, P.J.; Nademanee, A.P.; Maziarz, R.T.; Horwitz, M.E.; Stadtmauer, E.A.; Kaufman, J.L.; McCarty, J.M.; Vargo, R.; Cheverton, P.D.; et al. Plerixafor Plus Granulocyte Colony-Stimulating Factor for Patients with Non-Hodgkin’s Lymphoma and Multiple Myeloma: Long-Term Follow-Up Report. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 1187–1195. [Google Scholar] [CrossRef]
- Ghobadi, A.; Fiala, M.A.; Rettig, M.; Schroeder, M.; Uy, G.L.; Stockerl-Goldstein, K.; Westervelt, P.; Vij, R.; DiPersio, J.F. A Phase I Study of the Safety and Feasibility of Bortezomib in Combination With G-CSF for Stem Cell Mobilization in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e588–e593. [Google Scholar] [CrossRef]
- Forman, S.; Negrin, R.; Antin, J.; Appelbaum, F. Thomas’ Hematopoietic Cell Transplantation, 5th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Vaxman, I.; Muchtar, E.; Jacob, E.; Kapoor, P.; Kumar, S.; Dispenzieri, A.; Buadi, F.; Dingli, D.; Gonsalves, W.; Kourelis, T.; et al. The Efficacy and Safety of Chemotherapy-Based Stem Cell Mobilization in Multiple Myeloma Patients Who Are Poor Responders to Induction: The Mayo Clinic Experience. Transplant. Cell. Ther. 2021, 27, 770.e1–770.e7. [Google Scholar] [CrossRef]
- Wang, L.; Xiang, H.; Yan, Y.; Deng, Z.; Li, H.; Li, X.; Liu, J. Comparison of the Efficiency, Safety, and Survival Outcomes in Two Stem Cell Mobilization Regimens with Cyclophosphamide plus G-CSF or G-CSF Alone in Multiple Myeloma: A Meta-Analysis. Ann. Hematol. 2021, 100, 563–573. [Google Scholar] [CrossRef]
- Jeker, B.; Farag, S.; Taleghani, B.M.; Novak, U.; Mueller, B.U.; Li, Q.; Betticher, D.; Luethi, J.-M.; Farese, S.; Ruefer, A.; et al. A Randomized Evaluation of Vinorelbine versus Gemcitabine Chemotherapy Mobilization of Stem Cells in Myeloma Patients. Bone Marrow Transplant. 2020, 55, 2047–2051. [Google Scholar] [CrossRef]
- Shah, E.E.; Young, R.P.; Wong, S.W.; Damon, L.E.; Wolf, J.L.; Shah, N.D.; Leavitt, A.D.; Loeffler, P.; Martin, T.G. Impact of Plerixafor Use at Different Peripheral Blood CD34+ Thresholds on Autologous Stem Cell Collection in Patients with Multiple Myeloma. Biol. Blood Marrow Transplant. 2020, 26, 876–883. [Google Scholar] [CrossRef]
- Maechler, M.; Bacher, U.; Daskalakis, M.; Nilius, H.; Nagler, M.; Taleghani, B.M.; Jeker, B.; Pabst, T. Long-Term Safety of the Stem Cell Releasing Compound Plerixafor for Peripheral Stem Cell Collection in Myeloma Patients. Hematol. Oncol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Johnsrud, A.; Ladha, A.; Muffly, L.; Shiraz, P.; Goldstein, G.; Osgood, V.; Shizuru, J.A.; Johnston, L.; Arai, S.; Weng, W.-K.; et al. Stem Cell Mobilization in Multiple Myeloma: Comparing Safety and Efficacy of Cyclophosphamide +/− Plerixafor versus Granulocyte Colony-Stimulating Factor +/− Plerixafor in the Lenalidomide Era. Transplant. Cell. Ther. 2021, 27, 590.e1–590.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lazarus, H.M.; Dahi, P.B.; Avecilla, S.; Giralt, S.A. Getting Blood Out of a Stone: Identification and Management of Patients with Poor Hematopoietic Cell Mobilization. Blood Rev. 2021, 47, 100771. [Google Scholar] [CrossRef] [PubMed]
- Samaras, P.; Rütti, M.F.; Seifert, B.; Bachmann, H.; Schanz, U.; Eisenring, M.; Renner, C.; Müller, A.M.S.; Schmidt, A.; Mischo, A.; et al. Mobilization of Hematopoietic Progenitor Cells with Standard- or Reduced-Dose Filgrastim after Vinorelbine in Multiple Myeloma Patients: A Randomized Prospective Single-Center Phase II Study. Biol. Blood Marrow Transplant. 2018, 24, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Samaras, P.; Pfrommer, S.; Seifert, B.; Petrausch, U.; Mischo, A.; Schmidt, A.; Schanz, U.; Nair, G.; Bargetzi, M.; Taverna, C.; et al. Efficacy of Vinorelbine plus Granulocyte Colony-Stimulation Factor for CD34+ Hematopoietic Progenitor Cell Mobilization in Patients with Multiple Myeloma. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 74–80. [Google Scholar] [CrossRef]
- Keller, S.; Seipel, K.; Novak, U.; Mueller, B.U.; Taleghani, B.M.; Leibundgut, K.; Pabst, T. Neurotoxicity of Stem Cell Mobilization Chemotherapy with Vinorelbine in Myeloma Patients after Bortezomib Treatment. Leuk. Res. 2015, 39, 786–792. [Google Scholar] [CrossRef]
- Mahmoudpour, S.H.; Bandapalli, O.R.; da Silva Filho, M.I.; Campo, C.; Hemminki, K.; Goldschmidt, H.; Merz, M.; Försti, A. Chemotherapy-Induced Peripheral Neuropathy: Evidence from Genome-Wide Association Studies and Replication within Multiple Myeloma Patients. BMC Cancer 2018, 18, 820. [Google Scholar] [CrossRef]
- Montefusco, V.; Mussetti, A.; Salas, M.Q.; Martinelli, G.; Cerchione, C. Old and New Generation Proteasome Inhibitors in Multiple Myeloma. Panminerva Med. 2020, 62, 193–206. [Google Scholar] [CrossRef]
- Brayer, J.; Baz, R. The Potential of Ixazomib, a Second-Generation Proteasome Inhibitor, in the Treatment of Multiple Myeloma. Ther. Adv. Hematol. 2017, 8, 209–220. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Richardson, P.G.; Kumar, S.K.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; et al. Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2021, 39, 2430–2442. [Google Scholar] [CrossRef]
- Ghobadi, A.; Rettig, M.P.; Holt, M.S.; Ritchey, J.K.; Kennerly, K.; Chendamarai, E.; Eissenberg, L.; DiPersio, J.F. Ixazomib, an Oral Proteasome Inhibitor, Induces Rapid Mobilization of Hematopoietic Progenitor Cells in Mice. Blood 2018, 131, 2594–2596. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A.; Rettig, M.P.; Cooper, M.L.; Holt, M.S.; Ritchey, J.K.; Eissenberg, L.; DiPersio, J.F. Bortezomib Is a Rapid Mobilizer of Hematopoietic Stem Cells in Mice via Modulation of the VCAM-1/VLA-4 Axis. Blood 2014, 124, 2752–2754. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Takamatsu, Y.; Moriyama, H.; Terada, K.; Mori, M.; Ono, K.; Migita, K.; Hara, S. Bortezomib Enhances G-CSF-Induced Hematopoietic Stem Cell Mobilization by Decreasing CXCL12 Levels and Increasing Vascular Permeability. Exp. Hematol. 2021, 97, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Novella, E.; Giaretta, I.; Elice, F.; Madeo, D.; Piccin, A.; Castaman, G.; Rodeghiero, F. Fluorescent Polymerase Chain Reaction and Capillary Electrophoresis for IgH Rearrangement and Minimal Residual Disease Evaluation in Multiple Myeloma. Haematologica 2002, 87, 1157–1164. [Google Scholar]
- Luo, C.; Wu, G.; Huang, X.; Zhang, Y.; Ma, Y.; Huang, Y.; Huang, Z.; Li, H.; Hou, Y.; Chen, J.; et al. Efficacy of Hematopoietic Stem Cell Mobilization Regimens in Patients with Hematological Malignancies: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Stem Cell Res. Ther. 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Giralt, S.; Stadtmauer, E.A.; Harousseau, J.L.; Palumbo, A.; Bensinger, W.; Comenzo, R.L.; Lentzsch, S.; Munshi, N.; Niesvizky, R.; et al. Mobilization in Myeloma Revisited: IMWG Consensus Perspectives on Stem Cell Collection Following Initial Therapy with Thalidomide-, Lenalidomide-, or Bortezomib-Containing Regimens. Blood 2009, 114, 1729–1735. [Google Scholar] [CrossRef]
- Milone, G.; Leotta, S.; Indelicato, F.; Mercurio, S.; Moschetti, G.; Di Raimondo, F.; Tornello, A.; Consoli, U.; Guido, G.; Giustolisi, R. G-CSF Alone vs Cyclophosphamide plus G-CSF in PBPC Mobilization of Patients with Lymphoma: Results Depend on Degree of Previous Pretreatment. Bone Marrow Transplant. 2003, 31, 747–754. [Google Scholar] [CrossRef]
- Bakanay, Ş.M.; Demirer, T. Novel Agents and Approaches for Stem Cell Mobilization in Normal Donors and Patients. Bone Marrow Transplant. 2012, 47, 1154–1163. [Google Scholar] [CrossRef]
- Alegre, A.; Tomás, J.F.; Martínez-Chamorro, C.; Gil-Fernández, J.J.; Fernández-Villalta, M.J.; Arranz, R.; Díaz, M.A.; Granda, A.; Bernardo, M.R.; Escudero, A.; et al. Comparison of Peripheral Blood Progenitor Cell Mobilization in Patients with Multiple Myeloma: High-Dose Cyclophosphamide plus GM-CSF vs G-CSF Alone. Bone Marrow Transplant. 1997, 20, 211–217. [Google Scholar] [CrossRef]
- Hiwase, D.K.; Bollard, G.; Hiwase, S.; Bailey, M.; Muirhead, J.; Schwarer, A.P. Intermediate-Dose CY and G-CSF More Efficiently Mobilize Adequate Numbers of PBSC for Tandem Autologous PBSC Transplantation Compared with Low-Dose CY in Patients with Multiple Myeloma. Cytotherapy 2007, 9, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, R.A.; Reisbach, G.; Spitzer, E.; Thalmeier, K.; Dienemann, H.; Mergenthaler, H.G.; Dörmer, P. VLA-4 and VCAM-1 Are the Principal Adhesion Molecules Involved in the Interaction between Blast Colony-Forming Cells and Bone Marrow Stromal Cells. Br. J. Haematol. 1995, 91, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bagal, B.; Gokarn, A.; Punatar, S.; Das, S.; Bonda, A.; Nayak, L.; Chichra, A.; Kannan, S.; Mathew, L.J.; Tembhare, P.; et al. Bortezomib and Cyclophosphamide Based Chemo-Mobilization in Multiple Myeloma. Int. J. Hematol. 2020, 112, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, R.; Anttila, P.; Säily, M.; Lundan, T.; Heiskanen, J.; Siitonen, T.M.; Kakko, S.; Putkonen, M.; Ollikainen, H.; Terävä, V.; et al. A Randomized Phase II Study of Stem Cell Mobilization with Cyclophosphamide+G-CSF or G-CSF Alone after Lenalidomide-Based Induction in Multiple Myeloma. Bone Marrow Transplant. 2016, 51, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Innis-Shelton, R.; Costa, L.J. Chapter 5-Sources of Cells for Hematopoietic Cell Transplantation: Practical Aspects of Hematopoietic Cell Collection. In Hematopoietic Cell Transplantation for Malignant Conditions; Bashir, Q., Hamadani, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–84. ISBN 978-0-323-56802-9. [Google Scholar]
| Parameter | Results |
|---|---|
| Number of patients, n | 19 |
| Median age, years, n (range) | 65 (38–75) |
| Sex: male/female, n (%) | 15 (79%)/4 (21%) |
| Heavy chain subtype, n (%) | |
| IgG a | 14 (74%) |
| IgA | 4 (21%) |
| IgM | 0 (0%) |
| Light chain only Light chain subtype | 1 (5%) |
| Lambda | 7 (37%) |
| Kappa | 12 (63%) |
| Organ involvement Confirmed amyloidosis, n (%) Hypercalcemia (>2.6 mmol/L), n (%) Renal insufficiency (eGFR b <60 mL/min), n (%) Creatinine (µmol/L), median (range) Presence of osteolytic lesions, n (%) | 0 (0%) 2 (11%) 3 (16%) 82 (53–322) 16 (84%) |
| Anemia (hemoglobin < 110 g/L), n (%) | 12 (63%) |
| Hemoglobin, g/L, median (range) c | 97.0 (103.0–139.0) |
| Cytogenetic alterations, n (%) | |
| High-risk d | 6 (32%) |
| Standard-risk | 13 (68%) |
| β-2-Microglobulin (mg/L), median (range) | 3.36 (1.92–21) |
| Albumin (g/L), median (range) | 35 (20–44) |
| Stage, R-ISS e, n (%) | |
| I | 4 (21%) |
| II | 8 (42%) |
| III | 7 (37%) |
| Parameter | Results |
|---|---|
| Patients with newly diagnosed MM, n (%) Patients with relapsed/refractory MM, n (%) | |
| Induction regimen, n (%) | |
| VRd a | 15 (79%) |
| VD b | 1 (5%) |
| Bortezomib, Thalidomide, Dexamethasone | 1 (5%) |
| VD (3 cycles), Pegylated Liposomal Doxorubicin, Bortezomib, Dexamethasone (2 cycles) | 1 (5%) |
| VRd (2 cycles), VCd c (3 cycles) | 1 (5%) |
| Number of cycles, median (range) | 4 (3–6) |
| Symptomatic radiotherapy, n (%) | |
| Yes | 7 (37%) |
| No | 12 (63%) |
| Remission status previous to HDCT and ASCT, n (%) | |
| Stringent complete response | 0 (0%) |
| Complete response | 3 (16%) |
| Very good partial response | 6 (32%) |
| Partial response | 8 (42%) |
| Stable disease | 2 (11%) |
| Progressive disease | 0 (0%) |
| Parameter | Results |
|---|---|
| Stem Cell Mobilization | |
| Median age, years (range) | 65 (38–75) |
| Duration of G-CSF a administration before apheresis, days, median (range) | 5 (4–6) |
| Time interval from ixazomib administration to apheresis, days, median (range) | 1 (0–2) |
| Plerixafor use, n (%) | |
| Yes | 9 (47%) |
| No | 10 (53%) |
| Leukocytes count, G/L, median (range) | |
| before mobilization | 5.2 (2.3–9.5) |
| before ixazomib b | 31.4 (10.1–55.4) |
| at day 5 before apheresis | 42.2 (3.8–64.6) 41.7 (3.8–64.6) |
| Neutrophil granulocytes count, G/L, median (range) | |
| before mobilization | 2.7 (0.9–6.9) |
| before ixazomib b | 23.9 (49.6–7.3) |
| at day 5 c | 31.6 (2.6–55.2) |
| CD34+ cells count, 106/L, median (range) | |
| before ixazomib d | 14.0 (2.0–95.2) |
| at day 5 | 33.7 (1.8–177.0) |
| before apheresis | 33.0 (4.2–177.0) |
| Stem cell apheresis | |
| Duration of apheresis, minutes, median (range) | 313 (144–438) |
| Processed blood volume, ml, median (range) | 28,506 (15,387–57,489) |
| Number of collected CD34+ cells, 106/kg body weight, median (range) | 7.1 (2.9–21.6) |
| Second apheresis, n (%) | 2 (11%) |
| Duration of Hospitalization and Time to Hematologic Recovery | |
|---|---|
| Duration of hospitalization, days, median (range) | 21 (17–48) |
| Time to platelets recovery ≥ 20 G/L, days, median (range) | 14 (10–70) |
| Time to neutrophil granulocytes recovery ≥ 0.5 G/L, days, median (range) | 12 (10–16) |
| Patient requiring platelet transfusions, n (%) | 17 (89%) |
| Number of platelet concentrates, median (range) | 2 (0–13) |
| Patient requiring erythrocyte transfusion, n (%) | 12 (63%) |
| Number of erythrocyte concentrates, median (range) | 1 (0–8) |
| Infectious complications | |
| Febrile episodes, n (%) | 19 (100%) |
| Number of febrile episodes, median (range) | 1 (1–2) |
| Detected pathogen, n (%) | |
| Viral | 1 (5%) |
| Bacterial | 6 (32%) |
| Fungi | 0 (0%) |
| No pathogen detected | 12 (63%) |
| Positive blood cultures, n (%) | |
| Yes | 3 (16%) |
| No | 16 (84%) |
| Remission status after HDCT a, n (%) | |
| Stringent complete response | 8 (42%) |
| Complete response | 4 (21%) |
| Very good partial response | 2 (11%) |
| Partial response | 4 (21%) |
| Stable disease | 1 (5%) |
| Progressive disease | 0 (0%) |
| Maintenance therapy, n (%) | |
| Yes | 14 (74%) |
| No | 5 (26%) |
| Progression after HDCT and ASCT, n (%) | 7 (37%) |
| Time from transplantation to progression, months, median (range) | 9 (1–14) |
| Time from transplantation to next treatment, months, median (range) b Number of deaths Time from transplantation to death, months, median (range) | 12 (4–15) 3 (16%) 12 (11–14) |
| Follow-up, months, median (range) | 19 (11–24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bühler, S.; Akhoundova, D.; Jeker, B.; Legros, M.; Seipel, K.; Daskalakis, M.; Bacher, U.; Pabst, T. Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma. Cancers 2023, 15, 430. https://doi.org/10.3390/cancers15020430
Bühler S, Akhoundova D, Jeker B, Legros M, Seipel K, Daskalakis M, Bacher U, Pabst T. Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma. Cancers. 2023; 15(2):430. https://doi.org/10.3390/cancers15020430
Chicago/Turabian StyleBühler, Selina, Dilara Akhoundova, Barbara Jeker, Myriam Legros, Katja Seipel, Michael Daskalakis, Ulrike Bacher, and Thomas Pabst. 2023. "Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma" Cancers 15, no. 2: 430. https://doi.org/10.3390/cancers15020430
APA StyleBühler, S., Akhoundova, D., Jeker, B., Legros, M., Seipel, K., Daskalakis, M., Bacher, U., & Pabst, T. (2023). Stem Cell Mobilization with Ixazomib and G-CSF in Patients with Multiple Myeloma. Cancers, 15(2), 430. https://doi.org/10.3390/cancers15020430








