Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Population
2.3. Trial Design and Interventions
2.4. Study Endpoints and Assessments
2.5. PRO Assessment
2.6. Pharmacokinetics
2.7. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Efficacy
3.3. Safety
3.4. Patient-Reported Outcomes
3.5. Pharmacokinetics
3.6. PDGFR-α and PDGFR-β Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1L | First line |
| 2L | Second line |
| BOR | Best overall response |
| CI | Confidence interval |
| CONSORT | Consolidated Standards of Reporting Trials |
| CR | Complete response |
| D | Docetaxel |
| DCR | Disease control rate |
| DoR | Duration of response |
| ECOG | Eastern Cooperative Oncology Group |
| EORTC | European Organization for Research and Treatment of Cancer |
| G | Gemcitabine |
| HR | Hazard ratio |
| IHC | Immunohistochemistry |
| IRR | Infusion-related reaction |
| ITT | Intention to treat |
| IV | Intravenous |
| KM | Kaplan–Meier |
| LMS | Leiomyosarcoma |
| mBPI-sf | Modified Brief Pain Inventory-short form |
| NONMEM | Nonlinear Mixed Effects Modelling software |
| O | Olaratumab |
| ORR | Objective response rate |
| OS | Overall survival |
| PBO | Placebo |
| PDGF | Platelet-derived growth factor |
| PDGFR | Platelet-derived growth factor receptor |
| PFS | Progression-free survival |
| PK | Pharmacokinetics |
| PR | Partial response |
| PROs | Patient-reported outcomes |
| PS | Performance status |
| QLQ-C30 | Quality of life questionnaire |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SAE | Serious adverse event |
| SD | Stable disease |
| STS | Soft tissue sarcoma |
| TEAE | Treatment-emergent adverse event |
References
- D’Angelo, S.P.; Tap, W.D.; Schwartz, G.K.; Carvajal, R.D. Sarcoma Immunotherapy: Past Approaches and Future Directions. Sarcoma 2014, 2014, 391967. [Google Scholar] [CrossRef]
- Sharma, S.; Takyar, S.; Manson, S.C.; Powell, S.; Penel, N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: A systematic review. BMC Cancer 2013, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Linch, M.; Miah, A.B.; Thway, K.; Judson, I.R.; Benson, C. Systemic treatment of soft-tissue sarcoma—Gold standard and novel therapies. Nat. Rev. Clin. Oncol. 2014, 11, 187–202. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, F.K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Lorigan, P.; Verweij, J.; Papai, Z.; Rodenhuis, S.; Le Cesne, A.; Leahy, M.G.; Radford, J.A.; van Glabbeke, M.M.; Kirkpatrick, A.; Hogendoorn, P.C.; et al. Phase III Trial of Two Investigational Schedules of Ifosfamide Compared with Standard-Dose Doxorubicin in Advanced or Metastatic Soft Tissue Sarcoma: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 2007, 25, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Ryan, C.W.; Merimsky, O.; Agulnik, M.; Blay, J.-Y.; Schuetze, S.M.; van Tine, B.A.; Jones, R.L.; Elias, A.D.; Choy, E.; Alcindor, T.; et al. PICASSO III: A Phase III, Placebo-Controlled Study of Doxorubicin with or without Palifosfamide in Patients with Metastatic Soft Tissue Sarcoma. J. Clin. Oncol. 2016, 34, 3898–3905. [Google Scholar] [CrossRef]
- Tap, W.D.; Papai, Z.; van Tine, B.A.; Attia, S.; Ganjoo, K.N.; Jones, R.L.; Schuetze, S.; Reed, D.; Chawla, S.P.; Riedel, R.F.; et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): An international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1089–1103. [Google Scholar] [CrossRef]
- Raj, S.; Franco, V.I.; Lipshultz, S.E. Anthracycline-Induced Cardiotoxicity: A Review of Pathophysiology, Diagnosis, and Treatment. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 315. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk Factors for Doxorubicin-lnduced Congestive Heart Failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Accordino, M.K.; Neugut, A.I.; Hershman, D.L. Cardiac Effects of Anticancer Therapy in the Elderly. J. Clin. Oncol. 2014, 32, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Wagner, A.J.; Kawai, A.; Tamura, K.; Shahir, A.; van Tine, B.A.; Martín-Broto, J.; Peterson, P.M.; Wright, J.; Tap, W.D. Prospective Evaluation of Doxorubicin Cardiotoxicity in Patients with Advanced Soft-tissue Sarcoma Treated in the ANNOUNCE Phase III Randomized Trial. Clin. Cancer Res. 2021, 27, 3861–3866. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, K.; Kusaba, H.; Yoshihiro, T.; Fujiwara, T.; Setsu, N.; Endo, M.; Matsumoto, Y.; Imajima, T.; Shinohara, Y.; Ito, M.; et al. Eribulin as a first-line treatment for soft tissue sarcoma patients with contraindications for doxorubicin. Sci. Rep. 2020, 10, 20896. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Lennartsson, J. The PDGF/PDGFR pathway as a drug target. Mol. Asp. Med. 2018, 62, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Loizos, N.; Xu, Y.; Huber, J.; Liu, M.; Lu, D.; Finnerty, B.; Rolser, R.; Malikzay, A.; Persaud, A.; Corcoran, E.; et al. Targeting the platelet-derived growth factor receptor α with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: Implications as a potential therapeutic target. Mol. Cancer Ther. 2005, 4, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Miyake, K.; Sugisawa, N.; Oshiro, H.; Zhang, Z.; Razmjooei, S.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; et al. The combination of olaratumab with gemcitabine and docetaxel arrests a chemotherapy-resistant undifferentiated soft-tissue sarcoma in a patient-derived orthotopic xenograft mouse model. Cancer Chemother. Pharmacol. 2019, 83, 1075–1082. [Google Scholar] [CrossRef]
- Tap, W.D.; Wagner, A.J.; Schöffski, P.; Martin-Broto, J.; Krarup-Hansen, A.; Ganjoo, K.N.; Yen, C.-C.; Razak, A.R.A.; Spira, A.; Kawai, A.; et al. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients with Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA 2020, 323, 1266–1276. [Google Scholar] [CrossRef]
- Villalobos, V.M.; Redondo, A.; Van Tine, B.A.; Schwartz, G.K.; Dickson, M.A.; Chmielowski, B.; Peterson, P.; Cronier, D.; Wright, J.A.; Attia, S. Phase 1b/2 study of olaratumab plus gemcitabine and docetaxel for the treatment of advanced soft tissue sarcoma (STS) (ANNOUNCE 2): Phase 1b results. J. Clin. Oncol. 2018, 36, 11542. [Google Scholar] [CrossRef]
- Fayers, P.A.N.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC Quality of Life Group. In The EORTC QLQ C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Rowbotham, M.C. What is a ‘clinically meaningful’ reduction in pain? Pain 2001, 94, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Mo, G.; Baldwin, J.R.; Luffer-Atlas, D.; Ilaria, R.L., Jr.; Conti, I.; Heathman, M.; Cronier, D.M. Population Pharmacokinetic Modeling of Olaratumab, an Anti-PDGFRα Human Monoclonal Antibody, in Patients with Advanced and/or Metastatic Cancer. Clin. Pharmacokinet. 2017, 57, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.H.; Lee, C.-H.; Witten, D.M.; Gleason, B.C.; Edris, B.; Espinosa, I.; Zhu, S.; Li, R.; Montgomery, K.D.; Marinelli, R.J.; et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene 2009, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Bathan, A.J.; Constantinidou, A.; Pollack, S.M.; Jones, R.L. Diagnosis, prognosis, and management of leiomyosarcoma: Recognition of anatomic variants. Curr. Opin. Oncol. 2013, 25, 384–389. [Google Scholar] [CrossRef]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized Phase II Study of Gemcitabine and Docetaxel Compared with Gemcitabine Alone in Patients with Metastatic Soft Tissue Sarcomas: Results of Sarcoma Alliance for Research through Collaboration Study 002. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Floquet, A.; Penel, N.; Piperno-Neumann, S.; Isambert, N.; Rey, A.; Bompas, E.; Cioffi, A.; Delcambre, C.; Cupissol, D.; et al. Randomized Multicenter and Stratified Phase II Study of Gemcitabine Alone Versus Gemcitabine and Docetaxel in Patients with Metastatic or Relapsed Leiomyosarcomas: A Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 2012, 17, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
| n (%) | Olaratumab-Naïve | Olaratumab-Pretreated | ||
|---|---|---|---|---|
| Cohort | Investigational Arm (O + G + D) N = 81 | Control Arm (PBO + G + D) N = 86 | Investigational Arm (O + G + D) N = 46 | Control Arm (PBO + G + D) N = 43 |
| Sex, Female | 48 (59.3) | 58 (67.4) | 28 (60.9) | 28 (65.1) |
| Age, years, mean (SD) | 53.4 (13.4) | 53.7 (13.4) | 60.5 (11.6) | 57.1 (12.3) |
| <65 | 66 (81.5) | 66 (76.7) | 27 (58.7) | 30 (69.8) |
| ≥65 | 15 (18.5) | 20 (23.3) | 19 (41.3) | 13 (30.2) |
| Race, n (%) | ||||
| Asian | 3 (3.7) | 2 (2.3) | 2 (4.3) | 2 (4.7) |
| Black or African American | 1 (1.2) | 1 (1.2) | 8 (17.4) | 2 (4.7) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) |
| White | 61 (75.3) | 70 (81.4) | 30 (65.2) | 34 (79.1) |
| Other | 16 (19.8) | 13 (15.1) | 5 (10.9) | 5 (11.6) |
| ECOG PS a | ||||
| 0 | 43 (53.8) | 49 (57.6) | 26 (57.8) | 22 (51.2) |
| 1 | 37 (46.3) | 36 (42.4) | 19 (42.2) | 21 (48.8) |
| Pathological diagnosis type | ||||
| Leiomyosarcoma | 37 (45.7) | 38 (44.2) | 21 (45.7) | 20 (46.5) |
| Non-leiomyosarcoma | 44 (54.3) | 48 (55.8) | 25 (54.3) | 23 (53.5) |
| Study entry: Histopathological grade | ||||
| G1 | 2 (2.5) | 2 (2.3) | 0 (0.0) | 2 (4.7) |
| G2 | 1 (1.2) | 3 (3.5) | 3 (6.5) | 2 (4.7) |
| G3 | 15 (18.5) | 22 (25.6) | 17 (37.0) | 11 (25.6) |
| Other/unknown | 63 (77.8) | 59 (68.6) | 26 (56.5) | 28 (65.1) |
| Prior anticancer therapies | ||||
| Prior radiotherapy | 35 (43.2) | 44 (51.2) | 21 (45.7) | 21 (48.8) |
| Prior surgical procedure | 70 (86.4) | 80 (93.0) | 36 (78.3) | 39 (90.7) |
| Systemic therapy | 57 (70.4) | 65 (75.6) | 46 (100) | 43 (100) |
| Number of regimens | ||||
| 1 | 41 (50.6) | 41 (47.7) | 32 (69.6) | 32 (74.4) |
| 2 | 11 (13.6) | 12 (14.0) | 11 (23.9) | 11 (25.6) |
| 3 | 0 (0.0) | 1 (1.2) | 2 (4.3) | 0 (0.0) |
| n (%) | Olaratumab-Naïve | Olaratumab-Pretreated | ||||
|---|---|---|---|---|---|---|
| Arm | Investigational Arm (O + G + D) N = 81 | Control Arm (PBO + G + D) N = 86 | HR (95% CI) [p-Value] c | Investigational Arm (O + G + D) N = 46 | Control Arm (PBO + G + D) N = 43 | HR (95% CI) [p-Value] c, d |
| Best overall response a | ||||||
| Complete response (CR) | 1 (1.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) | ||
| Partial response (PR) | 25 (30.9) | 19 (22.1) | 14 (30.4) | 6 (14.0) | ||
| Stable disease (SD) | 34 (42.0) | 42 (48.8) | 17 (37.0) | 21 (48.8) | ||
| Progressive disease (PD) | 16 (19.8) | 21 (24.4) | 11 (23.9) | 11 (25.6) | ||
| Non-evaluable | 5 (6.2) | 3 (3.5) | 4 (8.7) | 5 (11.6) | ||
| ORR, n (CR+PR; 95% Cl b) | 26 (32.1; 22.2–43.4) | 20 (23.3; 14.8–33.6) | 0.19 | 14 (30.4; 17.7–45.8) | 6 (14.0; 5.3–27.9) | 0.06 |
| DCR, n (CR+PR+SD; 95% CI b) | 60 (74.1; 63.1–83.2) | 62 (72.1; 61.4–81.2) | 0.77 | 31 (67.4; 52.0–80.5) | 27 (62.8; 46.7–77.0) | 0.65 |
| Median DoR (95% CI c) | 5.6 (4.0–8.1) | 9.9 (5.2–) | 12.4 (3.0–15.9) | 8.3 (1.4–) | ||
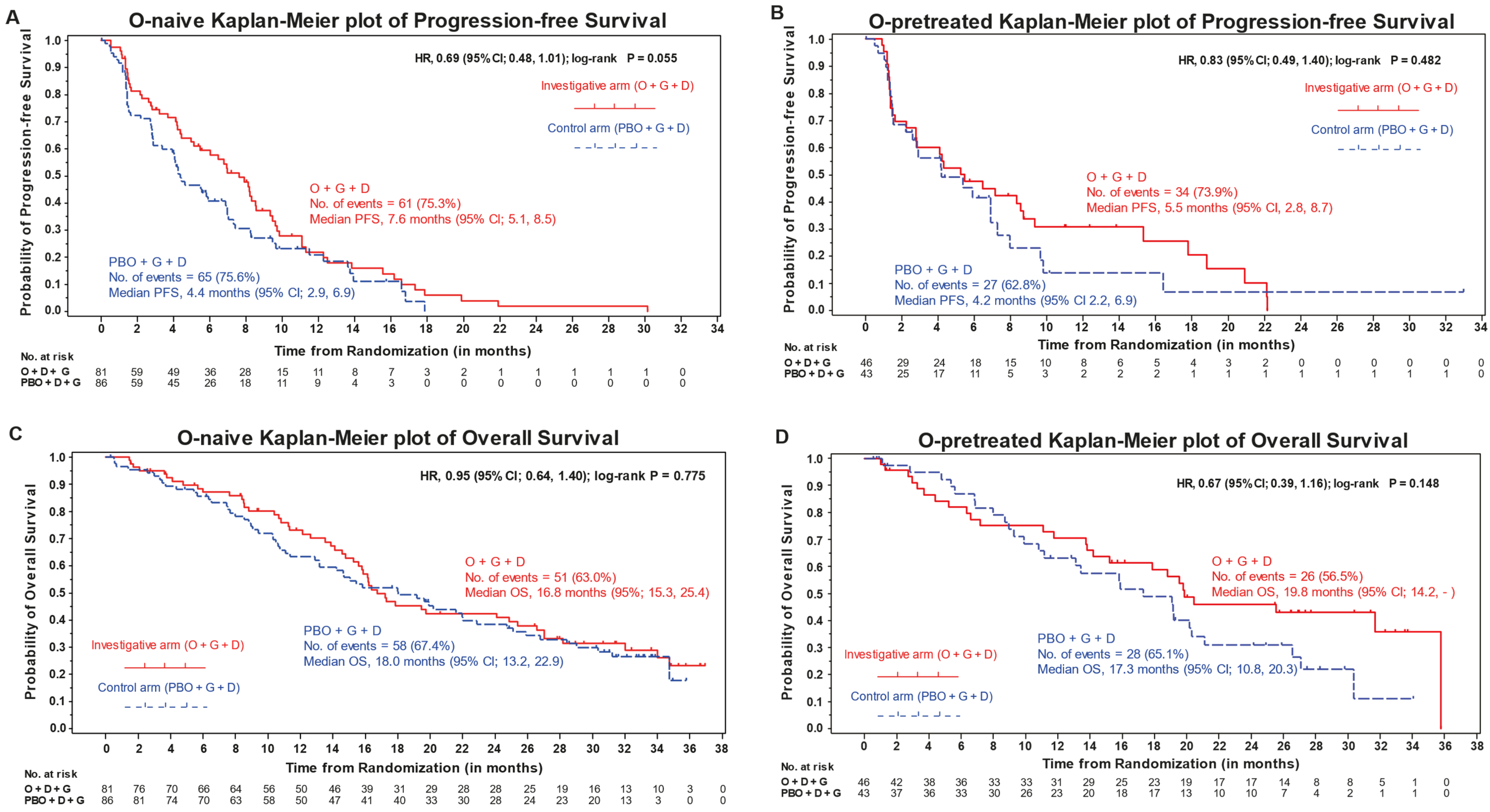
| PFS, median mos (95% CI) | 7.6 (5.1–8.5) | 4.4 (2.9–6.9) | 0.7 (0.48–1.01) [0.06] | 5.5 (2.76–8.71) | 4.2 (2.2–6.9) | 0.8 (0.5–1.4) [0.48] |
| OS, median mos (95% CI) | 16.8 (15.3–25.4) | 18.0 (13.2–22.9) | 0.9 (0.6–1.4) [0.78] | 19.8 (14.19–) | 17.3 (10.8–20.3) | 0.7 (0.4–1.2) [0.15] |
| Number of Patients a n (%) | Olaratumab-Naïve | Olaratumab-Pretreated | ||
|---|---|---|---|---|
| Cohort | Investigational Arm (O+G+D) N = 81 | Control Arm (PBO+G+D) N = 86 | Investigational Arm (O+G+D) N = 45 | Control Arm (PBO+G+D) N = 43 |
| Exposure to olaratumab or placebo | 81 (100.0) | 86 (100.0) | 45 (100.0) | 43 (100.0) |
| Duration of treatment, weeks, median (range) | 19.0 (3.0–134.9) | 18.5 (2.9–84.1) | 18.0 (3.0–99.0) | 12.0 (3.0–150.0) |
| Cycles received per patient a, median (range) | 6.0 (1.0–44.0) | 5.5 (1.0–27.0) | 6.0 (1.0–32.0) | 4.0 (1.0–48.0) |
| Exposure to gemcitabine | 79 (97.5) | 86 (100.0) | 45 (100.0) | 43 (100.0) |
| Duration of treatment, weeks, median (range) | 18.3 (3.0–94.1) | 15.0 (2.9–77.3) | 18.0 (3.0–99.0) | 12.0 (3.0–150.0) |
| Cycles received per patient a, median (range) | 6.0 (1.0–27.0) | 5.0 (1.0–24.0) | 6.0 (1.0–32.0) | 4.0 (1.0–48.0) |
| Exposure to docetaxel | 76 (93.8) | 83 (96.5) | 41 (91.1) | 41 (95.3) |
| Duration of treatment, weeks, median (range) | 15.8 (2.0–94.0) | 12.0 (2.0–59.9) | 17.0 (2.0–149.0) | 11.0 (2.0–149.0) |
| Cycles received per patient a, median (range) | 5.0 (1.0–29.0) | 4.0 (1.0–19.0) | 5.0 (1.0–32.0) | 4.0 (1.0–46.0) |
| Dose adjustments | ||||
| Patients with at least one dose adjustment | 73 (90.1) | 67 (77.9) | 38 (84.4) | 30 (69.8) |
| Patients with at least one dose reduction | 51 (63.0) | 39 (45.3) | 28 (62.2) | 18 (41.9) |
| Adverse events in the safety population | ||||
| Patients with ≥1 TEAE | 81 (100.0) | 83 (96.5) | 45 (100.0) | 43 (100.0) |
| Related to study treatment b | 77 (95.1) | 78 (90.7) | 43 (95.6) | 40 (93.0) |
| Patients with ≥1 grade ≥3 TEAE | 68 (84.0) | 63 (73.3) | 38 (84.4) | 35 (81.4) |
| Related to study treatment b | 59 (72.8) | 51 (59.3) | 32 (71.1) | 29 (67.4) |
| Patients with ≥1 SAE | 44 (54.3) | 38 (44.2) | 21 (46.7) | 23 (53.5) |
| Related to study treatment b | 33 (40.7) | 24 (27.9) | 13 (28.9) | 17 (39.5) |
| Patients who discontinued study treatment due to AE | 15 (18.5) | 13 (15.1) | 6 (13.3) | 10 (23.3) |
| Related to study treatment b | 12 (14.8) | 8 (9.3) | 5 (11.1) | 8 (18.6) |
| Patients who died due to AE on study treatment or within 30 days of discontinuation c | 0 (0.0) | 2 (2.3) | 1 (2.2) | 1 (2.3) |
| Related to study treatment b | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1 (2.3) |
| Number of Patients n (%) | Olaratumab-Naïve | Number of Patients n (%) | Olaratumab-Pretreated | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Investigational Arm (O+G+D) N = 81 | Control Arm (PBO+G+D) N = 86 | Investigational Arm (O+G+D) N = 45 | Control Arm (PBO+G+D) N = 43 | |||||
| MedDRA Preferred Term | All Grades a | ≥ Grade 3 | All Grades a | ≥ Grade 3 | MedDRA Preferred Term | All Grades a | ≥ Grade 3 | All Grades a | ≥ Grade 3 |
| Fatigue b | 61 (75.3) | 12 (14.8) | 56 (65.1) | 7 (8.1) | Anemia b | 34 (75.6) | 12 (26.7) | 20 (46.5) | 6 (14.0) |
| Musculoskeletal pain b | 41 (50.6) | 3 (3.7) | 38 (44.2) | 2 (2.3) | Fatigue b | 34 (75.6) | 7 (15.6) | 25 (58.1) | 4 (9.3) |
| Anemia b | 46 (56.8) | 18 (22.2) | 48 (55.8) | 18 (20.9) | Musculoskeletal pain b | 31 (68.9) | 2 (4.4) | 22 (51.2) | 4 (9.3) |
| Nausea | 38 (46.9) | 2 (2.5) | 40 (46.5) | 3 (3.5) | Neutropenia b | 27 (60.0) | 20 (44.4) | 8 (18.6) | 6 (14.0) |
| Oedema peripheral | 38 (46.9) | 3 (3.7) | 23 (26.7) | 1 (1.2) | Thrombocytopenia b | 22 (48.9) | 10 (22.2) | 13 (30.2) | 5 (11.6) |
| Diarrhea | 37 (45.7) | 5 (6.2) | 31 (36.0) | 1 (1.2) | Diarrhea | 21 (46.7) | 1 (2.2) | 14 (32.6) | 1 (2.3) |
| Neutropenia b | 34 (42.0) | 27 (33.3) | 37 (43.0) | 22 (25.6) | Oedema peripheral | 21 (46.7) | 1 (2.2) | 14 (32.6) | 1(2.3) |
| Alopecia | 29 (35.8) | 1 (1.2) | 32 (37.2) | 0 (0.0) | Nausea | 20 (44.4) | 0 (0.0) | 12 (27.9) | 0 (0.0) |
| Thrombocytopenia b | 28 (34.6) | 18 (22.2) | 26 (30.2) | 15 (17.4) | Dyspnea | 16 (35.6) | 3 (6.7) | 13 (30.2) | 3 (7.0) |
| Pyrexia | 28 (34.6) | 1 (1.2) | 28 (32.6) | 1 (1.2) | Cough | 13 (28.9) | 0 (0.0) | 9 (20.9) | 0 (0.0) |
| Vomiting | 25 (30.9) | 1 (1.2) | 13 (15.1) | 1 (1.2) | Constipation | 13 (28.9) | 1 (2.2) | 12 (27.9) | 0 (0.0) |
| Constipation | 21 (25.9) | 0 (0.0) | 20 (23.3) | 0 (0.0) | Dysgeusia | 13 (28.9) | 0 (0.0) | 14 (32.6) | 0 (0.0) |
| Decreased appetite | 21 (25.9) | 0 (0.0) | 15 (17.4) | 1 (1.2) | Leukopenia b | 13 (28.9) | 8 (17.8) | 5 (11.6) | 1 (2.3) |
| Dyspnea | 21 (25.9) | 1 (1.2) | 21 (24.4) | 4 (4.7) | - | - | - | - | - |
| AESI | AESI | ||||||||
| Infusion-related reactions c | Infusion-related reactions c | ||||||||
| Investigator reported | 15 (18.5) | 3 (3.7) | 5 (5.8) | 0 (0.0) | Investigator reported | 2 (4.4) | 0 (0.0) | 1 (2.3) | 0 (0.0) |
| Algorithm derived | 15 (18.5) | 3 (3.7) | 13 (15.1) | 0 (0.0) | Algorithm derived | 5 (11.1) | 0 (0.0) | 4 (9.3) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, S.; Villalobos, V.; Hindi, N.; Wagner, A.J.; Chmielowski, B.; Oakley, G.J., III; Peterson, P.M.; Ceccarelli, M.; Jones, R.L.; Dickson, M.A. Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas. Cancers 2023, 15, 4871. https://doi.org/10.3390/cancers15194871
Attia S, Villalobos V, Hindi N, Wagner AJ, Chmielowski B, Oakley GJ III, Peterson PM, Ceccarelli M, Jones RL, Dickson MA. Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas. Cancers. 2023; 15(19):4871. https://doi.org/10.3390/cancers15194871
Chicago/Turabian StyleAttia, Steven, Victor Villalobos, Nadia Hindi, Andrew J. Wagner, Bartosz Chmielowski, Gerard J. Oakley, III, Patrick M. Peterson, Matteo Ceccarelli, Robin L. Jones, and Mark A. Dickson. 2023. "Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas" Cancers 15, no. 19: 4871. https://doi.org/10.3390/cancers15194871
APA StyleAttia, S., Villalobos, V., Hindi, N., Wagner, A. J., Chmielowski, B., Oakley, G. J., III, Peterson, P. M., Ceccarelli, M., Jones, R. L., & Dickson, M. A. (2023). Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas. Cancers, 15(19), 4871. https://doi.org/10.3390/cancers15194871







