Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Objective
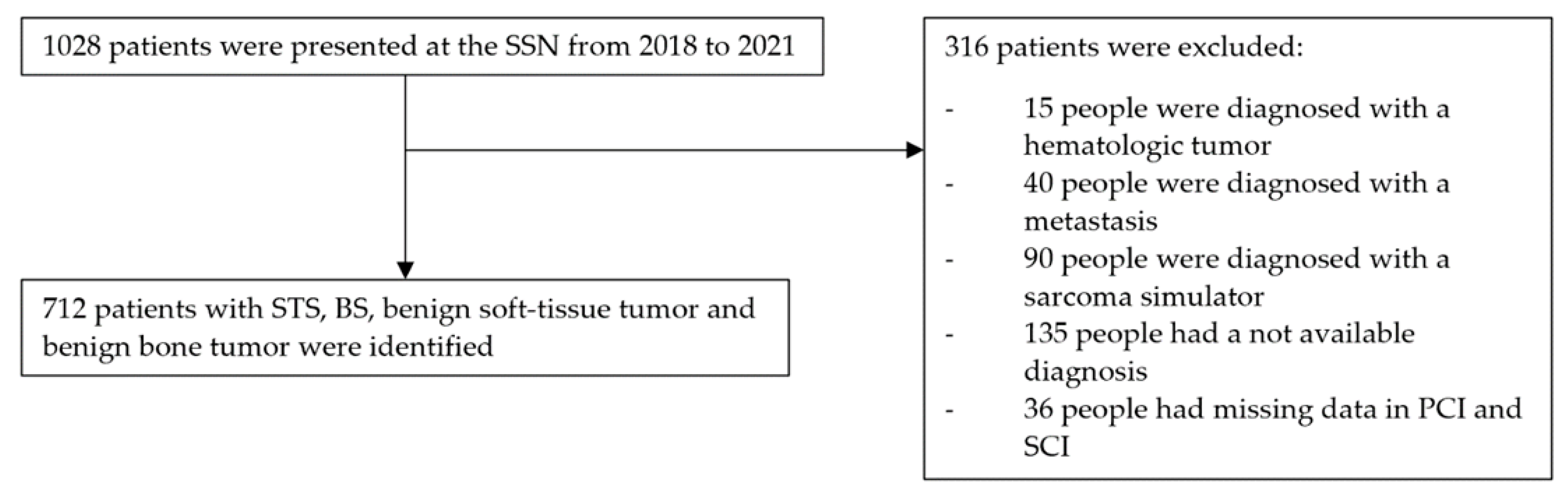
2.3. Selection Criteria
2.4. Data Collection
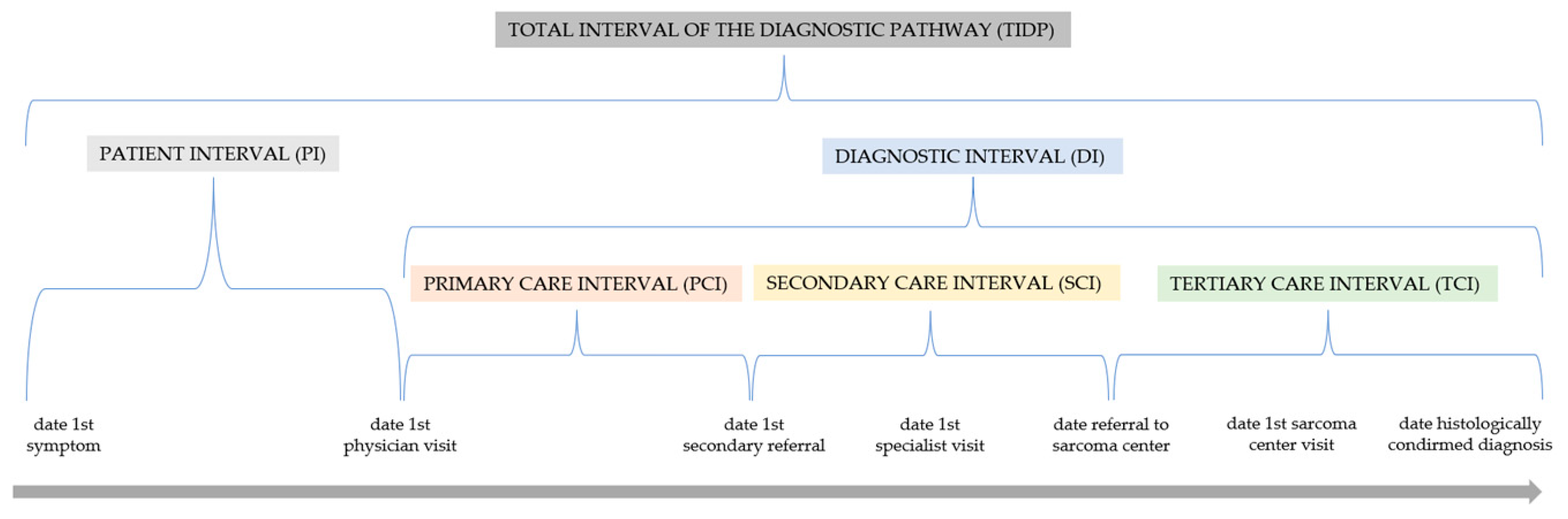
2.5. Definition of the Intervals
2.6. Statistical Analysis
3. Results
3.1. Diagnosis Probability Based on Patient and Tumor Traits (See Table 1)
3.2. Patient Interval (PI)
3.2.1. Length (See Table 2)
3.2.2. Influencing Parameters (See Table 3)
3.3. Diagnostic Interval (DI), Primary Care Interval (PCI)
3.3.1. Length (See Table 2)
3.3.2. Influencing Parameters (See Table 3)
3.4. Diagnostic Interval (DI), Secondary Care Interval (SCI)
3.4.1. Length (See Table 2)
3.4.2. Influencing Parameters (See Table 3)
3.5. Diagnostic Interval (DI), Tertiary Care Interval (TCI)
3.5.1. Length (See Table 2)
3.5.2. Influencing Parameters (See Table 3)
3.6. Total Interval (TI)
3.6.1. Length (See Table 2)
3.6.2. Influencing Factors (See Table 3)
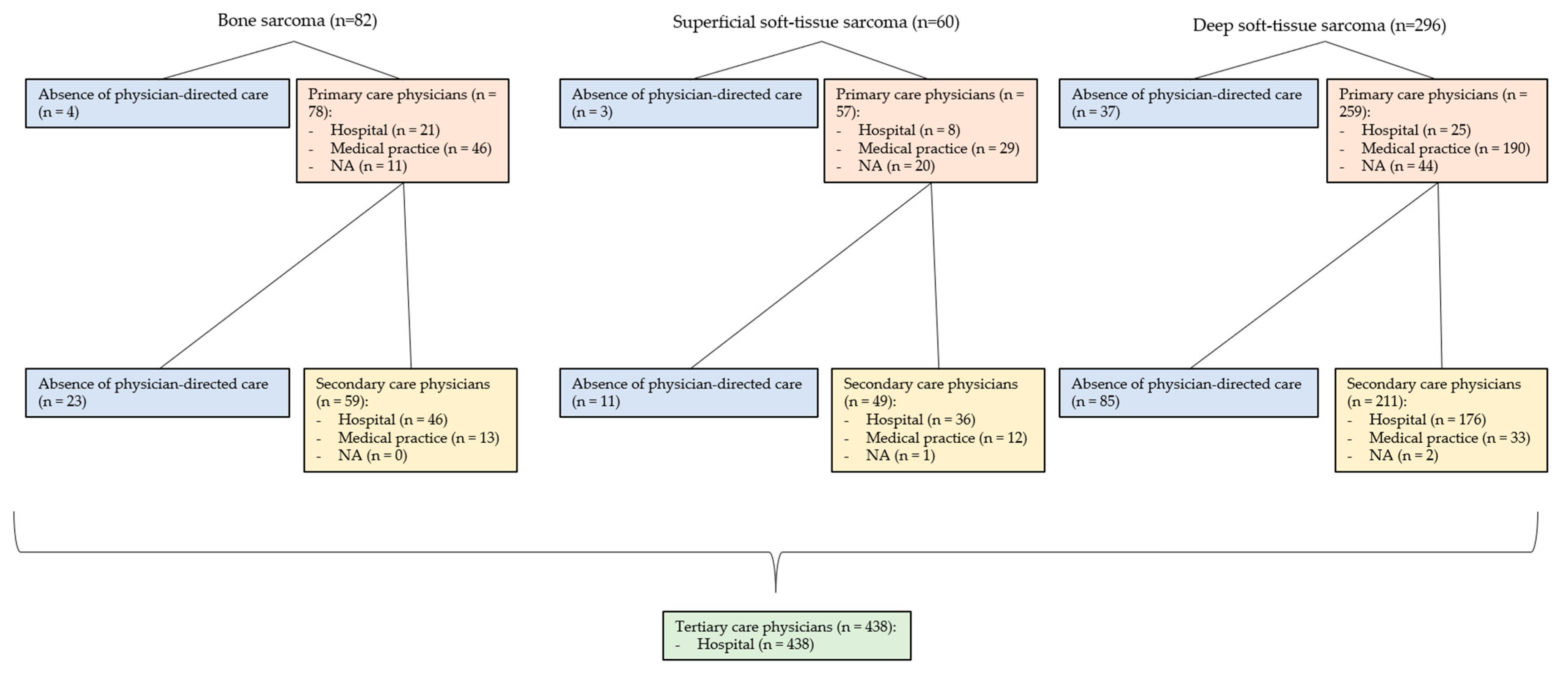
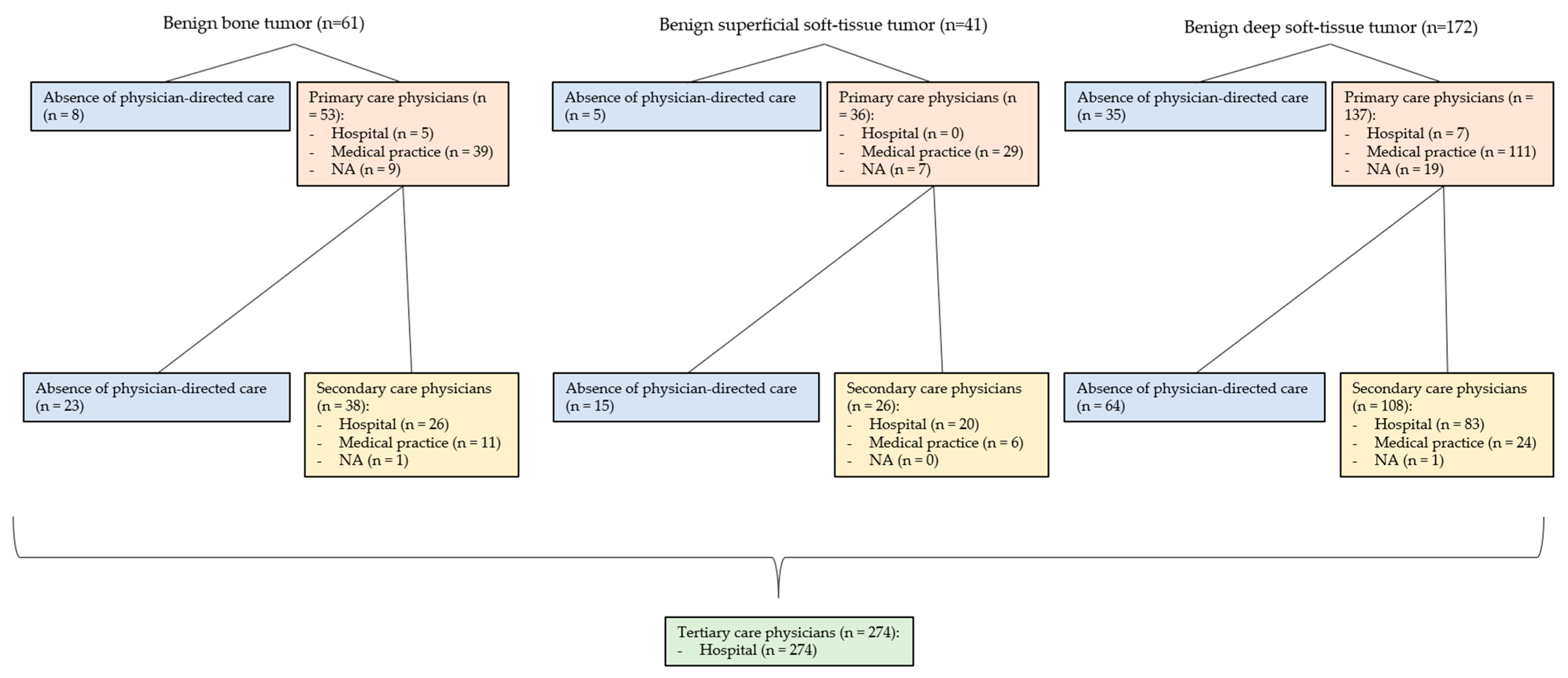
3.7. Involved Physicians in the Primary Care Interval (PCI) (See Figure 3 and Figure 4)
3.8. Involved Physicians in the Secondary Care Interval (SCI) (See Figure 3 and Figure 4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. OECD Health at a Glance 2021: OECD Indicators; OECD: Paris, France, 2021. [Google Scholar]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Kollár, A.; Rothermundt, C.; Klenke, F.; Bode, B.; Baumhoer, D.; Arndt, V.; Feller, A. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019, 63, 101596. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2020, 28, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet—A population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- Soomers, V.; Husson, O.; Young, R.; Desar, I.; Van der Graaf, W. The sarcoma diagnostic interval: A systematic review on length, contributing factors and patient outcomes. ESMO Open 2020, 5, e000592. [Google Scholar] [CrossRef]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Olesen, F.; Hansen, R.P.; Vedsted, P. Delay in diagnosis: The experience in Denmark. Br. J. Cancer 2009, 101 (Suppl. 2), S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Kissin, M.W.; Fisher, C.; Webb, A.J.; Westbury, G. Value of fine needle aspiration cytology in the diagnosis of soft tissue tumours: A preliminary study on the excised specimen. Br. J. Surg. 1987, 74, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Montesco, M.C.; Coindre, J.M.; Tos, A.P.D.; Lurkin, A.; Ranchère-Vince, D.; Vecchiato, A.; Decouvelaere, A.V.; Mathoulin-Pélissier, S.; Albert, S.; et al. Sarcoma: Concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann. Oncol. 2012, 23, 2442–2449. [Google Scholar] [CrossRef]
- Thway, K.; Wang, J.; Mubako, T.; Fisher, C. Histopathological Diagnostic Discrepancies in Soft Tissue Tumours Referred to a Specialist Centre: Reassessment in the Era of Ancillary Molecular Diagnosis. Sarcoma 2014, 2014, 686902. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Tos, A.P.D. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- McPhail, S.; Johnson, S.; Greenberg, D.; Peake, M.; Rous, B. Stage at diagnosis and early mortality from cancer in England. Br. J. Cancer 2015, 112, S108–S115. [Google Scholar] [CrossRef] [PubMed]
- Seinen, J.; Almquist, M.; Styring, E.; Rydholm, A.; Nilbert, M. Delays in the Management of Retroperitoneal Sarcomas. Sarcoma 2010, 2010, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Widhe, B.; Bauer, H.C.F. Diagnostic difficulties and delays with chest wall chondrosarcoma: A Swedish population based Scandinavian Sarcoma Group study of 106 patients. Acta Oncol. 2011, 50, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Roscoe, J.; Ryder, W.; Gattamaneni, H.; Eden, T. Symptom interval in young people with bone cancer. Eur. J. Cancer 2004, 40, 2280–2286. [Google Scholar] [CrossRef]
- Widhe, B.; Widhe, T.; Bauer, H.C.F. Ewing sarcoma of the rib—Initial symptoms and clinical features: Tumor missed at the first visit in 21 of 26 patients. Acta Orthop. 2007, 78, 840–844. [Google Scholar] [CrossRef]
- Brotzmann, M.; Hefti, F.; Baumhoer, D.; Krieg, A.H. Do Malignant Bone Tumors of the Foot Have a Different Biological Behavior than Sarcomas at Other Skeletal Sites? Sarcoma 2013, 2013, 767960. [Google Scholar] [CrossRef]
- Brasme, J.-F.; Chalumeau, M.; Oberlin, O.; Valteau-Couanet, D.; Gaspar, N. Time to Diagnosis of Ewing Tumors in Children and Adolescents Is Not Associated With Metastasis or Survival: A Prospective Multicenter Study of 436 Patients. J. Clin. Oncol. 2014, 32, 1935–1940. [Google Scholar] [CrossRef]
- Simpson, P.M.S.; Reid, R.; Porter, D. Ewing's Sarcoma of the Upper Extremity: Presenting Symptoms, Diagnostic Delay and Outcome. Sarcoma 2005, 9, 15–20. [Google Scholar] [CrossRef]
- Wurtz, L.D.; Peabody, T.D.; Simon, M.A. Delay in the Diagnosis and Treatment of Primary Bone Sarcoma of the Pelvis*. J. Bone Jt. Surg. 1999, 81, 317–325. [Google Scholar] [CrossRef]
- Nandra, R.; Hwang, N.; Matharu, G.; Reddy, K.; Grimer, R. One-year mortality in patients with bone and soft tissue sarcomas as an indicator of delay in presentation. Ind. Mark. Manag. 2015, 97, 425–433. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Grimer, R. Early symptoms of bone and soft tissue sarcomas: Could they be diagnosed earlier? Ind. Mark. Manag. 2012, 94, 261–266. [Google Scholar] [CrossRef]
- Smith, G.; Johnson, G.; Grimer, R.; Wilson, S. Trends in presentation of bone and soft tissue sarcomas over 25 years: Little evidence of earlier diagnosis. Ind. Mark. Manag. 2011, 93, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J. Size Matters for Sarcomas! Ind. Mark. Manag. 2006, 88, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mullen, C.A. Patterns of Diagnosis and Misdiagnosis in Pediatric Cancer and Relationship to Survival. J. Pediatr. Hematol. 2017, 39, e110–e115. [Google Scholar] [CrossRef] [PubMed]
- Lawrenz, J.M.; Styron, J.F.; Parry, M.; Grimer, R.J.; Mesko, N.W. Longer duration of symptoms at the time of presentation is not associated with worse survival in primary bone sarcoma. Bone Jt. J. 2018, 100-B, 652–661. [Google Scholar]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities or Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef]
- Balmant, N.V.; Silva, N.d.P.; Santos, M.d.O.; Reis, R.d.S.; de Camargo, B. Delays in the health care system for children, adolescents, and young adults with bone tumors in Brazil. J. De Pediatr. 2019, 95, 744–751. [Google Scholar] [CrossRef]
- Desandes, E.; Brugières, L.; Molinié, F.; Defossez, G.; Delafosse, P.; Jehannin-Ligier, K.; Velten, M.; Trétarre, B.; Amadéo, B.; Marrer, E.; et al. Adolescent and young adult oncology patients in France: Heterogeneity in pathways of care. Pediatr. Blood Cancer 2018, 65, e27235. [Google Scholar] [CrossRef]
- Yang, J.Y.K.; Cheng, F.W.T.; Wong, K.C.; Lee, V.; Leung, W.K.; Shing, M.M.K.; Kumta, S.M.; Li, C.K. Initial presentation and management of osteosarcoma, and its impact on disease outcome. Hong Kong Med. J. 2009, 15, 434–439. [Google Scholar]
- Rougraff, B.T.; Lawrence, J.; Davis, K. Length of Symptoms Before Referral: Prognostic Variable for High-grade Soft Tissue Sarcoma? Clin. Orthop. Relat. Res. 2012, 470, 706–711. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.V.C.; Barrett, A.; Reid, R. Premonitory Pain Preceding Swelling: A Distinctive Clinical Presentation of Synovial Sarcoma which may Prompt Early Detection. Sarcoma 2003, 7, 131–135. [Google Scholar] [CrossRef]
- Clark, M.; Thomas, J. Delay in referral to a specialist soft-tissue sarcoma unit. Eur. J. Surg. Oncol. (EJSO) 2005, 31, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.D.; Smith, G.; Dramis, A.; Grimer, R.J. Delays in Referral of Soft Tissue Sarcomas. Sarcoma 2008, 2008, 378574. [Google Scholar] [CrossRef]
- Bruun, J.P. Time lapse by diagnosis of oral cancer. Oral Surgery, Oral Med. Oral Pathol. 1976, 42, 139–149. [Google Scholar] [CrossRef]
- Chotel, F.; Unnithan, A.; Chandrasekar, C.R.; Parot, R.; Jeys, L.; Grimer, R.J. Variability in the presentation of synovial sarcoma in children. J. Bone Jt. Surg. 2008, 90-B, 1090–1096. [Google Scholar]
- Durve, D.; Kanegaonkar, R.; Albert, D.; Levitt, G. Paediatric rhabdomyosarcoma of the ear and temporal bone. Clin. Otolaryngol. 2004, 29, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Monnier, D.; Vidal, C.; Martin, L.; Danzon, A.; Pelletier, F.; Puzenat, E.; Algros, M.; Blanc, D.; Laurent, R.; Humbert, P.; et al. Dermatofibrosarcoma protuberans: A population-based cancer registry descriptive study of 66 consecutive cases diagnosed between 1982 and 2002. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1237–1242. [Google Scholar] [CrossRef]
- Martin, S.; Ulrich, C.; Munsell, M.; Taylor, S.; Lange, G.; Bleyer, A. Delays in Cancer Diagnosis in Underinsured Young Adults and Older Adolescents. Oncol. 2007, 12, 816–824. [Google Scholar] [CrossRef]
- Soomers, V.L.M.N.; Husson, O.; Desar, I.M.E.; van de Sande, M.A.J.; de Haan, J.J.; Verhoef, C.; Vriens, I.J.H.; van Houdt, W.J.; van de Poll-Franse, L.; van der Graaf, W.T.A. Patient and diagnostic intervals of survivors of sarcoma: Results from the SURVSARC study. Cancer 2020, 126, 5283–5292. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Saunders, C.L.; A Abel, G.; McPhail, S.; Neal, R.D.; Wardle, J.; Rubin, G.P. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br. J. Cancer 2015, 112 (Suppl. S1), S35–S40. [Google Scholar] [CrossRef]
- Sneppen, O.; Hansen, L.M. Presenting Symptoms and Treatment Delay in Osteosarcoma and Ewing's Sarcoma. Acta Radiol. Oncol. 1984, 23, 159–162. [Google Scholar] [CrossRef]
- Guerra, R.B.; Tostes, M.D.; Miranda, L.d.C.; de Camargo, O.P.; Baptista, A.M.; Caiero, M.T.; Machado, T.M.d.S.; Abadi, M.D.; de Oliveira, C.R.G.M.; Filippi, R.Z. Comparative analysis between osteosarcoma and ewing’s sarcoma: Evaluation of the time from onset of signs and symptoms until diagnosis. Clinics 2006, 61, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Parry, M.; Jeys, L.; Abudu, S.; Grimer, R. Synovial sarcoma: Do children do better? Eur. J. Surg. Oncol. (EJSO) 2019, 45, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Panchabhai, T.S.; Bajaj, N.S.; Patil, P.D.; Bunte, M.C. Primary pulmonary artery sarcoma: A close associate of pulmonary embolism—20-year observational analysis. J. Thorac. Dis. 2016, 8, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Miceli, R.; Casanova, M.; Meazza, C.; Favini, F.; Luksch, R.; Catania, S.; Fiore, M.; Morosi, C.; Mariani, L. The symptom interval in children and adolescents with soft tissue sarcomas. Cancer 2010, 116, 177–183. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, X.; Zhu, Z.; Fu, Y.; Hu, Y. How Patients with an Uncertain Diagnosis Experience Intolerance of Uncertainty: A Grounded Theory Study. Psychol. Res. Behav. Manag. 2021, 14, 1269–1279. [Google Scholar] [CrossRef]
- van Harten, W. Turning teams and pathways into integrated practice units: Appearance characteristics and added value. Int. J. Care Co-ord. 2018, 21, 113–116. [Google Scholar] [CrossRef]
- Porter, M.E.; Lee, T.H. Integrated Practice Units: A Playbook for Health Care Leaders. NEJM Catal. Innov. Care Deliv. 2021, 2. [Google Scholar] [CrossRef]
- Theus-Steinmann, C.; Fuchs, B.; Studer, G.; Bode, B.; Wellauer, H.; Frei, A.; Theus, C.; Schüpfer, G.; Plock, J.; Windegger, H.; et al. Development of a value-based healthcare delivery model for sarcoma patients. Swiss Med. Wkly. 2021, 151, w30047. [Google Scholar]
- Berwick, D.M.; Nolan, T.W.; Whittington, J. The Triple Aim: Care, Health, And Cost. Health Aff. 2008, 27, 759–769. [Google Scholar] [CrossRef]
| OVERALL | BONE SARCOMA | LIKELIHOOD OF BONE SARCOMA VS. SOFT- TISSUE SARCOMA a | SOFT-TISSUE SARCOMA | LIKELIHOOD OF SARCOMA VS. BENIGN TUMOR b | BENIGN BONE TUMOR | BENIGN SOFT-TISSUE TUMOR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | Deep and Superficial | Deep | Superficial | OR | 95% CI | p-Value | Deep and Superficial | Deep | Superficial | ||||
| n = 712 | n = 82 | n = 356 | n = 296 | n = 60 | n = 61 | n = 213 | n = 172 | n = 41 | |||||||
| Age, years | 56.0 (40.0, 68.0) | 44.0 (19.0, 65.0) | 1.03 | 1.02, 1.04 | <0.001 | 60.0 (46.0, 72.0) | 60.0 (46.0, 72.0) | 61.5 (42.3, 74.3) | 1.02 | 1.01, 1.02 | <0.001 | 34.0 (23.0, 45.0) | 55.0 (44.0, 63.0) | 56.0 (44.0, 65.0) | 54.0 (44.0, 61.0) |
| Female, (%) | 348 (48.9%) | 30 (36.6%) | 1.09 | 0.82, 1.47 | 0.5 | 178 (50.0%) | 145 (49.0%) | 33 (55.0%) | 0.87 | 0.64, 1.17 | 0.3 | 30 (49.2%) | 110 (51.6%) | 90 (52.3%) | 20 (48.8%) |
| Grade | not applicable | not applicable | not applicable | not applicable | |||||||||||
| G1, (%) | 74 (16.9%) | 7 (8.5%) | 67 (18.8%) | 52 (17.6%) | 15 (25.0%) | ||||||||||
| G2, (%) | 54 (12.3%) | 5 (6.1%) | 1.17 | 0.37, 4.07 | 0.8 | 49 (13.8%) | 41 (13.9%) | 8 (13.3%) | |||||||
| G3, (%) | 126 (28.8%) | 26 (31.7%) | 0.46 | 0.18, 1.03 | 0.07 | 100 (28.1%) | 85 (28.7%) | 15 (25.0%) | |||||||
| NA | 184 (42.0%) | 44 (53.7%) | 140 (39.3%) | 118 (39.8%) | 22 (36.7%) | ||||||||||
| Region | 2.91 | 2.11, 4.04 | <0.001 | 2.34 | 1.67, 3.30 | <0.001 | |||||||||
| appendicular | 469 (65.9%) | 65 (79.3%) | 193 (54.2%) | 165 (55.7%) | 28 (46.7%) | 50 (82.0%) | 161 (75.6%) | 131 (76.2%) | 30 (73.2%) | ||||||
| axial | 243 (34.1%) | 17 (20.7%) | 163 (45.8%) | 131 (44.3%) | 32 (53.3%) | 11 (18.0%) | 52 (24.4%) | 41 (23.8%) | 11 (26.8%) | ||||||
| Size, mm | 60.0 (34.3, 102.0) | 60.0 (39.5, 85.0) | 1.01 | 1.00, 1.01 | <0.001 | 70.0 (32.0, 124.0) | 86.0 (45.0, 130.0) | 28.0 (20.0, 44.0) | 1.00 | 1.00, 1.01 | 0.001 | 31.5 (11.5, 50.5) | 60.0 (38.3, 97.3) | 61.0 (39.0, 100.5) | 54.0 (35.5, 79.5) |
| 0–50 mm, n | 247 (34.7%) | 25 (30.5%) | 106 (29.8%) | 70 (23.6%) | 36 (60.0%) | 33 (54.1%) | 83 (39.0%) | 65 (37.8%) | 18 (43.9%) | ||||||
| 51–100 mm, n | 179 (25.1%) | 30 (36.6%) | 67 (18.8%) | 59 (19.9%) | 8 (13.3%) | 10 (16.4%) | 72 (33.8%) | 55 (32.0%) | 17 (41.5%) | ||||||
| 101–150 mm, n | 91 (12.8%) | 11 (13.4%) | 50 (14.0%) | 50 (16.9%) | 0 (0%) | 1 (1.6%) | 29 (13.6%) | 27 (15.7%) | 2 (4.9%) | ||||||
| >150 mm, n | 57 (8.0%) | 1 (1.2%) | 42 (11.8%) | 41 (13.9%) | 1 (1.7%) | 0 (0%) | 14 (6.6%) | 13 (7.5%) | 1 (2.4%) | ||||||
| NA | 138 (19.4%) | 15 (18.3%) | 91 (25.6%) | 76 (25.7%) | 15 (25.0%) | 17 (27.9%) | 15 (7.0%) | 12 (7.0%) | 3 (7.3%) | ||||||
| OVERALL | BONE SARCOMA | SOFT-TISSUE SARCOMA | BENIGN BONE TUMOR | BENIGN SOFT-TISSUE TUMOR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deep and Superficial | Deep | Superficial | Deep and Superficial | Deep | Superficial | ||||||||
| n = 712 | n = 82 | p-Value c | n = 356 | n = 296 | p- Value d | n = 60 | n = 61 | p- Value e | n = 213 | n = 172 | p- Value f | n = 41 | |
| Patient Interval, weeks | 90.0 (22.0, 284.0) | 7.8 (2.7, 27.5) | 0.46 | 8.8 (2.1, 29.0) | 8.3 (2.0, 24.4) | 0.01 | 20.7 (4.2, 130.6) | 19.1 (4.3, 52.1) | 0.17 | 21.6 (6.4, 109.6) | 19.8 (6.3, 75.1) | 0.22 | 29.9 (9.0, 176.4) |
| Diagnostic Interval, weeks | 46.0 (25.5, 95.5) | 7.6 (3.1, 14.2) | 0.89 | 6.7 (3.7, 13.3) | 6.9 (3.9, 13.7) | 0.22 | 5.7 (3.6, 9.3) | 19.8 (6.8, 79.7) | 0.005 | 6.0 (3.6, 13.4) | 6.0 (3.6, 14.6) | 0.35 | 5.6 (3.6, 9.5) |
| Primary Care Interval, weeks | 4.0 (0.0, 18.5) | 0.6 (0.1, 6.5) | 0.14 | 0.4 (0.0, 1.4) | 0.4 (0.0, 1.3) | 0.31 | 0.0 (0.0, 1.4) | 0.8 (0.0, 44.9) | 0.30 | 0.7 (0.0, 3.1) | 0.7 (0.0, 4.4) | 0.15 | 0.3 (0.0, 1.0) |
| Secondary Care Interval, weeks | 26.0 (12.0, 57.0) | 2.2 (0.9, 6.6) | 0.005 | 4.3 (2.1, 9.1) | 3.9 (1.9, 8.1) | 0.01 | 8.1 (4.9, 10.2) | 2.6 (1.0, 10.7) | 0.47 | 3.5 (1.6, 7.5) | 3.9 (1.7, 10.0) | 0.14 | 2.6 (1.5, 3.8) |
| Tertiary Care Interval, weeks | 14.0 (5.0, 26.3) | 2.1 (1.0, 3.7) | 0.006 | 1.3 (-0.6, 3.4) | 1.6 (-0.2, 3.6) | 0.01 | 0.9 (-3.3, 1.9) | 3.1 (2.0, 8.1) | 0.14 | 2.6 (1.8, 4.1) | 2.6 (1.7, 4.0) | 0.36 | 2.7 (1.9, 5.8) |
| Total Interval, weeks | 213.0 (84.0, 762.2) | 22.8 (11.9, 56.7) | 0.82 | 23.3 (10.4, 59.4) | 20.9 (10.4, 55.3) | 0.07 | 34.8 (12.3, 148.0) | 100.5 (48.1, 206.6) | 0.22 | 48.2 (17.7, 193.3) | 43.0 (14.7, 150.6) | 0.04 | 138.1 (29.1, 304.4) |
| PI g | DI h | TI l | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCI i | SCI j | TCI k | |||||||||||||
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |
| Overall (n = 712) | |||||||||||||||
| Age | 7.07 | 0.08, 14.05 | 0.047 | −0.91 | −3.36, 1.54 | 0.46 | 0.71 | −2.31, 3.73 | 0.65 | −0.28 | −1.77, 1.21 | 0.71 | 6.79 | −1.03, 14.61 | 0.09 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | 86.53 | −185.00, 358.05 | 0.53 | −45.41 | −142.57, 51.74 | 0.36 | 46.20 | −68.73, 161.13 | 0.43 | −11.31 | −67.84 45.22 | 0.69 | 168.90 | −132.39, 470.11 | 0.27 |
| Grade G1 | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| G2 | −392.10 | −1039.97, 255.67 | 0.24 | −17.17 | −258.50, 224.15 | 0.89 | 17.17 | −261.18, 295.52 | 0.90 | −28.42 | −149.59, 92.76 | 0.65 | −498.92 | −1140.89, 143.05 | 0.13 |
| G3 | −384.70 | −911.59, 142.23 | 0.15 | −77.84 | −261.66, 105.98 | 0.41 | −18.07 | −240.93, 204.78 | 0.87 | 116.81 | 17.25, 216.36 | 0.02 | −470.85 | −994.55, 52.85 | 0.08 |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | −177.51 | −463.87, 108.86 | 0.224 | −46.46 | −147.89, 54.97 | 0.37 | −21.42 | −141.14, 98.29 | 0.73 | −84.47 | −143.12, −25.82 | 0.41 | −264.11 | −583.97, 55.75 | 0.11 |
| Bone sarcoma (n = 82) | |||||||||||||||
| Age | 5.61 | −13.44, 24.66 | 0.56 | −1.48 | −10.59, 7.63 | 0.75 | −0.14 | −0.95, 0.68 | 0.74 | −0.13 | −0.39, 0.13 | 0.31 | 7.55 | −12.71, 27.80 | 0.46 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | 88.12 | −830.06, 1006.30 | 0.85 | −53.64 | −494.73, 387.45 | 0.81 | 2.72 | −36.48, 41.93 | 0.89 | −8.45 | −21.05, 4.14 | 0.19 | −42.57 | −1017.19, 932.04 | 0.93 |
| Grade G1 | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| G2 | −37.05 | −2366.05, 2291.95 | 0.98 | −107.67 | −1167.15, 951.82 | 0.84 | −37.95 | −129.28, 53.37 | 0.41 | −12.80 | −42.96, 17.36 | 0.40 | −122.40 | −2409.75, 2164.95 | 0.92 |
| G3 | 48.49 | −1679.16, 1776.14 | 0.96 | −90.88 | −724.04, 542.28 | 0.77 | −21.64 | −82.91, 39.62 | 0.48 | −16.05 | −38.39, 6.30 | 0.16 | −15.80 | −1815.48, 1783.88 | 0.99 |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | −187.90 | −1424.36, 1048.56 | 0.76 | 472.71 | −88.70, 1034.13 | 0.10 | −7.66 | −51.97, 36.65 | 0.73 | −2.06 | −16.31, 12.19 | 0.77 | −45.29 | −1259.59, 1169.00 | 0.94 |
| Soft-tissue sarcoma (n = 356) | |||||||||||||||
| Age | 4.67 | −6.17, 15.51 | 0.40 | 0.55 | −1.51, 2.60 | 0.60 | 0.78 | −3.91, 5.47 | 0.74 | 1.11 | −1.71, 3.93 | 0.44 | 3.90 | −6.60, 14.39 | 0.47 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | −42.41 | −427.41, 342.60 | 0.83 | −24.30 | −101.26, 52.67 | 0.53 | −22.46 | −189.49, 144.57 | 0.79 | −16.93 | −115.72, 81.87 | 0.74 | −18.80 | −391.39, 353.80 | 0.92 |
| Grade G1 | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| G2 | −430.19 | −1119.79, 259.41 | 0.22 | −5.59 | −157.29, 146.12 | 0.45 | 24.29 | −291.39, 339.96 | 0.88 | −28.36 | −192.80, 136.07 | 0.74 | −525.67 | −1148.01, 96.67 | 0.10 |
| G3 | −420.90 | −997.45, 155.64 | 0.15 | −73.77 | −194.34, 46.79 | 0.94 | −17.01 | −277.62, 243.60 | 0.90 | 134.37 | −3.76, 272.50 | 0.06 | −498.96 | −1015.67, 17.75 | 0.06 |
| Localizationappendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | −220.20 | −604.58, 164.21 | 0.26 | −84.99 | −160.21, −9.77 | 0.03 | −109.96 | −275.68, 55.75 | 0.19 | −112.59 | −210.99, −14.18 | 0.03 | −297.40 | −672.92, 78.11 | 0.12 |
| Deep soft-tissue sarcoma (n = 296) | |||||||||||||||
| Age | 5.03 | −6.50, 16.57 | 0.39 | 1.43 | −0.97, 3.84 | 0.24 | 0.72 | −4.95, 6.38 | 0.80 | 0.63 | −2.83, 4.10 | 0.72 | 4.06 | −7.91, 16.02 | 0.51 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | −77.25 | −479.40, 324.89 | 0.71 | −40.20 | −128.01, 47.61 | 0.37 | −18.11 | −213.53, 177.31 | 0.86 | −40.66 | −158.05, 76.72 | 0.50 | −66.69 | −481.55, 348.17 | 0.75 |
| Grade G1 | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| G2 | −222.90 | −966.79, 520.90 | 0.56 | −32.83 | −218.91, 153.24 | 0.73 | 32.40 | −354.26, 419.07 | 0.87 | −54.82 | −251.40, 141.76 | 0.58 | −384.41 | −1088.57, 319.74 | 0.28 |
| G3 | −302.30 | −928.03, 323.33 | 0.34 | −114.18 | −260.68, 32.32 | 0.13 | −21.76 | −339.89, 296.37 | 0.89 | 141.65 | −24.05, 307.35 | 0.09 | −477.53 | −1060.61, 105.54 | 0.11 |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | −292.70 | −694.86, 109.40 | 0.15 | −83.18 | −169.70, 3.33 | 0.06 | −124.82 | −319.22, 69.58 | 0.21 | −128.24 | −245.63, −10.84 | 0.03 | −314.20 | −735.48, 107.05 | 0.14 |
| Superficial soft-tissue sarcoma (n = 60) | |||||||||||||||
| Age | 9.08 | −22.59, 40.75 | 0.56 | −3.20 | −6.91, 0.52 | 0.09 | 0.13 | −1.18, 1.43 | 0.84 | 2.71 | −0.64, 6.06 | 0.11 | 3.61 | −18.69, 25.91 | 0.75 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | −244.50 | −1550.78, 1061.81 | 0.71 | 62.06 | −98.57, 222.68 | 0.43 | −21.07 | −73.30, 31.16 | 0.41 | 87.35 | −47.66, 222.35 | 0.20 | 109.40 | −765.23, 984.12 | 0.80 |
| Grade G1 | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| G2 | −1031.80 | −3045.04, 981.47 | 0.30 | 36.96 | −205.08, 279.01 | 0.75 | −18.82 | −95.17, 57.53 | 0.61 | 102.78 | −128.78, 334.35 | 0.38 | −1004.50 | −2403.36, 394.36 | 0.16 |
| G3 | −414.60 | −2092.34, 1263.09 | 0.62 | −9.45 | −224.29, 205.39 | 0.93 | −1.07 | −68.84, 66.70 | 0.97 | 93.13 | −100.01, 286.27 | 0.34 | −352.70 | −1545.67, 840.24 | 0.56 |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | −1.20 | −1214.74, 1212.34 | 0.998 | −91.68 | −243.05, 59.69 | 0.22 | −11.61 | −61.93, 38.71 | 0.64 | −52.58 | −187.69, 82.53 | 0.44 | −371.90 | −1235.52, 491.64 | 0.39 |
| Benign bone tumor (n = 61) | |||||||||||||||
| Age | 12.03 | 0.31, 23.75 | 0.045 | 0.3656 | −17.12, 17.85 | 0.97 | −12.059 | −32.23, 8.11 | 0.23 | −5.180 | −16.54, 6.18 | 0.35 | −11.95 | −46.97, 23.06 | 0.48 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| Female | 30.04 | −300.40, 360.48 | 0.86 | −367.90 | −798.01, 62.17 | 0.09 | 337.80 | −253.09, 928.69 | 0.25 | 271.00 | −145.74, 687.74 | 0.19 | 314.00 | −1011.63, 1639.63 | 0.63 |
| Grade | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | 169.01 | −229.74, 567.76 | 0.002 | −211.10 | −797.88, 375.62 | 0.46 | −89.73 | −908.39, 728.93 | 0.82 | −119.60 | −575.95, 336.65 | 0.59 | −314.90 | −1676.93, 1047.06 | 0.63 |
| Benign soft-tissue tumor (n = 213) | |||||||||||||||
| Age | 16.29 | 0.42, 32.15 | 0.04 | 0.756 | −6.20, 7.715 | 0.83 | 5.24 | −2.40, 12.87 | 0.18 | 0.2511 | −0.84, 1.34 | 0.65 | 26.53 | 8.84, 44.22 | 0.004 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | 280.60 | −243.33, 804.44 | 0.29 | 46.05 | −172.08, 264.18 | 0.68 | 114.86 | −124.11, 353.82 | 0.34 | −23.45 | −59.68, 12.79 | 0.20 | 515.70 | −100.63, 1132.03 | 0.10 |
| Grade | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | 21.60 | −594.30, 637.50 | 0.95 | −17.69 | −268.97, 233.60 | 0.89 | 234.58 | −42.02, 511.18 | 0.10 | 5.99 | −37.39, 49.38 | 0.79 | 180.70 | −569.78, 931.19 | 0.64 |
| Benign deep soft-tissue tumor (n = 172) | |||||||||||||||
| Age | 13.01 | −2.12, 28.13 | 0.09 | 2.38 | −3.62, 8.37 | 0.43 | 5.54 | −3.47, 14.54 | 0.23 | 0.37 | −0.94, 1.67 | 0.58 | 26.59 | 8.57, 44.61 | 0.004 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | −1.82 | −524.09, 520.46 | 0.995 | 144.49 | −44.13, 333.12 | 0.13 | 127.00 | −157.82, 411.82 | 0.38 | −30.23 | −75.17, 14.71 | 0.19 | 382.50 | −285.27, 1050.29 | 0.26 |
| Grade | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable |
| Localizationappendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | 59.61 | −569.86, 689.07 | 0.85 | 48.07 | −174.99, 271.13 | 0.67 | 291.60 | −39.66, 622.89 | 0.08 | 8.75 | −45.72, 63.22 | 0.75 | 422.40 | −398.51, 1243.35 | 0.31 |
| Benign superficial soft-tissue tumor (n = 41) | |||||||||||||||
| Age | 43.61 | −17.13, 104.36 | 0.15 | −5.39 | −36.71, 25.93 | 0.72 | −0.16 | −0.97, 0.64 | 0.67 | −0.29 | −1.11, 0.52 | 0.47 | 30.60 | −23.82, 85.01 | 0.26 |
| Gender male | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| female | 1364.80 | −196.65, 2926.25 | 0.08 | −316.80 | −1170.00, 536.33 | 0.44 | 6.39 | −15.79, 28.57 | 0.55 | 6.41 | −16.34, 29.15 | 0.57 | 1137.60 | −350.82, 2625.95 | 0.13 |
| Grade | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable | not applicable |
| Localization appendicular | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference | reference |
| axial (head, neck, trunk) | −302.60 | −2074.28, 1469.01 | 0.73 | −263.20 | −1218.54, 692.14 | 0.56 | −9.32 | −33.62, 14.99 | 0.42 | −6.88 | −32.90, 19.14 | 0.59 | −657.90 | −2405.68, 1089.96 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyes, M.; Heesen, P.; Schelling, G.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B., on behalf of the Swiss Sarcoma Network. Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis. Cancers 2023, 15, 4892. https://doi.org/10.3390/cancers15194892
Elyes M, Heesen P, Schelling G, Bode-Lesniewska B, Studer G, Fuchs B on behalf of the Swiss Sarcoma Network. Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis. Cancers. 2023; 15(19):4892. https://doi.org/10.3390/cancers15194892
Chicago/Turabian StyleElyes, Maria, Philip Heesen, Georg Schelling, Beata Bode-Lesniewska, Gabriela Studer, and Bruno Fuchs on behalf of the Swiss Sarcoma Network. 2023. "Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis" Cancers 15, no. 19: 4892. https://doi.org/10.3390/cancers15194892
APA StyleElyes, M., Heesen, P., Schelling, G., Bode-Lesniewska, B., Studer, G., & Fuchs, B., on behalf of the Swiss Sarcoma Network. (2023). Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis. Cancers, 15(19), 4892. https://doi.org/10.3390/cancers15194892







