Simple Summary
In the tumor microenvironment, cancer-associated fibroblasts (CAFs) have multiple tumor-promoting functions in drug resistance, regulation of the niche of cancer stem cells and formation of the immunosuppressive network. Multiple mechanisms are involved, including production of growth factors, cytokines, and chemokines, as well as extracellular matrix remodeling. While cancer treatment generally targets cancer cells, elucidation of the tumor microenvironment has allowed for successful targeting of cells other than cancer cells involved in the construction of the tumor microenvironment. Cancer cells are prone to develop drug resistance, whereas resistance to treatments targeting the tumor microenvironment may be less likely to develop. Therefore, various treatment strategies against CAFs have attracted attention as possible novel cancer treatments.
Abstract
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer death worldwide. The most common lung cancer is non-small cell lung cancer (NSCLC), with an overall 5-year survival rate of around 20% because NSCLC is a metastatic disease. A better understanding of the mechanism underlying lung cancer metastasis is therefore urgently needed. The tumor microenvironment involves different types of stromal cells and functions as key components in the progression of NSCLC. Through epithelial–mesenchymal transition (EMT), in which epithelial cells lose their polarity and acquire mesenchymal potential, cancer cells acquire metastatic abilities, as well as cancer stem-cell-like potential. We previously reported that cancer-associated fibroblasts (CAFs) interact with lung cancer cells to allow for the acquisition of malignancy and treatment resistance by paracrine loops via EMT signals in the tumor microenvironment. Furthermore, CAFs regulate the cytotoxic activity of immune cells via various cytokines and chemokines, creating a microenvironment of immune tolerance. Regulation of CAFs can therefore affect immune responses. Recent research has shown several roles of CAFs in NSCLC tumorigenesis, owing to their heterogeneity, so molecular markers of CAFs should be elucidated to better classify tumor-promoting subtypes and facilitate the establishment of CAF-specific targeted therapies. CAF-targeted cancer treatments may suppress EMT and regulate the niche of cancer stem cells and the immunosuppressive network and thus may prove useful for NSCLC treatment through multiple mechanisms.
1. Introduction
Survival rates for non-small cell lung cancer (NSCLC) patients after diagnosis have improved substantially in this century, owing to the implementation of chemotherapeutic regimens, novel molecularly targeted therapies, and immunotherapies; however, mortality rates remain high [1]. The main reason for this problem is that NSCLC is a highly metastatic disease, and curative systemic therapies remain lacking. Furthermore, the occurrence of drug resistance represents a major obstacle to successful treatment for NSCLC patients that requires urgent attention [2,3].
The tumor microenvironment comprises different types of stromal cells, including vascular lymphatic networks, fibroblastic cells, inflammatory immune cells, mesenchymal stem cells (MSCs), and extracellular matrix (ECM). These represent key components in tumor progression, and stromal components have been speculated to be functionally organized to promote cancer cell survival, promoting aggressive neoplastic phenotypes and the acquisition of drug resistance [4,5]. A specific subset of stromal cells, termed cancer-associated fibroblasts (CAFs), constitutes a major stromal component [6]. CAFs were initially described as myofibroblasts that are distinct from normal fibroblasts because of their expression of α-smooth muscle actin (α-SMA) and are also shown to synthesize important amounts of collagen and other ECM components [7]. Recently, CAFs have been speculated to have important functions in epithelial solid tumor biology, such as neoplastic progression, tumor growth, and metastasis, via secretion of factors that promote tumorigenesis by stimulating angiogenesis, cancer cell proliferation, and invasion, in addition to recruiting macrophages and suppressing T-cell antitumor immunity [6,8]. CAFs are genetically stable and influence tumor progression and sensitivity to antitumor therapy through the secretion of ECM, cytokines, and growth factors. Recently, many reports have shown that CAFs can modulate the immune system via paracrine signaling, juxtacrine interactions, and ECM remodeling [9,10,11]. In NSCLC, CAFs have been implicated in enhanced stemness; migration; tumor growth; and resistance to chemotherapy, radiotherapy, tyrosine kinase inhibitors (TKIs), and immunotherapy [12,13,14,15]. Therapeutic targeting of CAFs in the NSCLC tumor microenvironment thus represents an intriguing possibility for improving outcomes in NSCLC patients.
Before antitumor roles for CAFs can be proposed, markers to allow for the definition of CAF subsets need to be identified [16,17]. Two commonly used molecular markers are α-SMA and fibroblast activation protein (FAP); however, these markers do not allow for precise detection of CAFs. CAFs are reported to account for 50–70% of all cells in the tumor microenvironment of breast cancer [18]. While they are also most prominent in the lung cancer tumor microenvironment [19], it is unclear what percentage of stromal cells consists of CAFs because of a lack of specific markers. In addition, not all CAFs demonstrate these features. Several studies have presented details showing the wide heterogeneity among CAFs, probably due to multiple types of originating cells of origin, suggesting that specific CAF populations could be exploited as therapeutic targets [20,21,22].
CAFs are negative for epithelial, endothelial, and leukocyte markers and show an elongated morphology and a lack of mutations in cancer cells. Although CAFs are known as a type of activated fibroblast in the tumor microenvironment, they remain poorly defined due to their heterogeneity and their lack of highly specific markers. This review provides an overview of the current knowledge base regarding the roles of CAFs in NSCLC and the implications of these findings for NSCLC treatment.
2. CAF Biomarkers and Heterogeneity in NSCLC
2.1. Biomarkers for CAFs in NSCLC
Fibroblasts are easily digested and cultured in plastic flasks, whereas other types of cells are not [23]. We defined fibroblasts obtained from NSCLC tumors as CAFs and those from normal lung tissue as lung normal fibroblasts (LNFs) [24]. A passage number from 1 to 6 is suitable for cultured CAFs in experiments to keep their characteristics. Primary CAFs should be negative for epithelial cell adhesion molecule (EpCAM), cluster of differentiation (CD) 31, and CD45. In practice, traditional CAF biomarkers are typically combined with lineage exclusion to identify CAFs [9]. Vimentin is a marker of quiescent CAFs, whereas α-SMA, fibroblast-specific protein 1 (FSP-1), FAP, platelet-derived growth factor (PDGF) receptor (PDGFR)-α, PDGFR-β, tenascin-C, periostin, podoplanin, CD90 (known as Thy-1), integrin β1, caveolin-1, adipocyte enhancer-binding protein, and endoglin are markers of activated CAFs [7,25,26,27] (Table 1).

Table 1.
List of biomarkers for CAFs in non-small cell lung cancer.
Each protein has a biological function. Although α-SMA expression is the hallmark of mature myofibroblasts and implicated in contraction and remodeling of the extracellular matrix (ECM), its role in fibroblast function remains poorly understood [28]. FSP1 belongs to the S100 superfamily, also identified as S100A4, and has been implicated in microtubule dynamics, cytoskeletal membrane interactions, calcium signal transduction, and cell cycle regulation, as well as cell growth and differentiation [29,30]. It is also associated with tissue fibrosis; therefore, it is a useful marker for fibroblasts in pulmonary fibrosis [31]. While the initial description of a cell surface antigen notes its expression by reactive stromal fibroblasts associated with epithelial cancer, it was found to be not expressed by normal fibroblasts and therefore termed “fibroblast activation” protein (FAP). This is a type II transmembrane serine protease that shows enzymatic activity, and research conducted with a catalytically mutant FAP has suggested that FAP can have a functional impact independent of its enzymatic activity [32]. As described above, each expressed protein has a complex biological function. Furthermore, each marker defines a different cell population with partial overlapping, although populations also show distinct expression profiles. As a result, there is no definitive single marker that can be used to identify CAFs.
CAFs have different markers and functions according to their heterogeneity [33]. Irvine et al. performed a meta-analysis of all published markers of CAFs in NSCLC to characterize their heterogeneity [34]. They found that five proteins, namely podoplanin, carbonic anhydrase IX, α-SMA, periostin, and FAP, were suitable for meta-analysis and showed that CAF expression of podoplanin or α-SMA was consistently associated with poor prognosis in NSCLC patients. They also concluded that studies linking CAF protein markers to the cellular processes crucial to CAF function are critical to understanding the biology of CAFs.
2.2. Heterogeneity of CAFs in NSCLC
Evidence increasingly suggests wide heterogeneity among CAFs due to varying stages of differentiation from cells that have diverse origins such as tissue-resident fibroblasts, endothelial cells, pericytes, adipocytes, and bone-marrow-derived MSCs, as well as epithelial cells and cancer cells [35,36,37]. Recent single-cell RNA sequencing (scRNA-seq) studies of NSCLC have suggested that CAFs represent a collection of cells with diverse molecular features [38,39,40,41,42]. Studies using scRNA-seq have demonstrated various molecular phenotypes among CAFs and have led to the development of new strategies for investigating CAF biology (Table 2). Lambrechts et al. analyzed stromal cells derived from resected NSCLC tumor tissues and non-tumor lung tissues via scRNA-seq, identifying seven molecular subpopulations of fibroblasts [40]. They proposed limited marker genes for each subpopulation, resulting in the identification of five types of fibroblasts in cancer tissues [40]. Whereas one of these five clusters showed an epithelial–mesenchymal transition (EMT) signal in line with the expression of transforming growth factor (TGF)-β-associated genes, one of the other phenotypes expressed ACTA2 (the gene for α-SMA) with high expression of other genes involved in myogenesis and angiogenesis. Two of the five clusters were highly similar, with lower myogenesis and signature high mechanistic target of rapamycin (mTOR) expression, but expression of glycolysis-related genes, as well as the location in the tumor differed between them. Hu et al. identified three functional subtypes in NSCLC according to hepatocyte growth factor (HGF) and fibroblast growth factor (FGF) 7 expression using a living biobank of CAFs from NSCLC patients [43]. While subtype I and II CAFs have high HGF and FGF7 expressions and protect cancer cells by activating tyrosine kinase receptor-mediated signaling, subtype III CAFs are associated with better clinical response and immune cell migration via the production of chemoattractants for immune cells with enhanced migration of tumor-infiltrating lymphocytes. These functional differences among CAFs are regulated by TGF-β signaling, which suppresses HGF and FGF7 expression. Kim et al. identified subpopulations of CAFs isolated from human lung adenocarcinomas via scRNA-seq cell trajectory analysis and revealed two branch points, allowing the definition of the following subpopulations of lung CAFs: immunosuppressive, neoantigen-presenting, myofibroblastic, and proliferative CAFs [44]. They also showed that expression of karyopherin subunit alpha 2 on CAFs, as a protein involved in the nucleocytoplasmic transport pathway for a variety of tumor-associated proteins and representing one of the neoantigen-presenting CAF-specific markers, influenced tumorigenesis and metastasis of lung cancer cells in a murine model, suggesting that CAF subtype markers may offer therapeutic targets in the tumor microenvironment. Su and colleagues found that CD10 + GPR77 + CAFs promote tumor formation and chemoresistance by providing a niche for cancer stem cells through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [45].

Table 2.
Phenotypic and functional heterogeneity of CAFs in non-small cell lung cancer.
CAFs should therefore be investigated based on the notion that some CAF subtypes promote tumor growth and others suppress tumor growth in order to clarify novel populations of CAFs relevant as therapeutic targets in NSCLC.
3. CAF-Related Signaling Pathways in NSCLC
3.1. Signaling Pathways between CAFs and NSCLC
Cancer initiates fibroblast transition with acquisition of CAF phenotypes via cancer-derived growth factors, as well as cytokines that regulate the TGF-β and NF-κB signaling pathways [46]. We have previously shown that TGF-β secreted from NSCLC cancer cells activates fibroblasts in the tumor microenvironment [15]. CAFs secrete growth factors, including TGF-β, FGF2/7, PDGF, and HGF, as well as vascular endothelial growth factor (VEGF), which promote cancer cell proliferation [47]. Resident fibroblasts are also activated and change to proinflammatory CAFs during the early preneoplastic stages of tumorigenesis [6]. Activated CAFs produce higher amounts of stromal cell-derived factor 1 (SDF-1) than LNFs, and SDF-1 facilitates cancer cell proliferation and chemoresistance via the CXC chemokine receptor 4 (CXCR4)-mediated signaling pathway, which involves NF-κB and B-cell lymphoma-extra large [48]. Mitogen-activated protein kinase, phosphatidylinositol 3-kinase (PI3K)/mTOR, and Wnt/β-catenin signaling are also activated in cancer cells in response to CAF-derived growth factors and cytokines. The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway are activated by CAF-derived interleukin (IL)-6, IL-10, IL-11, and IL-22 [10]. We previously reported that NSCLC cells underwent EMT and acquired stem-cell-like properties when cocultured with CAFs isolated from surgical exploration [24]. We also found that IL-6 from CAFs enhanced EMT and chemoresistance in NSCLC cells, suggesting a role of IL-6 in the maintenance of a paracrine loop that functions as part of the communication between CAFs and NSCLC cells [15,49]. Similarly, CAF-derived C-X-C motif chemokine ligand (CXCL)1, IL-6, and cyclooxygenase-2, known targets of the NF-κB transcription factor, reportedly correlate with tumor-promoting inflammation and tumor invasiveness [50,51]. Iwai et al. reported altered gene expression in NSCLC CAFs compared to LNFs using capped analysis of gene expression to comprehensively analyze promoter activity in CAFs [52]. Among 390 genes highly expressed in NSCLC CAFs, they identified collagen type XI α1, integrin α11, and collagen type I α1 as CAF-specific genes that promote CAFs migration toward collagen type I and fibronectin via extracellular signal-regulated kinase 1/2. Thus, several different signaling pathways in CAF-mediated cancer progression have been extensively explored to determine their roles in the tumor microenvironment (Figure 1). All these signaling pathways in CAFs have potential as targets for blocking crosstalk between CAFs and cancer cells.
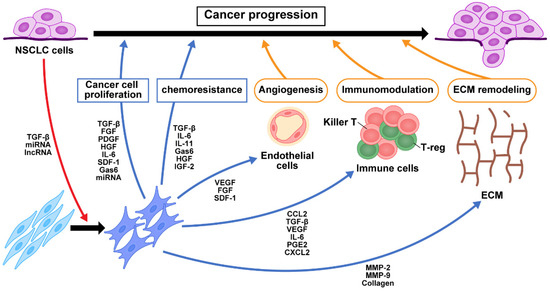
Figure 1.
Crosstalk of signaling pathways among CAFs, NSCLC cells, and immune cells. TGF-β and exosomes secreted from NSCLC cancer cells activate fibroblasts into CAFs in the tumor microenvironment. The production of growth factors, cytokines, chemokines, and exosomes from CAFs contribute to NSCLC cell proliferation, chemoresistance, angiogenesis, immunomodulation, and ECM remodeling. These in turn change the tumor microenvironment and contribute to NSCLC progression. NFs, normal fibroblasts; CAFs, cancer-associated fibroblasts; NSCLC, non-small cell lung cancer; miRNA, microRNA; lncRNA, long non-coding RNA; TGF-β, transforming growth factor-β; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; HGF, hepatocyte growth factor; IL-6, interleukin-6; SDF-1, stromal cell-derived factor-1; Gas6, growth arrest-specific 6; IL-11, interleukin-11; IGF2, insulin-like growth factor 2; VEGF, vascular endothelial growth factor; CCL2, C-C motif chemokine 2; PGE2, prostaglandin E2; CXCL2, chemokine (C-X-C motif) ligand 2; MMP, matrix metalloproteinase; ECM, extracellular matrix.
3.2. Role of CAFs in Resistance to Antitumor Therapy
We previously demonstrated that EMT resulted in increased malignant potential and reduced sensitivity to chemotherapy in NSCLC cells [53]. Furthermore, chronic exposure to anticancer drugs or radiation resulted in cells forming therapy-resistant sublines that underwent EMT via interactions between NSCLC cells and CAFs through the TGF-β, and IL-6 pathways [15]. Exposure to anticancer drugs enhances IL-11 secretion by CAFs, which promotes chemoresistance of cancer cells through the STAT3 signaling pathway [51,54]. We also demonstrated that CAF expression of growth arrest-specific 6 (Gas6), a natural ligand of tumor-associated macrophage (TAM) receptors with high affinity for the receptor tyrosine kinase Axl, increases during chemotherapy and promotes proliferation and migration of NSCLC cells [55]. Tumoral Axl activation induces EMT and promotes cell survival and chemoresistance [56]. In addition, CAFs enhanced chemoresistance by repression of caspase-3 and caspase-8 through the activation of the annexin A3/JNK pathway [57]. CAFs also induce acquired chemoresistance through the insulin-like growth factor (IGF) 2/IGF receptor (IGFR)-1 paracrine pathway, which activates IGF2/AKT/Sox2/ATP-binding cassette B1 signaling and upregulation of P-glycoprotein expression in NSCLC cells [58]. Such data suggest that CAFs, in concert with tumor cells and other components of the tumor microenvironment, abet resistance to treatment [59].
3.3. Role of CAFs in Oncogene Addicted NSCLC
Numerous driver mutations have been identified in NSCLC, and oncogene addiction provides rationale for molecular targeted therapy [60]. Pellinen et al. recently explored associations of EGFR mutations and CAF subtypes using multiplex fluorescence immunohistochemistry; their findings indicated that gene alterations may affect the properties of CAFs in the tumor microenvironment [61]. That microenvironment provides sustained resistance to molecular targeted therapy. CAFs have a critical role in the resistance of lung cancer to epidermal growth factor receptor (EGFR) TKIs through the induction of EMT via CAF-mediated signaling pathways [62,63]. Yoshida et al. also found that lung adenocarcinoma cell lines became more resistant to EGFR TKIs when cocultured with CAFs expressing podoplanin, suggesting that podoplanin-positive CAFs may be useful for predicting response to EGFR TKIs [14]. CAF-released IL-6 mediates NSCLC acquired resistance to EGFR TKIs through the JAK1/STAT3 pathway [64]. In addition, MET activation is an important mechanism for acquisition of resistance to TKIs [65]. Treatment of EGFR- or MET-addicted NSCLC cancer cells was shown to cause a metabolic shift toward increased glycolysis and lactate production, which develop chemoresistance in NSCLC cells [66]. Another study demonstrated that CAFs increase the expression and phosphorylation of annexin A2 by secretion of HGF and IGF-1, which regulate EMT and EGFR TKI resistance in a paracrine manner [63]. Together, these findings suggest that cotreatment targeting CAFs may further improve the antitumor efficacy of molecular targeted therapy.
3.4. Role of Extracellular Vesicles in Communication between CAFs and NSCLC
Exosomes carry and transfer a variety of cargo, including small non-coding RNAs, also known as microRNAs (miRNAs), and long non-coding RNAs (lncRNAs), which have essential roles in cellular communication. Shen et al. compared miRNA expression profiles between CAFs from NSCLC and LNFs from matched healthy lungs and showed downregulation of miR-1 and miR-206 and upregulation of miR-31 in CAFs [67]. CAF-derived miRNAs also contribute to the transformation of LNFs into CAFs [68]. Furthermore, miR-210 in the exosome secreted by CAFs was taken up by NSCLC cells and promoted EMT by targeting upstream frameshift 1, a key factor in a variety of RNA decay pathways, and activating the phosphatase and tensin homolog (PTEN)/PI3K pathway in cancer cells, thereby promoting NSCLC migration and invasion [69]. CAF-derived exosomes also exhibited miR-20a upregulation and promoted NSCLC cell proliferation and chemoresistance via PTEN downregulation following activation of the PI3K/AKT pathway [70]. Cancer cell-derived TGF-β1 activates miR-21 expression in LNFs and induces differentiation to CAFs, which promote the proliferation of cancer cells through the secretion of calumenin [71]. CAF-specific miR-196a promotes NSCLC progression via C-C motif chemokine ligand 2 (CCL2) secretion by directly targeting ANXA1 (the gene for annexin-A1) which has anti-inflammatory properties [72]. LncRNAs also participate in activation of LNFs to CAFs, whereas activated CAFs can change gene expression and secretion characteristics of NSCLC cells through lncRNAs [73]. Liu et al. screened fibroblast-specific lncRNAs using RAN-seq data and identified LINC01614 promoting the secretion of IL-6 from cancer cells that upregulates LINC01614 in CAFs, constituting a feedforward loop between CAFs and NSCLC cells [74].
Together, the dysregulation of exosomal miRNA and lncRNAs is involved in the dynamic crosstalk between CAFs and NSCLC cells.
4. Role of CAFs in the Immune Environment in NSCLC
4.1. Impact of CAFs on Immunosuppressive Activity in NSCLC
CAFs represent a major source of immunosuppressive activity in the tumor microenvironment [75]. Immune regulation by fibroblasts originally attracted attention in other fields of research, particularly the field of autoimmune diseases [27]. CAFs have also been shown to exert strong immunomodulatory function in the tumor microenvironment [76]. Nazareth et al. established culture of stromal cells from primary NSCLCs and revealed that CAFs provide multiple complex regulatory signals, which suppress tumor-associated T-cell functions [77].
Fibroblasts recruit bone-marrow-derived monocytes and dendritic cell precursors via secretion of the CCL2/monocyte chemoattractant protein 1 [78,79]. Xiang et al. showed that lung squamous cell carcinoma patient-derived CAFs promoted recruitment of C-C chemokine receptor 2 (CCR2) + monocytes via CCL2 [80]. Interactions between CCL2/CCR2 recruit immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and metastasis-promoting monocytes, resulting in the suppression of autologous CD8+ T-cell proliferation and interferon (IFN)-γ production. Inhibition of CAF function reduced the infiltration of immune suppressor cells into tumor stroma. CAFs overexpressing immunoregulatory cytokines such as TGF-β and VEGF may play important roles in the induction of forkhead box P3 (FOXP3) regulatory T cells (Tregs), and the coexistence of Tregs with CAFs correlate with poor outcomes for NSCLC [81]. Infiltration of immune suppressor cell types, including Tregs and MDSCs, in lung cancer stroma was effectively decreased through reductions in SDF-1, prostaglandin (PG)E2, and TGF-β via inhibition of CAF function in vivo [82]. CAFs can directly enhance the differentiation of Tregs through the production of TGF-β, PGE2 and IL-6 [83]. Furthermore, CAF-conditioned medium induced the expression of programmed death-ligand 1 (PD-L1) in NSCLC cells through CXCL2 secretion [84]. Some CAF subsets can directly deactivate the immune system via the expression of PD-L1 to reduce the activation of T cells, and PD-L1 expression on CAFs represents an independent prognostic factor [85]. CAFs cross-present antigens complexed with major histocompatibility complex class I to antigen-specific CD8+ T cells to directly contribute to the suppression of antitumor T-cell responses in an antigen-specific, antigen-dependent manner via PD-L2 and CD95 (known as FAS) ligand engagement, suggesting that CAFs play a role in immunosuppressive activity within the tumor microenvironment by a mechanism dependent on immune checkpoint activation [86].
4.2. ECM Production and CAF Barrier Function in NSCLC
ECM dysregulation is associated with the presence of CAFs and the activity of TGF-β and is linked to immunosuppression [87]. Activated fibroblasts modify the composition of ECM by increasing the deposition of new matrix components such as type I collagen and by modulating the expression of matrix metalloproteinases [88]. CAFs impose a physical barrier around the tumor and inhibit the ability of T cells to reach the tumor stroma [89]. Whereas loose fibronectin and collagen regions enhance active T-cell motility in NSCLC, T cells migrate poorly through areas of dense ECM. This was clarified in a real-time imaging study [90]. The resulting immune-excluding phenotype could be counteracted by remodeling the stromal ECM through treatment with collagenase D. A transgenic mouse in which FAP-expressing cells can be ablated showed decreases in both ECM production and T-cell retention in the stroma of Lewis lung carcinomas by a process involving IFN-γ and tumor necrosis factor-α [91]. In this manner, CAFs contribute to the formation of an immunosuppressed microenvironment through the production and remodeling of ECM components.
5. Tumor-Suppressing CAF Phenotypes
CAFs facilitate tumorigenesis and cancer development and have also been shown to be involved in tumor suppression [92]. With varying degrees of activation, CAFs are produced with tumor suppression characteristics and are considered to be more prevalent in early-stage cancer, indicating that greater numbers of tumor-promoting CAFs should be involved in advanced-stage cancer. The underlying mechanisms that lead to tumor promotion or tumor inhibition effects of CAFs are largely unknown [93].
Results obtained with transgenic mouse models with depletion of CAFs in the tumor stroma showed that depletion causes rapid tumor progression rather than tumor suppression, providing direct evidence of the protective effects of CAFs against cancer by secretion of tumor-suppressive cytokines, chemokines, growth factors, and miRNAs [94]. In addition, Rix et al. described a mechanism of paracrine effects for NSCLC cells via specific lung CAF populations through secretion of pro- and antitumor proteins such as IGF1/2 and IGF-binding proteins, respectively [95]. The depletion of CAFs accelerates tumor progression, raising caution regarding nonspecific CAF-depletion therapies. CAFs thus play immunoactivating or immunosuppressive roles in the tumor microenvironment due to their heterogeneity, similar to the direct effects on lung cancer cells.
6. CAF-Mediated Anticancer Therapies
6.1. Targeting CAFs
Genetic deletion and pharmacological inhibition of FAP inhibited tumor growth in an endogenous mouse model of lung cancer driven by the K-rasG12D mutant [96]. High stromal expression of FAP was identified as an independent marker of poor prognosis in patients with NSCLC [97]. FAP+ cells can both promote tumor progression directly and present a barrier to immunotherapies through the production of ECM and direct signaling pathways [75]. As a result, FAP has been targeted in tumors for imaging and therapies using several approaches, including antibodies, FAP inhibitors, vaccines, and chimeric antigen receptor (CAR) T cells [59]. Targeting FAP in human cancer patients with the humanized monoclonal antibody sibrotuzumab, which shows high affinity for binding to FAP [98], or the FAP enzyme inhibitor talabostat [99], which has not demonstrated clinical efficacy in NSCLC patients. This is probably because binding those drugs to tumor cells in the stroma did not cause sufficient drug-dependent cell cytolysis. A DNA vaccine directed against FAP suppressed tumor proliferation by stimulating a CD8+ T-cell-mediated immune response [100]. Duperret et al. presented a DNA vaccine targeting FAP that drives both CD4 and CD8 T cells [101]. Mice vaccinated with recombinant adenoviral vectors containing FAP cDNA produced FAP-specific cytotoxic T lymphocytes capable of destroying FAP-expressing CAFs, suggesting FAP as a potential target for elimination of CAFs that may develop immunogenic tumor vaccines [102]. CARs are genetically engineered to express CAR molecules targeting tumor antigens and recognize and kill tumor cells. FAP-specific CAR T cells were generated and evaluated in vitro and in vivo, showing that FAP-targeted CAR T cells inhibited the proliferation of NSCLC cells by eliminating FAP-positive CAFs [103,104]. While FAP-targeted CAR-T cells caused severe side effects such as bone marrow toxicity and cachexia [105], in a phase I clinical trial using anti-FAP CAR T-cell therapies for malignant pleural mesothelioma patients, a single infusion of anti-FAP CAR T cells was shown to be safe as long as local administration was used [106]. Further genetic modifications to CAR designs are still necessary to reduce the incidence of treatment-related toxicities [107].
Another CAF-depleting therapeutic strategy depends on surface markers of CAFs. CAF markers offer a potential target for antitumor treatments as CAF-targeted therapies, but such approaches will not exert curative effects against solid tumors. As mentioned above, CAF subsets and phenotypes recently discovered through scRNA-seq have provided glimpses of the possibility of actively targeting these subsets, which represent a minor fraction in the tumor microenvironment [108].
6.2. Targeting the Signaling Pathways of CAFs
TGF-β plays an important role in the activation of CAFs and interactions between CAFs and immune cells, indicating TGF-β inhibition therapy as a potentially attractive therapy targeting CAFs [23,109]. We previously reported that CAF mediated secretion of IL-6-induced EMT via enhanced TGF-β signaling in NSCLC, which was attenuated by IL-6 blocking antibody [15]. Song et al. presented the antitumor effect of an IL-6-neutralizing antibody, siltuximab, in mouse xenograft models of NSCLC [110]. In preclinical and phase I and II trials of ALD518, a humanized monoclonal antibody targeting of IL-6 appeared to be well-tolerated and ameliorated NSCLC-related anemia and cachexia by controlling oncogene-associated inflammatory pathways [111]. Tocilizumab, a monoclonal antibody specific to the IL-6 receptor, is expected to control tumor-related symptoms and reduce drug resistance and immune-related adverse events following immune checkpoint inhibitor therapy in NSCLC patients [112]. Thus, IL-6 may present an attractive therapeutic target for the treatment of NSCLC. The FGF/FGFR tyrosine kinase signaling pathway also regulates multiple biological events between CAFs and NSCLC cells, and an FGFR blocker significantly suppressed tumor progression and tumor stroma formation [113]. The chemokine receptor CXCR4 and its ligand, SDF-1 (known as CXCL12), are expressed in a variety of NSCLC and play important roles in the activation and immune suppression of CAFs; thus, the CXCL12/CXCR4 axis may be a target for immune intervention [114]. AMD3100, a specific antagonist of CXCR4, is the most potent small-molecule non-peptide inhibitor of the CXCR4/CXCL12 axis via immune modulation in the tumor microenvironment [115,116,117].
JQ1, a bromodomain and extraterminal domain protein inhibitor, shows therapeutic efficacy against various malignancies. JQ1 attenuated the activation of CAFs via the inhibition of Hedgehog and TGF-β pathways [118]. JQ1 also downregulates glutathione peroxidase 8 (GPX8) expression, which is overexpressed in CAFs and is associated with CAF infiltration in the tumor microenvironment of NSCLC [119]. Furthermore, aberrant expression of histone deacetylases (HDACs) is frequent in human cancers and may be involved in the progression of fibrosis in idiopathic pulmonary fibrosis, as well as NSCLC progression [120]. The HDAC inhibitor CUDC-907 suppressed cancer cell proliferation, collagen production, and ECM deposition and inhibited the migration and invasion properties of fibroblasts and CAFs in an animal NSCLC model [121].
6.3. Antifibrotic Therapy to Normalize the Tumor Microenvironment
Fibrosis is a critical feature of the tumor microenvironment of many types of solid tumors. Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial pulmonary disease and is known to increase the risk of NSCLC; thus, there are multiple common genetic, molecular, and cellular processes that connect lung fibrosis with NSCLC, including fibroblast activation [122]. CAF-targeted treatments designed to block the progression of fibrosis are in development [123]. Pirfenidone is clinically used to treat IPF as an antifibrotic agent [124,125]. We previously showed that pirfenidone inhibited not only fibroblast activity but also crosstalk between NSCLC cells and CAFs by a synergistic potential for inhibiting both IL-6 and TGF-β signaling [126]. Periostin is an ECM protein secreted from CAFs and IPF-activated fibroblasts [127,128]. We reported that periostin plays critical roles in the proliferation of NSCLC cells, and the inhibition of periostin–receptor (integrin β3) interactions attenuated the aggressiveness of NSCLC with IPF [129]. Periostin with CAFs impaired lymphatic endothelial barriers and consequently enhanced metastasis [130]. Periostin has variant C-terminal splicing forms, so pharmacological inhibition of cancer-specific or CAF-specific variants of periostin may effectively suppress tumor expansion and overcome chemoresistance [131]. Nintedanib (BIBF1120) is a small-molecule inhibitor of receptor tyrosine kinases, including FGFR, PDGFR, and VEGFR, and has been approved for treatment of lung adenocarcinoma, as well as IPF [132,133]. Kato et al. reported that nintedanib enhances antitumor immunity by targeting CAFs and thereby improves response to an immune checkpoint blockade [134]. Those findings indicate that antifibrotic drugs may be useful as CAF-targeted agents.
6.4. Targeting Immunomodulation of CAFs
Immunotherapy does not work well in some NSCLC patients. One major reason for cancers failing to respond to anti-PD-1/PD-L immune checkpoint therapy is that CD8+ T cells cannot infiltrate into the tumor [135]. CAF-rich tumors exhibit an immunologically cold tumor microenvironment by forming a physical barrier [136,137]. Furthermore, CAFs directly attenuate the activation and proliferation of CD8+ and CD4+ T cells, promote the differentiation of Tregs, and recruit MDSCs for tumor progression and treatment resistance. Altogether, CAFs represent an attractive clinical target for improving response rates to immunotherapy in solid tumors.
6.5. New Concepts for Targeted Therapies Using CAFs
Using CAFs as a drug delivery tool is another interesting concept. Leucine-rich-repeat-containing 15 (LRRC15) was induced by TGF-β on activated α-SMA+ CAFs. ABBV-085, a LRRC15 antibody drug conjugate used to deliver the potent antimitotic drug monomethyl auristatin E (MMAE), demonstrated preclinical efficacy against LRRC15 in in vivo models of NSCLC, resulting in localization of the MMAE at high levels in the tumor microenvironment and killing tumor cells by the cell-permeable effects of MMAE [138]. A novel class of CAR T cells was engineered to express an enzyme that activates a systemically administered small-molecule prodrug in situ at a tumor site [139]. CAR T cells against CAFs localize drugs in the TME via this delivery ability and reshape the TME for favorable antitumor responses [140].
6.6. Clinical Trials Targeting CAFs in NSCLC
The tumor-promoting functions of CAFs make them promising targets for anticancer therapy, providing attractive emerging therapeutic strategies that can be categorized into blockage of CAF recruitment and activation, depletion of CAF populations, blockage of CAF-mediated signals, and remodeling of the ECM (Figure 2).
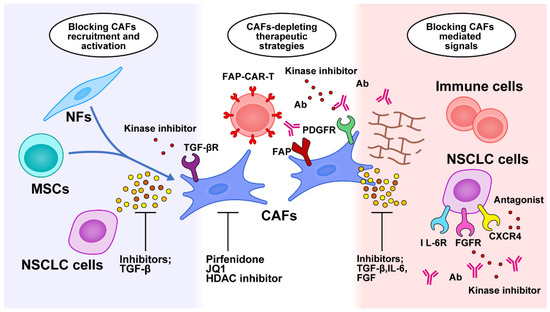
Figure 2.
Targeted therapy against CAF-related crosstalk in the tumor microenvironment. Therapeutic strategies are categorized into blocking CAF recruitment and activation, depleting the CAF population, blocking CAF-mediated signals, and remodeling the ECM. NFs, normal fibroblasts; CAFs, cancer-associated fibroblasts; NSCLC, non-small cell lung cancer; MSCs, mesenchymal stem cells; FAP, fibroblast activation protein; CAR-T, chimeric antigen receptor T cell; PDGFR, platelet-derived growth factor receptor; HDAC, histone deacetylase; Ab, antibody; TGF-β, transforming growth factor-β; IL-6, interleukin-6; IL-6R, interleukin-6 receptor; FGFR, fibroblast growth factor; CXCR4, CXC chemokine receptor 4.
However, many proposed strategies have failed to show promising clinical outcomes, probably because of the current incomplete understanding of fibroblast heterogeneity. Several clinical trials targeting CAF-related signals in a variety of malignant diseases are ongoing; recent clinical trials involving NSCLC patients are summarized in Table 3.

Table 3.
Clinical trials targeting CAF-related signals in non-small cell lung cancer.
7. Future Perspectives and Conclusion
CAF-targeted cancer treatments may suppress tumor progression and regulate the niche of cancer stem cells and immunosuppressive networks, providing utility in NSCLC treatment through multiple mechanisms. Key signaling pathways provided by CAFs and CAF-derived factors have great potential for targeted therapy. We have shown that IL-6 contributes to the maintenance of a paracrine loop, which functions as part of the communication between CAFs and NSCLC cells. Anti-IL-6 therapies have been used clinically to treat various diseases; thus, a study to reveal an inhibitor of the IL-6/JAK/STAT3 signaling pathway may be valuable for investigations of targeted therapy [15,141,142]. As mentioned before, FAP is preferentially expressed by CAF and is one of the biomarkers used for their identification; thus, a method for targeting FAP was developed to overcome challenges in the tumor microenvironment [143]. Additionally, FAP inhibitors (FAPI) have recently been developed for positron emission tomography (PET) imaging and radioligand therapy, which will be useful for exploring clinical applications in several types of cancer [144]. FAPI PET findings can also be used to predetermine the candidacy for an FAP-expressing CAF-targeted therapy, as well as evaluation of its efficacy.
However, the tumor-promoting or tumor-suppressive status of CAFs may cancel each other out, depending on the tumor microenvironment; thus, analysis of their lineage is necessary. New research technologies, including scRNA-seq, which allows access to changes among stromal, immune, and cancer cells involved in tumor progression, as multiplex spatial analysis methods, have recently been developed [145]. These technologies can provide valuable insight into the effects of cellular crosstalk dynamics and heterogeneity on cancer patient prognosis and response to various treatments [146].
In conclusion, to develop treatment strategies against CAFs, which have attracted attention as potent novel NSCLC treatments, we should understand the subset of functions of CAFs in the tumor microenvironment and identify targeted CAFs in a more specific manner.
Author Contributions
Y.S. is the corresponding author and wrote this article. T.K. (Toru Kimura), S.F., N.O., T.K. (Takashi Kanou) and E.F. discussed the content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Desmouliere, A.; Guyot, C.; Gabbiani, G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int. J. Dev. Biol. 2004, 48, 509–517. [Google Scholar] [CrossRef]
- Eyden, B. The myofibroblast: Phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J. Cell Mol. Med. 2008, 12, 22–37. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nature reviews. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Q.; Yamada, T.; Matsumoto, K.; Matsumoto, I.; Oda, M.; Watanabe, G.; Kayano, Y.; Nishioka, Y.; Sone, S.; et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6630–6638. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Ho, C.C.; Chang, Y.L.; Chen, H.Y.; Lin, C.A.; Ling, T.Y.; Yu, S.L.; Yuan, S.S.; Chen, Y.J.; Lin, C.Y.; et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 2014, 5, 3472. [Google Scholar] [CrossRef]
- Yoshida, T.; Ishii, G.; Goto, K.; Neri, S.; Hashimoto, H.; Yoh, K.; Niho, S.; Umemura, S.; Matsumoto, S.; Ohmatsu, H.; et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Fujiwara, A.; Kimura, T.; Kawamura, T.; Funaki, S.; Minami, M.; Okumura, M. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, T.; Yin, R. Biomarkers for cancer-associated fibroblasts. Biomark. Res. 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-alpha as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11, 648187. [Google Scholar] [CrossRef]
- Domen, A.; Quatannens, D.; Zanivan, S.; Deben, C.; Van Audenaerde, J.; Smits, E.; Wouters, A.; Lardon, F.; Roeyen, G.; Verhoeven, Y.; et al. Cancer-Associated Fibroblasts as a Common Orchestrator of Therapy Resistance in Lung and Pancreatic Cancer. Cancers 2021, 13, 987. [Google Scholar] [CrossRef]
- Gieniec, K.A.; Butler, L.M.; Worthley, D.L.; Woods, S.L. Cancer-associated fibroblasts-heroes or villains? Br. J. Cancer 2019, 121, 293–302. [Google Scholar] [CrossRef]
- Guerrero-Juarez, C.F.; Dedhia, P.H.; Jin, S.; Ruiz-Vega, R.; Ma, D.; Liu, Y.; Yamaga, K.; Shestova, O.; Gay, D.L.; Yang, Z.; et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nature reviews. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Abulaiti, A.; Kimura, T.; Funaki, S.; Nakagiri, T.; Inoue, M.; Sawabata, N.; Minami, M.; Morii, E.; Okumura, M. Pulmonary fibroblasts induce epithelial mesenchymal transition and some characteristics of stem cells in non-small cell lung cancer. Ann. Thorac. Surg. 2013, 96, 425–433. [Google Scholar] [CrossRef]
- Paauwe, M.; Schoonderwoerd, M.J.A.; Helderman, R.; Harryvan, T.J.; Groenewoud, A.; van Pelt, G.W.; Bor, R.; Hemmer, D.M.; Versteeg, H.H.; Snaar-Jagalska, B.E.; et al. Endoglin Expression on Cancer-Associated Fibroblasts Regulates Invasion and Stimulates Colorectal Cancer Metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 6331–6344. [Google Scholar] [CrossRef]
- Sugai, M.; Yanagawa, N.; Shikanai, S.; Hashimoto, M.; Saikawa, H.; Osakabe, M.; Saito, H.; Maemondo, M.; Sugai, T. Correlation of tumor microenvironment-related markers with clinical outcomes in patients with squamous cell carcinoma of the lung. Transl. Lung Cancer Res. 2022, 11, 975–990. [Google Scholar] [CrossRef]
- Harryvan, T.J.; Verdegaal, E.M.E.; Hardwick, J.C.H.; Hawinkels, L.; van der Burg, S.H. Targeting of the Cancer-Associated Fibroblast-T-Cell Axis in Solid Malignancies. J. Clin. Med. 2019, 8, 1989. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef]
- Donato, R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1999, 1450, 191–231. [Google Scholar] [CrossRef]
- Lawson, W.E.; Polosukhin, V.V.; Zoia, O.; Stathopoulos, G.T.; Han, W.; Plieth, D.; Loyd, J.E.; Neilson, E.G.; Blackwell, T.S. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2005, 171, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, R.; Pietras, K. Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci. 2020, 111, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.F.; Waise, S.; Green, E.W.; Stuart, B.; Thomas, G.J. Characterising cancer-associated fibroblast heterogeneity in non-small cell lung cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 3727. [Google Scholar] [CrossRef]
- Nair, N.; Calle, A.S.; Zahra, M.H.; Prieto-Vila, M.; Oo, A.K.K.; Hurley, L.; Vaidyanath, A.; Seno, A.; Masuda, J.; Iwasaki, Y.; et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci. Rep. 2017, 7, 6838. [Google Scholar] [CrossRef]
- Strong, A.L.; Pei, D.T.; Hurst, C.G.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity Enhances the Conversion of Adipose-Derived Stromal/Stem Cells into Carcinoma-Associated Fibroblast Leading to Cancer Cell Proliferation and Progression to an Invasive Phenotype. Stem Cells Int. 2017, 2017, 9216502. [Google Scholar] [CrossRef]
- Aoto, K.; Ito, K.; Aoki, S. Complex formation between platelet-derived growth factor receptor beta and transforming growth factor beta receptor regulates the differentiation of mesenchymal stem cells into cancer-associated fibroblasts. Oncotarget 2018, 9, 34090–34102. [Google Scholar] [CrossRef]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lovrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwe, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Olbrecht, S.; Boeckx, B.; Vos, H.; Laoui, D.; Etlioglu, E.; Wauters, E.; Pomella, V.; Verbandt, S.; Busschaert, P.; et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 2020, 30, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021, 39, 1531–1547.e1510. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.S.; Cheon, I.; Kim, S.R.; Chun, S.H.; Kim, J.J.; Lee, S.; Yoon, J.S.; Hong, S.A.; Won, H.S.; et al. Identification and Characterization of Cancer-Associated Fibroblast Subpopulations in Lung Adenocarcinoma. Cancers 2022, 14, 3486. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e816. [Google Scholar] [CrossRef] [PubMed]
- Denys, H.; Derycke, L.; Hendrix, A.; Westbroek, W.; Gheldof, A.; Narine, K.; Pauwels, P.; Gespach, C.; Bracke, M.; De Wever, O. Differential impact of TGF-beta and EGF on fibroblast differentiation and invasion reciprocally promotes colon cancer cell invasion. Cancer Lett. 2008, 266, 263–274. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yashiro, M.; Nishii, T.; Matsuoka, J.; Fuyuhiro, Y.; Morisaki, T.; Fukuoka, T.; Shimizu, K.; Shimizu, T.; Miwa, A.; et al. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling. Int. J. Cancer 2014, 134, 1785–1795. [Google Scholar] [CrossRef]
- Li, J.; Guan, J.; Long, X.; Wang, Y.; Xiang, X. mir-1-mediated paracrine effect of cancer-associated fibroblasts on lung cancer cell proliferation and chemoresistance. Oncol. Rep. 2016, 35, 3523–3531. [Google Scholar] [CrossRef]
- Abulaiti, A.; Shintani, Y.; Funaki, S.; Nakagiri, T.; Inoue, M.; Sawabata, N.; Minami, M.; Okumura, M. Interaction between non-small-cell lung cancer cells and fibroblasts via enhancement of TGF-beta signaling by IL-6. Lung Cancer 2013, 82, 204–213. [Google Scholar] [CrossRef]
- Erez, N.; Glanz, S.; Raz, Y.; Avivi, C.; Barshack, I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013, 437, 397–402. [Google Scholar] [CrossRef]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Tulafu, M.; Togo, S.; Kawaji, H.; Kadoya, K.; Namba, Y.; Jin, J.; Watanabe, J.; Okabe, T.; Hidayat, M.; et al. Cancer-associated fibroblast migration in non-small cell lung cancers is modulated by increased integrin alpha11 expression. Mol. Oncol. 2021, 15, 1507–1527. [Google Scholar] [CrossRef]
- Shintani, Y.; Okimura, A.; Sato, K.; Nakagiri, T.; Kadota, Y.; Inoue, M.; Sawabata, N.; Minami, M.; Ikeda, N.; Kawahara, K.; et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann. Thorac. Surg. 2011, 92, 1794–1804; discussion 1804. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 2016, 6, 38408. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, R.; Naito, H.; Kise, K.; Takara, K.; Eino, D.; Minami, M.; Shintani, Y.; Funaki, S.; Kawamura, T.; Kimura, T.; et al. Gas6 derived from cancer-associated fibroblasts promotes migration of Axl-expressing lung cancer cells during chemotherapy. Sci. Rep. 2017, 7, 10613. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Huang, R.Y. AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res. 2017, 77, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Ren, Y.; Geng, H.; Zhang, Q.; Cao, L.; Meng, Z.; Wu, X.; Xu, M.; Xu, K. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019, 110, 1609–1620. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Bai, J.; Ren, J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018, 109, 944–955. [Google Scholar] [CrossRef]
- De, P.; Aske, J.; Sulaiman, R.; Dey, N. Bete Noire of Chemotherapy and Targeted Therapy: CAF-Mediated Resistance. Cancers 2022, 14, 1519. [Google Scholar] [CrossRef]
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef]
- Pellinen, T.; Paavolainen, L.; Martin-Bernabe, A.; Papatella Araujo, R.; Strell, C.; Mezheyeuski, A.; Backman, M.; La Fleur, L.; Bruck, O.; Sjolund, J.; et al. Fibroblast subsets in non-small cell lung cancer: Associations with survival, mutations, and immune features. J. Natl. Cancer Inst. 2022, djac178. [Google Scholar] [CrossRef]
- Choe, C.; Shin, Y.S.; Kim, C.; Choi, S.J.; Lee, J.; Kim, S.Y.; Cho, Y.B.; Kim, J. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. Onco Targets Ther. 2015, 8, 3665–3678. [Google Scholar] [CrossRef]
- Yi, Y.; Zeng, S.; Wang, Z.; Wu, M.; Ma, Y.; Ye, X.; Zhang, B.; Liu, H. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Shien, K.; Papadimitrakopoulou, V.A.; Ruder, D.; Behrens, C.; Shen, L.; Kalhor, N.; Song, J.; Lee, J.J.; Wang, J.; Tang, X.; et al. JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2017, 16, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, S.; Wang, K.; Sun, S.Y. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J. Hematol. Oncol. 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernandez-Perez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018, 28, 848–865.e846. [Google Scholar] [CrossRef]
- Shen, H.; Yu, X.; Yang, F.; Zhang, Z.; Shen, J.; Sun, J.; Choksi, S.; Jitkaew, S.; Shu, Y. Reprogramming of Normal Fibroblasts into Cancer-Associated Fibroblasts by miRNAs-Mediated CCL2/VEGFA Signaling. PLoS Genet. 2016, 12, e1006244. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Qin, X.; Yan, M.; Li, R.R.; Chen, G.; Zhang, J.J.; Chen, W.T. Cancer-associated fibroblasts promote cancer cell growth through a miR-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget 2017, 8, 1290–1303. [Google Scholar] [CrossRef]
- Yang, F.; Yan, Y.; Yang, Y.; Hong, X.; Wang, M.; Yang, Z.; Liu, B.; Ye, L. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell. Signal. 2020, 73, 109675. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, W.; Huang, Y.; Zhuo, L.; Wang, S.; Chen, S.; Zhang, B.; Ke, B. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e989. [Google Scholar] [CrossRef]
- Kunita, A.; Morita, S.; Irisa, T.U.; Goto, A.; Niki, T.; Takai, D.; Nakajima, J.; Fukayama, M. MicroRNA-21 in cancer-associated fibroblasts supports lung adenocarcinoma progression. Sci. Rep. 2018, 8, 8838. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, J.H.; Kim, J.S.; Yoon, J.S.; Chun, S.H.; Hong, S.A.; Kim, E.J.; Kang, K.; Lee Kang, J.; Ko, Y.H.; et al. Cancer-associated fibroblasts activated by miR-196a promote the migration and invasion of lung cancer cells. Cancer Lett. 2021, 508, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ti, W.; Wang, J.; Cheng, Y. The Interaction Between Long Non-Coding RNAs and Cancer-Associated Fibroblasts in Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 714125. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, C.; Fang, P.; Ma, Z.; Wang, X.; Chen, H.; Wang, S.; Meng, F.; Wang, C.; Zhang, E.; et al. Cancer-associated fibroblast-specific lncRNA LINC01614 enhances glutamine uptake in lung adenocarcinoma. J. Hematol. Oncol. 2022, 15, 141. [Google Scholar] [CrossRef]
- Barrett, R.L.; Pure, E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife 2020, 9, 7243. [Google Scholar] [CrossRef] [PubMed]
- Mhaidly, R.; Mechta-Grigoriou, F. Role of cancer-associated fibroblast subpopulations in immune infiltration, as a new means of treatment in cancer. Immunol. Rev. 2021, 302, 259–272. [Google Scholar] [CrossRef]
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J., Jr.; Yokota, S.J.; Bankert, R.B. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J. Immunol. 2007, 178, 5552–5562. [Google Scholar] [CrossRef]
- Galindo, M.; Santiago, B.; Rivero, M.; Rullas, J.; Alcami, J.; Pablos, J.L. Chemokine expression by systemic sclerosis fibroblasts: Abnormal regulation of monocyte chemoattractant protein 1 expression. Arthritis Rheum. 2001, 44, 1382–1386. [Google Scholar] [CrossRef]
- Vanbervliet, B.; Homey, B.; Durand, I.; Massacrier, C.; Ait-Yahia, S.; de Bouteiller, O.; Vicari, A.; Caux, C. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: Possible role at inflamed epithelial surfaces. Eur. J. Immunol. 2002, 32, 231–242. [Google Scholar] [CrossRef]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer-Associated Fibroblasts Promote Immunosuppression by Inducing ROS-Generating Monocytic MDSCs in Lung Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ishii, G.; Hiraoka, N.; Hirayama, S.; Yamauchi, C.; Aokage, K.; Hishida, T.; Yoshida, J.; Nagai, K.; Ochiai, A. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci. 2013, 104, 409–415. [Google Scholar] [CrossRef]
- Ohshio, Y.; Teramoto, K.; Hanaoka, J.; Tezuka, N.; Itoh, Y.; Asai, T.; Daigo, Y.; Ogasawara, K. Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine. Cancer Sci. 2015, 106, 134–142. [Google Scholar] [CrossRef]
- Baker, A.T.; Abuwarwar, M.H.; Poly, L.; Wilkins, S.; Fletcher, A.L. Cancer-Associated Fibroblasts and T Cells: From Mechanisms to Outcomes. J. Immunol. 2021, 206, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Inoue, C.; Miki, Y.; Saito, R.; Hata, S.; Abe, J.; Sato, I.; Okada, Y.; Sasano, H. PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells. Cancers 2019, 11, 1257. [Google Scholar] [CrossRef]
- Teramoto, K.; Igarashi, T.; Kataoka, Y.; Ishida, M.; Hanaoka, J.; Sumimoto, H.; Daigo, Y. Clinical significance of PD-L1-positive cancer-associated fibroblasts in pN0M0 non-small cell lung cancer. Lung Cancer 2019, 137, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef] [PubMed]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 924. [Google Scholar] [CrossRef]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830. [Google Scholar] [CrossRef]
- Feng, B.; Wu, J.; Shen, B.; Jiang, F.; Feng, J. Cancer-associated fibroblasts and resistance to anticancer therapies: Status, mechanisms, and countermeasures. Cancer Cell Int. 2022, 22, 166. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Tan, Y.; Tang, Y.; Ye, J.; Yuan, B.; Yu, W. Cancer-Associated Fibroblasts Suppress Cancer Development: The Other Side of the Coin. Front. Cell Dev. Biol. 2021, 9, 613534. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Remsing Rix, L.L.; Sumi, N.J.; Hu, Q.; Desai, B.; Bryant, A.T.; Li, X.; Welsh, E.A.; Fang, B.; Kinose, F.; Kuenzi, B.M.; et al. IGF-binding proteins secreted by cancer-associated fibroblasts induce context-dependent drug sensitization of lung cancer cells. Sci. Signal. 2022, 15, eabj5879. [Google Scholar] [CrossRef]
- Santos, A.M.; Jung, J.; Aziz, N.; Kissil, J.L.; Pure, E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Investig. 2009, 119, 3613–3625. [Google Scholar] [CrossRef]
- Moreno-Ruiz, P.; Corvigno, S.; Te Grootenhuis, N.C.; La Fleur, L.; Backman, M.; Strell, C.; Mezheyeuski, A.; Hoelzlwimmer, G.; Klein, C.; Botling, J.; et al. Stromal FAP is an independent poor prognosis marker in non-small cell lung adenocarcinoma and associated with p53 mutation. Lung Cancer 2021, 155, 10–19. [Google Scholar] [CrossRef]
- Scott, A.M.; Wiseman, G.; Welt, S.; Adjei, A.; Lee, F.T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 1639–1647. [Google Scholar]
- Eager, R.M.; Cunningham, C.C.; Senzer, N.; Richards, D.A.; Raju, R.N.; Jones, B.; Uprichard, M.; Nemunaitis, J. Phase II trial of talabostat and docetaxel in advanced non-small cell lung cancer. Clin. Oncol. (R. Coll. Radiol.) 2009, 21, 464–472. [Google Scholar] [CrossRef]
- Loeffler, M.; Kruger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef]
- Duperret, E.K.; Trautz, A.; Ammons, D.; Perales-Puchalt, A.; Wise, M.C.; Yan, J.; Reed, C.; Weiner, D.B. Alteration of the Tumor Stroma Using a Consensus DNA Vaccine Targeting Fibroblast Activation Protein (FAP) Synergizes with Antitumor Vaccine Therapy in Mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yuan, S.; Peng, L.; Li, H.; Niu, L.; Xu, H.; Guo, X.; Yang, M.; Duan, F. Antitumor immunity targeting fibroblast activation protein-alpha in a mouse Lewis lung carcinoma model. Oncol. Lett. 2020, 20, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kakarla, S.; Chow, K.K.; Mata, M.; Shaffer, D.R.; Song, X.T.; Wu, M.F.; Liu, H.; Wang, L.L.; Rowley, D.R.; Pfizenmaier, K.; et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Tran, E.; Chinnasamy, D.; Yu, Z.; Morgan, R.A.; Lee, C.C.; Restifo, N.P.; Rosenberg, S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013, 210, 1125–1135. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Britschgi, C.; Schuberth, P.; Bankel, L.; Nguyen-Kim, T.D.L.; Gulati, P.; Weder, W.; Opitz, I.; Lauk, O.; Caviezel, C.; et al. Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: First report of FAPME, a phase I clinical trial. Ann. Oncol. 2021, 32, 120–121. [Google Scholar] [CrossRef]
- Xiao, B.F.; Zhang, J.T.; Zhu, Y.G.; Cui, X.R.; Lu, Z.M.; Yu, B.T.; Wu, N. Chimeric Antigen Receptor T-Cell Therapy in Lung Cancer: Potential and Challenges. Front. Immunol. 2021, 12, 782775. [Google Scholar] [CrossRef]
- Saw, P.E.; Chen, J.; Song, E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer 2022, 8, 527–555. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Song, L.; Smith, M.A.; Doshi, P.; Sasser, K.; Fulp, W.; Altiok, S.; Haura, E.B. Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2014, 9, 974–982. [Google Scholar] [CrossRef]
- Bayliss, T.J.; Smith, J.T.; Schuster, M.; Dragnev, K.H.; Rigas, J.R. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin. Biol. Ther. 2011, 11, 1663–1668. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, L.; Dai, Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac. Cancer 2020, 11, 835–839. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Kameyama, N.; Gao, J.; Kagawa, S.; Yasuda, H.; Soejima, K.; Yin, Y.; Guzy, R.D.; Nakamura, Y.; et al. Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts. J. Pathol. 2019, 249, 193–205. [Google Scholar] [CrossRef]
- Wald, O.; Izhar, U.; Amir, G.; Kirshberg, S.; Shlomai, Z.; Zamir, G.; Peled, A.; Shapira, O.M. Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: Role in non-small cell lung cancer tumor proliferation. J. Thorac. Cardiovasc. Surg. 2011, 141, 1503–1512. [Google Scholar] [CrossRef]
- Chen, Y.; Ramjiawan, R.R.; Reiberger, T.; Ng, M.R.; Hato, T.; Huang, Y.; Ochiai, H.; Kitahara, S.; Unan, E.C.; Reddy, T.P.; et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015, 61, 1591–1602. [Google Scholar] [CrossRef]
- Song, J.S.; Chang, C.C.; Wu, C.H.; Dinh, T.K.; Jan, J.J.; Huang, K.W.; Chou, M.C.; Shiue, T.Y.; Yeh, K.C.; Ke, Y.Y.; et al. A highly selective and potent CXCR4 antagonist for hepatocellular carcinoma treatment. Proc. Natl. Acad. Sci. USA 2021, 118, 3118. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Xu, N.; Yu, M.; Tu, X.; Chen, Z.; Lin, M.; Xie, B.; Fu, J.; Han, L. AMD3100 inhibits brain-specific metastasis in lung cancer via suppressing the SDF-1/CXCR4 axis and protecting blood-brain barrier. Am. J. Transl. Res. 2017, 9, 5259–5274. [Google Scholar] [PubMed]
- Yamamoto, K.; Tateishi, K.; Kudo, Y.; Hoshikawa, M.; Tanaka, M.; Nakatsuka, T.; Fujiwara, H.; Miyabayashi, K.; Takahashi, R.; Tanaka, Y.; et al. Stromal remodeling by the BET bromodomain inhibitor JQ1 suppresses the progression of human pancreatic cancer. Oncotarget 2016, 7, 61469–61484. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Yuan, L.W.; Jiang, X.M.; Su, M.X.; Huang, M.Y.; Chen, Y.C.; Zhang, L.L.; Chen, X.; Zhu, H.; Lu, J.J. Glutathione peroxidase 8 expression on cancer cells and cancer-associated fibroblasts facilitates lung cancer metastasis. MedComm 2022, 3, e152. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Jalal, S.I. Histone Deacetylase Inhibition in Non-small Cell Lung Cancer: Hype or Hope? Front. Cell Dev. Biol. 2020, 8, 582370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Tu, T.; Schmull, S.; Han, Y.; Wang, W.; Li, H. Dual inhibition of HDAC and tyrosine kinase signaling pathways with CUDC-907 attenuates TGFbeta1 induced lung and tumor fibrosis. Cell Death Dis. 2020, 11, 765. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Taguchi, Y.; Ogura, T.; Ebina, M.; Taniguchi, H.; Kondoh, Y.; Suga, M.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir. Res. 2011, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Gili, E.; Fagone, E.; Fruciano, M.; Iemmolo, M.; Vancheri, C. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 58, 13–19. [Google Scholar] [CrossRef]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Moore, B.B. The role of periostin in lung fibrosis and airway remodeling. Cell. Mol. Life Sci. CMLS 2017, 74, 4305–4314. [Google Scholar] [CrossRef]
- Ratajczak-Wielgomas, K.; Kmiecik, A.; Grzegrzolka, J.; Piotrowska, A.; Gomulkiewicz, A.; Partynska, A.; Pawelczyk, K.; Nowinska, K.; Podhorska-Okolow, M.; Dziegiel, P. Prognostic Significance of Stromal Periostin Expression in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2020, 21, 7025. [Google Scholar] [CrossRef]
- Yamato, H.; Kimura, K.; Fukui, E.; Kanou, T.; Ose, N.; Funaki, S.; Minami, M.; Shintani, Y. Periostin secreted by activated fibroblasts in idiopathic pulmonary fibrosis promotes tumorigenesis of non-small cell lung cancer. Sci. Rep. 2021, 11, 21114. [Google Scholar] [CrossRef]
- Wei, W.F.; Chen, X.J.; Liang, L.J.; Yu, L.; Wu, X.G.; Zhou, C.F.; Wang, Z.C.; Fan, L.S.; Hu, Z.; Liang, L.; et al. Periostin(+) cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol. Oncol. 2021, 15, 210–227. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Taniyama, Y.; Sanada, F.; Morishita, R.; Nakamori, S.; Morimoto, K.; Yeung, K.T.; Yang, J. Periostin blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci. Rep. 2018, 8, 4013. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Haratani, K.; Hayashi, H.; Sakai, K.; Sakai, H.; Kawakami, H.; Tanaka, K.; Takeda, M.; Yonesaka, K.; Nishio, K.; et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: Potential role of cancer-associated fibroblasts. Br. J. Cancer 2021, 124, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Parpal, S.; Van Moer, K.; Xiao, M.; Yu, Y.; Viklund, J.; De Milito, A.; Hasmim, M.; Andersson, M.; Amaravadi, R.K.; et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci. Adv. 2020, 6, eaax7881. [Google Scholar] [CrossRef]
- Jenkins, L.; Jungwirth, U.; Avgustinova, A.; Iravani, M.; Mills, A.; Haider, S.; Harper, J.; Isacke, C.M. Cancer-Associated Fibroblasts Suppress CD8+ T-cell Infiltration and Confer Resistance to Immune-Checkpoint Blockade. Cancer Res. 2022, 82, 2904–2917. [Google Scholar] [CrossRef]
- Pure, E.; Lo, A. Can Targeting Stroma Pave the Way to Enhanced Antitumor Immunity and Immunotherapy of Solid Tumors? Cancer Immunol. Res. 2016, 4, 269–278. [Google Scholar] [CrossRef]
- Purcell, J.W.; Tanlimco, S.G.; Hickson, J.; Fox, M.; Sho, M.; Durkin, L.; Uziel, T.; Powers, R.; Foster, K.; McGonigal, T.; et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody-Drug Conjugates. Cancer Res. 2018, 78, 4059–4072. [Google Scholar] [CrossRef]
- Gardner, T.J.; Lee, J.P.; Bourne, C.M.; Wijewarnasuriya, D.; Kinarivala, N.; Kurtz, K.G.; Corless, B.C.; Dacek, M.M.; Chang, A.Y.; Mo, G.; et al. Engineering CAR-T cells to activate small-molecule drugs in situ. Nat. Chem. Biol. 2022, 18, 216–225. [Google Scholar] [CrossRef]
- Kankeu Fonkoua, L.A.; Sirpilla, O.; Sakemura, R.; Siegler, E.L.; Kenderian, S.S. CAR T cell therapy and the tumor microenvironment: Current challenges and opportunities. Mol. Ther. Oncolytics 2022, 25, 69–77. [Google Scholar] [CrossRef]
- Jia, C.; Wang, G.; Wang, T.; Fu, B.; Zhang, Y.; Huang, L.; Deng, Y.; Chen, G.; Wu, X.; Chen, J.; et al. Cancer-associated Fibroblasts induce epithelial-mesenchymal transition via the Transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in Hepatocellular Carcinoma. Int. J. Biol.Sci. 2020, 16, 2542–2558. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moreno, V.; Gaggioli, C.; Yeo, M.; Albrengues, J.; Wallberg, F.; Viros, A.; Hooper, S.; Mitter, R.; Feral, C.C.; Cook, M.; et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 2011, 20, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Bughda, R.; Dimou, P.; D’Souza, R.R.; Klampatsa, A. Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: Launching an Attack on Tumor Stroma. Immunotargets Ther. 2021, 10, 313–323. [Google Scholar] [CrossRef]
- Gilardi, L.; Airo Farulla, L.S.; Demirci, E.; Clerici, I.; Omodeo Sale, E.; Ceci, F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines 2022, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, S.; Baars, M.J.D.; Vercoulen, Y. Multiplex Tissue Imaging: Spatial Revelations in the Tumor Microenvironment. Cancers 2022, 14, 3170. [Google Scholar] [CrossRef]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).