Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology
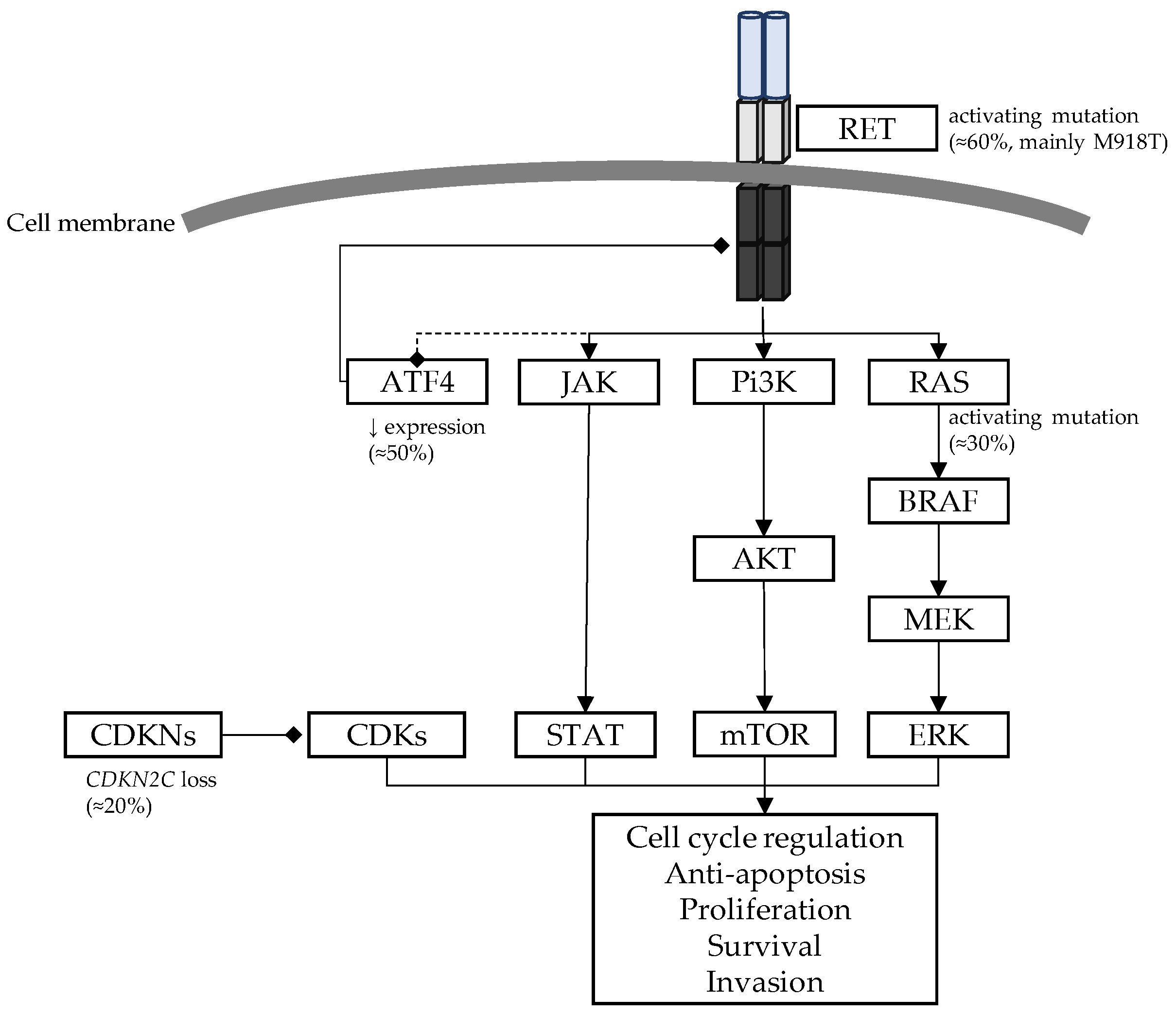
3. Molecular Basis of the RET Pathway
- RET proto-oncogene and RET protein structure
- b.
- RET canonical signalling pathway
- c.
- RET oncogene
4. Genetic Drivers of Hereditary and Sporadic MTC
- Hereditary MTC
- b.
- Sporadic MTC
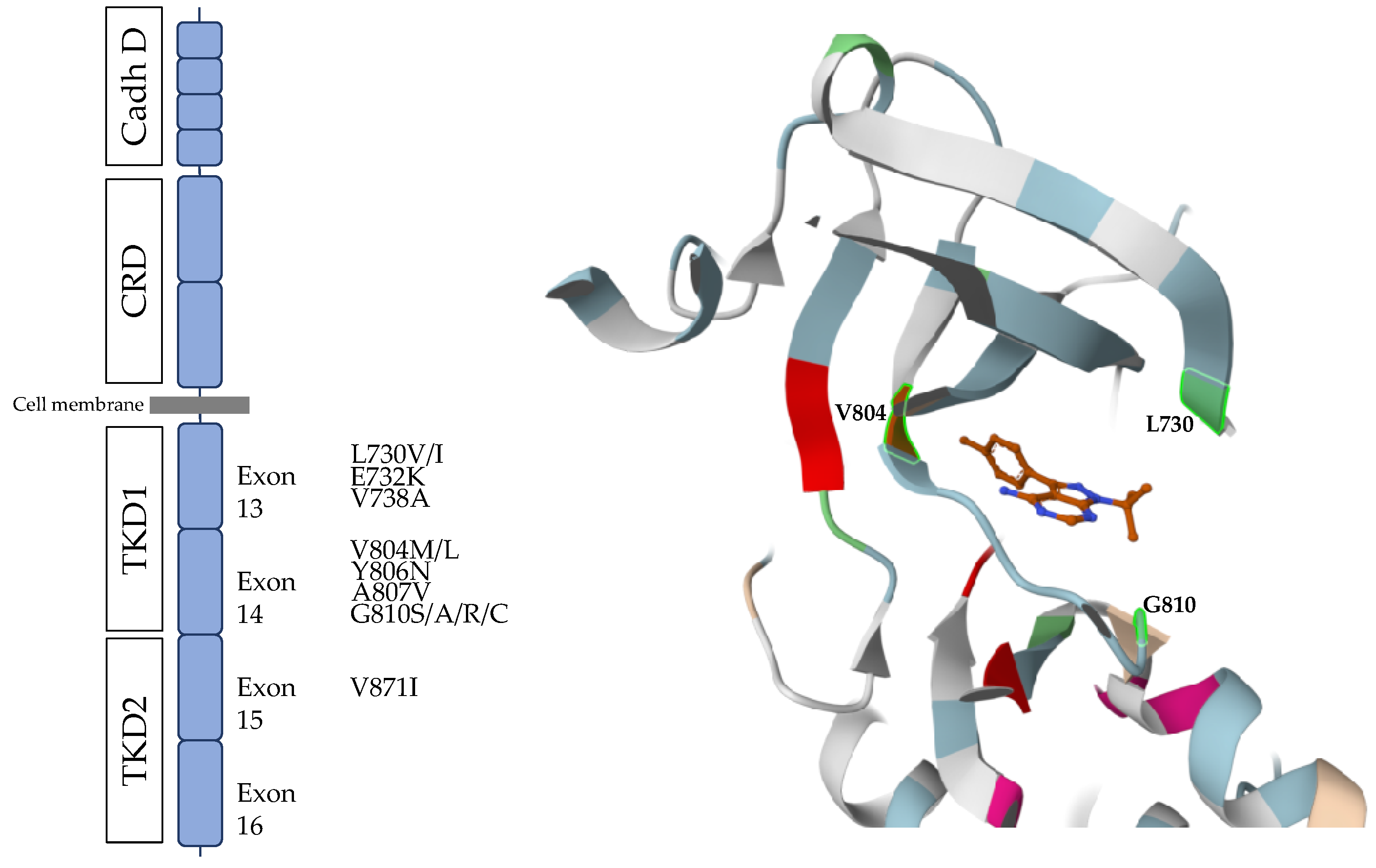
- Somatic RET mutations are a major factor in sporadic MTC tumourigenesis.
- RAS oncogene in sporadic MTC
- CDKNs and tumour suppressor genes in sporadic MTCs
- Other genetic abnormalities in sporadic MTC
5. Modifiers of the Natural History of MTC
- RET single nucleotide polymorphisms and MTC
- RET G691S/S904S (rs1799939/rs1800863)
- RET L769L (rs1800861)
- RET S836S (rs1800862)
- Multiple RET polymorphisms
- Other RET polymorphisms
- b.
- Multiple mutations
- c.
- Somatic mutation in MEN2-associated MTC
- RET somatic alterations in MEN2-associated MTCs
- Non-RET mutations could modify MEN2-related MTC outcome
- d.
- Epigenetic alterations in MTC
- e.
- Parent-of-origin effects as phenotype modifiers in MEN2-related MTC
6. Targeting RET in MTC: A New Therapeutic Paradigm
- Multikinase inhibitors
- Vandetanib (ZD6474)
- Cabozantinib (XL184)
- b.
- RET specific inhibitor
- Pralsetinib (BLU-667)
- Selpercatinib (LOXO-292)
- Zeteletinib (BOS172738)
- c.
- TKI limitations
- d.
- Future perspectives and alternative therapies in MTC
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 10 August 2023).
- Hazard, J.B.; Hawk, W.A.; Crile, G. Medullary (Solid) Carcinoma of the Thyroid—A Clinicopathologic Entity. J. Clin. Endocrinol. Metab. 1959, 19, 152–161. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Schytte, S.; Londero, S.C.; Pedersen, H.B.; et al. Incidence and Prevalence of Sporadic and Hereditary MTC in Denmark 1960–2014: A Nationwide Study. Endocr. Connect. 2018, 7, 829–839. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; MacHens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Marsh, D.J.; Mulligan, L.M.; Eng, C. RET Proto-Oncogene Mutations in Multiple Endocrine Neoplasia Type 2 and Medullary Thyroid Carcinoma. Horm. Res. Paediatr. 1997, 47, 168–178. [Google Scholar] [CrossRef]
- Elisei, R.; Cosci, B.; Romei, C.; Bottici, V.; Renzini, G.; Molinaro, E.; Agate, L.; Vivaldi, A.; Faviana, P.; Basolo, F.; et al. Prognostic Significance of Somatic RET Oncogene Mutations in Sporadic Medullary Thyroid Cancer: A 10-Year Follow-up Study. J. Clin. Endocrinol. Metab. 2008, 93, 682–687. [Google Scholar] [CrossRef]
- Takahashi, M.; Ritz, J.; Cooper, G.M. Activation of a Novel Human Transforming Gene, Ret, by DNA Rearrangement. Cell 1985, 42, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Cooper, G.M. Ret Transforming Gene Encodes a Fusion Protein Homologous to Tyrosine Kinases. Mol. Cell. Biol. 1987, 7, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.M.; Eng, C.; Ponder, B.A.; Mulligan, L.M. Characterization of RET Proto-Oncogene 3′ Splicing Variants and Polyadenylation Sites: A Novel C-Terminus for RET. Oncogene 1995, 11, 2039–2045. [Google Scholar]
- Takahashi, M.; Asai, N.; Iwashita, T.; Isomura, T.; Miyazaki, K.; Matsuyama, M. Characterization of the RET Proto-Oncogene Products Expressed in Mouse L Cells. Oncogene 1993, 8, 2925–2929. [Google Scholar] [PubMed]
- Kawai, K.; Takahashi, M. Intracellular RET Signaling Pathways Activated by GDNF. Cell Tissue Res. 2020, 382, 113–123. [Google Scholar] [CrossRef]
- Jing, S.; Wen, D.; Yu, Y.; Holst, P.L.; Luo, Y.; Fang, M.; Tamir, R.; Antonio, L.; Hu, Z.; Cupples, R.; et al. GDNF-Induced Activation of the RET Protein Tyrosine Kinase Is Mediated by GDNFR-Alpha, a Novel Receptor for GDNF. Cell 1996, 85, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kjær, S.; Ibáñez, C.F. Identification of a Surface for Binding to the GDNF-GFR Alpha 1 Complex in the First Cadherin-like Domain of RET. J. Biol. Chem. 2003, 278, 47898–47904. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Carlomagno, F.; Romano, A.; Bottaro, D.P.; Dathan, N.A.; Grieco, M.; Fusco, A.; Vecchio, G.; Maťoškova, B.; Kraus, M.H.; et al. Activation of RET as a Dominant Transforming Gene by Germline Mutations of MEN2A and MEN2B. Science 1995, 267, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Borrello, M.G.; Smith, D.P.; Pasini, B.; Bongarzone, I.; Greco, A.; Lorenzo, M.J.; Arighi, E.; Miranda, C.; Eng, C.; Alberti, L. RET Activation by Germline MEN2A and MEN2B Mutations. Oncogene 1995, 11, 2419–2427. [Google Scholar]
- Chappuis-Flament, S.; Pasini, A.; De Vita, G.; Ségouffin-Cariou, C.; Fusco, A.; Attié, T.; Lenoir, G.M.; Santoro, M.; Billaud, M. Dual Effect on the RET Receptor of MEN 2 Mutations Affecting Specific Extracytoplasmic Cysteines. Oncogene 1998, 17, 2851–2861. [Google Scholar] [CrossRef][Green Version]
- Rossel, M.; Pasini, A.; Chappuis, S.; Geneste, O.; Fournier, L.; Schuffenecker, I.; Takahashi, M.; Van Grunsven, L.A.; Urdiales, J.L.; Rudkin, B.B.; et al. Distinct Biological Properties of Two RET Isoforms Activated by MEN 2A and MEN 2B Mutations. Oncogene 1997, 14, 265–275. [Google Scholar] [CrossRef]
- Carlson, K.M.; Dou, S.; Chi, D.; Scavarda, N.; Toshima, K.; Jackson, C.E.; Wells, S.A.; Goodfellow, P.J.; Donis-Keller, H. Single Missense Mutation in the Tyrosine Kinase Catalytic Domain of the RET Protooncogene Is Associated with Multiple Endocrine Neoplasia Type 2B. Proc. Natl. Acad. Sci. USA 1994, 91, 1579–1583. [Google Scholar] [CrossRef]
- Gujral, T.S.; Singh, V.K.; Jia, Z.; Mulligan, L.M. Molecular Mechanisms of RET Receptor-Mediated Oncogenesis in Multiple Endocrine Neoplasia 2B. Cancer Res. 2006, 66, 10741–10749. [Google Scholar] [CrossRef]
- Runeberg-Roos, P.; Virtanen, H.; Saarma, M. RET(MEN 2B) Is Active in the Endoplasmic Reticulum before Reaching the Cell Surface. Oncogene 2007, 26, 7909–7915. [Google Scholar] [CrossRef] [PubMed]
- Sipple, J.H. The Association of Pheochromocytoma with Carcinoma of the Thyroid Gland. Am. J. Med. 1961, 31, 163–166. [Google Scholar] [CrossRef]
- Steiner, A.L.; Goodman, A.D.; Powers, S.R. Study of a Kindred with Pheochromocytoma, Medullary Thyroid Carcinoma, Hyperparathyroidism and Cushing’s Disease: Multiple Endocrine Neoplasia, Type 2. Medicine 1968, 47, 371–409. [Google Scholar] [CrossRef]
- Wermer, P. Genetic Aspects of Adenomatosis of Endocrine Glands. Am. J. Med. 1954, 16, 363–371. [Google Scholar] [CrossRef]
- Mulligan, L.M.; Kwok, J.B.J.; Healey, C.S.; Elsdon, M.J.; Eng, C.; Gardner, E.; Love, D.R.; Mole, S.E.; Moore, J.K.; Papi, L.; et al. Germ-Line Mutations of the RET Proto-Oncogene in Multiple Endocrine Neoplasia Type 2A. Nature 1993, 363, 458–460. [Google Scholar] [CrossRef]
- Mulligan, L.M.; Marsh, D.J.; Robinson, B.G.; Schuffenecker, I.; Zedenius, J.; Lips, C.J.M.; Gagel, R.F.; Takai, S.I.; Noll, W.W.; Fink, M.; et al. Genotype-Phenotype Correlation in Multiple Endocrine Neoplasia Type 2: Report of the International RET Mutation Consortium. J. Intern. Med. 1995, 238, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Clayton, D.; Schuffenecker, I.; Lenoir, G.; Cote, G.; Gagel, R.F.; Amstel, H.K.P.v.; Lips, C.J.M.; Nishisho, I.; Takai, S.-I.; et al. The Relationship Between Specific RET Proto-Oncogene Mutations and Disease Phenotype in Multiple Endocrine Neoplasia Type 2: International RET Mutation Consortium Analysis. JAMA 1996, 276, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, R.M.W.; Landsvater, R.M.; Ceccherini, I.; Stulp, R.P.; Stelwagen, T.; Luo, Y.; Pasini, B.; Hoppener, J.W.M.; Van Amstel, H.K.P.; Romeo, G.; et al. A Mutation in the RET Proto-Oncogene Associated with Multiple Endocrine Neoplasia Type 2B and Sporadic Medullary Thyroid Carcinoma. Nature 1994, 367, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Donis-Keller, H.; Dou, S.; Chi, D.; Carlson, K.M.; Toshima, K.; Lairmore, T.C.; Howe, J.R.; Moley, J.F.; Goodfellow, P.; Wells, S.A. Mutations in the RET Proto-Oncogene Are Associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993, 2, 851–856. [Google Scholar] [CrossRef]
- Qi, X.P.; Jin, B.Y.; Li, P.F.; Wang, S.; Zhao, Y.H.; Cao, Z.L.; Yu, X.H.; Cheng, J.; Fang, X.D.; Zhao, J.Q. RET S409Y Germline Mutation and Associated Medullary Thyroid Carcinoma. Thyroid 2019, 29, 1447–1456. [Google Scholar] [CrossRef]
- Álvares Da Silva, A.M.; Maciel, R.M.B.; Dias Da Silva, M.R.; Toledo, S.R.C.; De Carvalho, M.B.; Cerutti, J.M. A Novel Germ-Line Point Mutation in RET Exon 8 (Gly(533)Cys) in a Large Kindred with Familial Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 5438–5443. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Snow-Bailey, K.; Highsmith, W.E.; Sun, W.; Fenwick, R.G.; Mao, R. Nine Novel Germline Gene Variants in the RET Proto-Oncogene Identified in Twelve Unrelated Cases. J. Mol. Diagn. 2005, 7, 283–288. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, D.J.; Kim, J.Y.; Park, S.Y.; Choi, S.H.; Song, Y.D.; Ki, C.S.; Chung, J.H. A Rare Extracellular D631Y Germline Mutation of the RET Proto-Oncogene in Two Korean Families with Multiple Endocrine Neoplasia 2A. Thyroid 2006, 16, 609–614. [Google Scholar] [CrossRef]
- Peppa, M.; Boutati, E.; Kamakari, S.; Pikounis, V.; Peros, G.; Panayiotides, I.G.; Economopoulos, T.; Raptis, S.A.; Hadjidakis, D. Multiple Endocrine Neoplasia Type 2A in Two Families with the Familial Medullary Thyroid Carcinoma Associated G533C Mutation of the RET Proto-Oncogene. Eur. J. Endocrinol. 2008, 159, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Smith, D.P.; Mulligan, L.M.; Healey, C.S.; Zvelebil, M.J.; Stonehouse, T.J.; Ponder, M.A.; Jackson, C.E.; Waterfield, M.D.; Ponder, B.A. A Novel Point Mutation in the Tyrosine Kinase Domain of the RET Proto-Oncogene in Sporadic Medullary Thyroid Carcinoma and in a Family with FMTC—PubMed. Oncogene 1995, 10, 509–513. [Google Scholar]
- Fattoruso, O.; Quadro, L.; Libroia, A.; Verga, U.; Lupoli, G.; Cascone, E.; Colantuoni, V. A GTG to ATG Novel Point Mutation at Codon 804 in Exon 14 of the RET Proto-Oncogene in Two Families Affected by Familial Medullary Thyroid Carcinoma. Hum. Mutat. 1998, 11 (Suppl. S1), S167–S171. [Google Scholar] [CrossRef] [PubMed]
- Bolino, A.; Schuffenecker, I.; Luo, Y.; Seri, M.; Silengo, M.; Tocco, T.; Chabrier, G.; Houdent, C.; Murat, A.; Schlumberger, M.; et al. RET Mutations in Exons 13 and 14 of FMTC Patients. Oncogene 1995, 10, 2415–2419. [Google Scholar]
- Berndt, I.; Reuter, M.; Saller, B.; Frank-Raue, K.; Groth, P.; Grußendorf, M.; Raue, F.; Ritter, M.M.; Höppner, W. A New Hot Spot for Mutations in the RET Protooncogene Causing Familial Medullary Thyroid Carcinoma and Multiple Endocrine Neoplasia Type 2A. J. Clin. Endocrinol. Metab. 1998, 83, 770–774. [Google Scholar] [CrossRef]
- Smith, D.P.; Houghton, C.; Ponder, B.A.J. Germline Mutation of RET Codon 883 in Two Cases of de Novo MEN 2B. Oncogene 1997, 15, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Margraf, R.L.; Crockett, D.K.; Krautscheid, P.M.F.; Seamons, R.; Calderon, F.R.O.; Wittwer, C.T.; Mao, R. Multiple Endocrine Neoplasia Type 2 RET Protooncogene Database: Repository of MEN2-Associated RET Sequence Variation and Reference for Genotype/Phenotype Correlations. Hum. Mutat. 2009, 30, 548–556. [Google Scholar] [CrossRef] [PubMed]
- ARUP Scientific Resource for Research and Education: MEN2 Database | University of Utah. Available online: https://arup.utah.edu/database/MEN2/MEN2_welcome.php (accessed on 7 June 2023).
- Frank-Raue, K.; Raue, F. Hereditary Medullary Thyroid Cancer Genotype-Phenotype Correlation. Recent Results Cancer Res. 2015, 204, 139–156. [Google Scholar] [CrossRef]
- Voss, R.K.; Feng, L.; Lee, J.E.; Perrier, N.D.; Graham, P.H.; Hyde, S.M.; Nieves-Munoz, F.; Cabanillas, M.E.; Waguespack, S.G.; Cote, G.J.; et al. Medullary Thyroid Carcinoma in MEN2A: ATA Moderate- or High-Risk RET Mutations Do Not Predict Disease Aggressiveness. J. Clin. Endocrinol. Metab. 2017, 102, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Whollk, N. Medullary Thyroid Carcinoma in MEN2A: ATA Moderate- or High-Risk RET Mutations Do Not Predict Disease Aggressiveness. J. Clin. Endocrinol. Metab. 2017, 102, 3557–3558. [Google Scholar] [CrossRef][Green Version]
- Raue, F.; Bruckner, T.; Frank-Raue, K. Long-Term Outcomes and Aggressiveness of Hereditary Medullary Thyroid Carcinoma: 40 Years of Experience at One Center. J. Clin. Endocrinol. Metab. 2019, 104, 4264–4272. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Lymph Node Metastasis in Hereditary Medullary Thyroid Cancer Is Independent of the Underlying RET Germline Mutation. Eur. J. Surg. Oncol. 2021, 47, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, A.; Zgubieński, K.; Chmielewski, G.; Hińcza-Nowak, K.; Kowalik, A.; Jaskulski, J.; Kowalska, A. Late-Onset Medullary Thyroid Cancer in a Patient with a Germline RET Codon C634R Mutation. Diagnostics 2021, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.J.; Li, Y.S.; Shan, Z.Y. Genetic Analysis and Clinical Investigation of a Pedigree with Multiple Endocrine Neoplasia Type 2A: A Case Report. Oncol. Lett. 2016, 12, 2657–2659. [Google Scholar] [CrossRef]
- Marsh, D.J.; Learoyd, D.L.; Andrew, S.D.; Krishnan, L.; Pojer, R.; Richardson, A.L.; Delbridge, L.; Eng, C.; Robinson, B.G. Somatic Mutations in the RET Proto-Oncogene in Sporadic Medullary Thyroid Carcinoma. Clin. Endocrinol. 1996, 44, 249–257. [Google Scholar] [CrossRef]
- Romei, R.; Elisei, R.; Pinchera, A.; Ceccherini, I.; Molinaro, E.; Mancusi, F.; Martino, E.; Romeo, G.; Pacini, F. Somatic Mutations of the RET Protooncogene in Sporadic Medullary Thyroid Carcinoma Are Not Restricted to Exon 16 and Are Associated with Tumor Recurrence. J. Clin. Endocrinol. Metab. 1996, 81, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yokogoshi, Y.; Saito, S.; Yoshimoto, K. Mutations in the Cysteine-Rich Region of the RET Proto-Oncogene in Patients Diagnosed as Having Sporadic Medullary Thyroid Carcinoma. Endocr. J. 1995, 42, 517–525. [Google Scholar] [CrossRef][Green Version]
- Dvorakova, S.; Vaclavikova, E.; Sykorova, V.; Vcelak, J.; Novak, Z.; Duskova, J.; Ryska, A.; Laco, J.; Cap, J.; Kodetova, D.; et al. Somatic Mutations in the RET Proto-Oncogene in Sporadic Medullary Thyroid Carcinomas. Mol. Cell. Endocrinol. 2008, 284, 21–27. [Google Scholar] [CrossRef]
- Dvorǎká, Š.; Václavíková, E.; Sýkorová, V.; Dušková, J.; Vlček, P.; Ryška, A.; Novák, Z.; Bendlová, B. New Multiple Somatic Mutations in the RET Proto-Oncogene Associated with a Sporadic Medullary Thyroid Carcinoma. Thyroid 2006, 16, 311–316. [Google Scholar] [CrossRef]
- Schilling, T.; Bürck, J.; Sinn, H.-P.; Clemens, A.; Otto, H.F.; Höppner, W.; Herfarth, C.; Ziegler, R.; Schwab, M.; Raue, F. Prognostic Value of Codon 918 (ATG→ACG) RET Proto-Oncogene Mutations in Sporadic Medullary Thyroid Carcinoma. Int. J. Cancer 2001, 95, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef]
- Vuong, H.G.; Odate, T.; Ngo, H.T.T.; Pham, T.Q.; Tran, T.T.K.; Mochizuki, K.; Nakazawa, T.; Katoh, R.; Kondo, T. Clinical Significance of RET and RAS Mutations in Sporadic Medullary Thyroid Carcinoma: A Meta-Analysis. Endocr. Relat. Cancer 2018, 25, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Casella, F.; Tacito, A.; Bottici, V.; Valerio, L.; Viola, D.; Cappagli, V.; Matrone, A.; Ciampi, R.; Piaggi, P.; et al. New Insights in the Molecular Signature of Advanced Medullary Thyroid Cancer: Evidence of a Bad Outcome of Cases with Double RET Mutations. J. Med. Genet. 2016, 53, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Ugolini, C.; Cosci, B.; Torregrossa, L.; Vivaldi, A.; Ciampi, R.; Tacito, A.; Basolo, F.; Materazzi, G.; Miccoli, P.; et al. Low Prevalence of the Somatic M918T RET Mutation in Micro-Medullary Thyroid Cancer. Thyroid 2012, 22, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Ciampi, R.; Matrone, A.; Prete, A.; Gambale, C.; Ramone, T.; Simeakis, G.; Materazzi, G.; Torregrossa, L.; Ugolini, C.; et al. Somatic RET Indels in Sporadic Medullary Thyroid Cancer: Prevalence and Response to Selpercatinib. J. Clin. Endocrinol. Metab. 2022, 107, 2195–2202. [Google Scholar] [CrossRef]
- Jhiang, S.M.; Fithian, L.; Weghorst, C.M.; Clark, O.H.; Falko, J.M.; O’Dorisio, T.M.; Mazzaferri, E.L. RET Mutation Screening in MEN2 Patients and Discovery of a Novel Mutation in a Sporadic Medullary Thyroid Carcinoma. Thyroid 1996, 6, 115–121. [Google Scholar] [CrossRef]
- Alemi, M.; Lucas, S.D.; Sällström, J.F.; Akerström, G.; Wilander, E. A Novel Deletion in the RET Proto-Oncogene Found in Sporadic Medullary Thyroid Carcinoma. Anticancer Res. 1996, 16, 2619–2622. [Google Scholar]
- Alemi, M.; Lucas, S.D.; Sällström, J.F.; Bergholm, U.; Åkerström, G.; Wilander, E. A Complex Nine Base Pair Deletion in RET Exon 11 Common in Sporadic Medullary Thyroid Carcinoma. Oncogene 1997, 14, 2041–2045. [Google Scholar] [CrossRef]
- Ceccherini, I.; Pasini, B.; Pacini, F.; Gullo, M.; Bongarzone, I.; Romei, C.; Santamaria, G.; Matera, I.; Mondellini, P.; Scopsi, L.; et al. Somatic in Frame Deletions Not Involving Juxtamembranous Cysteine Residues Strongly Activate the RET Proto-Oncogene. Oncogene 1997, 14, 2609–2612. [Google Scholar] [CrossRef][Green Version]
- Marsh, D.J.; Andrew, S.D.; Learoyd, D.L.; Pojer, R.; Eng, C.; Robinson, B.G. Deletion-Insertion Mutation Encompassing RET Codon 634 Is Associated with Medullary Thyroid Carcinoma. Hum. Mutat. 1998, 11 (Suppl. S1), S3–S4. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.; Frilling, A. 27-Bp Deletion in the RET Proto-Oncogene as a Somatic Mutation Associated with Medullary Thyroid Carcinoma. J. Mol. Med. 1998, 76, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Wijewardene, A.; Bastard, K.; Wang, B.; Gild, M.; Luxford, C.; Gill, A.; Robinson, B.; Bullock, M.; Clifton-Bligh, R. A Case Report of Poor Response to Selpercatinib in the Presence of a 632_633 RET Deletion. Thyroid 2023, 33, 119–125. [Google Scholar] [CrossRef]
- Koch, C.A.; Huang, S.C.; Moley, J.F.; Azumi, N.; Chrousos, G.P.; Gagel, R.F.; Zhuang, Z.; Pacak, K.; Vortmeyer, A.O. Allelic Imbalance of the Mutant and Wild-Type RET Allele in MEN 2A-Associated Medullary Thyroid Carcinoma. Oncogene 2001, 20, 7809–7811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramone, T.; Mulè, C.; Ciampi, R.; Bottici, V.; Cappagli, V.; Prete, A.; Matrone, A.; Piaggi, P.; Torregrossa, L.; Basolo, F.; et al. RET Copy Number Alteration in Medullary Thyroid Cancer Is a Rare Event Correlated with RET Somatic Mutations and High Allelic Frequency. Genes 2020, 12, 35. [Google Scholar] [CrossRef]
- Ciampi, R.; Romei, C.; Cosci, B.; Vivaldi, A.; Bottici, V.; Renzini, G.; Ugolini, C.; Tacito, A.; Basolo, F.; Pinchera, A.; et al. Chromosome 10 and RET Gene Copy Number Alterations in Hereditary and Sporadic Medullary Thyroid Carcinoma. Mol. Cell. Endocrinol. 2012, 348, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Mulè, C.; Ciampi, R.; Ramone, T.; Prete, A.; Matrone, A.; Cappagli, V.; Torregrossa, L.; Basolo, F.; Elisei, R.; Romei, C. Higher RET Gene Expression Levels Do Not Represent AnAlternative RET Activation Mechanism in Medullary Thyroid Carcinoma. Biomolecules 2021, 11, 1542. [Google Scholar] [CrossRef]
- Plowman, S.J.; Hancock, J.F. Ras Signaling from Plasma Membrane and Endomembrane Microdomains. Biochim. Biophys. Acta 2005, 1746, 274–283. [Google Scholar] [CrossRef]
- Lau, K.S.; Haigis, K.M. Non-Redundancy within the RAS Oncogene Family: Insights into Mutational Disparities in Cancer. Mol. Cells 2009, 28, 315–320. [Google Scholar] [CrossRef]
- Agrawal, N.; Jiao, Y.; Sausen, M.; Leary, R.; Bettegowda, C.; Roberts, N.J.; Bhan, S.; Ho, A.S.; Khan, Z.; Bishop, J.; et al. Exomic Sequencing of Medullary Thyroid Cancer Reveals Dominant and Mutually Exclusive Oncogenic Mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013, 98, E364–E369. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Romei, C.; Pieruzzi, L.; Tacito, A.; Molinaro, E.; Agate, L.; Bottici, V.; Casella, F.; Ugolini, C.; Materazzi, G.; et al. Classical Point Mutations of RET, BRAF and RAS Oncogenes Are Not Shared in Papillary and Medullary Thyroid Cancer Occurring Simultaneously in the Same Gland. J. Endocrinol. Invest. 2017, 40, 55–62. [Google Scholar] [CrossRef]
- Moura, M.M.; Cavaco, B.M.; Pinto, A.E.; Leite, V. High Prevalence of RAS Mutations in RET-Negative Sporadic Medullary Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2011, 96, E863–E868. [Google Scholar] [CrossRef]
- Oczko-Wojciechowska, M.; Pfeifer, A.; Rusinek, D.; Pawlaczek, A.; Zebracka-Gala, J.; Kowalska, M.; Kowal, M.; Swierniak, M.; Krajewska, J.; Gawlik, T.; et al. The Prevalence of Somatic RAS Mutations in Medullary Thyroid Cancer—A Polish Population Study. Endokrynol. Pol. 2015, 66, 121–125. [Google Scholar] [CrossRef]
- Boichard, A.; Croux, L.; Al Ghuzlan, A.; Broutin, S.; Dupuy, C.; Leboulleux, S.; Schlumberger, M.; Bidart, J.M.; Lacroix, L. Somatic RAS Mutations Occur in a Large Proportion of Sporadic RET-Negative Medullary Thyroid Carcinomas and Extend to a Previously Unidentified Exon. J. Clin. Endocrinol. Metab. 2012, 97, E2031–E2035. [Google Scholar] [CrossRef]
- Shi, X.; Sun, Y.; Shen, C.; Zhang, Y.; Shi, R.; Zhang, F.; Liao, T.; Lv, G.; Zhu, Z.; Jiao, L.; et al. Integrated Proteogenomic Characterization of Medullary Thyroid Carcinoma. Cell Discov. 2022, 8, 120. [Google Scholar] [CrossRef]
- Grubbs, E.G.; Williams, M.D.; Scheet, P.; Vattathil, S.; Perrier, N.D.; Lee, J.E.; Gagel, R.F.; Hai, T.; Feng, L.; Cabanillas, M.E.; et al. Role of CDKN2C Copy Number in Sporadic Medullary Thyroid Carcinoma. Thyroid 2016, 26, 1553–1562. [Google Scholar] [CrossRef]
- Maxwell, J.E.; Gule-Monroe, M.K.; Subbiah, V.; Hu, M.; Perrier, N.D.; Cabanillas, M.E.; Lee, J.E.; Graham, P.H.; Cote, G.J.; Busaidy, N.L.; et al. Novel Use of a Clinical Laboratory Improvements Amendments (CLIA)-Certified Cyclin-Dependent Kinase N2C (CDKN2C) Loss Assay in Sporadic Medullary Thyroid Carcinoma. Surgery 2020, 167, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Van Veelen, W.; Van Gasteren, C.J.R.; Acton, D.S.; Franklin, D.S.; Berger, R.; Lips, C.J.M.; Höppener, J.W.M. Synergistic Effect of Oncogenic RET and Loss of P18 on Medullary Thyroid Carcinoma Development. Cancer Res. 2008, 68, 1329–1337. [Google Scholar] [CrossRef]
- Van Veelen, W.; Klompmaker, R.; Gloerich, M.; Van Gasteren, C.J.R.; Kalkhoven, E.; Berger, R.; Lips, C.J.M.; Medema, R.H.; Höppener, J.W.M.; Acton, D.S. P18 Is a Tumor Suppressor Gene Involved in Human Medullary Thyroid Carcinoma and Pheochromocytoma Development. Int. J. Cancer 2009, 124, 339–345. [Google Scholar] [CrossRef]
- Barbieri, R.B.; Bufalo, N.E.; Secolin, R.; Assumpção, L.V.M.; Maciel, R.M.B.; Cerutti, J.M.; Ward, L.S. Polymorphisms of Cell Cycle Control Genes Influence the Development of Sporadic Medullary Thyroid Carcinoma. Eur. J. Endocrinol. 2014, 171, 761–767. [Google Scholar] [CrossRef]
- Mishra, V.; Kowtal, P.; Rane, P.; Sarin, R. Modulatory Role of Single Nucleotide Polymorphisms of Distinct Genetic Pathways on Clinical Behavior of Medullary Thyroid Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 1289–1293. [Google Scholar] [CrossRef]
- Pasquali, D.; Circelli, L.; Faggiano, A.; Pancione, M.; Renzullo, A.; Elisei, R.; Romei, C.; Accardo, G.; Coppola, V.R.; De Palma, M.; et al. CDKN1B V109G Polymorphism a New Prognostic Factor in Sporadic Medullary Thyroid Carcinoma. Eur. J. Endocrinol. 2011, 164, 397–404. [Google Scholar] [CrossRef]
- Mishra, V.; Kowtal, P.; Rane, P.; Sarin, R. Genetic Risk Association of CDKN1A and RET Gene SNPs with Medullary Thyroid Carcinoma: Results from the Largest MTC Cohort and Meta-Analysis. Cancer Med. 2019, 8, 6151–6161. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the C-Met Receptor Tyrosine Kinase in Carcinogenesis and Associated Therapeutic Opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef]
- Trovato, M.; Villari, D.; Bartolone, L.; Spinella, S.; Simone, A.; Violi, M.A.; Trimarchi, F.; Batolo, D.; Benvenga, S. Expression of the Hepatocyte Growth Factor and C-Met in Normal Thyroid, Non-Neoplastic, and Neoplastic Nodules. Thyroid 1998, 8, 125–131. [Google Scholar] [CrossRef]
- Ruco, L.P.; Stoppacciaro, A.; Ballarini, F.; Prat, M.; Scarpino, S. Met Protein and Hepatocyte Growth Factor (HGF) in Papillary Carcinoma of the Thyroid: Evidence for a Pathogenetic Role in Tumourigenesis. J. Pathol. 2001, 194, 4–8. [Google Scholar] [CrossRef]
- Nardone, H.C.; Ziober, A.F.; LiVolsi, V.A.; Mandel, S.J.; Baloch, Z.W.; Weber, R.S.; Mick, R.; Ziober, B.L. C-Met Expression in Tall Cell Variant Papillary Carcinoma of the Thyroid. Cancer 2003, 98, 1386–1393. [Google Scholar] [CrossRef]
- Ruco, L.; Scarpino, S. The Pathogenetic Role of the HGF/c-Met System in Papillary Carcinoma of the Thyroid. Biomedicines 2014, 2, 263–274. [Google Scholar] [CrossRef]
- Pekova, B.; Sykorova, V.; Dvorakova, S.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Astl, J.; Vlcek, P.; Kodetova, D.; Vcelak, J.; et al. RET, NTRK, ALK, BRAF, and MET Fusions in a Large Cohort of Pediatric Papillary Thyroid Carcinomas. Thyroid 2020, 30, 1771–1780. [Google Scholar] [CrossRef]
- Ramirez, R.; Hsu, D.; Patel, A.; Fenton, C.; Dinauer, C.; Tuttle, R.M.; Francis, G.L. Over-Expression of Hepatocyte Growth Factor/Scatter Factor (HGF/SF) and the HGF/SF Receptor (CMET) Are Associated with a High Risk of Metastasis and Recurrence for Children and Young Adults with Papillary Thyroid Carcinoma. Clin. Endocrinol. 2000, 53, 635–644. [Google Scholar] [CrossRef]
- Schmidt, L.; Duh, F.-M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and Somatic Mutations in the Tyrosine Kinase Domain of the MET Proto-Oncogene in Papillary Renal Carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Ma, P.C.; Tretiakova, M.S.; MacKinnon, A.C.; Ramnath, N.; Johnson, C.; Dietrich, S.; Seiwert, T.; Christensen, J.G.; Jagadeeswaran, R.; Krausz, T.; et al. Expression and Mutational Analysis of MET in Human Solid Cancers. Genes Chromosomes Cancer 2008, 47, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Wasenius, V.M.; Hemmer, S.; Karjalainen-Lindsberg, M.L.; Nupponen, N.N.; Franssila, K.; Joensuu, H. MET Receptor Tyrosine Kinase Sequence Alterations in Differentiated Thyroid Carcinoma. Am. J. Surg. Pathol. 2005, 29, 544–549. [Google Scholar] [CrossRef]
- Papotti, M.; Olivero, M.; Volante, M.; Negro, F.; Prat, M.; Comoglio, P.M.; Direnzo, M.F. Expression of Hepatocyte Growth Factor (HGF) and Its Receptor (MET) in Medullary Carcinoma of the Thyroid. Endocr. Pathol. 2000, 11, 19–30. [Google Scholar] [CrossRef]
- Bagheri-Yarmand, R.; Williams, M.D.; Grubbs, E.G.; Gagel, R.F. ATF4 Targets RET for Degradation and Is a Candidate Tumor Suppressor Gene in Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2017, 102, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Yarmand, R.; Sinha, K.M.; Gururaj, A.E.; Ahmed, Z.; Rizvi, Y.Q.; Huang, S.C.; Ladbury, J.E.; Bogler, O.; Williams, M.D.; Cote, G.J.; et al. A Novel Dual Kinase Function of the RET Proto-Oncogene Negatively Regulates Activating Transcription Factor 4-Mediated Apoptosis. J. Biol. Chem. 2015, 290, 11749–11761. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, H.; Li, W.; Yan, Z.; Luo, X.; Lu, P. The Correlation between the Expression of ATF4 and Procalcitonin Combined with the Detection of RET Mutation and the Pathological Stage and Clinical Prognosis of Medullary Thyroid Carcinoma. Can. J. Physiol. Pharmacol. 2022, 100, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Ma, J.; Grubbs, E.G.; Gagel, R.F.; Bagheri-Yarmand, R. ATF4 Loss of Heterozygosity Is Associated with Poor Overall Survival in Medullary Thyroid Carcinoma. Am. J. Cancer Res. 2021, 11, 3227. [Google Scholar]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Ciampi, R.; Nikiforov, Y.E. Alterations of the BRAF Gene in Thyroid Tumors. Endocr. Pathol. 2005, 16, 163–171. [Google Scholar] [CrossRef]
- Robbins, R.J.; Thomas, J.S.; Osuna, P.M.; Shakil, J. A BRAF V600E Mutation in RET-Negative Medullary Thyroid Cancer. Case Rep. Endocrinol. 2020, 2020, 7641940. [Google Scholar] [CrossRef] [PubMed]
- Goutas, N.; Vlachodimitropoulous, D.; Bouka, M.; Lazaris, A.C.; Nasioulas, G.; Gazouli, M. BRAF and K-RAS Mutation in a Greek Papillary and Medullary Thyroid Carcinoma Cohort. Anticancer. Res. 2008, 28, 305–308. [Google Scholar]
- Fibbi, B.; Pinzani, P.; Salvianti, F.; Rossi, M.; Petrone, L.; De Feo, M.L.; Panconesi, R.; Vezzosi, V.; Bianchi, S.; Simontacchi, G.; et al. Synchronous Occurrence of Medullary and Papillary Carcinoma of the Thyroid in a Patient with Cutaneous Melanoma: Determination of BRAFV600E in Peripheral Blood and Tissues. Report of a Case and Review of the Literature. Endocr. Pathol. 2014, 25, 324–331. [Google Scholar] [CrossRef]
- Schulten, H.J.; Al-Maghrabi, J.; Al-Ghamdi, K.; Salama, S.; Al-muhayawi, S.; Chaudhary, A.; Hamour, O.; Abuzenadah, A.; Gari, M.; Al-Qahtani, M. Mutational Screening of RET, HRAS, KRAS, NRAS, BRAF, AKT1, and CTNNB1 in Medullary Thyroid Carcinoma. Anticancer Res. 2011, 31, 4179–4183. [Google Scholar]
- Araujo, A.N.; Camacho, C.P.; Mendes, T.B.; Lindsey, S.C.; Moraes, L.; Miyazawa, M.; Delcelo, R.; Pellegrino, R.; Mazzotti, D.R.; Maciel, R.M.d.B.; et al. Comprehensive Assessment of Copy Number Alterations Uncovers Recurrent AIFM3 and DLK1 Copy Gain in Medullary Thyroid Carcinoma. Cancers 2021, 13, 218. [Google Scholar] [CrossRef]
- Elisei, R.; Cosci, B.; Romei, C.; Bottici, V.; Sculli, M.; Lari, R.; Barale, R.; Pacini, F.; Pinchera, A. RET Exon 11 (G691S) Polymorphism Is Significantly More Frequent in Sporadic Medullary Thyroid Carcinoma than in the General Population. J. Clin. Endocrinol. Metab. 2004, 89, 3579–3584. [Google Scholar] [CrossRef] [PubMed]
- Robledo, M.; Gil, L.; Polla, M.; Cebria, A.; Rui, S.; Azan, M.; Benitez, J.; Mena, J.; Rojas, M. Polymorphisms G691S/S904S of RET as Genetic Modifiers of MEN 2A. Cancer Res. 2003, 63, 1814–1817. [Google Scholar]
- Sheikholeslami, S.; Yeganeh, M.Z.; Rad, L.H.; Ghadaksaz, H.G.; Hedayati, M. Haplotype Frequency of G691S/S904S in the RET Proto-Onco-Gene in Patients with Medullary Thyroid Carcinoma. Iran. J. Public Health 2014, 43, 235–240. [Google Scholar] [PubMed]
- Cebrian, A.; Lesueur, F.; Martin, S.; Leyland, J.; Ahmed, S.; Luccarini, C.; Smith, P.L.; Luben, R.; Whittaker, J.; Pharoah, P.D.; et al. Polymorphisms in the Initiators of RET (Rearranged during Transfection) Signaling Pathway and Susceptibility to Sporadic Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2005, 90, 6268–6274. [Google Scholar] [CrossRef] [PubMed]
- Lantieri, F.; Caroli, F.; Ceccherini, I.; Griseri, P. The Involvement of the RET Variant G691S in Medullary Thyroid Carcinoma Enlightened by a Meta-Analysis Study. Int. J. Cancer 2013, 132, 2808–2819. [Google Scholar] [CrossRef]
- Fugazzola, L.; Muzza, M.; Mian, C.; Cordella, D.; Barollo, S.; Alberti, L.; Cirello, V.; Dazzi, D.; Girelli, M.E.; Opocher, G.; et al. RET Genotypes in Sporadic Medullary Thyroid Cancer: Studies in a Large Italian Series. Clin. Endocrinol. 2008, 69, 418–425. [Google Scholar] [CrossRef]
- Borrello, M.G.; Aiello, A.; Peissel, B.; Rizzetti, M.G.; Mondellini, P.; Degl’Innocenti, D.; Catalano, V.; Gobbo, M.; Collini, P.; Bongarzone, I.; et al. Functional Characterization of the MTC-Associated Germline RET-K666E Mutation: Evidence of Oncogenic Potential Enhanced by the G691S Polymorphism. Endocr. Relat. Cancer 2011, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Minna, E.; Rizzetti, M.G.; Romeo, P.; Lecis, D.; Persani, L.; Mondellini, P.; Pierotti, M.A.; Greco, A.; Fugazzola, L.; et al. The Modifier Role of RET-G691S Polymorphism in Hereditary Medullary Thyroid Carcinoma: Functional Characterization and Expression/Penetrance Studies. Orphanet J. Rare Dis. 2015, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Shifrin, A.L.; Ogilvie, J.B.; Stang, M.T.; Fay, A.M.; Kuo, Y.H.; Matulewicz, T.; Xenachis, C.Z.; Vernick, J.J. Single Nucleotide Polymorphisms Act as Modifiers and Correlate with the Development of Medullary and Simultaneous Medullary/Papillary Thyroid Carcinomas in 2 Large, Non-Related Families with the RET V804M Proto-Oncogene Mutation. Surgery 2010, 148, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Cardot-Bauters, C.; Leteurtre, E.; Leclerc, L.; Vantyghem, M.C.; Do Cao, C.; Wemeau, J.L.; D’Herbomez, M.; Carnaille, B.; Barbu, V.; Pinson, S.; et al. Does the RET Variant G691S Influence the Features of Sporadic Medullary Thyroid Carcinoma? Clin. Endocrinol. 2008, 69, 506–510. [Google Scholar] [CrossRef]
- Fernández, R.M.; Peciña, A.; Antiñolo, G.; Navarro, E.; Borrego, S. Analysis of RET Polymorphisms and Haplotypes in the Context of Sporadic Medullary Thyroid Carcinoma. Thyroid 2006, 16, 411–417. [Google Scholar] [CrossRef]
- Sharma, B.P.; Saranath, D. RET Gene Mutations and Polymorphisms in Medullary Thyroid Carcinomas in Indian Patients. J. Biosci. 2011, 36, 603–611. [Google Scholar] [CrossRef]
- Machens, A.; Frank-Raue, K.; Lorenz, K.; Rondot, S.; Raue, F.; Dralle, H. Clinical Relevance of RET Variants G691S, L769L, S836S and S904S to Sporadic Medullary Thyroid Cancer. Clin. Endocrinol. 2012, 76, 691–697. [Google Scholar] [CrossRef]
- Magalhães, P.K.R.; De Castro, M.; Elias, L.L.K.; Soares, E.G.; Maciel, L.M.Z. Polymorphisms in the RET Proto-Oncogene and the Phenotypic Presentation of Familial Medullary Thyroid Carcinoma. Thyroid 2004, 14, 848–852. [Google Scholar] [CrossRef]
- Wiench, M.; Wygoda, Z.; Gubala, E.; Wloch, J.; Lisowska, K.; Krassowski, J.; Scieglinska, D.; Fiszer-Kierzkowska, A.; Lange, D.; Kula, D.; et al. Estimation of Risk of Inherited Medullary Thyroid Carcinoma in Apparent Sporadic Patients. J. Clin. Oncol. 2001, 19, 1374–1380. [Google Scholar] [CrossRef]
- Sromek, M.; Czetwertyńska, M.; Skasko, E.; Zielińska, J.; Czapczak, D.; Steffen, J. The Frequency of Selected Polymorphic Variants of the RET Gene in Patients with Medullary Thyroid Carcinoma and in the General Population of Central Poland. Endocr. Pathol. 2010, 21, 178–185. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Lang, R.; Wagner, L.; Heinze, G.; Niederle, B.; Kaserer, K.; Waldhäasl, W.; Vierhapper, H. Polymorphisms in Exon 13 and Intron 14 of the RET Protooncogene: Genetic Modifiers of Medullary Thyroid Carcinoma? J. Clin. Endocrinol. Metab. 2005, 90, 6232–6236. [Google Scholar] [CrossRef]
- Gursoy, A.; Erdogan, M.F.; Erdogan, G. Significance of the RET Proto-Oncogene Polymorphisms in Turkish Sporadic Medullary Thyroid Carcinoma Patients. J. Endocrinol. Invest. 2006, 29, 858–862. [Google Scholar] [CrossRef]
- Gemignani, F.; Romei, C.; Ciampi, R.; Corrado, A.; Melaiu, O.; Figlioli, G.; Bonotti, A.; Foddis, R.; Cristaudo, A.; Pellegrini, G.; et al. Polymorphisms Within the RET Proto-Oncogene and Risk of Sporadic Medullary Thyroid Carcinoma. Thyroid 2020, 30, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Chen, X.; Huang, S.; Li, J. Quantitative Assessment of the Association between L769L and S836S Polymorphisms at RET Gene and Medullary Thyroid Carcinoma Risk. Tumor Biol. 2014, 35, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Gimm, O.; Neuberg, D.S.; Marsh, D.J.; Dahia, P.L.M.; Hoang-Vu, C.; Raue, F.; Hinze, R.; Dralle, H.; Eng, C. Over-Representation of a Germline RET Sequence Variant in Patients with Sporadic Medullary Thyroid Carcinoma and Somatic RET Codon 918 Mutation. Oncogene 1999, 18, 1369–1373. [Google Scholar] [CrossRef]
- Ruiz, A.; Antiñolo, G.; Fernández, R.M.; Eng, C.; Marcos, I.; Borrego, S. Germline Sequence Variant S836S in the RET Proto-Oncogene Is Associated with Low Level Predisposition to Sporadic Medullary Thyroid Carcinoma in the Spanish Population. Clin. Endocrinol. 2001, 55, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, D.R.; Romitti, M.; Da Rocha, A.P.; Ceolin, L.; Meotti, C.; Estivalet, A.; Puñales, M.K.; Maia, A.L. The RET Polymorphic Allele S836S Is Associated with Early Metastatic Disease in Patients with Hereditary or Sporadic Medullary Thyroid Carcinoma. Endocr. Relat. Cancer 2010, 17, 953–963. [Google Scholar] [CrossRef]
- Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Romitti, M.; Maia, S.C.; Leiria, L.; Crispim, D.; Prolla, P.A.; Maia, A.L. Additive Effect of RET Polymorphisms on Sporadic Medullary Thyroid Carcinoma Susceptibility and Tumor Aggressiveness. Eur. J. Endocrinol. 2012, 166, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.M.; Robledo, M.; Antiñolo, G.; Peciña, A.; Ruiz-Llorente, S.; Eng, C.; Borrego, S. The RET IVS1-126G>T Variant Is Strongly Associated with the Development of Sporadic Medullary Thyroid Cancer. Thyroid 2004, 14, 329–331. [Google Scholar] [CrossRef]
- Tamanaha, R.; Camacho, C.P.; Pereira, A.C.; Da Silva, A.M.Á.; MacIel, R.M.B.; Cerutti, J.M. Evaluation of RET Polymorphisms in a Six-Generation Family with G533C RET Mutation: Specific RET Variants May Modulate Age at Onset and Clinical Presentation. Clin. Endocrinol. 2009, 71, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Fitze, G.; Schierz, M.; Kuhlisch, E.; Schreiber, M.; Ziegler, A.; Roesner, D.; Schackert, H.K. Novel Intronic Polymorphisms in the RET Proto-Oncogene and Their Association with Hirschsprung Disease. Hum. Mutat. 2003, 22, 177. [Google Scholar] [CrossRef]
- Ceolin, L.; Siqueira, D.R.; Romitti, M.; Ferreira, C.V.; Maia, A.L. Molecular Basis of Medullary Thyroid Carcinoma: The Role of RET Polymorphisms. Int. J. Mol. Sci. 2012, 13, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, X.; Gross, N.; Kou, X.; Bai, Y.; Feng, Y.; Wang, B.; Zafereo, M.E.; Li, G.; Sun, C.; et al. The Synergy of Germline C634Y and V292M RET Mutations in a Northern Chinese Family with Multiple Endocrine Neoplasia Type 2A. J. Cell. Mol. Med. 2020, 24, 13163–13170. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Verrienti, A.; Magendra Rao, D.; Sponziello, M.; Fabbro, D.; Muthu, M.; Durante, C.; Maranghi, M.; Damante, G.; Pizzolitto, S.; et al. A Novel de Novo Germline V292M Mutation in the Extracellular Region of RET in a Patient with Phaeochromocytoma and Medullary Thyroid Carcinoma: Functional Characterization. Clin. Endocrinol. 2010, 73, 529–534. [Google Scholar] [CrossRef]
- Marsh, D.J.; Andrew, S.D.; Eng, C.; Learoyd, D.L.; Capes, A.G.; Pojer, R.; Richardson, A.L.; Houghton, C.; Mulligan, L.M.; Ponder, B.A.J.; et al. Germline and Somatic Mutations in an Oncogene: RET Mutations in Inherited Medullary Thyroid Carcinoma. Cancer Res. 1996, 56, 1241–1243. [Google Scholar]
- Eng, C.; Mulligan, L.M.; Healey, C.S.; Houghton, C.; Frilling, A.; Raue, F.; Thomas, G.A.; Ponder, B.A. Heterogeneous Mutation of the RET Proto-Oncogene in Subpopulations of Medullary Thyroid Carcinoma. Cancer Res. 1996, 56, 2167–2170. [Google Scholar] [PubMed]
- Lombardo, F.; Baudin, E.; Chiefari, E.; Arturi, F.; Bardet, S.; Caillou, B.; Conte, C.; Dallapiccola, B.; Giuffrida, D.; Bidart, J.M.; et al. Familial Medullary Thyroid Carcinoma: Clinical Variability and Low Aggressiveness Associated with RET Mutation at Codon 804. J. Clin. Endocrinol. Metab. 2002, 87, 1674–1680. [Google Scholar] [CrossRef][Green Version]
- Sahakian, N.; Romanet, P.; Mirebeau-Prunier, D.; Paladino, C.; Castinetti, F.; Barlier, A. Letter to the Editor: The Somatic RET M918T Variant May Modify the Natural History of Germline RET L790F MEN2-Related Medullary Thyroid Carcinoma. Thyroid 2023. In press. [Google Scholar] [CrossRef]
- Quadro, L.; Fattoruso, O.; Cosma, M.P.; Verga, U.; Porcellini, A.; Libroia, A.; Colantuoni, V. Loss of Heterozygosity at the RET Protooncogene Locus in a Case of Multiple Endocrine Neoplasia Type 2A. J. Clin. Endocrinol. Metab. 2001, 86, 239–244. [Google Scholar] [CrossRef]
- Huang, S.C.; Torres-Cruz, J.; Pack, S.D.; Koch, C.A.; Vortmeyer, A.O.; Mannan, P.; Lubensky, I.A.; Gagel, R.F.; Zhuang, Z. Amplification and Overexpression of Mutant RET in Multiple Endocrine Neoplasia Type 2-Associated Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.A.; Brouwers, F.M.; Vortmeyer, A.O.; Tannapfel, A.; Libutti, S.K.; Zhuang, Z.; Pacak, K.; Neumann, H.P.H.; Paschke, R. Somatic VHL Gene Alterations in MEN2-Associated Medullary Thyroid Carcinoma. BMC Cancer 2006, 6, 131. [Google Scholar] [CrossRef]
- Bim, L.V.; Navarro, F.C.P.; Valente, F.O.F.; Lima-Junior, J.V.; Delcelo, R.; Dias-Da-Silva, M.R.; Maciel, R.M.B.; Galante, P.A.F.; Cerutti, J.M. Retroposed Copies of RET Gene: A Somatically Acquired Event in Medullary Thyroid Carcinoma. BMC Med. Genomics 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.S.; Nielsen, S.G.; Rasmussen, Å.K.; Kiss, K.; Wadt, K.; Hermann, A.P.; Nielsen, M.F.; Larsen, S.R.; Brusgaard, K.; Frederiksen, A.L.; et al. Variability in Medullary Thyroid Carcinoma in RET L790F Carriers: A Case Comparison Study of Index Patients. Front. Endocrinol. 2020, 11, 251. [Google Scholar] [CrossRef]
- Joo, L.J.S.; Zhao, J.T.; Gild, M.L.; Glover, A.R.; Sidhu, S.B. Epigenetic Regulation of RET Receptor Tyrosine Kinase and Non-Coding RNAs in MTC. Mol. Cell. Endocrinol. 2018, 469, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Draht, M.X.G.; Smits, K.M.; Tournier, B.; Jooste, V.; Chapusot, C.; Carvalho, B.; Cleven, A.H.G.; Derks, S.; Wouters, K.A.D.; Belt, E.J.T.; et al. Promoter CpG Island Methylation of RET Predicts Poor Prognosis in Stage II Colorectal Cancer Patients. Mol. Oncol. 2014, 8, 679–688. [Google Scholar] [CrossRef]
- Shakiba, E.; Movahedi, M.; Majd, A.; Hedayati, M. Investigating the Expression and Promoter Methylation of RET Gene in Patients with Medullary Thyroid Cancer with Unmutated RET. J. Cell. Physiol. 2019, 234, 16304–16311. [Google Scholar] [CrossRef]
- Ceolin, L.; Goularte, A.P.P.; Ferreira, C.V.; Romitti, M.; Maia, A.L. Global DNA Methylation Profile in Medullary Thyroid Cancer Patients. Exp. Mol. Pathol. 2018, 105, 110–114. [Google Scholar] [CrossRef]
- Sponziello, M.; Durante, C.; Boichard, A.; Dima, M.; Puppin, C.; Verrienti, A.; Tamburrano, G.; Di Rocco, G.; Redler, A.; Lacroix, L.; et al. Epigenetic-Related Gene Expression Profile in Medullary Thyroid Cancer Revealed the Overexpression of the Histone Methyltransferases EZH2 and SMYD3 in Aggressive Tumours. Mol. Cell. Endocrinol. 2014, 392, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, S.; Zangari, J.; Popa, A.; Ilie, M.; Hofman, V.; Long, E.; Patey, M.; Tissier, F.; Belléannée, G.; Trouette, H.; et al. MicroRNA-375/SEC23A as Biomarkers of the in Vitro Efficacy of Vandetanib. Oncotarget 2016, 7, 30461–30478. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.M.; Bracamontes, J.; Jackson, C.E.; Clark, R.; Lacroix, A.; Wells, S.A.; Goodfellow, P.J. Parent-of-Origin Effects in Multiple Endocrine Neoplasia Type 2B. Am. J. Hum. Genet. 1994, 55, 1076–1082. [Google Scholar]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Sex Differences in MEN 2A Penetrance and Expression According to Parental Inheritance. Eur. J. Endocrinol. 2022, 186, 469–476. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Medullary Thyroid Cancer and Pheochromocytoma in MEN2A: Are There Parent of Origin Effects on Disease Expression? Fam. Cancer 2022, 21, 473–478. [Google Scholar] [CrossRef]
- Hadoux, J.; Pacini, F.; Tuttle, R.M.; Schlumberger, M. Management of Advanced Medullary Thyroid Cancer. Lancet Diabetes Endocrinol. 2016, 4, 64–71. [Google Scholar] [CrossRef]
- Hadoux, J.; Schlumberger, M. Chemotherapy and Tyrosine-Kinase Inhibitors for Medullary Thyroid Cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 335–347. [Google Scholar] [CrossRef]
- Thornton, K.; Kim, G.; Maher, V.E.; Chattopadhyay, S.; Tang, S.; Moon, Y.J.; Song, P.; Marathe, A.; Balakrishnan, S.; Zhu, H.; et al. Vandetanib for the Treatment of Symptomatic or Progressive Medullary Thyroid Cancer in Patients with Unresectable Locally Advanced or Metastatic Disease: U.S. Food and Drug Administration Drug Approval Summary. Clin. Cancer Res. 2012, 18, 3722–3730. [Google Scholar] [CrossRef]
- Wedge, S.R.; Ogilvie, D.J.; Dukes, M.; Kendrew, J.; Chester, R.; Jackson, J.A.; Boffey, S.J.; Valentine, P.J.; Curwen, J.O.; Musgrove, H.L.; et al. ZD6474 Inhibits Vascular Endothelial Growth Factor Signaling, Angiogenesis, and Tumor Growth Following Oral Administration. Cancer Res. 2002, 62, 4645–4655. [Google Scholar] [PubMed]
- Ryan, A.J.; Wedge, S.R. ZD6474—A Novel Inhibitor of VEGFR and EGFR Tyrosine Kinase Activity. Br. J. Cancer 2005, 92 (Suppl. S1), S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, F.; Vitagliano, D.; Guida, T.; Ciardiello, F.; Tortora, G.; Vecchio, G.; Ryan, A.J.; Fontanini, G.; Fusco, A.; Santoro, M. ZD6474, an Orally Available Inhibitor of KDR Tyrosine Kinase Activity, Efficiently Blocks Oncogenic RET Kinases. Cancer Res. 2002, 62, 7284–7290. [Google Scholar] [PubMed]
- Vitagliano, D.; De Falco, V.; Tamburrino, A.; Coluzzi, S.; Troncone, G.; Chiappetta, G.; Ciardiello, F.; Tortora, G.; Fagin, J.A.; Ryan, A.J.; et al. The Tyrosine Kinase Inhibitor ZD6474 Blocks Proliferation of RET Mutant Medullary Thyroid Carcinoma Cells. Endocr. Relat. Cancer 2010, 18, 1–11. [Google Scholar] [CrossRef]
- Wells, S.A.; Gosnell, J.E.; Gagel, R.F.; Moley, J.; Pfister, D.; Sosa, J.A.; Skinner, M.; Krebs, A.; Vasselli, J.; Schlumberger, M. Vandetanib for the Treatment of Patients with Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. J. Clin. Oncol. 2010, 28, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.G.; Paz-Ares, L.; Krebs, A.; Vasselli, J.; Haddad, R. Vandetanib (100 Mg) in Patients with Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Chougnet, C.N.; Borget, I.; Leboulleux, S.; De La Fouchardiere, C.; Bonichon, F.; Criniere, L.; Niccoli, P.; Bardet, S.; Schneegans, O.; Zanetta, S.; et al. Vandetanib for the Treatment of Advanced Medullary Thyroid Cancer Outside a Clinical Trial: Results from a French Cohort. Thyroid 2015, 25, 386–391. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, J.H.; Ahn, J.; Jeon, M.J.; Kim, H.K.; Lim, D.J.; Kang, H.-C.; Kim, I.J.; Shong, Y.K.; Kim, T.Y.; et al. Vandetanib for the Management of Advanced Medullary Thyroid Cancer: A Real-World Multicenter Experience. Endocrinol. Metab. 2020, 35, 587–594. [Google Scholar] [CrossRef]
- Koehler, V.F.; Adam, P.; Frank-Raue, K.; Raue, F.; Berg, E.; Hoster, E.; Allelein, S.; Schott, M.; Kroiss, M.; Spitzweg, C. Real-World Efficacy and Safety of Cabozantinib and Vandetanib in Advanced Medullary Thyroid Cancer. Thyroid 2021, 31, 459–469. [Google Scholar] [CrossRef]
- Bentzien, F.; Zuzow, M.; Heald, N.; Gibson, A.; Shi, Y.; Goon, L.; Yu, P.; Engst, S.; Zhang, W.; Huang, D.; et al. In Vitro and in Vivo Activity of Cabozantinib (XL184), an Inhibitor of RET, MET, and VEGFR2, in a Model of Medullary Thyroid Cancer. Thyroid 2013, 23, 1569–1577. [Google Scholar] [CrossRef]
- Kurzrock, R.; Sherman, S.I.; Ball, D.W.; Forastiere, A.A.; Cohen, R.B.; Mehra, R.; Pfister, D.G.; Cohen, E.E.W.; Janisch, L.; Nauling, F.; et al. Activity of XL184 (Cabozantinib), an Oral Tyrosine Kinase Inhibitor, in Patients with Medullary Thyroid Cancer. J. Clin. Oncol. 2011, 29, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall Survival Analysis of EXAM, a Phase III Trial of Cabozantinib in Patients with Radiographically Progressive Medullary Thyroid Carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I.; Clary, D.O.; Elisei, R.; Schlumberger, M.J.; Cohen, E.E.W.; Schöffski, P.; Wirth, L.J.; Mangeshkar, M.; Aftab, D.T.; Brose, M.S. Correlative Analyses of RET and RAS Mutations in a Phase 3 Trial of Cabozantinib in Patients with Progressive, Metastatic Medullary Thyroid Cancer. Cancer 2016, 122, 3856–3864. [Google Scholar] [CrossRef]
- Capdevila, J.; Klochikhin, A.; Leboulleux, S.; Isaev, P.; Badiu, C.; Robinson, B.; Hughes, B.G.M.; Keam, B.; Parnis, F.; Elisei, R.; et al. A Randomized, Double-Blind Noninferiority Study to Evaluate the Efficacy of the Cabozantinib Tablet at 60 Mg Per Day Compared with the Cabozantinib Capsule at 140 Mg Per Day in Patients with Progressive, Metastatic Medullary Thyroid Cancer. Thyroid 2022, 32, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Pralsetinib: First Approval. Drugs 2020, 80, 1865–1870. [Google Scholar] [CrossRef]
- Kim, J.; Bradford, D.; Larkins, E.; Pai-Scherf, L.H.; Chatterjee, S.; Mishra-Kalyani, P.S.; Wearne, E.; Helms, W.S.; Ayyoub, A.; Bi, Y.; et al. FDA Approval Summary: Pralsetinib for the Treatment of Lung and Thyroid Cancers With RET Gene Mutations or Fusions. Clin. Cancer Res. 2021, 27, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for Patients with Advanced or Metastatic RET-Altered Thyroid Cancer (ARROW): A Multi-Cohort, Open-Label, Registrational, Phase 1/2 Study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Subbiah, V.; Velcheti, V.; Tuch, B.B.; Ebata, K.; Busaidy, N.L.; Cabanillas, M.E.; Wirth, L.J.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET Kinase Inhibition for Patients with RET-Altered Cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Wirth, L.J.; Brose, M.S.; Elisei, R.; Capdevila, J.; Hoff, A.O.; Hu, M.I.; Tahara, M.; Robinson, B.; Gao, M.; Xia, M.; et al. LIBRETTO-531: A Phase III Study of Selpercatinib in Multikinase Inhibitor-Naïve RET-Mutant Medullary Thyroid Cancer. Future Oncol. 2022, 18, 3143–3150. [Google Scholar] [CrossRef]
- Schoffski, P.; Cho, B.C.; Italiano, A.; Loong, H.H.F.; Massard, C.; Medina Rodriguez, L.; Shih, J.-Y.; Subbiah, V.; Verlingue, L.; Andreas, K.; et al. BOS172738, a Highly Potent and Selective RET Inhibitor, for the Treatment of RET -Altered Tumors Including RET -Fusion+ NSCLC and RET -Mutant MTC: Phase 1 Study Results. J. Clin. Oncol. 2021, 39, 3008. [Google Scholar] [CrossRef]
- Safety, Efficacy, and Tolerability of BOS172738 in Patients with Advanced Rearranged During Transfection (RET) Gene-Altered Tumors—Full Text View. Available online: clinicaltrials.gov (accessed on 29 June 2023).
- Bruce, J.Y.; Bible, K.C.; Chintakuntlawar, A.V. Emergence of Resistant Clones in Medullary Thyroid Cancer May Not Be Rescued by Subsequent Salvage Highly Selective Rearranged During Transfection-Inhibitor Therapy. Thyroid 2021, 31, 332–333. [Google Scholar] [CrossRef]
- Subbiah, V.; Shen, T.; Terzyan, S.S.; Liu, X.; Hu, X.; Patel, K.P.; Hu, M.; Cabanillas, M.; Behrang, A.; Meric-Bernstam, F.; et al. Structural Basis of Acquired Resistance to Selpercatinib and Pralsetinib Mediated by Non-Gatekeeper RET Mutations. Ann. Oncol. 2021, 32, 261–268. [Google Scholar] [CrossRef]
- Plaza-Menacho, I. Structure and Function of RET in Multiple Endocrine Neoplasia Type 2. Endocr. Relat. Cancer 2018, 25, T79–T90. [Google Scholar] [CrossRef]
- Liu, X.; Shen, T.; Mooers, B.H.M.; Hilberg, F.; Wu, J. Drug Resistance Profiles of Mutations in the RET Kinase Domain. Br. J. Pharmacol. 2018, 175, 3504–3515. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Tan, L.; Lin, J.J.; Wong, S.Q.; Hollizeck, S.; Ebata, K.; Tuch, B.B.; Yoda, S.; Gainor, J.F.; Sequist, L.V.; et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J. Thorac. Oncol. 2020, 15, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Gumà, J.; Peña, K.B.; Riu, F.; Lucia-Gozálvez, C.; Vidaller, A.; Maldonado, V.; Parada, D. Blood Liquid Biopsy in an Advanced Medullary Thyroid Carcinoma: A Case Study with Rearranged during Transfection Heterogeneity. Pathobiology 2023, 90, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Saltiki, K.; Simeakis, G.; Karapanou, O.; Paschou, S.A.; Alevizaki, M. Metastatic Medullary Thyroid Carcinoma (MTC): Disease Course, Treatment Modalities and Factors Predisposing for Drug Resistance. Endocrine 2023, 80, 570–579. [Google Scholar] [CrossRef]
- Saltiki, K.; Simeakis, G.; Karapanou, O.; Alevizaki, M. Management Of Endocrine Disease: Medullary Thyroid Cancer: From Molecular Biology and Therapeutic Pitfalls to Future Targeted Treatment Perspectives. Eur. J. Endocrinol. 2022, 187, R53–R63. [Google Scholar] [CrossRef] [PubMed]
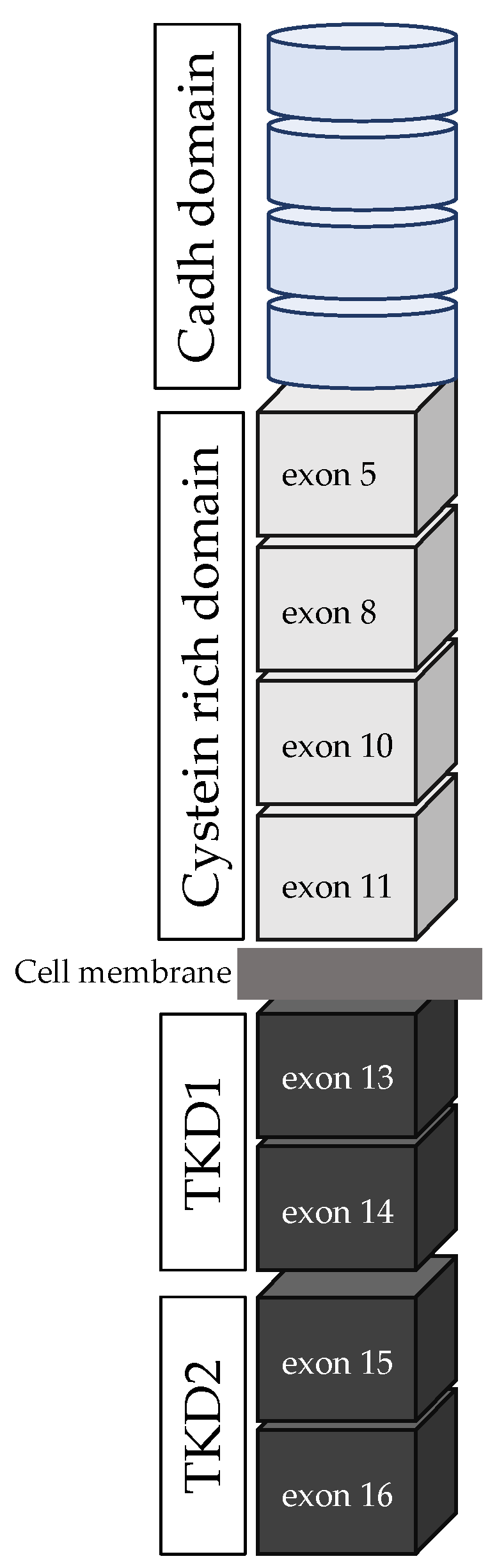
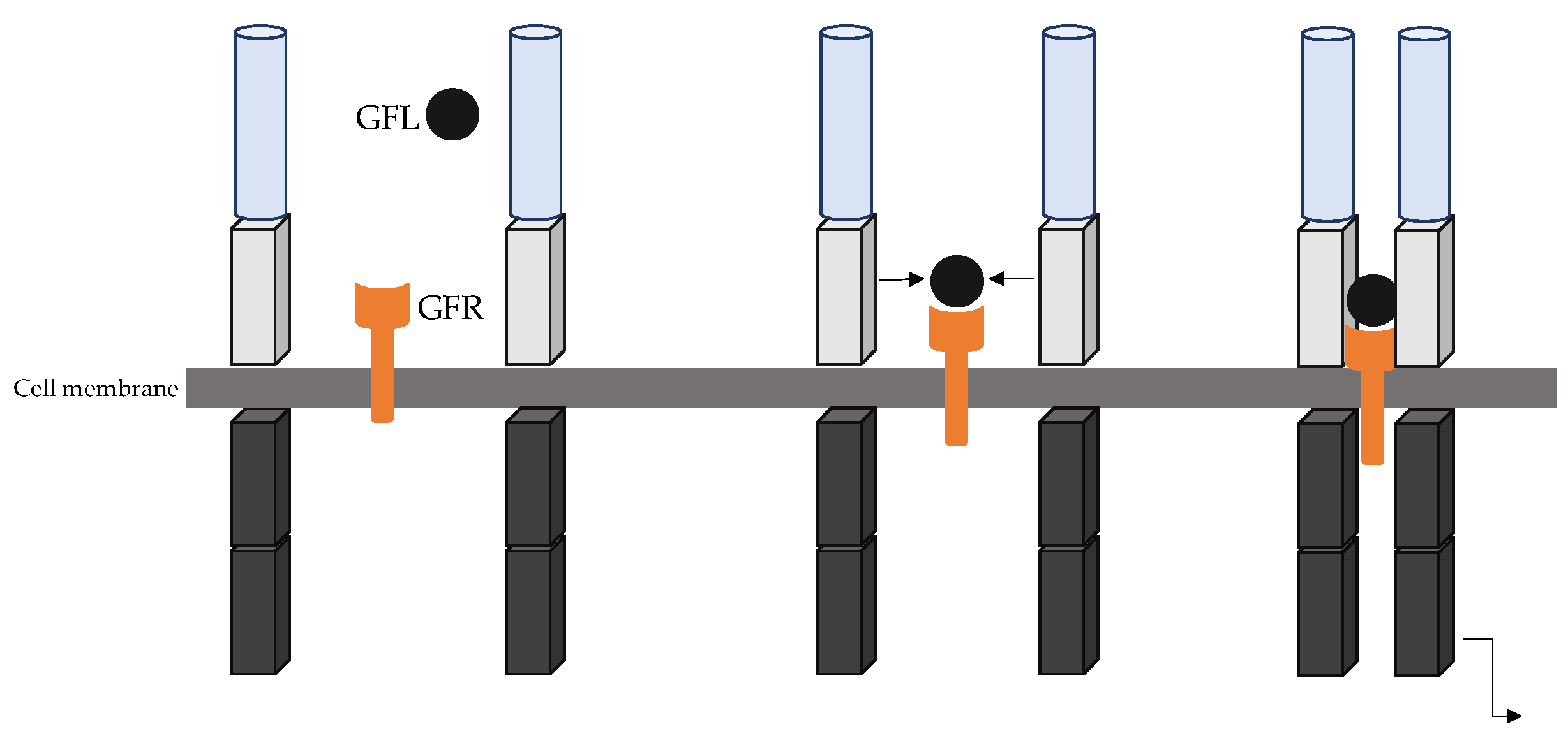
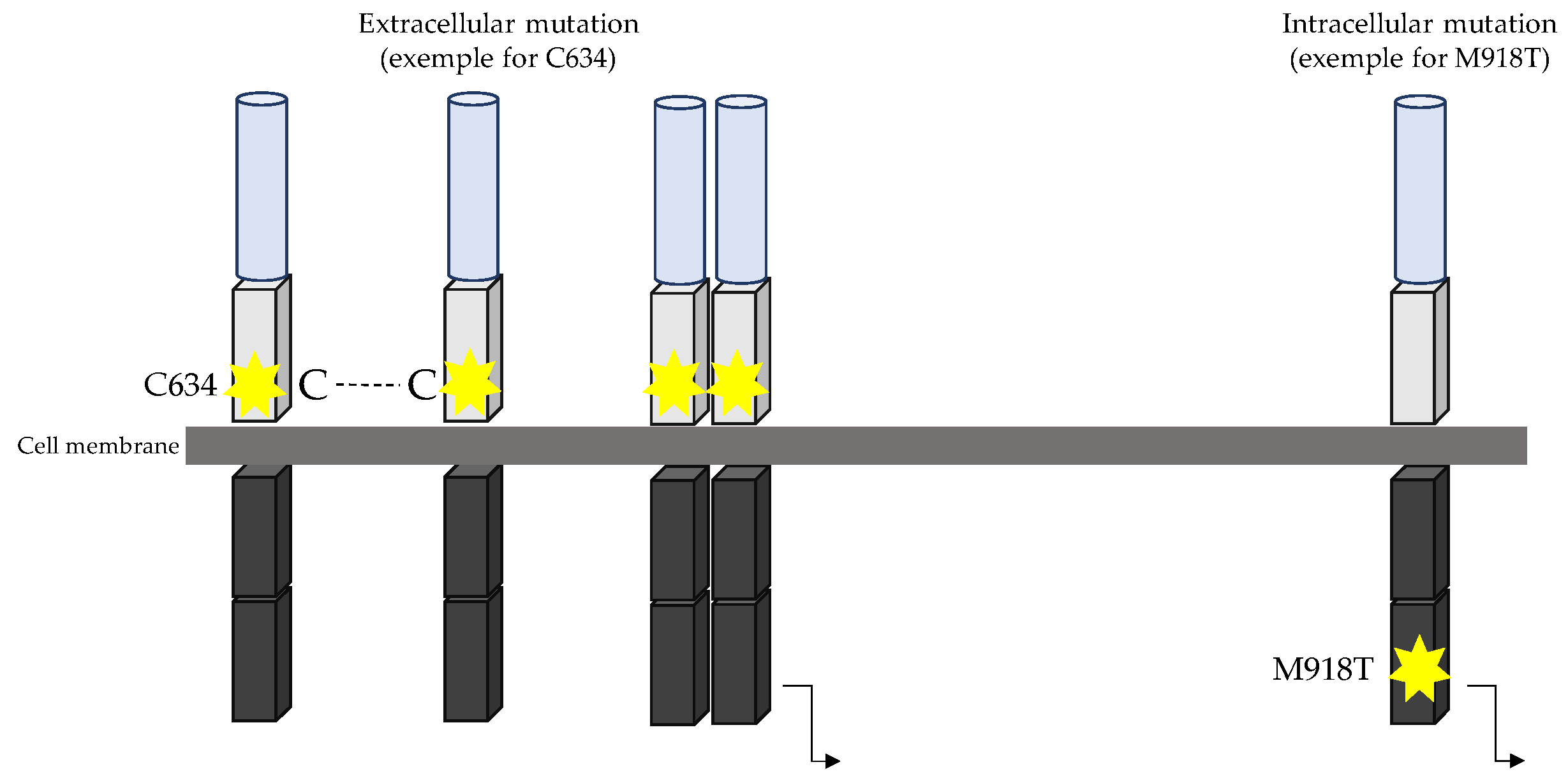
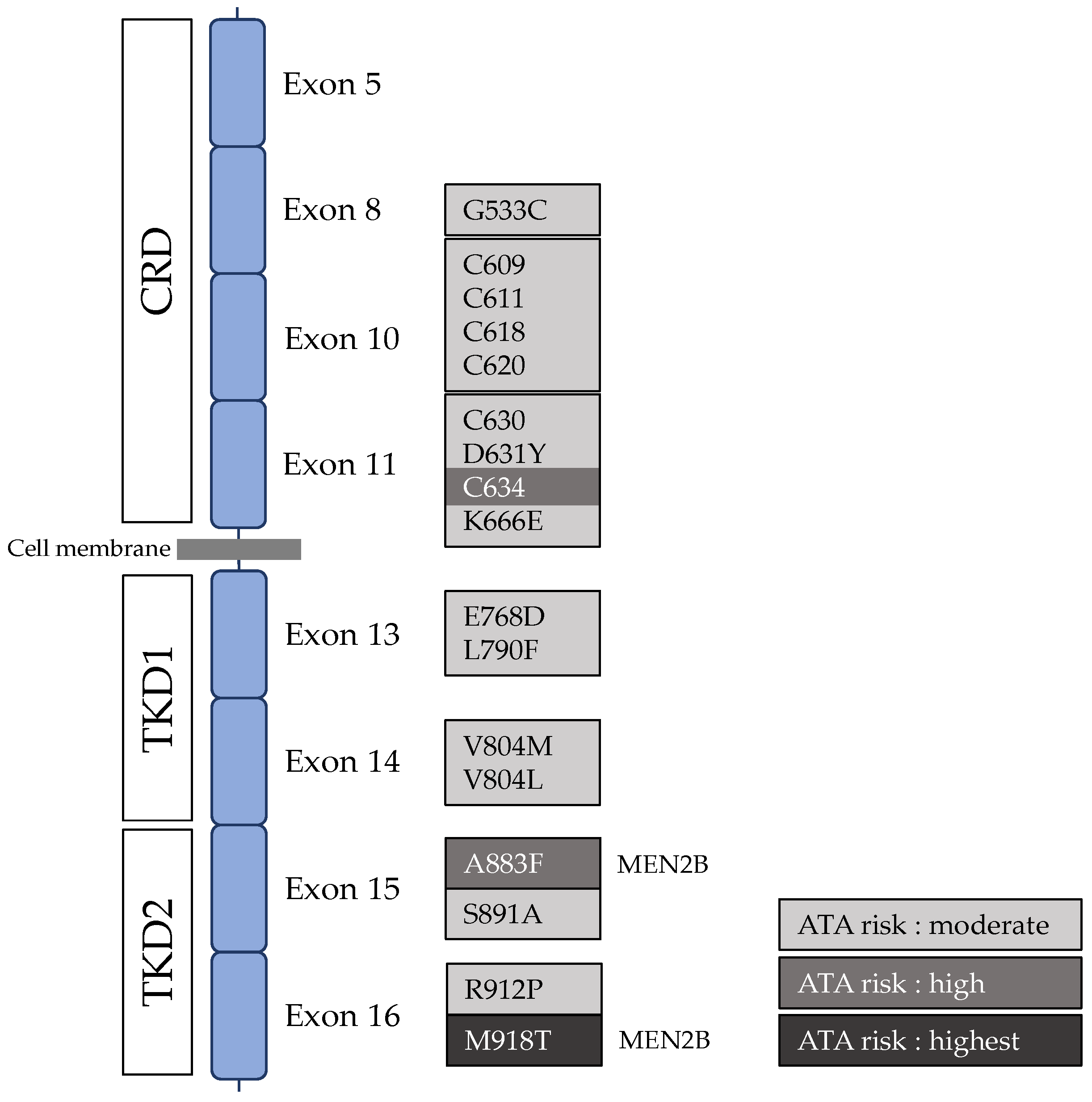
| Ref | RET Mutation | Clinical Features |
|---|---|---|
| [28] | 6-bp del involving codon 630 | No clinical data |
| [50] | 6-bp del involving codon 632 to 634 | 94 yo man, T3N0M0 MTC (post mortem data) |
| [36] | 6-bp del in exon 11 (codons 630–631) | No clinical data |
| [34] | 6-bp del in exon 11 (codons 630–631) | No clinical data |
| [59] | 6-bp del in exon 11 (codons 632–633) | 22 yo man, TxN1 MTC, |
| [60] | 9-bp del in exon 11 (codons 633 to 635) | No clinical data |
| [61] | 9-bp del in exon 11 (codons 633 to 635) | 58 yo man, no MTC data |
| [62] | 48-bp del in exon 10 (codons 592 to 607) | 28 yo woman, T3N1M0 MTC, node progression |
| [62] | 6-bp del in exon 11 (codons 632–633) | 32 yo, T4N1b MTC, metastatic progression |
| [63] | 24-bp deletion including codon 634 combined with a 6-bp insertion | No clinical data |
| [64] | 27-bp somatic heterozygous deletion (codons 611–619 or 612–620) in exon 10 | 46 yo woman, T1NxMx MTC |
| [51] | 6-bp del in exon 11 (codons 632–633) | 61 yo woman, T4N1Mx MTC, metastatic progression at 6 months 43 yo man, T4N1Mx MTC, node progression at 12 months 59 yo woman, T4N1Mx MTC, node progression at 36 months |
| [54] | T636_V637delinsCRT E898_E901del L629_D631delinsH E632_C634del | No clinical data |
| [58] | c.2694_2705delTGTTTATGAAGA c.2647_2648delGCinsT c.1894_1899delGAGCTG c.1899_1900delGTinsTG | 31 patients. MTC showed a rather aggressive clinical behaviour at the time of diagnosis and more frequent metastatic disease during the follow-up. 2 MTC exhibited an excellent response to TKI |
| [65] | 6-bp del in exon 11 (codons 632–633) | 35 yo man, aggressive metastatic MTC, poor response to TKI |
| # dbSNP | Genomic Coordinates (GRCh37) | HGVS Nomenclature (NM_020975.4) | Total Allelic Frequency (gnomAD v2.1, Last Accessed 4 July 2023) |
|---|---|---|---|
| rs2565206 | Chr10:43595781 | Intron 1 c.74-126G>T | 32.5% |
| rs1800858 | Chr10:43595968 | exon 2 c.135A>G, p.Ala45= | 73.6% |
| rs3026782 | Chr10:43624105 | 3′ UTR c. * 388G>A | 18.4% |
| rs754105711 | Chr10:43607759 | Intron 8 c.1648+88delC * | 74.9% |
| rs3026750 | Chr10:43607756 | Intron 8 c.1648+84G>A | 74.0% |
| rs1799939 | Chr10:43610119 | exon 11, c.2071G>A, p.Gly691Ser | 20.5% |
| rs1800861 | Chr10:43613843 | exon 13, c.2307G>T, p.Leu769= | 74.4% |
| rs1800862 | Chr10:43615094 | exon 14, c.2508C>T, pSer836= | 4.4% |
| rs2472737 | Chr10:43615505 | Intron 14 c.2608-24G>A | 20.4% |
| rs1800863 | Chr10:43615633 | exon 15, c.2712C>G, p.Ser904= | 20.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahakian, N.; Castinetti, F.; Romanet, P. Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET. Cancers 2023, 15, 4865. https://doi.org/10.3390/cancers15194865
Sahakian N, Castinetti F, Romanet P. Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET. Cancers. 2023; 15(19):4865. https://doi.org/10.3390/cancers15194865
Chicago/Turabian StyleSahakian, Nicolas, Frédéric Castinetti, and Pauline Romanet. 2023. "Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET" Cancers 15, no. 19: 4865. https://doi.org/10.3390/cancers15194865
APA StyleSahakian, N., Castinetti, F., & Romanet, P. (2023). Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET. Cancers, 15(19), 4865. https://doi.org/10.3390/cancers15194865




