A Subset of PD-1-Expressing CD56bright NK Cells Identifies Patients with Good Response to Immune Checkpoint Inhibitors in Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Population and Inclusion and Exclusion Criteria
2.3. Study Protocol
2.4. Sample Type and Processing
2.4.1. Sample Processing
2.4.2. Staining, Antibody Panels and Flow Cytometry
2.5. Determination of Cell Populations in Peripheral Blood
2.6. PDL-1 Expression in Lung Biopsies
2.7. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.1.1. Patient and Tumor Disease Characteristics
3.1.2. Determinations in Lymphocyte Populations
3.1.3. Determinations in NK Cell Populations
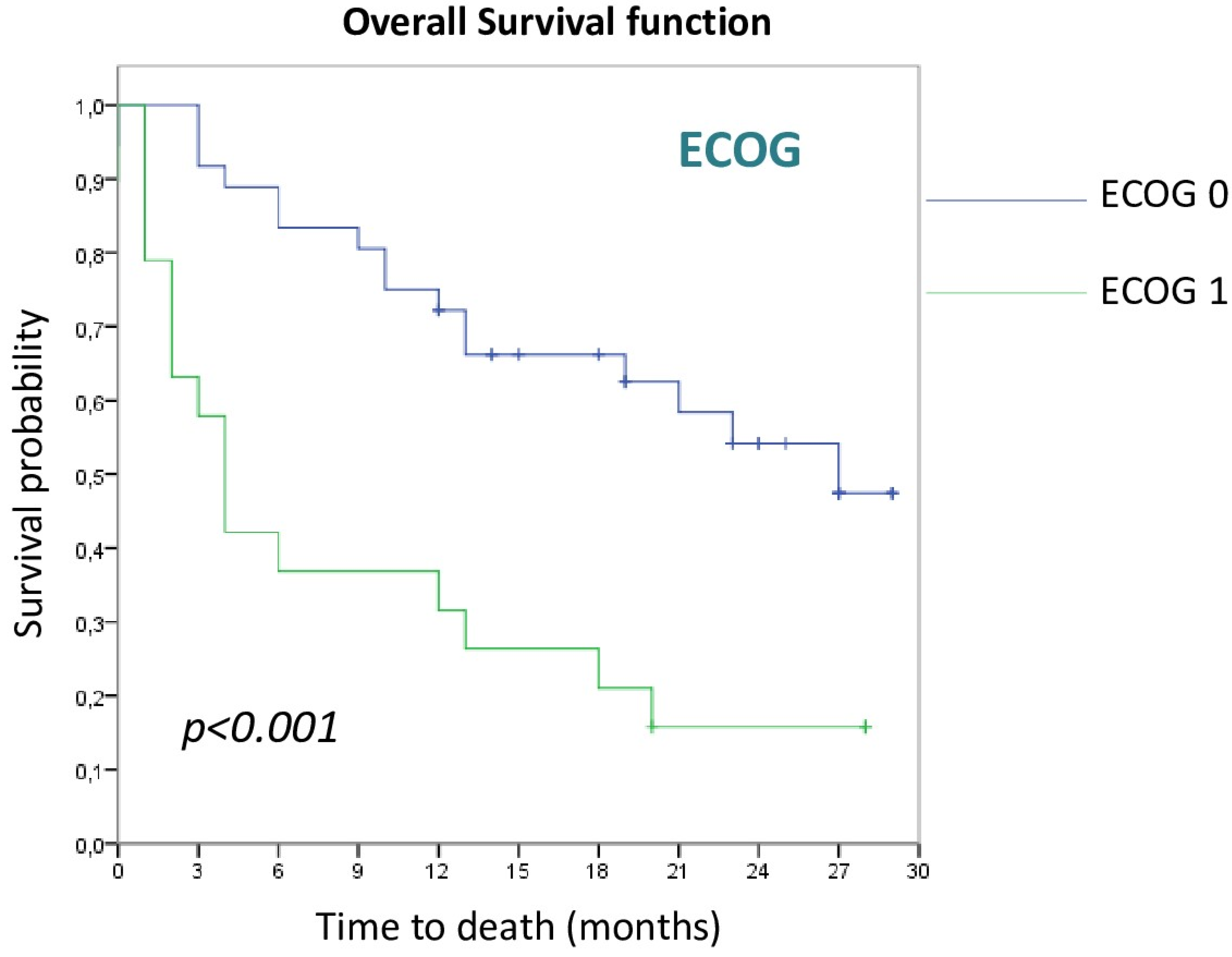
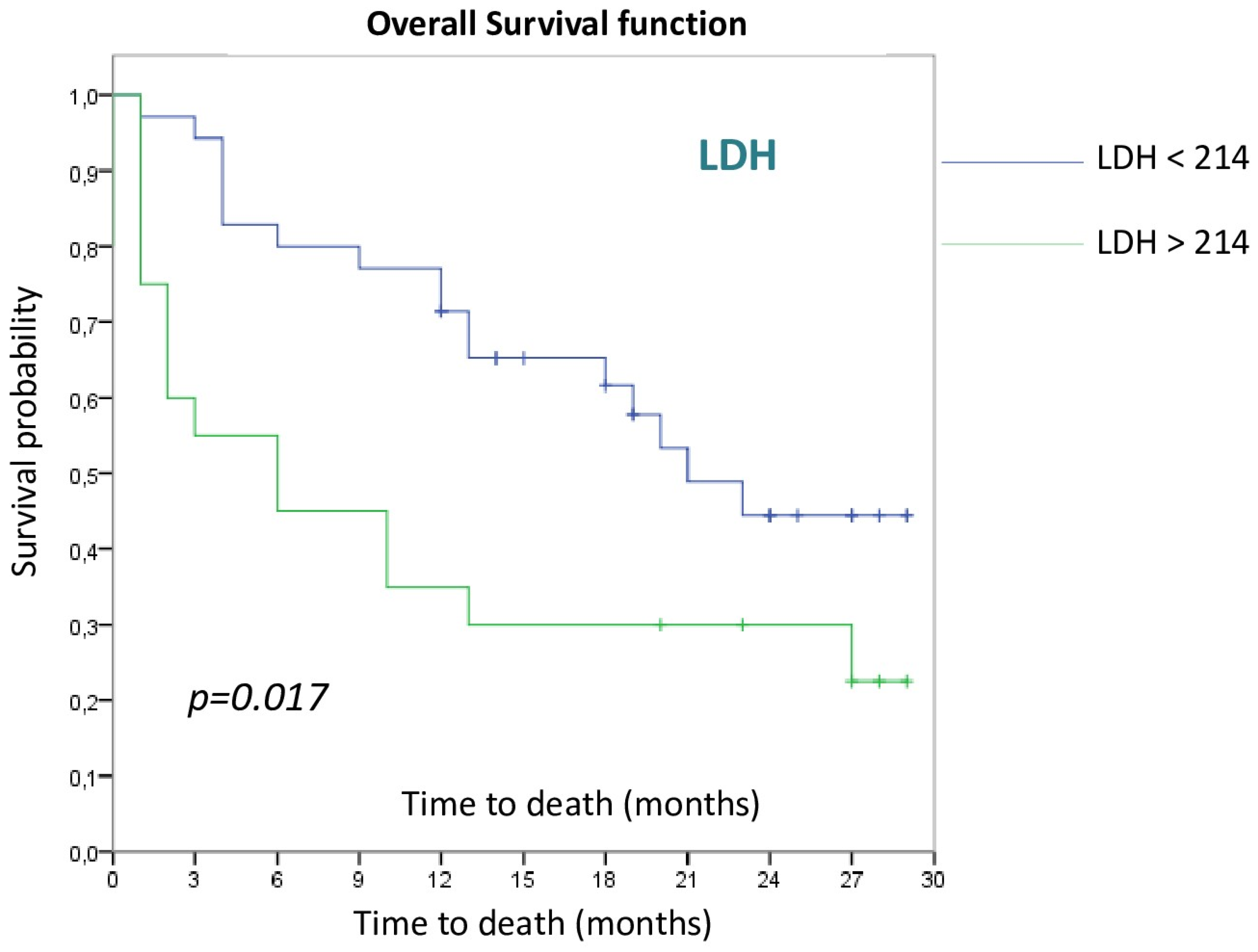
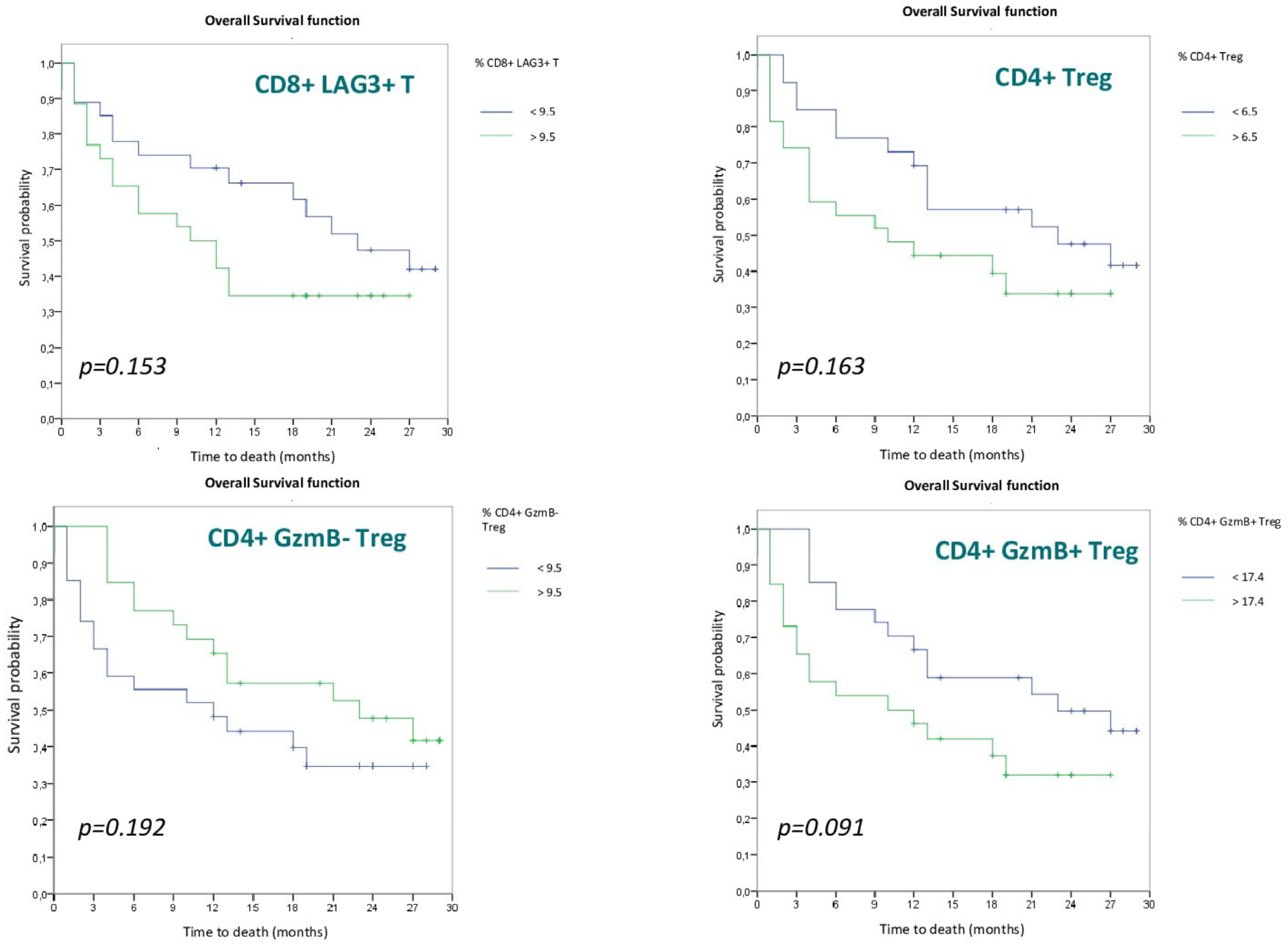
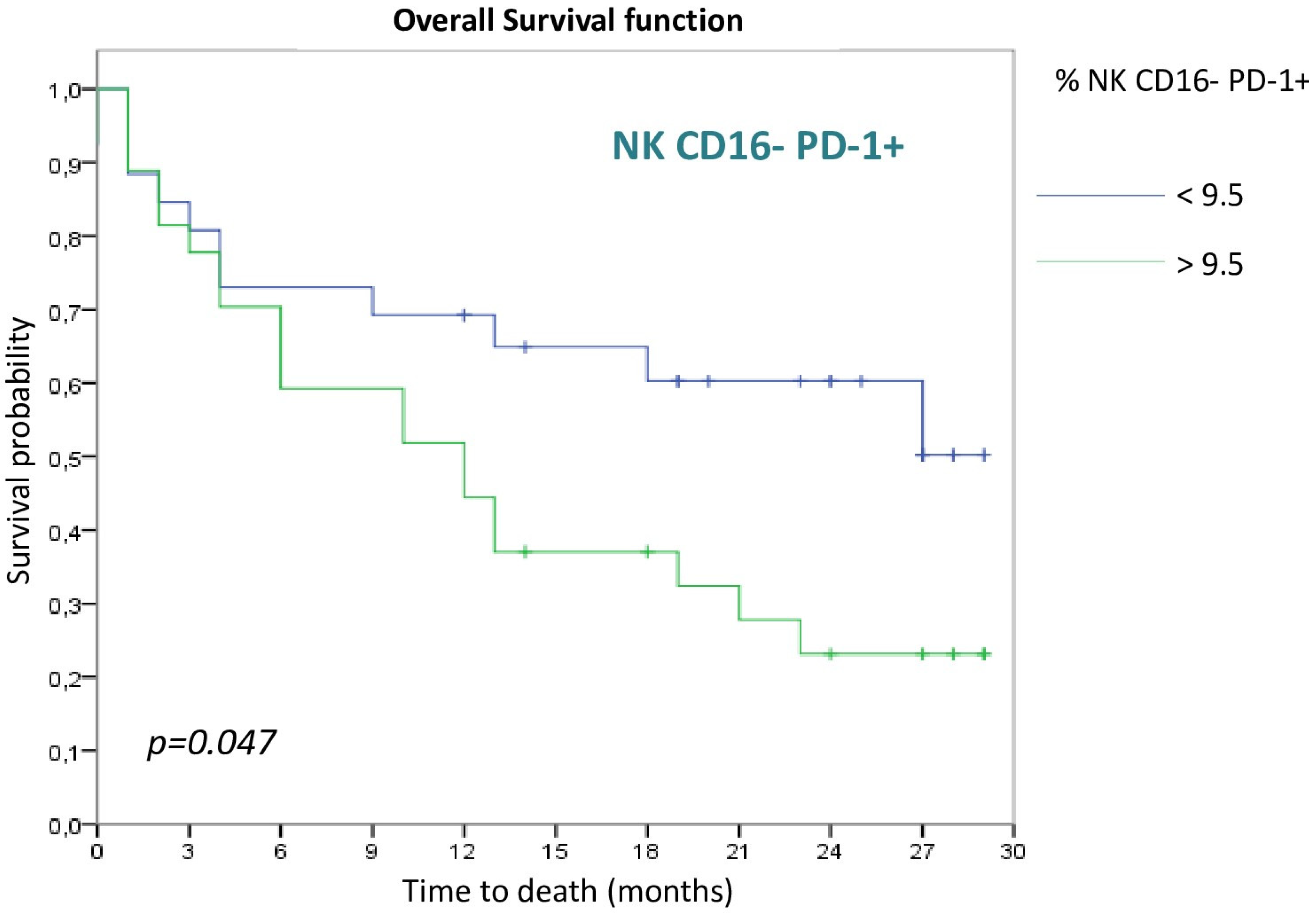
3.2. Survival Analysis
3.2.1. Patient and Tumor Disease Characteristics
3.2.2. Lymphocyte Populations
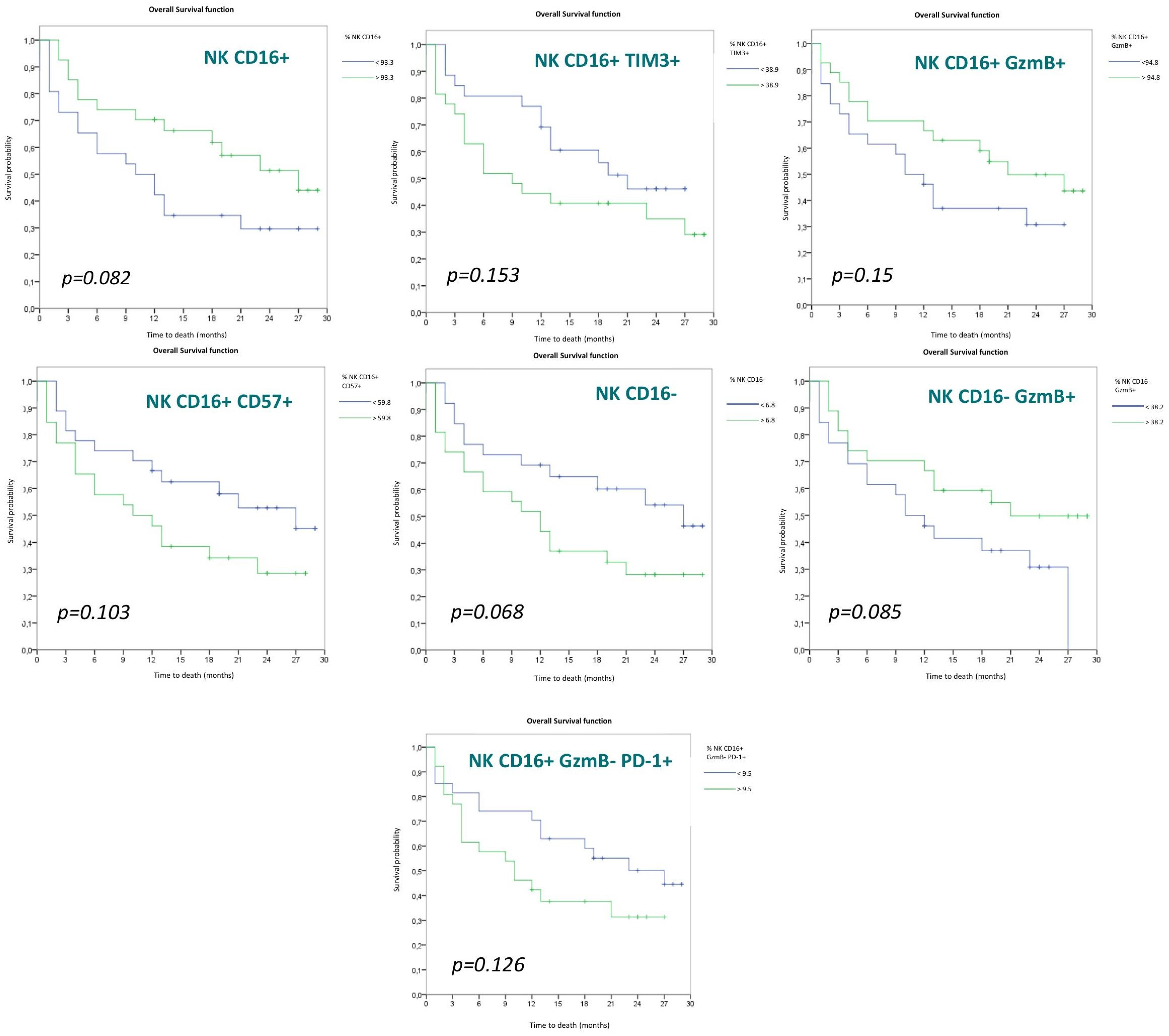
3.2.3. NK Cell Populations
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Provencio-Pulla, M.; Nadal, E.; Larriba, J.L.G.; Martinez-Marti, A.; Bernabé, R.; Bosch-Barrera, J.; Casal, J.; Calvo, V.; Insa, A.; Aix, S.P.; et al. Nivolumab + chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: Primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. J. Clin. Oncol. 2022, 40, 8501. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badalamenti, G.; Fanale, D.; Incorvaia, L.; Barraco, N.; Listi, A.; Maragliano, R.; Vincenzi, B.; Calo, V.; Iovanna, J.L.; Bazan, V.; et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell. Immunol. 2019, 343, 103753. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lostao, L. Estructura y organización del sistema inmunitario: Inmunidad innata y adaptativa. In Inmunología Tumoral e Inmunoterapia del Cáncer, 1st ed.; Antón, A., Ed.; AmazingBooks: Zaragoza, Spain, 2018; pp. 15–44. [Google Scholar]
- Lee, G.R. Phenotypic and Functional Properties of Tumor-Infiltrating Regulatory T Cells. Mediat. Inflamm. 2017, 2017, 5458178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.S.; Lanier, L.L. Natural Killer Cells in Cancer Immunotherapy. Annu. Rev. Cancer Biol. 2019, 3, 77–103. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, S.; Okamoto, Y.; Yoshino, I.; Nakayama, T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin. Immunol. 2011, 140, 167–176. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.; Martinez-Lostao, L.; Santiago, L.; Ferrandez, A.; Granville, D.J.; Pardo, J. The Untold Story of Granzymes in Oncoimmunology: Novel Opportunities with Old Acquaintances. Trends Cancer 2017, 3, 407–422. [Google Scholar] [CrossRef]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Wang, L.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, J. Mecanismos de citotoxicidad del sistema inmunitario. In Inmunología Tumoral e Inmunoterapia del Cáncer, 1st ed.; Antón, A., Ed.; Amazingbooks: Zaragoza, Spain, 2018; pp. 105–120. [Google Scholar]
- de Miguel, D.; Ramirez-Labrada, A.; Uranga, I.; Hidalgo, S.; Santiago, L.; Galvez, E.M.; Arias, M.; Pardo, J. Inflammatory cell death induced by cytotoxic lymphocytes: A dangerous but necessary liaison. FEBS J. 2022, 289, 4398–4415. [Google Scholar] [CrossRef] [PubMed]
- Lievense, L.A.; Sterman, D.H.; Cornelissen, R.; Aerts, J.G. Checkpoint Blockade in Lung Cancer and Mesothelioma. Am. J. Respir. Crit. Care Med. 2017, 196, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.G.; Maggio, I.; Massucci, M.; Mollica, V.; Fragomeno, B.; Ardizzoni, A. ECOG performance status ≥ 2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer 2020, 145, 95–104. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Giroux, D.J.; Chansky, K.; Crowley, J.; Asamura, H.; Goldstraw, P. The IASLC lung cancer staging project: The new database to inform the eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2014, 9, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Majem, M.; Juan, O.; Insa, A.; Reguart, N.; Trigo, J.M.; Carcereny, E.; García-Campelo, R.; García, Y.; Guirado, M.; Provencio, M. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin. Transl. Oncol. 2019, 21, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Ribas, A.; Shin, D.S.; Zaretsky, J.; Frederiksen, J.; Cornish, A.; Avramis, E.; Seja, E.; Kivork, C.; Siebert, J.; Kaplan-Lefko, P.; et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol. Res. 2016, 4, 194–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, M.; Fernández-Villar, A.; Ruano-Raviña, A. Lung Cancer Unrelated to Smoking. Arch. Bronconeumol. 2018, 54, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Lobefaro, R.; Viscardi, G.; Di Liello, R.; Massa, G.; Iacovino, M.L.; Sparano, F.; Della Corte, C.M.; Ferrara, R.; Signorelli, D.; Proto, C.; et al. Immunotherapy in advanced Non-Small Cell Lung Cancer patients with poor performance status: The role of clinical-pathological variables and inflammatory biomarkers. Lung Cancer 2021, 152, 165–173. [Google Scholar] [CrossRef]
- Huemer, F.; Lang, D.; Westphal, T.; Gampenrieder, S.P.; Hutarew, G.; Weiss, L.; Hackl, H.; Lamprecht, B.; Rinnerthaler, G.; Greil, R. Baseline Absolute Lymphocyte Count and ECOG Performance Score Are Associated with Survival in Advanced Non-Small Cell Lung Cancer Undergoing PD-1/PD-L1 Blockade. J. Clin. Med. 2019, 8, 1014. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015, 54, 961–970. [Google Scholar] [CrossRef]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Kazandjian, D.; Gong, Y.; Keegan, P.; Pazdur, R.; Blumenthal, G.M. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1481–1485. [Google Scholar] [CrossRef]
- Nabet, B.Y.; Esfahani, M.S.; Moding, E.J.; Hamilton, E.G.; Chabon, J.J.; Rizvi, H.; Steen, C.B.; Chaudhuri, A.A.; Liu, C.L.; Hui, A.B.; et al. Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition. Cell 2020, 183, 363–376. [Google Scholar] [CrossRef]
- Ottonello, S.; Genova, C.; Cossu, I.; Fontana, V.; Rijavec, E.; Rossi, G.; Biello, F.; Dal Bello, M.G.; Tagliamento, M.; Alama, A.; et al. Association Between Response to Nivolumab Treatment and Peripheral Blood Lymphocyte Subsets in Patients With Non-small Cell Lung Cancer. Front. Immunol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieg, C.; Nowicka, M.; Guglietta, S.; Schindler, S.; Hartmann, F.J.; Weber, L.M.; Dummer, R.; Robinson, M.D.; Levesque, M.P.; Becher, B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 2018, 24, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct pd-1 + cd8 + t cell pool with predictive potential in non-small-cell lung cancer treated with pd-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4(+) T-cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Qin, P.; Fu, X.; Zhang, G.; Yan, X.; Zhang, M.; Zhang, X.; Yang, J.; Wang, H.; Ma, Z. Associations between peripheral blood lymphocyte subsets and clinical outcomes in patients with lung cancer treated with immune checkpoint inhibitor. Ann. Palliat. Med. 2021, 10, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Laza-Briviesca, R.; Cruz-Bermúdez, A.; Nadal, E.; Insa, A.; García-Campelo, M.D.R.; Huidobro, G.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Blood biomarkers associated to complete pathological response on NSCLC patients treated with neoadjuvant chemoimmunotherapy included in NADIM clinical trial. Clin. Transl. Med. 2021, 11, e491. [Google Scholar] [CrossRef]
- Rutkowski, J.; Cyman, M.; Ślebioda, T.; Bemben, K.; Rutkowska, A.; Gruchała, M.; Kmieć, Z.; Pliszka, A.; Zaucha, R. Evaluation of peripheral blood T lymphocyte surface activation markers and transcription factors in patients with early stage non-small cell lung cancer. Cell. Immunol. 2017, 322, 26–33. [Google Scholar] [CrossRef]
- Saavedra, D.; García, B.; Lorenzo-Luaces, P.; González, A.; Popa, X.; Fuentes, K.P.; Mazorra, Z.; Crombet, T.; Neninger, E.; Lage, A. Biomarkers related to immunosenescence: Relationships with therapy and survival in lung cancer patients. Cancer Immunol. Immunother. 2016, 65, 37–45. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Facchinetti, F.; Missale, G.; Canetti, D.; Madeddu, D.; Zecca, A.; Veneziani, M.; Gelsomino, F.; Goldoni, M.; Buti, S.; et al. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer 2019, 127, 153–163. [Google Scholar] [CrossRef]
- de Jonge, K.; Ebering, A.; Nassiri, S.; Hajjami, M.E.; Ouertatani-Sakouhi, H.; Baumgaertner, P.; Speiser, D.E. Circulating CD56(bright) NK cells inversely correlate with survival of melanoma patients. Sci. Rep. 2019, 9, 4487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavilla, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Godet, Y.; Laheurte, C.; Dosset, M.; Galaine, J.; Beziaud, L.; Loyon, R.; Boullerot, L.; Lauret Marie Joseph, E.; Spehner, L.; et al. Circulating NKp46(+) Natural Killer cells have a potential regulatory property and predict distinct survival in Non-Small Cell Lung Cancer. Oncoimmunology 2018, 8, e1527498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, C.M.; White, M.J.; Goodier, M.R.; Riley, E.M. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front. Immunol. 2013, 4, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.J.; Kuo, M.L.; Hsiao, H.S.; Lee, P.T.; Lee, W.I.; Chen, J.Y.; Huang, J.L. Cytotoxic Function and Cytokine Production of Natural Killer Cells and Natural Killer T-Like Cells in Systemic Lupus Erythematosis Regulation with Interleukin-15. Mediat. Inflamm. 2019, 2019, 4236562. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, N.; Ji, N.; Hurez, V.; Curiel, T.J.; Montgomery, M.O.; Braun, A.J.; Nicolas, M.; Aguilera, M.; Kaushik, D.; Liu, Q.; et al. Intratumoral CD56(bright) natural killer cells are associated with improved survival in bladder cancer. Oncotarget 2018, 9, 36492–36502. [Google Scholar] [CrossRef]
| Variable | N (Total = 55) | % |
|---|---|---|
| Sex | ||
| Female | 16 | 29.10% |
| Male | 39 | 70.90% |
| Age | ||
| <75 years | 47 | 85% |
| >75 years | 8 | 15% |
| Smoking habit | ||
| Never smoker | 2 | 3.60% |
| Former smoker/Current smoker | 53 | 96.40% |
| ECOG | ||
| ECOG 0 | 36 | 65.50% |
| ECOG 1 | 19 | 34.50% |
| Histology | ||
| Squamous | 22 | 40% |
| Adenocarcinomas | 33 | 60% |
| Tumor stage | ||
| Stage III | 16 | 29.10% |
| Stage IV | 39 | 70.90% |
| Treatment indication | ||
| Locally advanced | 14 | 25.50% |
| First line | 18 | 32.70% |
| Second line or more | 23 | 41.80% |
| ICI drug | ||
| Durvalumab | 14 | 25.50% |
| Pembrolizumab | 21 | 38.20% |
| Atezolizumab | 18 | 32.70% |
| Nivolumab | 2 | 3.60% |
| Objective Response | ||
| Complete response | 10 | 18.80% |
| Partial response | 13 | 23.60% |
| Stable disease | 12 | 21.8% |
| Progressive disease | 15 | 27.30% |
| Not evaluable | 5 | 9.10% |
| LDH | 36.40% | |
| High (>202U/L) | 20 | 36.40% |
| Normal (<202U/L) | 35 | 63.60% |
| PD-L1 | ||
| <1% | 10 | 18.20% |
| 1–49% | 21 | 38.20% |
| >50% | 16 | 29.10% |
| Unknow | 8 | 14.50% |
| Lymphocyte Population | Median (%) | Standard Deviation (%) |
|---|---|---|
| CD8+ T | 28.5 | 25.8 |
| CD8+ PD-1+ T | 9.5 | 18 |
| CD8+ TIM3+ T | 4.1 | 7.2 |
| CD8+ LAG3 + T | 9.5 | 18.4 |
| CD8+ GzmB+ T | 31.7 | 25.5 |
| CD8+ GzmB+ PD-1+ T | 1.3 | 10.7 |
| CD8+ GzmB+ TIM3+ T | 1.1 | 4.6 |
| CD8+ GzmB+ LAG3+ T | 2.1 | 8.8 |
| CD8+ GzmB− T | 68.3 | 25.4 |
| CD8+ GzmB− TIM3+ T | 2.2 | 5.3 |
| CD8+ GzmB− LAG3+ T | 6.5 | 9.6 |
| CD8+ GzmB− PD-1+ T | 4.7 | 6.7 |
| CD8+ Tem | 3.5 | 9.5 |
| CD4+ T | 29.6 | 24.4 |
| CD4+ Treg | 6.5 | 8.8 |
| CD4+ GzmB− Treg | 82.6 | 24.4 |
| CD4+ GzmB+Treg | 17.4 | 24.4 |
| Coeficient CD8+ T/CD4+ T | 1 | 2.3 |
| NK Cell Populations | Median (%) | Standard Deviation (%) |
|---|---|---|
| NK (CD56+CD3−) | 15.7 | 11.4 |
| NKT (CD56+CD3+) | 7.5 | 9.2 |
| NK CD16+ | 93.3 | 17.5 |
| NK CD16+ TIM3+ | 38.9 | 24.3 |
| NK CD16+ LAG3+ | 16.6 | 25 |
| NK CD16+ PD-1+ | 5.9 | 15.3 |
| NK CD16+ GzmB+ | 94.8 | 18.6 |
| NK CD16+ GzmB+ TIM3+ | 32.3 | 20.7 |
| NK CD16+ GzmB+ LAG3+ | 21.3 | 19.1 |
| NK CD16+ GzmB+ PD-1+ | 5.3 | 17.8 |
| NK CD16+ GzmB− | 4.1 | 17.4 |
| NK CD16+ GzmB− TIM3+ | 1 | 8.1 |
| NK CD16+ GzmB− LAG3+ | 0.1 | 8.6 |
| NK CD16+ GzmB− PD-1+ | 0.2 | 5.4 |
| NK CD16+ CD57+ | 59.8 | 17.9 |
| NK CD16+ NKp46+ | 74.2 | 23.4 |
| NK CD16+ NKG2D+ | 69 | 33 |
| NK CD16+ NKG2A+ | 45.9 | 21.9 |
| NK CD16− | 6.8 | 17.5 |
| NK CD16− GzmB+ | 38.2 | 39.7 |
| NK CD16− PD-1+ | 9.5 | 15.3 |
| NK CD16− LAG3+ | 5 | 13.8 |
| NK CD16− TIM3+ | 40.7 | 22.5 |
| Covariates | HR | CI (HR) 95% | p-Value |
|---|---|---|---|
| ECOG 1 | 3.247 | 1.606–6.562 | 0.001 |
| Stage IV | 6.539 | 1.983–21.565 | 0.002 |
| TI locally advanced | - | - | 0.001 |
| TI first line | 5.063 | 1.108–23.126 | 0.036 |
| TI second line and more | 12.840 | 2.962–55.656 | 0.001 |
| Durvalumab | - | - | 0.001 |
| Nivolumab | 3.336 | 0.302–36.831 | 0.325 |
| Pembrolizumab | 5.668 | 1.266–25.376 | 0.023 |
| Atezolizumab | 15.635 | 3.543–68.922 | <0.001 |
| Complete response | - | - | <0.001 |
| Partial response | 4.582 | 0.534–39.348 | 0.165 |
| Stable disease | 8.384 | 1.026–68.509 | 0.047 |
| Progressive disease | 37.880 | 4.830–297.063 | 0.001 |
| Not evaluable | 377.216 | 33.191–4287.125 | <0.001 |
| High LDH > 214 U/L | 2.262 | 1.124–4.551 | 0.022 |
| NK CD16- PD-1+ (>9.5) | 2.052 | 0.980–4.295 | 0.056 |
| Covariates | HR | HR 95% CI | p-Value |
|---|---|---|---|
| ECOG 1 | 2.496 | 1.155–5.391 | 0.02 |
| Stage IV | 9.929 | 1.193–82.625 | 0.034 |
| Durvalumab | 0.454 | 0.037–5.540 | 0.536 |
| Nivolumab | 0.199 | 0.024–1.657 | 0.135 |
| Pembrolizumab | 0.233 | 0.098–0.557 | 0.001 |
| Atezolizumab | - | - | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gascón-Ruiz, M.; Ramírez-Labrada, A.; Lastra, R.; Martínez-Lostao, L.; Paño-Pardo, J.R.; Sesma, A.; Zapata-García, M.; Moratiel, A.; Quílez, E.; Torres-Ramón, I.; et al. A Subset of PD-1-Expressing CD56bright NK Cells Identifies Patients with Good Response to Immune Checkpoint Inhibitors in Lung Cancer. Cancers 2023, 15, 329. https://doi.org/10.3390/cancers15020329
Gascón-Ruiz M, Ramírez-Labrada A, Lastra R, Martínez-Lostao L, Paño-Pardo JR, Sesma A, Zapata-García M, Moratiel A, Quílez E, Torres-Ramón I, et al. A Subset of PD-1-Expressing CD56bright NK Cells Identifies Patients with Good Response to Immune Checkpoint Inhibitors in Lung Cancer. Cancers. 2023; 15(2):329. https://doi.org/10.3390/cancers15020329
Chicago/Turabian StyleGascón-Ruiz, Marta, Ariel Ramírez-Labrada, Rodrigo Lastra, Luis Martínez-Lostao, J. Ramón Paño-Pardo, Andrea Sesma, María Zapata-García, Alba Moratiel, Elisa Quílez, Irene Torres-Ramón, and et al. 2023. "A Subset of PD-1-Expressing CD56bright NK Cells Identifies Patients with Good Response to Immune Checkpoint Inhibitors in Lung Cancer" Cancers 15, no. 2: 329. https://doi.org/10.3390/cancers15020329
APA StyleGascón-Ruiz, M., Ramírez-Labrada, A., Lastra, R., Martínez-Lostao, L., Paño-Pardo, J. R., Sesma, A., Zapata-García, M., Moratiel, A., Quílez, E., Torres-Ramón, I., Yubero, A., Domingo, M. P., Esteban, P., Gálvez, E. M., Pardo, J., & Isla, D. (2023). A Subset of PD-1-Expressing CD56bright NK Cells Identifies Patients with Good Response to Immune Checkpoint Inhibitors in Lung Cancer. Cancers, 15(2), 329. https://doi.org/10.3390/cancers15020329









