Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Cultures
2.2. Preparation of Macrophages Derived from Peripheral Blood Monocytes
2.3. Direct Co-Culture Assay of ESCC Cells and Macrophages
2.4. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Western Blot Analysis
2.6. Overexpression of IL-7R in ESCC Cell Lines
2.7. IL-7R Knockdown in ESCC Cell Lines Using Small Interfering (si) RNA
2.8. Cell Proliferation Assay
2.9. Transwell Migration Assay
2.10. Wound Healing Assay
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Tissue Samples
2.13. Immunohistochemistry
2.14. Statistical Analyses
3. Results
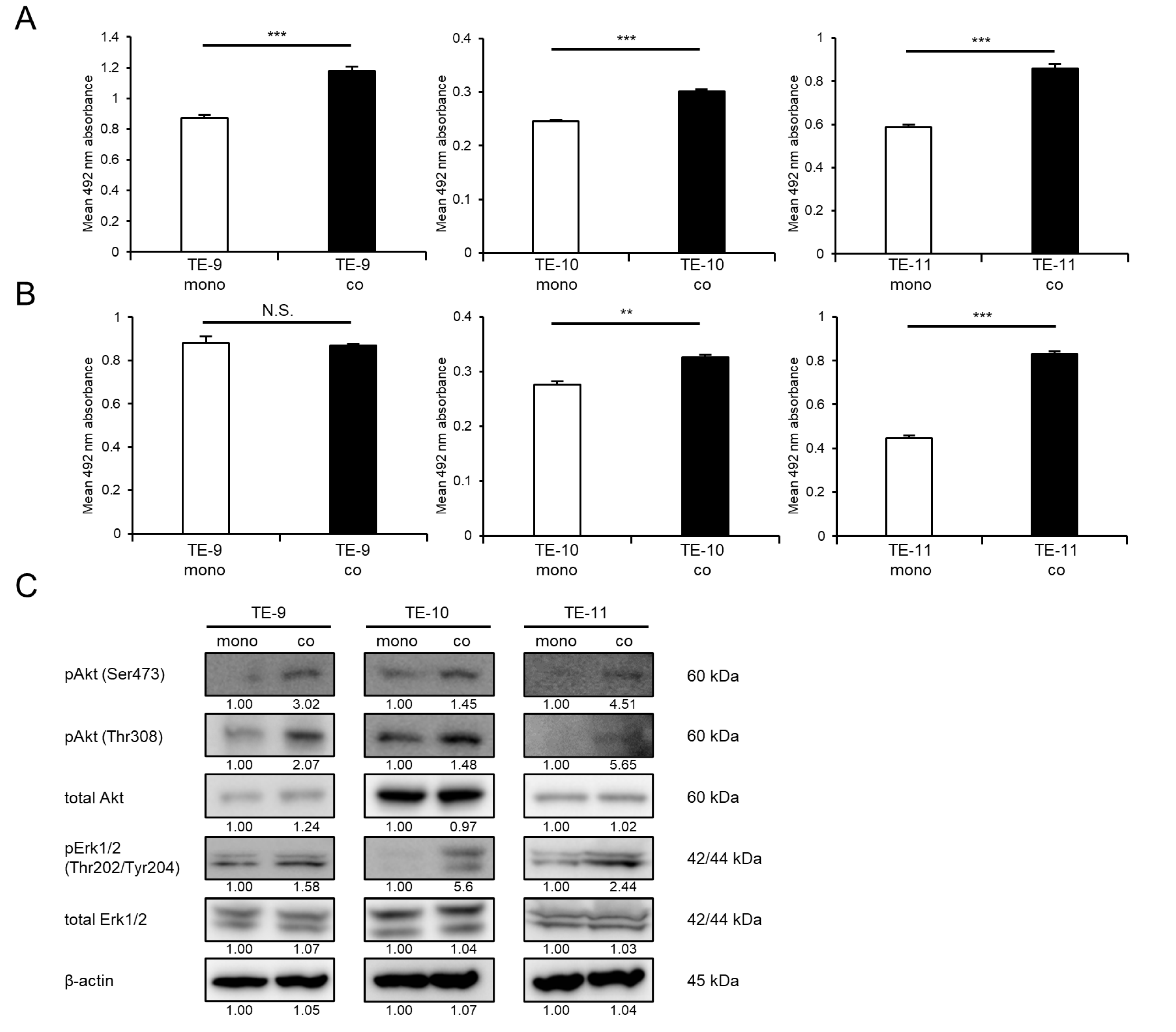
3.1. Direct Co-Culture with Macrophages Promotes the Survival and Growth of ESCC Cells through the Activation of Akt and Erk1/2 Signaling
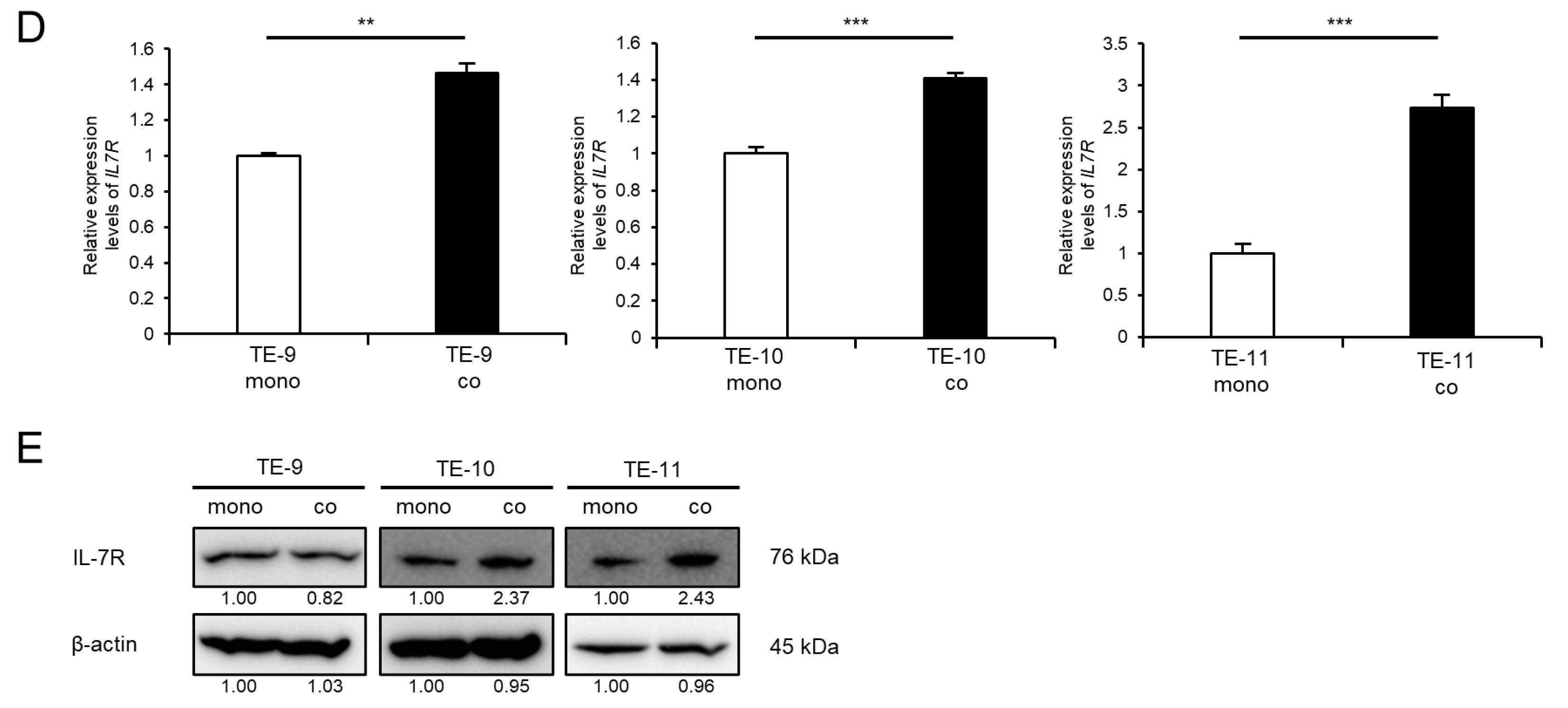
3.2. IL-7R Expression Is Upregulated in ESCC Cells following Direct Co-Culture with Macrophages
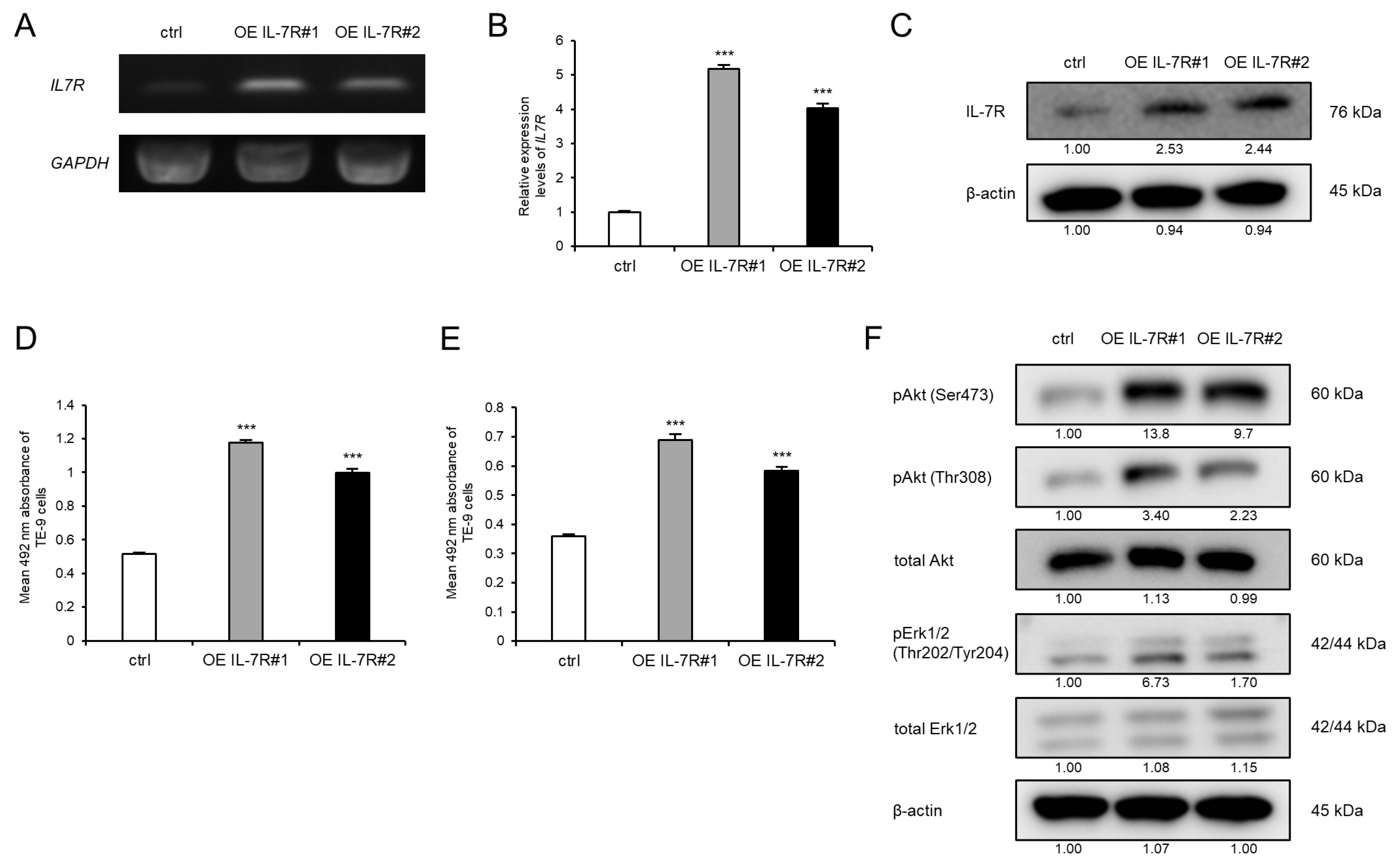
3.3. IL-7R Overexpression in ESCC Cells Markedly Promotes Cell Survival and Growth
3.4. Knockdown of IL-7R in ESCC Cells Markedly Suppresses Cell Survival and Growth
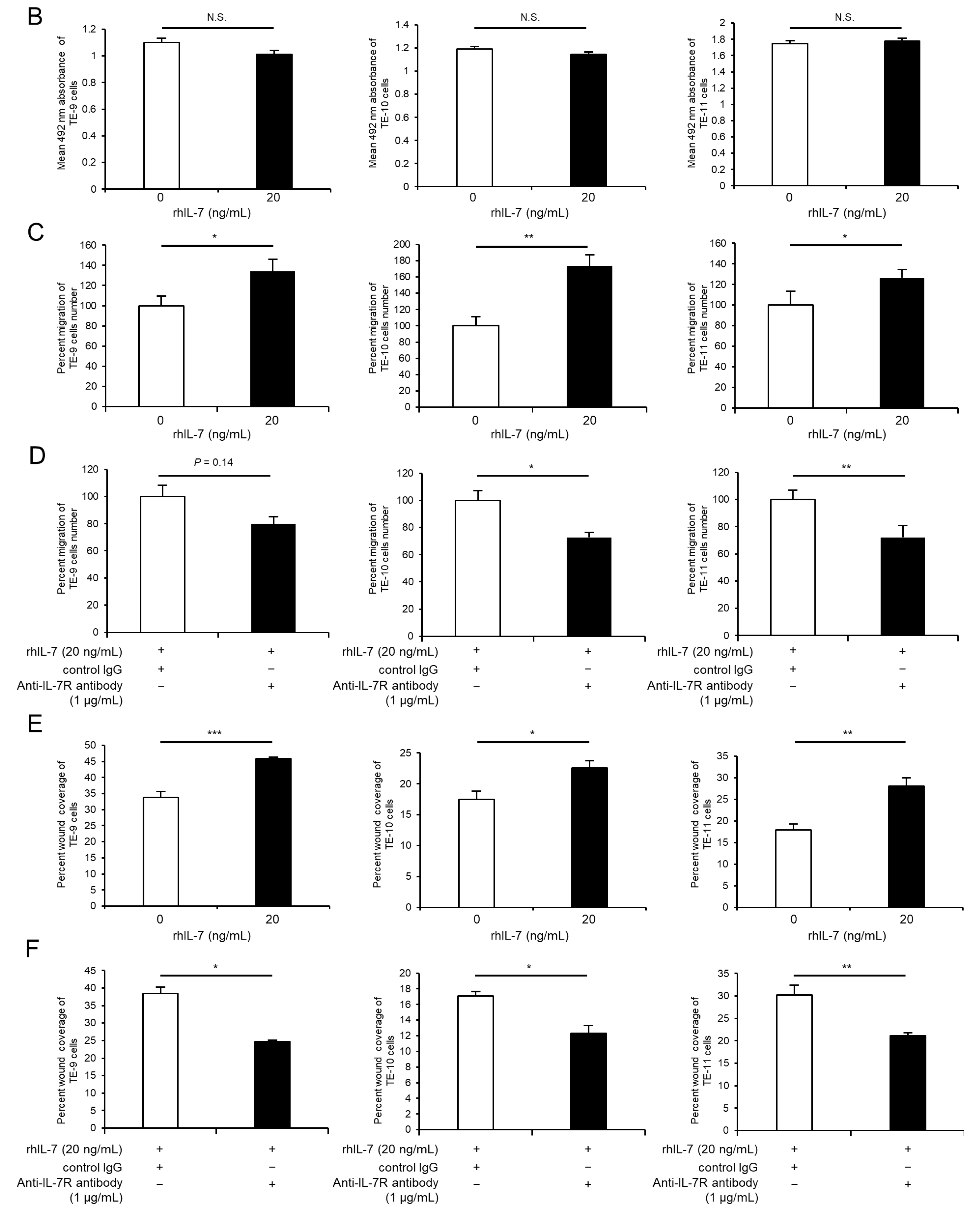
3.5. Exogeneous IL-7 Promotes ESCC Cell Migration Ability via the Akt and Erk1/2 Signaling Pathways
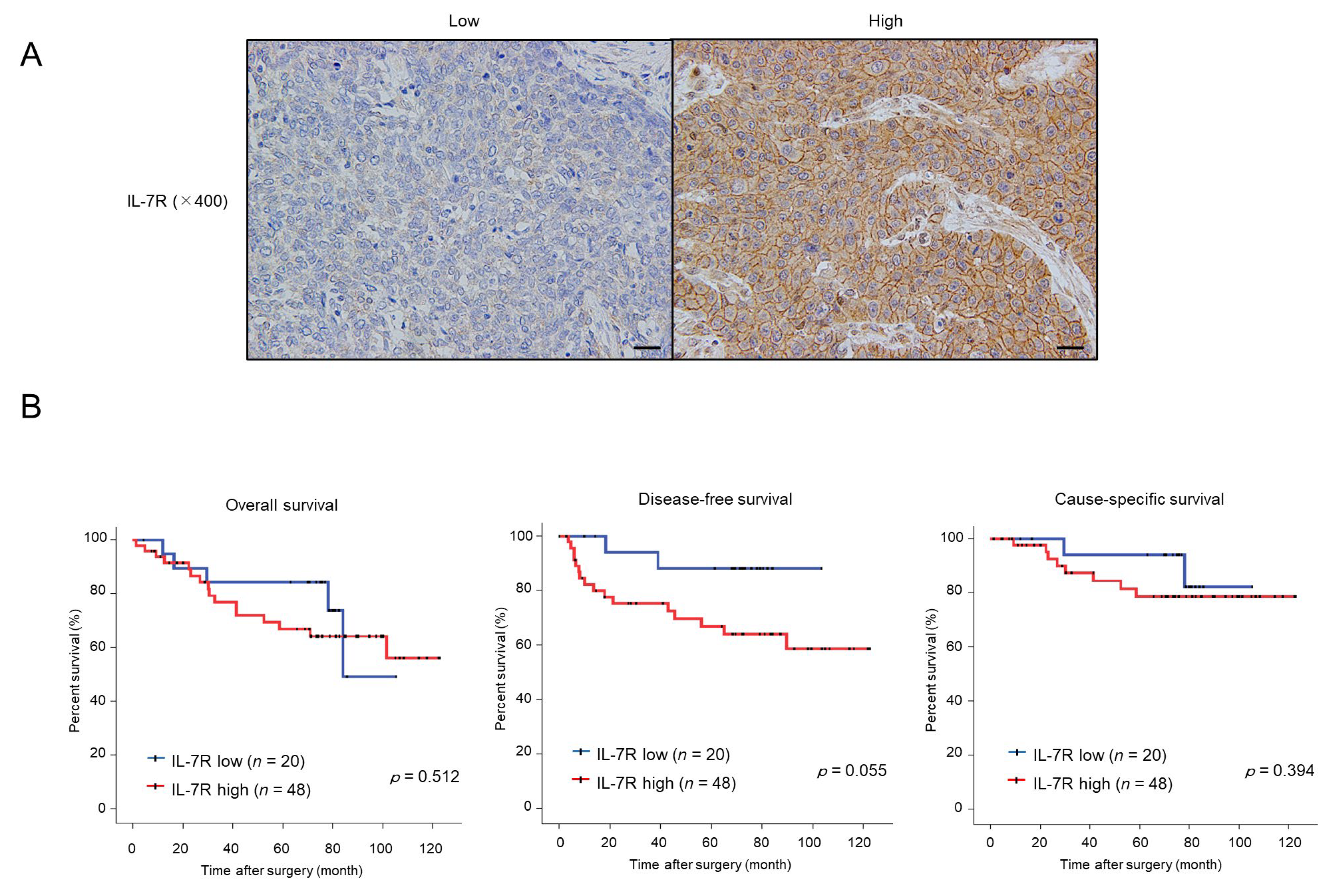
3.6. High Expression of IL-7R in Cancer Nests Is Associated with the Infiltration Levels of Tumor-Associated Macrophages (TAMs) or Cancer-Associated Fibroblasts (CAFs) and Tends to Be Correlated with Poor Prognosis in ESCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A breakthrough: Macrophage-directed cancer immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Komohara, Y.; Oshiumi, H. The role of macrophages in anti-tumor immune responses: Pathological significance and potential as therapeutic targets. Hum. Cell 2021, 34, 1031–1039. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Yokozaki, H.; Koma, Y.I.; Shigeoka, M.; Nishio, M. Cancer as a tissue: The significance of cancer-stromal interactions in the development, morphogenesis and progression of human upper digestive tract cancer. Pathol. Int. 2018, 68, 334–352. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Biswas, S.K.; Sica, A.; Lewis, C.E. Plasticity of macrophage function during tumor progression: Regulation by distinct molecular mechanisms. J. Immunol. 2008, 180, 2011–2017. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Shigeoka, M.; Urakawa, N.; Nakamura, T.; Nishio, M.; Watajima, T.; Kuroda, D.; Komori, T.; Kakeji, Y.; Semba, S.; Yokozaki, H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1112–1119. [Google Scholar] [CrossRef]
- Tanigawa, K.; Tsukamoto, S.; Koma, Y.I.; Kitamura, Y.; Urakami, S.; Shimizu, M.; Fujikawa, M.; Kodama, T.; Nishio, M.; Shigeoka, M.; et al. S100A8/A9 induced by interaction with macrophages in esophageal squamous cell carcinoma promotes the migration and invasion of cancer cells via Akt and p38 MAPK pathways. Am. J. Pathol. 2022, 192, 536–552. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, S.; Liu, T.; Liu, Q.; Chen, Y.; Tan, D.; Ma, R.; Lu, X. IL-1β from M2 macrophages promotes migration and invasion of ESCC cells enhancing epithelial-mesenchymal transition and activating NF-κB signaling pathway. J. Cell. Biochem. 2018, 119, 7040–7052. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Li, S.Y.; Hao-Bin, Y.; Lin-Yan, X.; Sheng, X. IL-11 activated by lnc-ATB promotes cell proliferation and invasion in esophageal squamous cell cancer. Biomed. Pharmacother. 2019, 114, 108835. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Krzystek-Korpacka, M. Local and systemic interleukin-32 in esophageal, gastric, and colorectal cancers: Clinical and diagnostic significance. Diagnostics 2020, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: Parts II and III. Esophagus 2009, 6, 71–94. [Google Scholar] [CrossRef]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Higashino, N.; Koma, Y.I.; Hosono, M.; Takase, N.; Okamoto, M.; Kodaira, H.; Nishio, M.; Shigeoka, M.; Kakeji, Y.; Yokozaki, H. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab. Investig. 2019, 99, 777–792. [Google Scholar] [CrossRef]
- Yeung, O.W.; Lo, C.M.; Ling, C.C.; Qi, X.; Geng, W.; Li, C.X.; Ng, K.T.; Forbes, S.J.; Guan, X.Y.; Poon, R.T.; et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 2015, 62, 607–616. [Google Scholar] [CrossRef]
- Chen, S.; Morine, Y.; Tokuda, K.; Yamada, S.; Saito, Y.; Nishi, M.; Ikemoto, T.; Shimada, M. Cancer-associated fibroblast-induced M2-polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor-1 pathway. Int. J. Oncol. 2021, 59, 59. [Google Scholar] [CrossRef]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, P.; Sun, R.; Li, J.; Hu, Z.; Xin, H.; Luo, C.; Zhou, J.; Fan, J.; Zhou, S. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J. Immunother. Cancer 2021, 9, e001946. [Google Scholar] [CrossRef]
- Urakawa, N.; Utsunomiya, S.; Nishio, M.; Shigeoka, M.; Takase, N.; Arai, N.; Kakeji, Y.; Koma, Y.I.; Yokozaki, H. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab. Investig. 2015, 95, 491–503. [Google Scholar] [CrossRef]
- Kodaira, H.; Koma, Y.I.; Hosono, M.; Higashino, N.; Suemune, K.; Nishio, M.; Shigeoka, M.; Yokozaki, H. ANXA10 induction by interaction with tumor-associated macrophages promotes the growth of esophageal squamous cell carcinoma. Pathol. Int. 2019, 69, 135–147. [Google Scholar] [CrossRef]
- Komohara, Y.; Horlad, H.; Ohnishi, K.; Fujiwara, Y.; Bai, B.; Nakagawa, T.; Suzu, S.; Nakamura, H.; Kuratsu, J.I.; Takeya, M. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci. 2012, 103, 2165–2172. [Google Scholar] [CrossRef]
- Shiraishi, D.; Fujiwara, Y.; Horlad, H.; Saito, Y.; Iriki, T.; Tsuboki, J.; Cheng, P.; Nakagata, N.; Mizuta, H.; Bekki, H.; et al. CD163 is required for protumoral activation of macrophages in human and murine sarcoma. Cancer Res. 2018, 78, 3255–3266. [Google Scholar] [CrossRef]
- Schluns, K.S.; Kieper, W.C.; Jameson, S.C.; Lefrançois, L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 2000, 1, 426–432. [Google Scholar] [CrossRef]
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat. Immunol. 2019, 20, 1584–1593. [Google Scholar] [CrossRef]
- Ming, J.; Zhang, Q.; Qiu, X.; Wang, E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: A mechanism of lymphangiogenesis in lung cancer. Eur. J. Cancer 2009, 45, 866–873. [Google Scholar] [CrossRef]
- Al-Rawi, M.A.; Rmali, K.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Aberrant expression of interleukin-7 (IL-7) and its signalling complex in human breast cancer. Eur. J. Cancer 2004, 40, 494–502. [Google Scholar] [CrossRef]
- Zhuang, W.; Sun, H.; Zhang, S.; Zhou, Y.; Weng, W.; Wu, B.; Ye, T.; Huang, W.; Lin, Z.; Shi, L.; et al. An immunogenomic signature for molecular classification in hepatocellular carcinoma. Mol. Ther. Nucleic Acids 2021, 25, 105–115. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.K.; Hong, S.H.; Eun, J.W.; Nam, S.W.; Han, J.W.; You, J.S. Oncogenic IL7R is downregulated by histone deacetylase inhibitor in esophageal squamous cell carcinoma via modulation of acetylated FOXO1. Int. J. Oncol. 2018, 53, 395–403. [Google Scholar] [CrossRef]
- Ming, J.; Jiang, G.; Zhang, Q.; Qiu, X.; Wang, E. Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer. Cancer Immunol. Immunother. 2012, 61, 79–88. [Google Scholar] [CrossRef]
- Kong, F.; Hu, W.; Zhou, K.; Wei, X.; Kou, Y.; You, H.; Zheng, K.; Tang, R. Hepatitis B virus X protein promotes interleukin-7 receptor expression via NF-κB and Notch1 pathway to facilitate proliferation and migration of hepatitis B virus-related hepatoma cells. J. Exp. Clin. Cancer Res. 2016, 35, 172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Wang, M.H.; Ren, H.J.; Qu, W.; Sun, L.M.; Zhang, Q.F.; Qiu, X.S.; Wang, E.H. Interleukin 7 signaling prevents apoptosis by regulating bcl-2 and bax via the p53 pathway in human non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 870–881. [Google Scholar] [PubMed]
- Al-Rawi, M.A.; Rmali, K.; Mansel, R.E.; Jiang, W.G. Interleukin 7 induces the growth of breast cancer cells through a wortmannin-sensitive pathway. Br. J. Surg. 2004, 91, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Zou, Z.; Pan, Z.; Zhang, T.; Deng, N.; Chen, G.; Wang, Z. IL-7/IL-7 receptor axis stimulates prostate cancer cell invasion and migration via AKT/NF-κB pathway. Int. Immunopharmacol. 2016, 40, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Alshyarba, M.; Otifi, H.; Al Fayi, M.; Dera, A.A.; Rajagopalan, P. Thymoquinone inhibits IL-7-induced tumor progression and metastatic invasion in prostate cancer cells by attenuating matrix metalloproteinase activity and Akt/NF-κB signaling. Biotechnol. Appl. Biochem. 2021, 68, 1403–1411. [Google Scholar] [CrossRef]
- Park, S.L.; Lee, E.J.; Kim, W.J.; Moon, S.K. p27KIP1 is involved in ERK1/2-mediated MMP-9 expression via the activation of NF-κB binding in the IL-7-induced migration and invasion of 5637 cells. Int. J. Oncol. 2014, 44, 1349–1356. [Google Scholar] [CrossRef]
- Mai, H.L.; Deshayes, S.; Nguyen, T.V.; Dehame, V.; Chéné, A.L.; Brouard, S.; Blanquart, C. IL-7 is expressed in malignant mesothelioma and has a prognostic value. Mol. Oncol. 2022, 16, 3606–3619. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, W.Q.; Aiello, F.B.; Mazzucchelli, R.; Asefa, B.; Khaled, A.R.; Durum, S.K. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005, 16, 513–533. [Google Scholar] [CrossRef]
- Seol, M.A.; Kim, J.H.; Oh, K.; Kim, G.; Seo, M.W.; Shin, Y.K.; Sim, J.H.; Shin, H.M.; Seo, B.Y.; Lee, D.S.; et al. Interleukin-7 contributes to the invasiveness of prostate cancer cells by promoting epithelial-mesenchymal transition. Sci. Rep. 2019, 9, 6917. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Peng, Y.; Chen, J.; Pan, L.; Pan, D. IL-7 splicing variant IL-7δ5 induces EMT and metastasis of human breast cancer cell lines MCF-7 and BT-20 through activation of PI3K/Akt pathway. Histochem. Cell Biol. 2014, 142, 401–410. [Google Scholar] [CrossRef]
- Heo, S.H.; Jang, S.I.; Kim, S.Y.; Choi, B.; Lee, D.K.; Lee, H.K.; Chang, E.J. Characterization of circulating IL-7R positive cell populations for early detection of pancreatic ductal adenocarcinoma. J. Clin. Med. 2021, 10, 4157. [Google Scholar] [CrossRef]
- Suzuki, K.; Kadota, K.; Sima, C.S.; Nitadori, J.; Rusch, V.W.; Travis, W.D.; Sadelain, M.; Adusumilli, P.S. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: Tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J. Clin. Oncol. 2013, 31, 490–498. [Google Scholar] [CrossRef]
- Jian, M.; Yunjia, Z.; Zhiying, D.; Yanduo, J.; Guocheng, J. Interleukin 7 receptor activates PI3K/Akt/mTOR signaling pathway via downregulation of Beclin-1 in lung cancer. Mol. Carcinog. 2019, 58, 358–365. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Ding, Z.; Ming, J. Interleukin 7 inhibit autophagy via P53 regulated AMPK/mTOR signaling pathway in non-small cell lung cancer. Sci. Rep. 2022, 12, 11208. [Google Scholar] [CrossRef]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef]
- Kröncke, R.; Loppnow, H.; Flad, H.D.; Gerdes, J. Human follicular dendritic cells and vascular cells produce interleukin-7: A potential role for interleukin-7 in the germinal center reaction. Eur. J. Immunol. 1996, 26, 2541–2544. [Google Scholar] [CrossRef]
- Boesch, M.; Onder, L.; Cheng, H.W.; Novkovic, M.; Mörbe, U.; Sopper, S.; Gastl, G.; Jochum, W.; Ruhstaller, T.; Knauer, M.; et al. Interleukin 7-expressing fibroblasts promote breast cancer growth through sustenance of tumor cell stemness. Oncoimmunology 2018, 7, e1414129. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhang, W.; Zhao, D.; Zhang, L.; Zhang, J.; Fan, J.; Zhan, Q. NOX5 mediates the crosstalk between tumor cells and cancer-associated fibroblasts via regulating cytokine network. Clin. Transl. Med. 2021, 11, e472. [Google Scholar] [CrossRef]



| Expression of IL-7R a | ||||
|---|---|---|---|---|
| Number | Low (n = 20) | High (n = 49) | p-Value | |
| Age | ||||
| <65 | 32 | 10 | 22 | 0.700 |
| ≥65 | 37 | 10 | 27 | |
| Sex | ||||
| Male | 55 | 19 | 36 | 0.044 * |
| Female | 14 | 1 | 13 | |
| Histological grade b | ||||
| HGIEN + WDSCC | 15 | 7 | 8 | 0.088 |
| MDSCC + PDSCC | 54 | 13 | 41 | |
| Depth of tumor invasion b | ||||
| T1 | 48 | 17 | 31 | 0.075 |
| T2 + T3 | 21 | 3 | 18 | |
| Lymphatic vessel invasion b | ||||
| Negative | 37 | 14 | 23 | 0.081 |
| Positive | 32 | 6 | 26 | |
| Blood vessel invasion b | ||||
| Negative | 43 | 16 | 27 | 0.053 |
| Positive | 26 | 4 | 22 | |
| Lymph node metastasis b | ||||
| Negative | 43 | 16 | 27 | 0.053 |
| Positive | 26 | 4 | 22 | |
| Stage c | ||||
| 0 + I | 38 | 14 | 24 | 0.111 |
| II + III + IV | 31 | 6 | 25 | |
| Expression of αSMA d | ||||
| Low | 36 | 15 | 21 | 0.015 * |
| High | 33 | 5 | 28 | |
| Expression of FAP d | ||||
| Low | 39 | 15 | 24 | 0.048 * |
| High | 30 | 5 | 25 | |
| Expression of CD68 e | ||||
| Low | 35 | 14 | 21 | 0.041 * |
| High | 34 | 6 | 28 | |
| Expression of CD163 e | ||||
| Low | 34 | 14 | 20 | 0.028 * |
| High | 35 | 6 | 29 | |
| Expression of CD204 e | ||||
| Low | 34 | 15 | 19 | 0.006 ** |
| High | 35 | 5 | 30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, Y.; Koma, Y.-i.; Tanigawa, K.; Tsukamoto, S.; Azumi, Y.; Miyako, S.; Urakami, S.; Kodama, T.; Nishio, M.; Shigeoka, M.; et al. Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma. Cancers 2023, 15, 394. https://doi.org/10.3390/cancers15020394
Kitamura Y, Koma Y-i, Tanigawa K, Tsukamoto S, Azumi Y, Miyako S, Urakami S, Kodama T, Nishio M, Shigeoka M, et al. Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma. Cancers. 2023; 15(2):394. https://doi.org/10.3390/cancers15020394
Chicago/Turabian StyleKitamura, Yu, Yu-ichiro Koma, Kohei Tanigawa, Shuichi Tsukamoto, Yuki Azumi, Shoji Miyako, Satoshi Urakami, Takayuki Kodama, Mari Nishio, Manabu Shigeoka, and et al. 2023. "Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma" Cancers 15, no. 2: 394. https://doi.org/10.3390/cancers15020394
APA StyleKitamura, Y., Koma, Y.-i., Tanigawa, K., Tsukamoto, S., Azumi, Y., Miyako, S., Urakami, S., Kodama, T., Nishio, M., Shigeoka, M., Kakeji, Y., & Yokozaki, H. (2023). Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma. Cancers, 15(2), 394. https://doi.org/10.3390/cancers15020394





