New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo- and Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Challenges in Colorectal Cancer: A Medical Point of View
3. Heterogeneity in Colorectal Cancer: Single-Cell Point of View
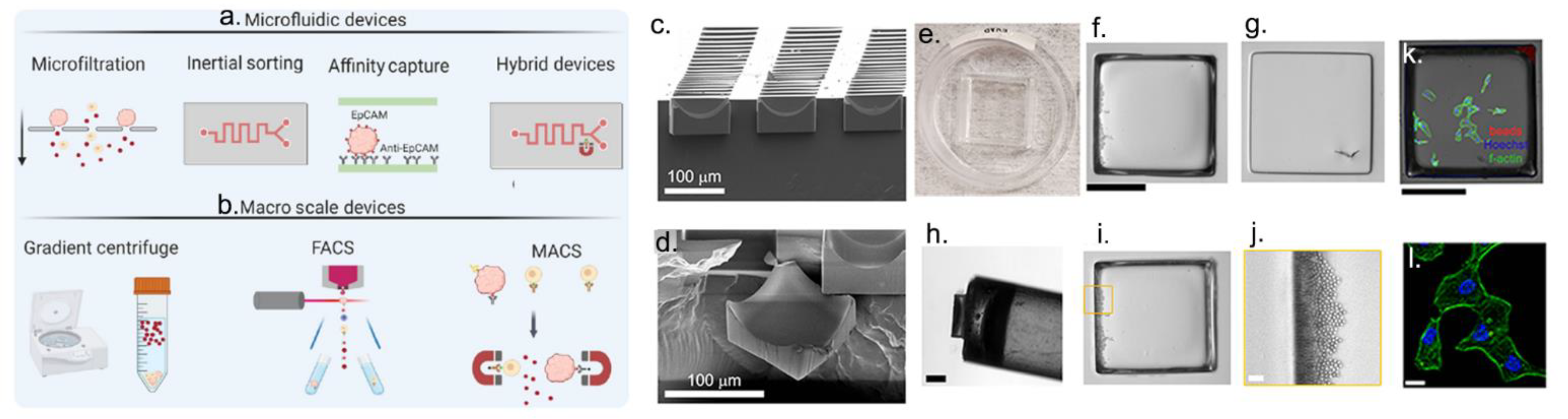
4. Nanoparticles in Radiotherapy
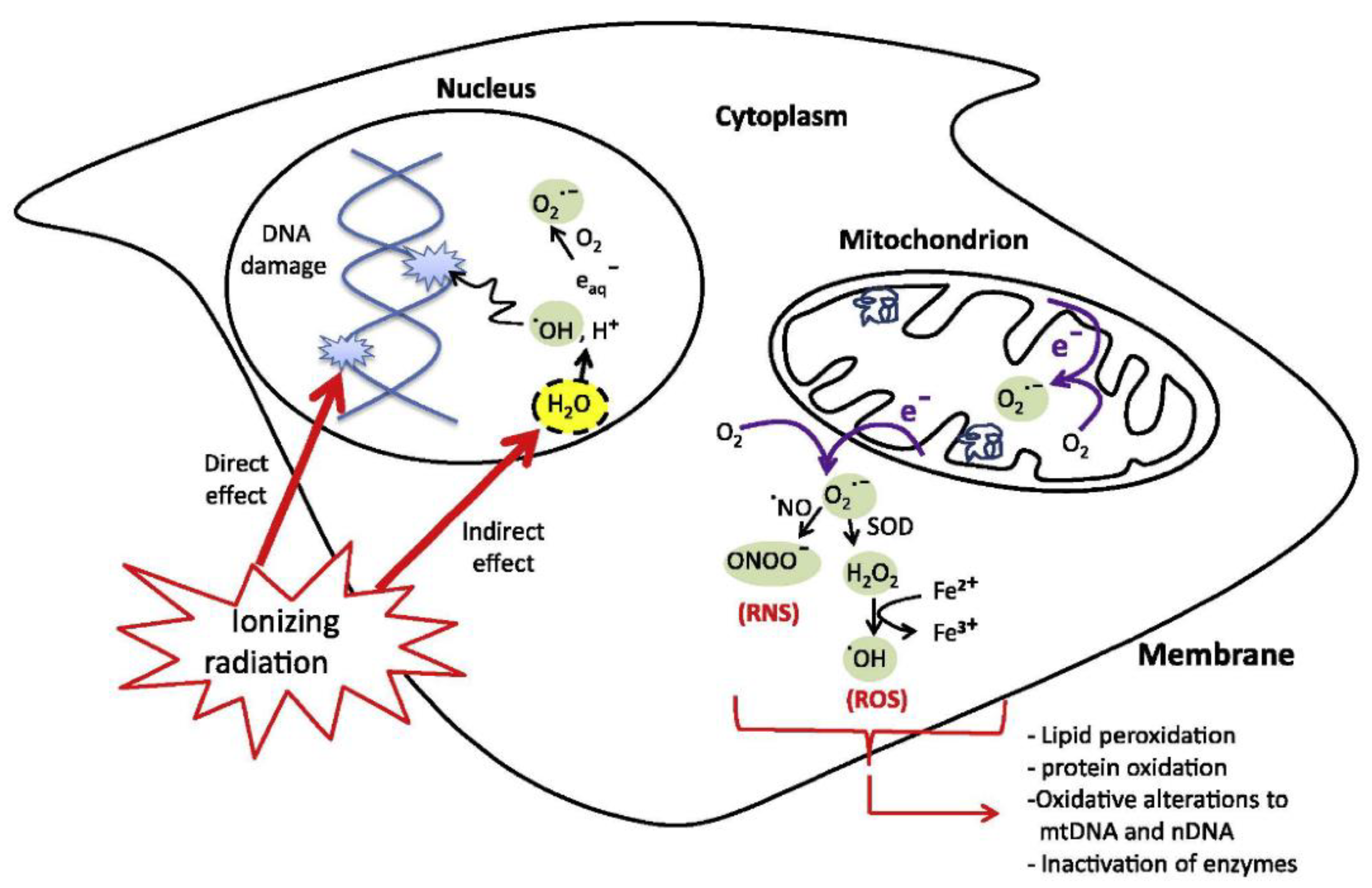
5. Nanoparticle-Based Photothermal Therapy
6. Photo- and Radiotherapy for Colorectal Cancer Treatment
6.1. In Vitro Studies
6.2. Three-dimensional Models
6.3. In Vivo Studies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Khot, M.I.; Andrew, H.; Svavarsdottir, H.S.; Armstrong, G.; Quyna, A.J.; Jaynea, D.G. A Review on the Scope of Photothermal Therapy–Based Nanomedicines in Preclinical Models of Colorectal Cancer. Clin. Color. Cancer 2019, 18, e200–e209. [Google Scholar] [CrossRef]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef] [PubMed]
- Brar, B.; Ranjan, K.; Palria, A.; Kumar, R.; Ghosh, M.; Sihag, S.; Minakshi, P. Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy. Front. Nanotechnol. 2021, 3, 1–21. [Google Scholar] [CrossRef]
- Soto, K.M.; Mendoza, S.; López-Romero, J.M.; Gasca-Tirado, J.R.; Manzano-Ramírez, A. Gold nanoparticles: Synthesis, application in colon cancer therapy and new approaches-review. Green Chem. Lett. Rev. 2021, 14, 665–678. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Cao, K.; Zhang, L.; Fang, X.; Chen, F.; Feng, S.; Yan, F. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021, 134, 621–632. [Google Scholar] [CrossRef]
- Burt, R.W.; Barthel, J.S.; Dunn, K.B.; David, D.S.; Drelichman, E.; Ford, J.M.; Giardiello, F.M.; Gruber, S.B.; Halverson, A.L.; Hamilton, S.R.; et al. Colorectal Cancer Screening. J. Natl. Compr. Cancer Netw. 2010, 8, 8–61. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Colorectal Cancer Early Detection, Diagnosis, and Staging. Available online: Cancer.org (accessed on 10 October 2022).
- Kaveh, S.; Ebrahimi, P.; Rezapour, A.; Mozafari, M.; Sayehmiri, K. Bevacizumab and erlotinib versus bevacizumab for colorectal cancer treatment: Systematic review and meta-analysis. Int. J. Clin. Pharm. 2019, 41, 30–41. [Google Scholar] [CrossRef]
- de Azevedo, J.M.; Vailati, B.B.; Julião, G.P.S.; Fernandez, L.M.; Perez, R.O. Current Surgical Strategies in the Management of Rectal Cancer. Curr. Color. Cancer Rep. 2019, 15, 18–27. [Google Scholar] [CrossRef]
- Nasir, I. Salvage surgery for local regrowths in Watch & Wait-Are we harming our patients by deferring the surgery? Eur. J. Surg. Oncol. 2019, 45, 1559–1566. [Google Scholar]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Reshmitha, T.R.; Shini, V.S.; Nisha, P. Nanotechnology Approaches for Colorectal Cancer Diagnosis and Therapy. In Colon Cancer Diagnosis and Therapy; Vishvakarma, N.K., Nagaraju, G.P., Shukla, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 2, pp. 171–186. [Google Scholar]
- Al-Joufi, F.A.; Setia, A.; Salem-Bekhit, M.M.; Sahu, R.K.; Alqahtani, F.Y.; Widyowati, R.; Aleanizy, F.S. Molecular Pathogenesis of Colorectal Cancer with an Emphasis on Recent Advances in Biomarkers, as Well as Nanotechnology-Based Diagnostic and Therapeutic Approaches. Nanomaterials 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Combes, G.F.; Vučković, A.-M.; Bakulić, M.P.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, S.; Li, W.; Yuan, D.; Song, J. Quantitative detection of the tumor-associated antigen large external antigen in colorectal cancer tissues and cells using quantum dot probe. Int. J. Nanomed. 2016, 11, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Granja, A.; Pinheiro, M.; Sousa, C.T.; Reis, S. Gold nanostructures as mediators of hyperthermia therapies in breast cancer. Biochem. Pharmacol. 2021, 190, 114639. [Google Scholar] [CrossRef]
- Li, W.; Belchior, A.; Beuve, M.; Chen, Y.; Di Maria, S.; Friedland, W.; Gervais, B.; Heide, B.; Hocine, N.; Ipatov, A.; et al. Intercomparison of dose enhancement ratio and secondary electron spectra for gold nanoparticles irradiated by X-rays calculated using multiple Monte Carlo simulation codes. Phys. Medica 2020, 69, 147–163. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef]
- AlMusawi, S.; Ahmed, M.; Nateri, A.S. Understanding cell-cell communication and signaling in the colorectal cancer microenvironment. Clin. Transl. Med. 2021, 11, e308. [Google Scholar] [CrossRef]
- Venkatachalapathy, S.; Jokhun, D.S.; Andhari, M.; Shivashankar, G.V. Single cell imaging-based chromatin biomarkers for tumor progression. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Mitra, A.K.; Mukherjee, U.K.; Harding, T.; Jang, J.S.; Stessman, H.; Li, Y.; Abyzov, A.; Jen, J.; Kumar, S.; Rajkumar, V.; et al. Single-cell analysis of targeted transcriptome predicts drug sensitivity of single cells within human myeloma tumors. Leukemia 2015, 30, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Crinier, A.; Escalière, B.; Ye, Y.; Wang, Z.; Zhang, T.; Batista, L.; Liu, H.; Hong, L.; Wu, N.; et al. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep. Med. 2021, 2, 100353. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, P.; Chen, T.; Gao, S.; Wu, Z.; Wang, X.; Li, J.; Marjani, S.L.; Costa, J.; Weissman, S.M.; et al. Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single-Cell Analysis of Transcriptome and Telomeres. Adv. Sci. 2021, 8, 2004320. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Srinivasan, T.; Lin, C.; Tung, K.-L.; Gao, Z.; Hsu, D.S.; Lipkin, S.M.; Shen, X. Single-Cell Transcriptomics Reveals Heterogeneity and Drug Response of Human Colorectal Cancer Organoids. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2378–2381. [Google Scholar] [CrossRef]
- Hinman, S.S.; Wang, Y.; Kim, R.; Allbritton, N.L. In vitro generation of self-renewing human intestinal epithelia over planar and shaped collagen hydrogels. Nat. Protoc. 2020, 16, 352–382. [Google Scholar] [CrossRef] [PubMed]
- Dutton, J.S.; Hinman, S.S.; Kim, R.; Wang, Y.; Allbritton, N.L. Primary Cell-Derived Intestinal Models: Recapitulating Physiology. Trends Biotechnol. 2018, 37, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gunasekara, D.B.; Attayek, P.J.; Reed, M.I.; DiSalvo, M.; Nguyen, D.L.; Dutton, J.S.; Lebhar, M.S.; Bultman, S.J.; Sims, C.E.; et al. In Vitro Generation of Mouse Colon Crypts. ACS Biomater. Sci. Eng. 2017, 3, 2502–2513. [Google Scholar] [CrossRef]
- Wang, Y.; Sims, C.E.; Allbritton, N.L. Human 2D Crypt Model for Assaying Intestinal Stem Cell Proliferation and Differentiation. Anal. Chem. 2022, 94, 9345–9354. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Weeber, F.; van de Wetering, M.; Hoogstraat, M.; Dijkstra, K.K.; Krijgsman, O.; Kuilman, T.; Gadellaa-van Hooijdonk, C.G.M.; van der Velden, D.L.; Peeper, D.S.; Cuppen, E.P.J.G.; et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl. Acad. Sci. USA 2015, 112, 13308–13311. [Google Scholar] [CrossRef]
- Qian, M.; Wang, D.C.; Chen, H.; Cheng, Y. Detection of single cell heterogeneity in cancer. Semin. Cell Dev. Biol. 2017, 64, 143–149. [Google Scholar] [CrossRef]
- Cortés-Llanos, B.; Wang, Y.; Sims, C.E.; Allbritton, N.L. A technology of a different sort: Microraft arrays. Lab Chip 2021, 21, 3204–3218. [Google Scholar] [CrossRef] [PubMed]
- LaBelle, C.A.; Massaro, A.; Cortés-Llanos, B.; Sims, C.E.; Allbritton, N.L. Image-Based Live Cell Sorting. Trends Biotechnol. 2020, 39, 613–623. [Google Scholar] [CrossRef] [PubMed]
- DiSalvo, M.; Cortés-Llanos, B.; LaBelle, C.A.; Murdoch, D.M.; Allbritton, N.L. Scalable Additive Construction of Arrayed Microstructures with Encoded Properties for Bioimaging. Micromachines 2022, 13, 1392. [Google Scholar] [CrossRef]
- Pan, J.; Wan, J. Methodological comparison of FACS and MACS isolation of enriched microglia and astrocytes from mouse brain. J. Immunol. Methods 2020, 486, 112834. [Google Scholar] [CrossRef] [PubMed]
- Enrico, L.; Veronica, Z.; Domenico, M.; Alessandra, R. FACS Analysis of Memory T Lymphocytes. In T-Cell Differentiation: Methods and Protocols; Luigi, E., Ed.; Springer: New York, NY, USA, 2017; pp. 31–47. [Google Scholar]
- Nowotarski, H.L.; Attayek, P.J.; Allbritton, N.L. Automated platform for cell selection and separation based on four-dimensional motility and matrix degradation. Analyst 2020, 145, 2731–2742. [Google Scholar] [CrossRef]
- Gracz, A.; Williamson, I.; Roche, K.C.; Johnston, M.J.; Wang, F.; Wang, Y.; Attayek, P.; Balowski, J.; Liu, X.F.; Laurenza, R.J.; et al. A high-throughput platform for stem cell niche co-cultures and downstream gene expression analysis. Nat. Cell Biol. 2015, 17, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.H.; Wilson, B.J.; Gold, J.S.; Frank, N.Y. Clinical Implications of Colorectal Cancer Stem Cells in the Age of Single-Cell Omics and Targeted Therapies. Gastroenterology 2021, 160, 1947–1960. [Google Scholar] [CrossRef]
- Mei, Y.; Xiao, W.; Hu, H.; Lu, G.; Chen, L.; Sun, Z.; Lü, M.; Ma, W.; Jiang, T.; Gao, Y.; et al. Single-cell analyses reveal suppressive tumor microenvironment of human colorectal cancer. Clin. Transl. Med. 2021, 11, e422. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Zhao, Z.; Yang, M.; Chen, M.; Liu, C.; Ji, J.; Zhu, D. Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Lett. 2019, 470, 84–94. [Google Scholar] [CrossRef]
- Bian, S.; Hou, Y.; Zhou, X.; Li, X.; Yong, J.; Wang, Y.; Wang, W.; Yan, J.; Hu, B.; Guo, H.; et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science 2018, 362, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.N.; Siu, H.C.; Ho, S.L.; Yue, S.S.K.; Gao, Y.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Wong, J.W.H.; Man, A.H.Y.; et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut 2020, 69, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andrä, I.; Andreata, F.; Annunziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef] [PubMed]
- Roerink, S.F.; Sasaki, N.; Lee-Six, H.; Young, M.D.; Alexandrov, L.B.; Behjati, S.; Mitchell, T.J.; Grossmann, S.; Lightfoot, H.; Egan, D.A.; et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 2018, 556, 457–462. [Google Scholar] [CrossRef]
- Radfar, P.; Es, H.A.; Salomon, R.; Kulasinghe, A.; Ramalingam, N.; Sarafraz-Yazdi, E.; Thiery, J.P.; Warkiani, M.E. Single-cell analysis of circulating tumour cells: Enabling technologies and clinical applications. Trends Biotechnol. 2022, 40, 1041–1060. [Google Scholar] [CrossRef]
- Ngwa, W.; Boateng, F.; Kumar, R.; Irvine, D.J.; Formenti, S.; Ngoma, T.; Herskind, C.; Veldwijk, M.R.; Hildenbrand, G.L.; Hausmann, M.; et al. Smart Radiation Therapy Biomaterials. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 624–637. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Dubrovska, A.; Peitzsch, C.; Ewe, A.; Aigner, A.; Schellenburg, S.; Muders, M.H.; Hampel, S.; Cirillo, G.; Iemma, F.; et al. Nanoparticles for radiooncology: Mission, vision, challenges. Biomaterials 2017, 120, 155–184. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Pallares, R.M.; Abergel, R.J. Nanoparticles for targeted cancer radiotherapy. Nano Res. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- West, C.M.L. Introduction to Radiobiology. Int. J. Radiat. Biol. 1992, 62, 125. [Google Scholar] [CrossRef]
- Hendry, J.H. Introduction to Radiobiology; Tubiana, M., Dutreix, J., Wambersie, A., Eds.; Wiley: Hoboken, NJ, USA, 1991; Volume 24, p. 339. [Google Scholar]
- Joiner, M.C.; van der Kogel, A.J. Basic Clinical Radiobiology; CRC Press: London, UK, 2009. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Campbell, J.M.; Deng, W.; Guller, A.; Nisar, S.; Liu, G.; Wilson, B.C.; Goldys, E.M. Mechanisms for Tuning Engineered Nanomaterials to Enhance Radiation Therapy of Cancer. Adv. Sci. 2020, 7, 2003584. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA A Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lawrenz, I.; Flentje, M. Moderately hypofractionated radiotherapy for localized prostate cancer: Long-term outcome using IMRT and volumetric IGRT. Strahlenther. Onkol. 2014, 190, 48–53. [Google Scholar] [CrossRef]
- Tekatli, H.; Hof, S.V.; Nossent, E.J.; Dahele, M.; Verbakel, W.F.; Slotman, B.J.; Senan, S. Use of Stereotactic Ablative Radiotherapy (SABR) in Non–Small Cell Lung Cancer Measuring More Than 5 cm. J. Thorac. Oncol. 2017, 12, 974–982. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Shao, Z.; Vang, J.; Kaidar-Person, O.; Wang, A.Z. Application of nanotechnology to cancer radiotherapy. Cancer Nanotechnol. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2019, 21, 273. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Zhai, S. The Rational Design and Biological Mechanisms of Nanoradiosensitizers. Nanomaterials 2020, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- Adams, F.H.; Norman, A.; Mello, R.S.; Bass, D. Effect of Radiation and Contrast Media on Chromosomes. Radiology 1977, 124, 823–826. [Google Scholar] [CrossRef]
- Goel, S.; Ni, D.; Cai, W. Harnessing the Power of Nanotechnology for Enhanced Radiation Therapy. ACS Nano 2017, 11, 5233–5237. [Google Scholar] [CrossRef]
- Allal, A.S.; Michel Richter, M.D.; Russo, M.; Rouzaud, M.; Dulguerov, P.; Kurtz, J.M. Dose Variation at Bone/Titanium Interfaces Using Titanium Hollow Screw Osseointegrating Reconstruction Plates. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 215–219. [Google Scholar] [CrossRef]
- Melian, E.; Fatyga, M.; Lam, P.; Steinberg, M.; Reddy, S.P.; Petruzzelli, G.J.; Glasgow, G.P. Effect of metal reconstruction plates on cobalt-60 dose distribution: A predictive formula and clinical implications. Int. J. Radiat. Oncol. 1999, 44, 725–730. [Google Scholar] [CrossRef]
- Crapanzano, R.; Secchi, V.; Villa, I. Co-Adjuvant Nanoparticles for Radiotherapy Treatments of Oncological Diseases. Appl. Sci. 2021, 11, 7073. [Google Scholar] [CrossRef]
- Hubbell, J.H. Radiation Physics, in Encyclopedia of Physical Science and Technology; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Tremi, I.; Spyratou, E.; Souli, M.; Efstathopoulos, E.; Makropoulou, M.; Georgakilas, A.; Sihver, L. Requirements for Designing an Effective Metallic Nanoparticle (NP)-Boosted Radiation Therapy (RT). Cancers 2021, 13, 3185. [Google Scholar] [CrossRef] [PubMed]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2017, 63, 02TR01. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [PubMed]
- L’Annunziata, M.F. Atomic Electron Radiation, in Radioactivity; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.; Jain, S.; Butterworth, K.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Mousavi, M.; Nedaei, H.A.; Khoei, S.; Eynali, S.; Khoshgard, K.; Robatjazi, M.; Rad, R.I. Enhancement of radiosensitivity of melanoma cells by pegylated gold nanoparticles under irradiation of megavoltage electrons. Int. J. Radiat. Biol. 2016, 93, 214–221. [Google Scholar] [CrossRef]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Cell-Specific Radiosensitization by Gold Nanoparticles at Megavoltage Radiation Energies. Int. J. Radiat. Oncol. 2011, 79, 531–539. [Google Scholar] [CrossRef]
- Detappe, A.; Kunjachan, S.; Drané, P.; Kotb, S.; Myronakis, M.; Biancur, D.E.; Ireland, T.; Wagar, M.; Lux, F.; Tillement, O.; et al. Key clinical beam parameters for nanoparticle-mediated radiation dose amplification. Sci. Rep. 2016, 6, 34040. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Schlathölter, T.; Eustache, P.; Porcel, E.; Salado, D.; Stefancikova, L.; Tillement, O.; Lux, F.; Mowat, P.; Biegun, A.K.; Van Goethem, M.J.; et al. Improving proton therapy by metal-containing nanoparticles: Nanoscale insights. Int. J. Nanomed. 2016, 11, 1549. [Google Scholar] [CrossRef]
- Martínez-Rovira, I.; Prezado, Y. Evaluation of the local dose enhancement in the combination of proton therapy and nanoparticles. Med. Phys. 2015, 42, 6703–6710. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef] [PubMed]
- Rieck, K.; Bromma, K.; Sung, W.; Bannister, A.; Schuemann, J.; Chithrani, D.B. Modulation of gold nanoparticle mediated radiation dose enhancement through synchronization of breast tumor cell population. Br. J. Radiol. 2019, 92, 20190283. [Google Scholar] [CrossRef]
- Butterworth, K.; McMahon, S.; Taggart, L.; Prise, K. Radiosensitization by gold nanoparticles: Effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013, 2, 269–279. [Google Scholar]
- Howard, D.; Sebastian, S.; Le, Q.V.-C.; Thierry, B.; Kempson, I. Chemical Mechanisms of Nanoparticle Radiosensitization and Radioprotection: A Review of Structure-Function Relationships Influencing Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 579. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Guerreiro, A.; Chatterton, N.; Crabb, E.M.; Golding, J.P. A comparison of the radiosensitisation ability of 22 different element metal oxide nanoparticles using clinical megavoltage X-rays. Cancer Nanotechnol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Liu, Y.; Wang, S.; Han, X.; Cheng, C. Research progress on nano-sensitizers for enhancing the effects of radiotherapy. Mater. Adv. 2022, 3, 3709–3725. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, H.; Doosti-Irani, A.; Bouraghi, H.; Nikzad, S. Investigation of Gold Nanoparticles Effects in Radiation Therapy of Cancer: A Systematic Review. J. Adv. Med. Biomed. Res. 2022, 30, 388–396. [Google Scholar] [CrossRef]
- Kotb, S.; Detappe, A.; Lux, F.; Appaix, F.; Barbier, E.; Tran, V.-L.; Plissonneau, M.; Gehan, H.; Lefranc, F.; Rodriguez-Lafrasse, C.; et al. Gadolinium-Based Nanoparticles and Radiation Therapy for Multiple Brain Melanoma Metastases: Proof of Concept before Phase I Trial. Theranostics 2016, 6, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: Recent advances and future challenges. J. Nanobiotechnol. 2020, 18, 1–17. [Google Scholar] [CrossRef]
- Mohammadian, M.; Minaei, S.E.; Dezfuli, A.S. Improve the cytotoxic effects of megavoltage radiation treatment by Fe3O4@Cus–PEG nanoparticles as a novel radiosensitizer in colorectal cancer cells. Cancer Nanotechnol. 2022, 13, 1–21. [Google Scholar] [CrossRef]
- Martínez-Esquivias, F.; Gutiérrez-Angulo, M.; Becerra-Ruiz, J.S.; Martinez-Perez, L.A.; de la Cruz-Ahumada, C.J.; Guzmán-Flores, J.M. Bioinformatic Analysis of the Effect of Silver Nanoparticles on Colorectal Cancer Cell Line. BioMed. Res. Int. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Habiba, K.; Aziz, K.; Sanders, K.; Santiago, C.M.; Mahadevan, L.S.K.; Makarov, V.; Weiner, B.R.; Morell, G.; Krishnan, S. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 1–30. [Google Scholar] [CrossRef]
- Cruz-Nova, P.; Ancira-Cortez, A.; Ferro-Flores, G.; Ocampo-García, B.; Gibbens-Bandala, B. Controlled-Release Nanosystems with a Dual Function of Targeted Therapy and Radiotherapy in Colorectal Cancer. Pharmaceutics 2022, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, F.; Peng, Y.; Xie, T.; Wang, Y.; Lan, Y. Current Progress in Cancer Treatment Using Nanomaterials. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Scher, N.; Bonvalot, S.; Le Tourneau, C.; Chajon, E.; Verry, C.; Thariat, J.; Calugaru, V. Review of clinical applications of radiation-enhancing nanoparticles. Biotechnol. Rep. 2020, 28, e00548. [Google Scholar] [CrossRef] [PubMed]
- Kempson, I. Mechanisms of nanoparticle radiosensitization. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1656. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Sangnier, A.P.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Z.; Huang, L.; Luo, S.; Dong, J.; Zhou, F.H.; Zhou, K.; Wang, L.; Kang, L. Photothermal therapy technology of metastatic colorectal cancer. Am. J. Transl. Res. 2020, 12, 3089–3115. [Google Scholar]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Mondal, S.; Nguyen, T.P.; Kim, H.; Phan, T.T.V.; Lee, K.D.; Oh, J. Biocompatible Chitosan Oligosaccharide Modified Gold Nanorods as Highly Effective Photothermal Agents for Ablation of Breast Cancer Cells. Polymers 2018, 10, 232. [Google Scholar] [CrossRef]
- Plan Sangnier, A.; Van de Walle, A.; Aufaure, R.; Fradet, M.; Motte, L.; Guénin, E.; Lalatonne, Y.; Wilhelm, C. Endosomal Confinement of Gold Nanospheres, Nanorods, and Nanoraspberries Governs Their Photothermal Identity and Is Beneficial for Cancer Cell Therapy. Adv. Biosyst. 2020, 4, 1900284. [Google Scholar] [CrossRef]
- Knights, O.; Freear, S.; McLaughlan, J.R. Improving Plasmonic Photothermal Therapy of Lung Cancer Cells with Anti-EGFR Targeted Gold Nanorods. Nanomaterials 2020, 10, 1307. [Google Scholar] [CrossRef]
- Goodrich, G.P.; Bao, L.; Gill-Sharp, K.; Sang, K.L.; Wang, J.; Payne, J.D. Photothermal therapy in a murine colon cancer model using near-infrared absorbing gold nanorods. J. Biomed. Opt. 2010, 15, 018001. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Khalidov, I.; Wang, Y.; Batt, C.A. Targeted near-IR hybrid magnetic nanoparticles for in vivo cancer therapy and imaging. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Ghavami, M.; Shanehsazzadeh, S.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf. B Biointerfaces 2016, 143, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bian, X.; Aliru, M.; Deorukhkar, A.A.; Ekpenyong, O.; Liang, S.; John, J.; Ma, J.; Gao, X.; Schwartz, J.; et al. Hypoxia-targeted gold nanorods for cancer photothermal therapy. Oncotarget 2018, 9, 26556–26571. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.; Norregaard, K.; Jørgensen, J.T.; Oddershede, L.B.; Kjaer, A. Fractionated photothermal therapy in a murine tumor model: Comparison with single dose. Int. J. Nanomed. 2019, 14, 5369–5379. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, L.; Perazzoli, G.; Mesas, C.; Jiménez-Luna, C.; Prados, J.; Rama, A.R.; Melguizo, C. Nanoparticles in Colorectal Cancer Therapy: Latest In Vivo Assays, Clinical Trials, and Patents. AAPS PharmSciTech 2020, 21, 1–15. [Google Scholar] [CrossRef]
- de la Encarnación, C. Multifunctional plasmonic-magnetic nanoparticles for bioimaging and hyperthermia. Adv. Drug Deliv. Rev. 2022, 189, 114484. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef]
- Ayala-Orozco, C.; Urban, C.; Bishnoi, S.; Urban, A.; Charron, H.; Mitchell, T.; Shea, M.; Nanda, S.; Schiff, R.; Halas, N.; et al. Sub-100nm gold nanomatryoshkas improve photo-thermal therapy efficacy in large and highly aggressive triple negative breast tumors. J. Control Release 2014, 191, 90–97. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, H.J.; Kim, S.Y.; Kwon, M.-K.; Kim, S.; Cho, A.; Yun, M.; Shin, J.-S.; Yoo, K.-H. Drug-loaded gold plasmonic nanoparticles for treatment of multidrug resistance in cancer. Biomaterials 2014, 35, 2272–2282. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, L.; Qian, H.; Li, R.; Jiang, X.; Liu, B. Multifunctional Nanocarriers for Cell Imaging, Drug Delivery, and Near-IR Photothermal Therapy. Langmuir 2010, 26, 5428–5434. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Atyabi, F.; Varnamkhasti, B.S.; Hosseinzadeh, R.; Ostad, S.N.; Ghahremani, M.H.; Dinarvand, R. SN38 conjugated hyaluronic acid gold nanoparticles as a novel system against metastatic colon cancer cells. Int. J. Pharm. 2017, 526, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Najafi, M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef] [PubMed]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’Yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Bozdoğan, E.; Chithrani, D. Combining Gold Nanoparticles with Other Radiosensitizing Agents for Unlocking the Full Potential of Cancer Radiotherapy. Pharmaceutics 2021, 13, 442. [Google Scholar] [CrossRef]
- Monaco, H.; Yokomizo, S.; Choi, H.S.; Kashiwagi, S. Quickly evolving near-infrared photoimmunotherapy provides multifaceted approach to modern cancer treatment. VIEW 2021, 3, 20200110. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef]
- Emami, F.; Banstola, A.; Vatanara, A.; Lee, S.; Kim, J.O.; Jeong, J.-H.; Yook, S. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol. Pharm. 2019, 16, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Shieh, M.-J. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- White, S.B.; Kim, D.-H.; Guo, Y.; Li, W.; Yang, Y.; Chen, J.; Gogineni, V.R.; Larson, A.C. Biofunctionalized Hybrid Magnetic Gold Nanoparticles as Catalysts for Photothermal Ablation of Colorectal Liver Metastases. Radiology 2017, 285, 809–819. [Google Scholar] [CrossRef]
- Kirui, D.K.; Rey, D.A.; Batt, C.A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology 2010, 21, 105105. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Kim, B.M.; Joe, A.; Han, H.W.; Chen, X.; Cheng, Z.; Jang, E.S. NIR-light-induced surface-enhanced Raman scattering for detection and photothermal/photodynamic therapy of cancer cells using methylene blue-embedded gold nanorod@SiO2 nanocomposites. Biomaterials 2014, 35, 3309–3318. [Google Scholar] [CrossRef]
- Wang, F.; Liu, P.; Sun, L.; Li, C.; Petrenko, V.A.; Liu, A. Bio-mimetic Nanostructure Self-assembled from Au@Ag Heterogeneous Nanorods and Phage Fusion Proteins for Targeted Tumor Optical Detection and Photothermal Therapy. Sci. Rep. 2014, 4, 6808. [Google Scholar] [CrossRef]
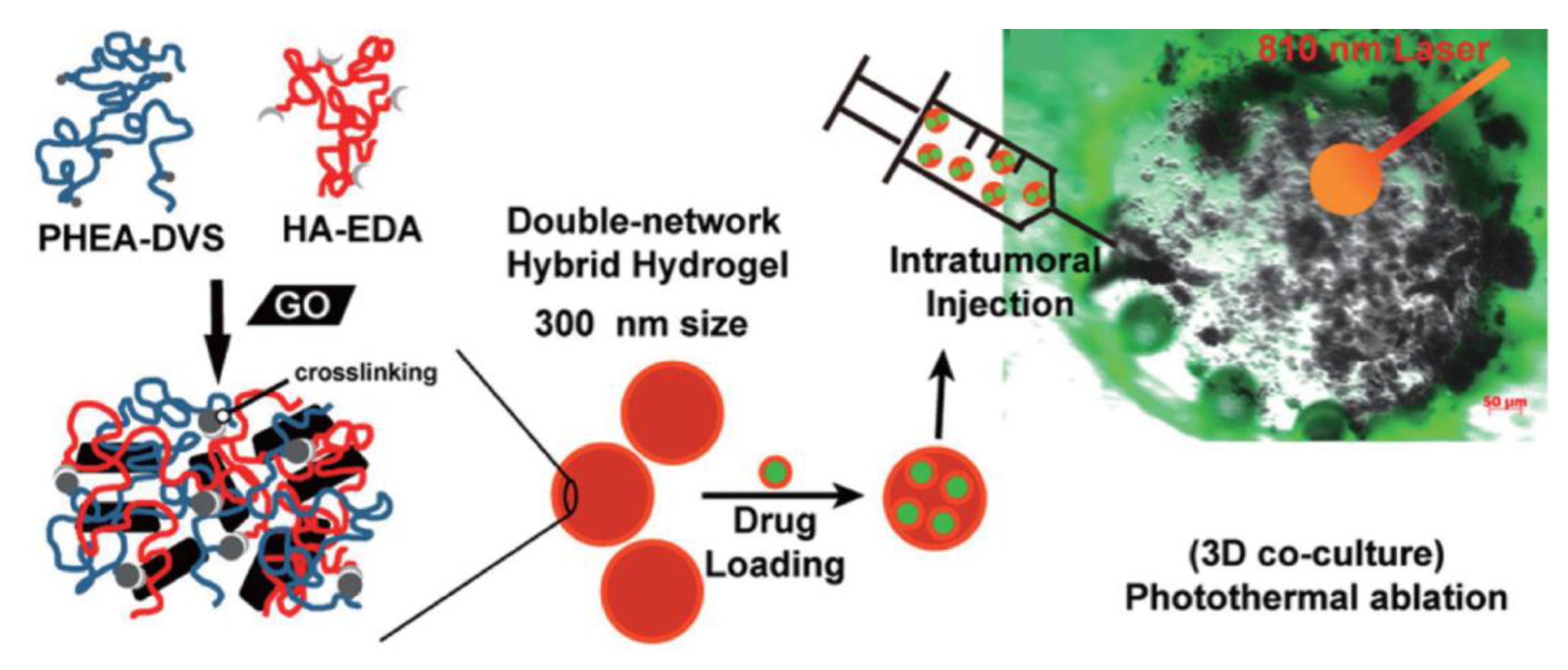
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef]
- Einafshar, E.; Asl, A.H.; Nia, A.H.; Mohammadi, M.; Malekzadeh, A.; Ramezani, M. New cyclodextrin-based nanocarriers for drug delivery and phototherapy using an irinotecan metabolite. Carbohydr. Polym. 2018, 194, 103–110. [Google Scholar] [CrossRef]
- Levi-Polyachenko, N.H.; Merkel, E.J.; Jones, B.T.; Carroll, D.L.; Stewart, I.J.H. Rapid Photothermal Intracellular Drug Delivery Using Multiwalled Carbon Nanotubes. Mol. Pharm. 2009, 6, 1092–1099. [Google Scholar] [CrossRef]
- Graham, E.G.; MacNeill, C.M.; Levi-Polyachenko, N.H. Quantifying folic acid-functionalized multi-walled carbon nanotubes bound to colorectal cancer cells for improved photothermal ablation. J. Nanoparticle Res. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Tan, A.; Madani, S.Y.; Rajadas, J.; Pastorin, G.; Seifalian, A.M. Synergistic photothermal ablative effects of functionalizing carbon nanotubes with a POSS-PCU nanocomposite polymer. J. Nanobiotechnol. 2012, 10, 34. [Google Scholar] [CrossRef]
- Koo, J.S.; Lee, S.Y.; Nam, S.; Azad, O.K.; Kim, M.; Kim, K.; Chae, B.-J.; Kang, W.-S.; Cho, H.-J. Preparation of cupric sulfate-based self-emulsifiable nanocomposites and their application to the photothermal therapy of colon adenocarcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 2471–2477. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper Selenide Nanocrystals for Photothermal Therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef]
- Obiweluozor, O.F.; Emechebe, A.G.; Tiwari, A.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Short duration cancer treatment: Inspired by a fast bio-resorbable smart nano-fiber device containing NIR lethal polydopamine nanospheres for effective chemo–photothermal cancer therapy. Int. J. Nanomed. 2018, 13, 6375–6390. [Google Scholar] [CrossRef]
- Kelkar, S.S.; McCabe-Lankford, E.; Albright, R.; Harrington, P.; Levi-Polyachenko, N.H. Dual wavelength stimulation of polymeric nanoparticles for photothermal therapy. Lasers Surg. Med. 2016, 48, 893–902. [Google Scholar] [CrossRef]
- MacNeill, C.M.; Coffin, R.C.; Carroll, D.L.; Levi-Polyachenko, N.H. Low Band Gap Donor-Acceptor Conjugated Polymer Nanoparticles and their NIR-mediated Thermal Ablation of Cancer Cells. Macromol. Biosci. 2012, 13, 28–34. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef]
- Darrigues, E.; Nima, Z.A.; Griffin, R.J.; Anderson, J.M.; Biris, A.S.; Rodriguez, A. 3D cultures for modeling nanomaterial-based photothermal therapy. Nanoscale Horiz. 2019, 5, 400–430. [Google Scholar] [CrossRef] [PubMed]
- Brüningk, S.C.; Rivens, I.; Box, C.; Oelfke, U.; ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Miranda, O.; Johnston, P.A.; Sant, S. Three dimensional engineered models to study hypoxia biology in breast cancer. Cancer Lett. 2020, 490, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2018, 116, 206–226. [Google Scholar] [CrossRef]
- Reidy, E.; Leonard, N.; Treacy, O.; Ryan, A. A 3D View of Colorectal Cancer Models in Predicting Therapeutic Responses and Resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Simelane, N.W.N.; Abrahamse, H. Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 12405. [Google Scholar] [CrossRef]
- Kiwaki, T.; Kataoka, H. Patient-Derived Organoids of Colorectal Cancer: A Useful Tool for Personalized Medicine. J. Pers. Med. 2022, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Pasch, C.A.; Favreau, P.F.; Yueh, A.E.; Babiarz, C.P.; Gillette, A.A.; Sharick, J.T.; Karim, M.R.; Nickel, K.P.; DeZeeuw, A.K.; Sprackling, C.M.; et al. Patient-Derived Cancer Organoid Cultures to Predict Sensitivity to Chemotherapy and Radiation. Clin. Cancer Res. 2019, 25, 5376–5387. [Google Scholar] [CrossRef]
- Hau, H.; Khanal, D.; Rogers, L.; Suchowerska, N.; Kumar, R.; Sridhar, S.; McKenzie, D.; Chrzanowski, W. Dose enhancement and cytotoxicity of gold nanoparticles in colon cancer cells when irradiated with kilo- and mega-voltage radiation. Bioeng. Transl. Med. 2016, 1, 94–102. [Google Scholar] [CrossRef] [PubMed]
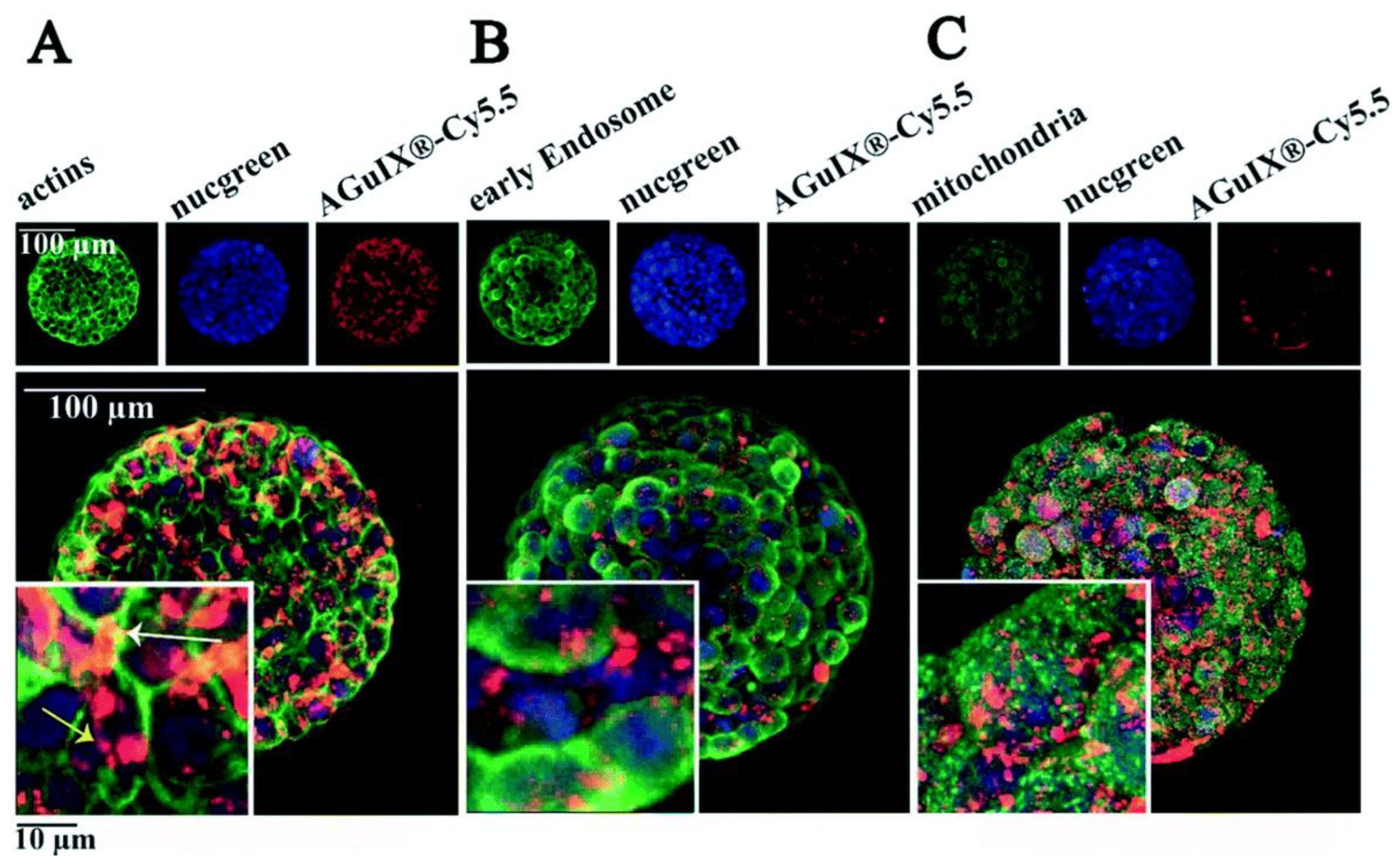
- Goodarzi, S.; Prunet, A.; Rossetti, F.; Bort, G.; Tillement, O.; Porcel, E.; Lacombe, S.; Wu, T.-D.; Guerquin-Kern, J.-L.; Delanoë-Ayari, H.; et al. Quantifying nanotherapeutic penetration using a hydrogel-based microsystem as a new 3D in vitro platform. Lab Chip 2021, 21, 2495–2510. [Google Scholar] [CrossRef]
- McCarthy, B.; Cudykier, A.; Singh, R.; Levi-Polyachenko, N.; Soker, S. Semiconducting polymer nanoparticles for photothermal ablation of colorectal cancer organoids. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Pombo, I.; Fernandes, A.; Baptista, P. Hyperthermia Induced by Gold Nanoparticles and Visible Light Photothermy Combined with Chemotherapy to Tackle Doxorubicin Sensitive and Resistant Colorectal Tumor 3D Spheroids. Int. J. Mol. Sci. 2020, 21, 8017. [Google Scholar] [CrossRef]
- Parchur, A.K.; Sharma, G.; Jagtap, J.M.; Gogineni, V.R.; LaViolette, P.S.; Flister, M.J.; White, S.B.; Joshi, A. Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano 2018, 12, 6597–6611. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Khateri, M.; Beik, J.; Ghoreishi, F.S.; Dezfuli, A.S.; Ghaznavi, H.; Shakeri-Zadeh, A. Enhancement of chemoradiation by co-incorporation of gold nanoparticles and cisplatin into alginate hydrogel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2658–2663. [Google Scholar] [CrossRef]
- Yun, W.S.; Shim, M.K.; Lim, S.; Song, S.; Kim, J.; Yang, S.; Hwang, H.S.; Kim, M.R.; Yoon, H.Y.; Lim, D.-K.; et al. Mesenchymal Stem Cell-Mediated Deep Tumor Delivery of Gold Nanorod for Photothermal Therapy. Nanomaterials 2022, 12, 3410. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, Y.; Mao, J.; Lei, X.; Yang, Q.; Cui, C. Dendrimer-modified gold nanorods as a platform for combinational gene therapy and photothermal therapy of tumors. J. Exp. Clin. Cancer Res. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Licciardi, M.; Varvarà, P.; Tranchina, L.; Puleio, R.; Cicero, L.; Cassata, G.; Giammona, G. In vivo efficacy of verteporfin loaded gold nanorods for combined photothermal/photodynamic colon cancer therapy. Int. J. Pharm. 2022, 625, 25. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, C.; Crisponi, G.; Nurchi, V.M.; Saba, L.; Cappai, R.; Cau, F.; Faa, G.; Van Eyken, P.; Scartozzi, M.; Floris, G.; et al. Gold Nanoparticles: A New Golden Era in Oncology? Pharmaceuticals 2020, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Moros, E.G.; Corry, P.M.; Orton, C.G. Thermoradiotherapy is underutilized for the treatment of cancer. Med. Phys. 2006, 34, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Charest, G.; Tippayamontri, T.; Shi, M.; Wehbe, M.; Anantha, M.; Bally, M.; Sanche, L. Concomitant Chemoradiation Therapy with Gold Nanoparticles and Platinum Drugs Co-Encapsulated in Liposomes. Int. J. Mol. Sci. 2020, 21, 4848. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.; Olmedo, I.; Clos, A.; Ramanujam, V.S.; Urayama, A.; Vergara, L.; Kogan, M.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, X.; Xu, J.; Sun, R.; Xia, H.; Ding, J.; Chin, Y.E.; Chai, Z.; Shi, H.; Gao, M. Furin Enzyme and pH Synergistically Triggered Aggregation of Gold Nanoparticles for Activated Photoacoustic Imaging and Photothermal Therapy of Tumors. Anal. Chem. 2021, 93, 9277–9285. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Nicolás-Boluda, A.; Pinto, A.; Balfourier, A.; Carn, F.; Silva, A.K.A.; Pocard, M.; Gazeau, F. Tumor-Selective Immune-Active Mild Hyperthermia Associated with Chemotherapy in Colon Peritoneal Metastasis by Photoactivation of Fluorouracil–Gold Nanoparticle Complexes. ACS Nano 2021, 15, 3330–3348. [Google Scholar] [CrossRef]
- Qin, X.; Yang, C.; Xu, H.; Zhang, R.; Zhang, D.; Tu, J.; Guo, Y.; Niu, B.; Kong, L.; Zhang, Z. Cell-Derived Biogenetic Gold Nanoparticles for Sensitizing Radiotherapy and Boosting Immune Response against Cancer. Small 2021, 17, e2103984. [Google Scholar] [CrossRef] [PubMed]
- Miladi, I.; Alric, C.; Dufort, S.; Mowat, P.; Dutour, A.; Mandon, C.; Laurent, G.; Bräuer-Krisch, E.; Herath, N.; Coll, J.-L.; et al. The In Vivo Radiosensitizing Effect of Gold Nanoparticles Based MRI Contrast Agents. Small 2014, 10, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Aldahhan, R.; Almohazey, D.; Alam Khan, F. Emerging trends in the application of gold nanoformulations in colon cancer diagnosis and treatment. Semin. Cancer Biol. 2022, 86, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Paquette, B.; Thippayamontri, T.; Gendron, L.; Guérin, B.; Sanche, L. Increased radiosensitivity of colorectal tumors with intra-tumoral injection of low dose of gold nanoparticles. Int. J. Nanomed. 2016, 11, 5323–5333. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, K.; Antaris, A.L.; Ma, X.; Yang, M.; Ramakrishnan, S.; Liu, G.; Lu, A.; Dai, H.; Tian, M.; et al. Hybrid anisotropic nanostructures for dual-modal cancer imaging and image-guided chemo-thermo therapies. Biomaterials 2016, 103, 265–277. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Wu, H.; Li, J.; Xie, P.; Xu, F.; Zhou, L.; Zhao, J.; Chen, H. Thermosensitive and tum or microenvironment activated nanotheranostics for the chemodynamic/photothermal therapy of colorectal tumor. J. Colloid Interface Sci. 2021, 612, 223–234. [Google Scholar] [CrossRef]
- Diagaradjane, P.; Shetty, A.; Wang, J.C.; Elliott, A.M.; Schwartz, J.; Shentu, S.; Park, H.C.; Deorukhkar, A.; Stafford, R.J.; Cho, S.H.; et al. Modulation of in Vivo Tumor Radiation Response via Gold Nanoshell-Mediated Vascular-Focused Hyperthermia: Characterizing an Integrated Antihypoxic and Localized Vascular Disrupting Targeting Strategy. Nano Lett. 2008, 8, 1492–1500. [Google Scholar] [CrossRef]


| Nanostructure | Preclinical Model | Application | PTT parameters | Ref. |
|---|---|---|---|---|
| PEGylated gold nanorods | CT26 tumor xenografts in vivo | PTT | Percutaneous irradiation with optical fiber; 808 nm; 3.5 W; 3 min | [121] |
| Immunotargeted gold-iron oxide NPs | SW1222 and HT29 tumor xenografts in vivo | PTT, MRI imaging | Laser; 808 nm; 5 W/cm2; 20 min | [122] |
| Gold-coated SPIONs functionalized with thiol derivatives | HT29 cells in vitro | PTT, MRI imaging | LED; 820 nm; 0.7 W/cm2; 2–8 min | [123] |
| Gold nanorods conjugated with carbonic anhydrase IX | HT29 cells in vitro and tumor xenografts in vivo | PTT | Laser; 760 nm; 12 W/cm2; 2–3 min | [124] |
| Silica-gold nanoshells | CT26 tumor xenografts in vivo | Fractionated PTT | Laser; 807 nm; 1.2 W/cm2, 5 min | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, S.C.; Sanderson, D.; Caspani, S.; Magalhães, R.; Cortés-Llanos, B.; Granja, A.; Reis, S.; Belo, J.H.; Azevedo, J.; Gómez-Gaviro, M.V.; et al. New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo- and Radiotherapy. Cancers 2023, 15, 383. https://doi.org/10.3390/cancers15020383
Freitas SC, Sanderson D, Caspani S, Magalhães R, Cortés-Llanos B, Granja A, Reis S, Belo JH, Azevedo J, Gómez-Gaviro MV, et al. New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo- and Radiotherapy. Cancers. 2023; 15(2):383. https://doi.org/10.3390/cancers15020383
Chicago/Turabian StyleFreitas, Sara C., Daniel Sanderson, Sofia Caspani, Ricardo Magalhães, Belén Cortés-Llanos, Andreia Granja, Salette Reis, João Horta Belo, José Azevedo, Maria Victoria Gómez-Gaviro, and et al. 2023. "New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo- and Radiotherapy" Cancers 15, no. 2: 383. https://doi.org/10.3390/cancers15020383
APA StyleFreitas, S. C., Sanderson, D., Caspani, S., Magalhães, R., Cortés-Llanos, B., Granja, A., Reis, S., Belo, J. H., Azevedo, J., Gómez-Gaviro, M. V., & Sousa, C. T. d. (2023). New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo- and Radiotherapy. Cancers, 15(2), 383. https://doi.org/10.3390/cancers15020383








