Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates
Abstract
Simple Summary
Abstract
1. Background
2. Three-Dimensional Models: An Alternative to In Vivo Models
3. The Need for 3D Spheroid Models
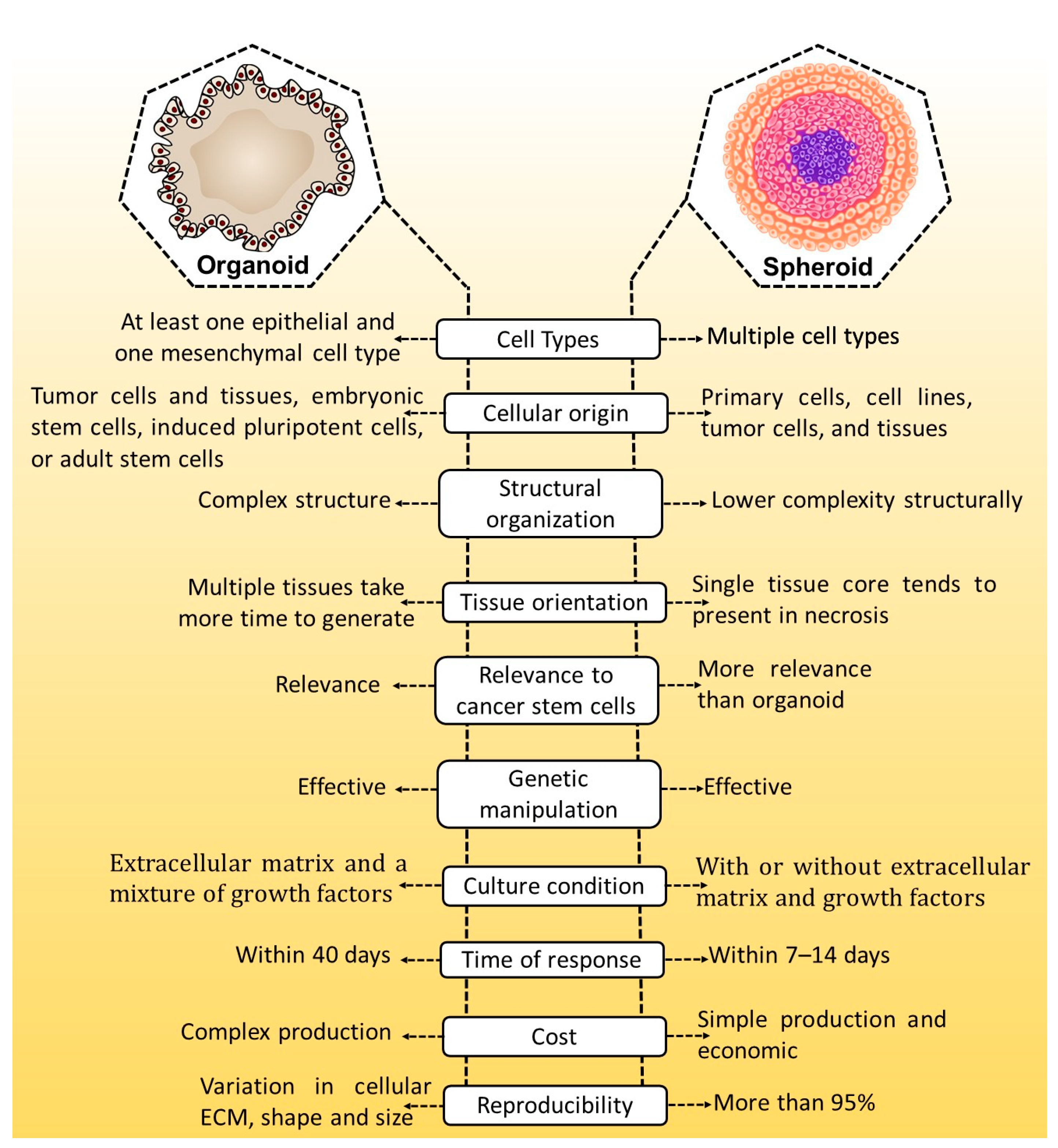
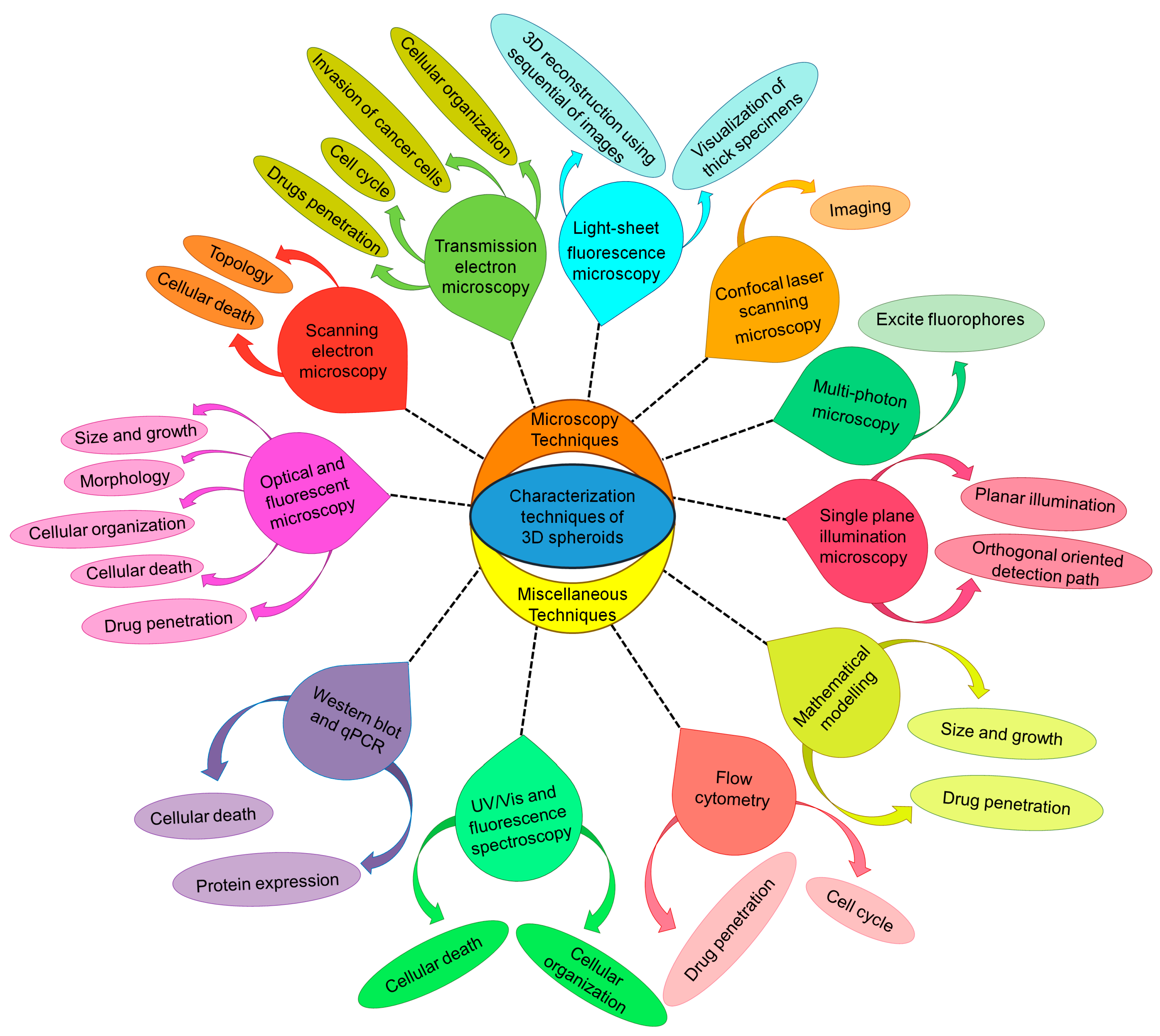
Fabrication and Characterization Technique for 3D Spheroids
4. Use of 3D Spheroid Models to Investigate Different Cancers
4.1. Prostate Cancer
4.2. Liver Cancer
4.3. Breast Cancer
4.4. Pancreatic Cancer
4.5. Thyroid Cancer
4.6. Lung Cancer
4.7. Ovarian Cancer
5. Three-dimensional Spheroid-Based Theragnostic Applications in Cancer Drug Discovery
5.1. Nuclear Medicine Therapy
5.2. Stem Cell Therapy
5.3. Photodynamic Therapy
5.4. Immune Therapy
6. Nanocarriers in 3D Spheroids Model
6.1. Dendrimers
6.2. Quantum Dots
6.3. Carbon Nanotubes
6.4. Liposomes
6.5. Polymeric Micelles
6.6. Silver Nanoparticles
6.7. Nanogels
6.8. Nanodiamonds
6.9. Polymeric Nanocarrier
6.10. Nanozymes
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Jarockyte, G.; Dapkute, D.; Karabanovas, V.; Daugmaudis, J.V.; Ivanauskas, F.; Rotomskis, R. 3D Cellular Spheroids as Tools for Understanding Carboxylated Quantum Dot Behavior in Tumors. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 914–923. [Google Scholar] [CrossRef]
- Jamieson, L.E.; Harrison, D.J.; Campbell, C.J. Chemical Analysis of Multicellular Tumour Spheroids. Analyst 2015, 140, 3910–3920. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Bin Three-Dimensional Tissue Culture Models in Cancer Biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Li, Y.; Lin, Y.; Liu, E.T.; Patnaik, A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018, 8, 1358–1365. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Sood, D.; Tang-Schomer, M.; Pouli, D.; Mizzoni, C.; Raia, N.; Tai, A.; Arkun, K.; Wu, J.; Black, L.D.; Scheffler, B.; et al. 3D Extracellular Matrix Microenvironment in Bioengineered Tissue Models of Primary Pediatric and Adult Brain Tumors. Nat. Commun. 2019, 10, 4529. [Google Scholar] [CrossRef]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H.; et al. A Bioprinted Human-Glioblastoma-on-a-Chip for the Identification of Patient-Specific Responses to Chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-Derived Organoids from Endometrial Disease Capture Clinical Heterogeneity and Are Amenable to Drug Screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Madoux, F.; Tanner, A.; Vessels, M.; Willetts, L.; Hou, S.; Scampavia, L.; Spicer, T.P. A 1536-Well 3D Viability Assay to Assess the Cytotoxic Effect of Drugs on Spheroids. SLAS Discov. 2017, 22, 516–524. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Rodrigues, I.C.P.; Perez, A.G.M.; Souto, E.M.B.; Gabriel, L.P.; Webster, T. Scaffolds for Tissue Engineering: A State-of-the-Art Review Concerning Types, Properties, Materials, Processing, and Characterization. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Blanco, I., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 647–676. [Google Scholar]
- Paradiso, F.; Serpelloni, S.; Francis, L.W.; Taraballi, F. Mechanical studies of the third dimension in cancer: From 2D to 3D model. Int. J. Mol. Sci. 2021, 22, 10098. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Das, V.; Bruzzese, F.; Konečný, P.; Iannelli, F.; Budillon, A.; Hajdúch, M. Pathophysiologically Relevant In Vitro Tumor Models for Drug Screening. Drug Discov. Today 2015, 20, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Malhotra, M.; Curtin, C.M.; O’Brien, F.J.; O’Driscoll, C.M. Life in 3D Is Never Flat: 3D Models to Optimise Drug Delivery. J. Control. Release 2015, 215, 39–54. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, E.L.; Porto, L.M.; Rambo, C.R. Nanotechnology Meets 3D in Vitro Models: Tissue Engineered Tumors and Cancer Therapies. Mater. Sci. Eng. C 2014, 34, 270–279. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular Tumor Spheroids: An Underestimated Tool Is Catching up Again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Holtfreter, J. A Study of the Mechanics of Gastrulation. J. Exp. Zool. 1944, 95, 171–212. [Google Scholar] [CrossRef]
- Sutherland, R.M.; Inch, W.R.; McCredie, J.A.; Kruuv, J. A Multi-Component Radiation Survival Curve Using an In Vitro Tumour Model. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1970, 18, 491–495. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chou, L.-F.; Chien, C.-C.M.; Chang, H.-Y. Dynamic Analysis of Hepatoma Spheroid Formation: Roles of E-Cadherin and Β1-Integrin. Cell Tissue Res. 2006, 324, 411–422. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Artegiani, B.; Hu, H.; Lopes, S.C.D.S.; Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 2021, 16, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as In Vitro Models to Mimic In Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Benien, P.; Swami, A. 3D Tumor Models: History, Advances and Future Perspectives. Future Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in Multicellular Spheroids Formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Huang, B.-W.; Gao, J.-Q. Application of 3D Cultured Multicellular Spheroid Tumor Models in Tumor-Targeted Drug Delivery System Research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-Chip: Recent Advances and Design Considerations in Microfluidic Platforms for Spheroid Formation and Culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kang, E.; Wilson, M.; Basso, T.; Chen, E.; Yu, Y.; Li, Y.R. 3D tumor spheroid and organoid to model tumor microenvironment for cancer immunotherapy. Organoids 2022, 1, 149–167. [Google Scholar] [CrossRef]
- De Grandis, R.A.; Dos Santos, P.W.D.S.; de Oliveira, K.M.; Machado, A.R.T.; Aissa, A.F.; Batista, A.A.; Antunes, L.M.G.; Pavan, F.R. Novel Lawsone-Containing Ruthenium(II) Complexes: Synthesis, Characterization and Anticancer Activity on 2D and 3D Spheroid Models of Prostate Cancer Cells. Bioorg. Chem. 2019, 85, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Arshad, F.; Hassan, I.U.; Naikoo, G.A.; Pedram, M.Z.; Zedegan, M.S.; Pourfarzad, H.; Aljabali, A.A.A.; Serrano-Aroca, Á.; Haggag, Y.; et al. Advances in Nanomaterial-Based Immunosensors for Prostate Cancer Screening. Biomed. Pharmacother. 2022, 155, 113649. [Google Scholar] [CrossRef]
- Xu, K.; Ganapathy, K.; Andl, T.; Wang, Z.; Copland, J.A.; Chakrabarti, R.; Florczyk, S.J. 3D Porous Chitosan-Alginate Scaffold Stiffness Promotes Differential Responses in Prostate Cancer Cell Lines. Biomaterials 2019, 217, 119311. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Limonta, P.; Gagliano, N. Epithelial-To-Mesenchymal Transition Markers and CD44 Isoforms Are Differently Expressed in 2D and 3D Cell Cultures of Prostate Cancer Cells. Cells 2019, 8, 143. [Google Scholar] [CrossRef]
- Khafaga, A.F.; Mousa, S.A.; Aleya, L.; Abdel-Daim, M.M. Three-dimensional (3D) cell culture: A valuable step in advancing treatments for human hepatocellular carcinoma. Cancer Cell Int. 2022, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of in vitro 3D cell model developed from human hepatocellular carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef]
- Bizjak, M.; Malavašič, P.; Pirkmajer, S.; Pavlin, M. Comparison of the Effects of Metformin on MDA-MB-231 Breast Cancer Cells in a Monolayer Culture and in Tumor Spheroids as a Function of Nutrient Concentrations. Biochem. Biophys. Res. Commun. 2019, 515, 296–302. [Google Scholar] [CrossRef]
- Minami, F.; Sasaki, N.; Shichi, Y.; Gomi, F.; Michishita, M.; Ohkusu-Tsukada, K.; Toyoda, M.; Takahashi, K.; Ishiwata, T. Morphofunctional analysis of human pancreatic cancer cell lines in 2-and 3-dimensional cultures. Sci. Rep. 2021, 11, 6775. [Google Scholar] [CrossRef]
- Lauri, C.; Chiurchioni, L.; Russo, V.M.; Zannini, L.; Signore, A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? J. Clin. Med. 2022, 11, 6590. [Google Scholar] [CrossRef]
- Oh, J.M.; Gangadaran, P.; Rajendran, R.L.; Hong, C.M.; Lee, J.; Ahn, B.-C. Different Expression of Thyroid-Specific Proteins in Thyroid Cancer Cells between 2-Dimensional (2D) and 3-Dimensional (3D) Culture Environment. Cells 2022, 11, 3559. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Wang, X.; Yousry, C.; Gupta, V. Scalable Production and In Vitro Efficacy of Inhaled Erlotinib Nanoemulsion for Enhanced Efficacy in Non-Small Cell Lung Cancer (NSCLC). Pharmaceutics 2023, 15, 996. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Kamboj, S.; Chen, C.; Wang, Z.; Kellouche, S.; Leroy-Dudal, J.; Carreiras, F.; Lambert, A.; Aimé, C. In Vitro Models of Ovarian Cancer: Bridging the Gap between Pathophysiology and Mechanistic Models. Biomolecules 2023, 13, 103. [Google Scholar] [CrossRef]
- Fiegl, H.; Hagenbuchner, J.; Kyvelidou, C.; Seeber, B.; Sopper, S.; Tsibulak, I.; Wieser, V.; Reiser, E.; Roessler, J.; Huhtinen, K.; et al. Dubious Effects of Methadone as an “Anticancer” Drug on Ovarian Cancer Cell-Lines and Patient-Derived Tumor-Spheroids. Gynecol. Oncol. 2022, 165, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The use of F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef] [PubMed]
- Amorim, B.J.; Schaarschmidt, B.M.; Grueneisen, J.; Tajmir, S.; Umutlu, L.; Signore, A.; Catalano, O.A. Nuclear Medicine Imaging of Infection/Inflammation by PET/CT and PET/MR. In Nuclear Medicine in Infectious Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 213–235. [Google Scholar] [CrossRef]
- Anzola, L.K.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Martinez, F.A.; Moreno, S.; Signore, A. Somatostatin Receptor Imaging by SPECT and PET in Patients with Chronic Inflammatory Disorders: A Systematic Review. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2496–2513. [Google Scholar] [CrossRef] [PubMed]
- Auletta, S.; Varani, M.; Horvat, R.; Galli, F.; Signore, A.; Hess, S. PET Radiopharmaceuticals for Specific Bacteria Imaging: A Systematic Review. J. Clin. Med. 2019, 8, 197. [Google Scholar] [CrossRef]
- Catalano, O.A.; Horn, G.L.; Signore, A.; Iannace, C.; Lepore, M.; Vangel, M.; Luongo, A.; Catalano, M.; Lehman, C.; Salvatore, M.; et al. PET/MR in Invasive Ductal Breast Cancer: Correlation between Imaging Markers and Histological Phenotype. Br. J. Cancer 2017, 116, 893–902. [Google Scholar] [CrossRef]
- Signore, A.; Galli, F.; Auletta, S.; Briganti, E.; Lauri, C. Molecular imaging of cancer microenvironment. Nucleus 2016, 1, 18–23. [Google Scholar]
- Signore, A.; Bonfiglio, R.; Varani, M.; Galli, F.; Campagna, G.; Desco, M.; Cussó, L.; Mattei, M.; Wunder, A.; Borri, F.; et al. Radioimmune Imaging of A4β7 Integrin and TNFα for Diagnostic and Therapeutic Applications in Inflammatory Bowel Disease. Pharmaceutics 2023, 15, 817. [Google Scholar] [CrossRef]
- Tornes, A.J.K.; Stenberg, V.Y.; Larsen, R.H.; Bruland, Ø.S.; Revheim, M.-E.; Juzeniene, A. Targeted Alpha Therapy with the 224Ra/212Pb-TCMC-TP-3 Dual Alpha Solution in a Multicellular Tumor Spheroid Model of Osteosarcoma. Front. Med. 2022, 9, 1058863. [Google Scholar] [CrossRef] [PubMed]
- Abramenkovs, A.; Hariri, M.; Spiegelberg, D.; Nilsson, S.; Stenerlöw, B. Ra-223 Induces Clustered DNA Damage and Inhibits Cell Survival in Several Prostate Cancer Cell Lines. Transl. Oncol. 2022, 26, 101543. [Google Scholar] [CrossRef] [PubMed]
- Ingargiola, M.; Runge, R.; Heldt, J.-M.; Freudenberg, R.; Steinbach, J.; Cordes, N.; Baumann, M.; Kotzerke, J.; Brockhoff, G.; Kunz-Schughart, L.A. Potential of a Cetuximab-Based Radioimmunotherapy Combined with External Irradiation Manifests in a 3-D Cell Assay. Int. J. Cancer 2014, 135, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shahverdi, K.; Huso, D.L.; Esaias, C.; Fox, J.; Liedy, A.; Zhang, Z.; Reilly, R.T.; Apostolidis, C.; Morgenstern, A.; et al. 213Bi (α-Emitter)–Antibody Targeting of Breast Cancer Metastases in the Neu-N Transgenic Mouse Model. Cancer Res. 2008, 68, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Lundsten, S.; Spiegelberg, D.; Stenerlow, B.; Nestor, M. The HSP90 Inhibitor Onalespib Potentiates 177Lu DOTATATE Therapy in Neuroendocrine Tumor Cells. Int. J. Oncol. 2019, 55, 1287–1295. [Google Scholar] [CrossRef]
- Kasten, B.B.; Gangrade, A.; Kim, H.; Fan, J.; Ferrone, S.; Ferrone, C.R.; Zinn, K.R.; Buchsbaum, D.J. 212Pb-Labeled B7-H3-Targeting Antibody for Pancreatic Cancer Therapy in Mouse Models. Nucl. Med. Biol. 2018, 58, 67–73. [Google Scholar] [CrossRef]
- Akil, H.; Quintana, M.; Raymond, J.H.; Billoux, T.; Benboubker, V.; Besse, S.; Auzeloux, P.; Delmas, V.; Petit, V.; Larue, L.; et al. Efficacy of Targeted Radionuclide Therapy Using [131I]ICF01012 in 3D Pigmented BRAF- and NRAS-Mutant Melanoma Models and In Vivo NRAS-Mutant Melanoma. Cancers 2021, 13, 1421. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; van der Meer, A.J.G.M.; Windmeijer, C.A.A.; Kouwenberg, J.J.M.; Morgenstern, A.; Bruchertseifer, F.; Sminia, P.; Denkova, A.G. The Therapeutic Potential of Polymersomes Loaded with 225Ac Evaluated in 2D and 3D in Vitro Glioma Models. Eur. J. Pharm. Biopharm. 2018, 127, 85–91. [Google Scholar] [CrossRef]
- Gaze, M.; Mairs, R.; Boyack, S.; Wheldon, T.; Barrett, A. 131I-Meta-Iodobenzylguanidine Therapy in Neuroblastoma Spheroids of Different Sizes. Br. J. Cancer 1992, 66, 1048–1052. [Google Scholar] [CrossRef][Green Version]
- Neshasteh-Riz, A.; Angerson, W.; Reeves, J.; Smith, G.; Rampling, R.; Mairs, R. Incorporation of Iododeoxyuridine in Multicellular Glioma Spheroids: Implications for DNA-Targeted Radiotherapy Using Auger Electron Emitters. Br. J. Cancer 1997, 75, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Campagna, G.; Aloisi, F.; Posa, A.; Iezzi, R.; Sirignano, P.; Taurino, M.; Signore, A. How to Combine CTA, 99mTc-WBC SPECT/CT, and [18F]FDG PET/CT in Patients with Suspected Abdominal Vascular Endograft Infections? Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3235–3250. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, D.; Carideo, L.; Russo, V.; Meucci, R.; Campagna, G.; Lastoria, S.; Signore, A. A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid. J. Clin. Med. 2023, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Signore, A.; Campagna, G.; Aloisi, F.; Taurino, M.; Sirignano, P. [18F]FDG Uptake in Non-Infected Endovascular Grafts: A Retrospective Study. Diagnostics 2023, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Lauri, C.; Bianchi, M.P.; Pelliccia, S.; Lenza, A.; Tetti, S.; Martini, M.L.; Franchi, G.; Trapasso, F.; De Biase, L.; et al. [18F]FDG PET/CT in Patients Affected by SARS-CoV-2 and Lymphoproliferative Disorders and Treated with Tocilizumab. J. Pers. Med. 2022, 12, 1839. [Google Scholar] [CrossRef]
- Silveri, G.G.; Chiurchioni, L.; Magi, L.; Ambrosini, V.; Pizzichini, P.; Russo, V.; Rinzivillo, M.; Panzuto, F.; Signore, A.; Prosperi, D. The impact of [18F]FDG PET/CT on clinical management in gastro-entero-pancreatic neuroendocrine tumors G1. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, S477. [Google Scholar]
- Magi, L.; Prosperi, D.; Lamberti, G.; Marasco, M.; Ambrosini, V.; Rinzivillo, M.; Campana, D.; Gentiloni, G.; Annibale, B.; Signore, A.; et al. Role of [18F]FDG PET/CT in the Management of G1 Gastro-Entero-Pancreatic Neuroendocrine Tumors. Endocrine 2022, 76, 484–490. [Google Scholar] [CrossRef]
- Kelly, C.J.; Hussien, K.; Muschel, R.J. 3D Tumour Spheroids as a Model to Assess the Suitability of [18F]FDG-PET as an Early Indicator of Response to PI3K Inhibition. Nucl. Med. Biol. 2012, 39, 986–992. [Google Scholar] [CrossRef]
- Purohit, N.K.; Shah, R.G.; Adant, S.; Hoepfner, M.; Shah, G.M.; Beauregard, J.-M. Potentiation of 177Lu-Octreotate Peptide Receptor Radionuclide Therapy of Human Neuroendocrine Tumor Cells by PARP Inhibitor. Oncotarget 2018, 9, 24693–24706. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Varani, M.; Lauri, C.; Ranieri, D.; Signore, A. Methods for Radiolabelling Nanoparticles: PET Use (Part 2). Biomolecules 2022, 12, 1517. [Google Scholar] [CrossRef]
- Varani, M.; Bentivoglio, V.; Lauri, C.; Ranieri, D.; Signore, A. Methods for Radiolabelling Nanoparticles: SPECT Use (Part 1). Biomolecules 2022, 12, 1522. [Google Scholar] [CrossRef] [PubMed]
- Jahandar, M.; Zarrabi, A.; Shokrgozar, M.A.; Mousavi, H. Synthesis, Characterization and Application of Polyglycerol Coated Fe3O4 Nanoparticles as a Nano-Theranostics Agent. Mater. Res. Express 2015, 2, 125002. [Google Scholar] [CrossRef]
- Ünak, P.; Yasakçı, V.; Tutun, E.; Karatay, K.B.; Walczak, R.; Wawrowicz, K.; Żelechowska-Matysiak, K.; Majkowska-Pilip, A.; Bilewicz, A. Multimodal Radiobioconjugates of Magnetic Nanoparticles Labeled with 44Sc and 47Sc for Theranostic Application. Pharmaceutics 2023, 15, 850. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Mehta, P.; Ward, M.R.; Bregenzer, M.E.; Fleck, E.M.A.; Tan, L.; McLean, K.; Buckanovich, R.J.; Mehta, G. Personalized Medicine–Based Approach to Model Patterns of Chemoresistance and Tumor Recurrence Using Ovarian Cancer Stem Cell Spheroids. Clin. Cancer Res. 2017, 23, 6934–6945. [Google Scholar] [CrossRef]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer Stem Cells. New Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Páez, D.; Labonte, M.J.; Bohanes, P.; Zhang, W.; Benhanim, L.; Ning, Y.; Wakatsuki, T.; Loupakis, F.; Lenz, H.-J. Cancer Dormancy: A Model of Early Dissemination and Late Cancer Recurrence. Clin. Cancer Res. 2012, 18, 645–653. [Google Scholar] [CrossRef]
- Varzideh, F.; Mahmoudi, E.; Pahlavan, S. Coculture with Noncardiac Cells Promoted Maturation of Human Stem Cell–Derived Cardiomyocyte Microtissues. J. Cell Biochem. 2019, 120, 16681–16691. [Google Scholar] [CrossRef]
- Wessely, A.; Waltera, A.; Reichert, T.E.; Stöckl, S.; Grässel, S.; Bauer, R.J. Induction of ALP and MMP9 Activity Facilitates Invasive Behavior in Heterogeneous Human BMSC and HNSCC 3D Spheroids. FASEB J. 2019, 33, 11884–11893. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Dong, D.L.; Jang, T.S.; Knowles, J.C.; Kim, H.W.; Jin, G.Z.; Xuan, Y. 3D culture technologies of cancer stem cells: Promising ex vivo tumor models. J. Tissue Eng. 2020, 11, 2041731420933407. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, W.; Lei, M.; He, Y.; Yan, M.; Zhang, X.; Zhao, C. Laser-Triggered Intraocular Implant to Induce Photodynamic Therapy for Posterior Capsule Opacification Prevention. Int. J. Pharm. 2016, 498, 1–11. [Google Scholar] [CrossRef]
- Sharma, S. The Cost-Effectiveness of Photodynamic Therapy for Fellow Eyes with Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Ophthalmology 2001, 108, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Basu, U.; Blacque, O.; Chao, H.; Gasser, G. Polymeric Encapsulation of Novel Homoleptic Bis(Dipyrrinato) Zinc(II) Complexes with Long Lifetimes for Applications as Photodynamic Therapy Photosensitisers. Angew. Chem. Int. Ed. 2019, 58, 14334–14340. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rompicharla, S.V.K.; Bhatt, H.; Ghosh, B.; Biswas, S. Development of chlorin e6-conjugated poly (ethylene glycol)-poly (d, l-lactide) nanoparticles for photodynamic therapy. Nanomedicine 2019, 14, 819–834. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Valente, M.; Cerase, A.; Lofiego, M.F.; Piazzini, F.; Calabrò, L.; Gambale, E.; Covre, A.; Maio, M. Immunotherapy of Brain Metastases: Breaking a “Dogma”. J. Exp. Clin. Cancer Res. 2019, 38, 419. [Google Scholar] [CrossRef] [PubMed]
- Herter, S.; Morra, L.; Schlenker, R.; Sulcova, J.; Fahrni, L.; Waldhauer, I.; Lehmann, S.; Reisländer, T.; Agarkova, I.; Kelm, J.M.; et al. A novel three-dimensional heterotypic spheroid model for the assessment of the activity of cancer immunotherapy agents. Cancer Immunol. Immunother. 2017, 66, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D Cell Culture Models: Utility in Novel Drug Discovery and Delivery Research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2021, 2021, 31–142. [Google Scholar]
- Charron, D.M.; Chen, J.; Zheng, G. Theranostic Lipid Nanoparticles for Cancer Medicine. Nanotechnol. Based Precis. Tools Detect. Treat. Cancer 2015, 2015, 103–127. [Google Scholar]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional Nanoparticles for Drug Delivery and Molecular Imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef]
- Khan, I.; Khan, M.; Umar, M.N.; Oh, D. Nanobiotechnology and Its Applications in Drug Delivery System: A Review. IET Nanobiotechnol. 2015, 9, 396–400. [Google Scholar] [CrossRef]
- Gupta, N.; Al-Saikhan, F.I.; Patel, B.; Rashid, J.; Ahsan, F. Fasudil and SOD Packaged in Peptide-Studded-Liposomes: Properties, Pharmacokinetics and Ex-Vivo Targeting to Isolated Perfused Rat Lungs. Int. J. Pharm. 2015, 488, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Figarol, A.; Gibot, L.; Golzio, M.; Lonetti, B.; Mingotaud, A.-F.; Rols, M.-P. A Journey from the Endothelium to the Tumor Tissue: Distinct Behavior between PEO-PCL Micelles and Polymersomes Nanocarriers. Drug Deliv. 2018, 25, 1766–1778. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Jones, M.K.; Szabo, S.; Tarnawski, A.S. Aberrant, Ectopic Expression of VEGF and VEGF Receptors 1 and 2 in Malignant Colonic Epithelial Cells. Implications for These Cells Growth via an Autocrine Mechanism. Biochem. Biophys. Res. Commun. 2013, 437, 515–520. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Alabdollah, F.; Sadeghi, F.; Mohammadi, M.; Abnous, K.; Ramezani, M.; Hadizadeh, F. Dextran-b-Poly(Lactide-Co-Glycolide) Polymersome for Oral Delivery of Insulin: In Vitro and in Vivo Evaluation. J. Control. Release 2016, 227, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chi, J.; Zhang, H.; Fan, Q.; Zhao, Y.; Ye, F. Development of Cell Spheroids by Advanced Technologies. Adv. Mater. Technol. 2020, 2020, 2000183. [Google Scholar] [CrossRef]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug Delivery to Solid Tumors: The Predictive Value of the Multicellular Tumor Spheroid Model for Nanomedicine Screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and Challenges for Use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Walenta, S.; Mueller-Klieser, W. Efficacy of Catumaxomab in Tumor Spheroid Killing Is Mediated by Its Trifunctional Mode of Action. Cancer Immunol. Immunother. 2010, 59, 1675–1684. [Google Scholar] [CrossRef]
- Patra, B.; Peng, C.-C.; Liao, W.-H.; Lee, C.-H.; Tung, Y.-C. Drug Testing and Flow Cytometry Analysis on a Large Number of Uniform Sized Tumor Spheroids Using a Microfluidic Device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.A.; Lemera, J.; D’Souza, G.G.M. Development of an in Vitro Tumor Spheroid Culture Model Amenable to High-Throughput Testing of Potential Anticancer Nanotherapeutics. J. Liposome Res. 2016, 26, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Ma, H.; Huang, K.; Liu, J.; Wei, T.; Jin, S.; Zhang, J.; He, S.; Liang, X.-J. Superior Penetration and Retention Behavior of 50 Nm Gold Nanoparticles in Tumors. Cancer Res. 2013, 73, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological Overviews from Bench to Bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The Effect of Co-Delivery of Paclitaxel and Curcumin by Transferrin-Targeted PEG-PE-Based Mixed Micelles on Resistant Ovarian Cancer in 3-D Spheroids and in Vivo Tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef]
- Sarisozen, C.; Dhokai, S.; Tsikudo, E.G.; Luther, E.; Rachman, I.M.; Torchilin, V.P. Nanomedicine Based Curcumin and Doxorubicin Combination Treatment of Glioblastoma with ScFv-Targeted Micelles: In Vitro Evaluation on 2D and 3D Tumor Models. Eur. J. Pharm. Biopharm. 2016, 108, 54–67. [Google Scholar] [CrossRef]
- Wang, X.; Tang, H.; Wang, C.; Zhang, J.; Wu, W.; Jiang, X. Phenylboronic Acid-Mediated Tumor Targeting of Chitosan Nanoparticles. Theranostics 2016, 6, 1378–1392. [Google Scholar] [CrossRef]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic Dual-Ligand Doxorubicin Liposomes Improve Targeting and Therapeutic Efficacy of Brain Glioma in Animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef]
- Kim, T.-H.; Mount, C.W.; Gombotz, W.R.; Pun, S.H. The Delivery of Doxorubicin to 3-D Multicellular Spheroids and Tumors in a Murine Xenograft Model Using Tumor-Penetrating Triblock Polymeric Micelles. Biomaterials 2010, 31, 7386–7397. [Google Scholar] [CrossRef]
- Saluja, V.; Mishra, Y.; Mishra, V.; Giri, N.; Nayak, P. Dendrimers Based Cancer Nanotheranostics: An Overview. Int. J. Pharm. 2021, 600, 120485. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, M.; Nayak, P. Smart Functionalised-Dendrimeric Medicine in Cancer Therapy. In Dendrimers in Nanomedicine; CRC Press: Boca Raton, FL, USA, 2021; pp. 233–253. [Google Scholar]
- Mishra, V.; Yadav, N.; Saraogi, G.K.; Tambuwala, M.M.; Giri, N. Dendrimer Based Nanoarchitectures in Diabetes Management: An Overview. Curr. Pharm. Des. 2019, 25, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, M.; Mishra, Y.; Charbe, N.; Nayak, P.; Sudhakar, K.; Aljabali, A.A.A.; Shahcheraghi, S.H.; Bakshi, H.; Serrano-Aroca, Á.; et al. Nanoarchitectures in Management of Fungal Diseases: An Overview. Appl. Sci. 2021, 11, 7119. [Google Scholar] [CrossRef]
- Jain, N.K.; Tare, M.S.; Mishra, V.; Tripathi, P.K. The Development, Characterization and in Vivo Anti-Ovarian Cancer Activity of Poly(Propylene Imine) (PPI)-Antibody Conjugates Containing Encapsulated Paclitaxel. Nanomedicine 2015, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Suttee, A.; Singh, G.; Yadav, N.; Pratap Barnwal, R.; Singla, N.; Prabhu, K.S.; Mishra, V. A Review on Status of Nanotechnology in Pharmaceutical Sciences. Int. J. Drug Deliv. Technol. 2019, 9, 98–103. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Biotin Functionalized PEGylated Poly(Amidoamine) Dendrimer Conjugate for Active Targeting of Paclitaxel in Cancer. Int. J. Pharm. 2019, 557, 329–341. [Google Scholar] [CrossRef]
- Riyaz, B.; Sudhakar, K.; Mishra, V. Quantum Dot-Based Drug Delivery for Lung Cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 311–326. [Google Scholar]
- Mishra, V.; Gurnany, E.; Mansoori, M.H. Quantum Dots in Targeted Delivery of Bioactives and Imaging. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 427–450. [Google Scholar]
- Mangeolle, T.; Yakavets, I.; Lequeux, N.; Pons, T.; Bezdetnaya, L.; Marchal, F. The Targeting Ability of Fluorescent Quantum Dots to the Folate Receptor Rich Tumors. Photodiagnosis Photodyn. Ther. 2019, 26, 150–156. [Google Scholar] [CrossRef]
- Singh, M.; Nayak, P.; Mishra, V. Carbon Nanotubes in Treatment of Arthritis: An Overview. Eur. J. Mol. Clin. Med. 2020, 7, 4366–4372. [Google Scholar]
- Mishra, V.; Singh, M.; Nayak, P.; Sriram, P.; Suttee, A. Carbon Nanotubes as Emerging Nanocarriers in Drug Delivery: An Overview. Int. J. Pharm. Qual. 2020, 11, 373–378. [Google Scholar]
- Mishra, V.; Kesharwani, P.; Jain, N.K. Biomedical Applications and Toxicological Aspects of Functionalized Carbon Nanotubes. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 293–330. [Google Scholar] [CrossRef]
- Kesharwani, P.; Mishra, V.; Jain, N.K. Validating the Anticancer Potential of Carbon Nanotube-Based Therapeutics through Cell Line Testing. Drug Discov. Today 2015, 20, 1049–1060. [Google Scholar] [CrossRef]
- Kabadi, P.K.; Rodd, A.L.; Simmons, A.E.; Messier, N.J.; Hurt, R.H.; Kane, A.B. A Novel Human 3D Lung Microtissue Model for Nanoparticle-Induced Cell-Matrix Alterations. Part Fibre. Toxicol. 2019, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59, e16150477. [Google Scholar] [CrossRef]
- Rodallec, A.; Sicard, G.; Giacometti, S.; Carré, M.; Pourroy, B.; Bouquet, F.; Savina, A.; Lacarelle, B.; Ciccolini, J.; Fanciullino, R. From 3D Spheroids to Tumor Bearing Mice: Efficacy and Distribution Studies of Trastuzumab-Docetaxel Immunoliposome in Breast Cancer. Int. J. Nanomed. 2018, 13, 6677–6688. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing Amphiphilic Polymeric Micelles for Diagnostic and Therapeutic Applications: Breakthroughs and Bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in Polymeric Micelles for Drug Delivery and Tumor Targeting. Nanomedicine 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Patil, A.; Mishra, V.; Thakur, S.; Riyaz, B.; Kaur, A.; Khursheed, R.; Patil, K.; Sathe, B. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Nanosci. 2018, 15, 137–146. [Google Scholar] [CrossRef]
- Kumari, P.; Jain, S.; Ghosh, B.; Zorin, V.; Biswas, S. Polylactide-Based Block Copolymeric Micelles Loaded with Chlorin E6 for Photodynamic Therapy: In Vitro Evaluation in Monolayer and 3D Spheroid Models. Mol. Pharm. 2017, 14, 3789–3800. [Google Scholar] [CrossRef]
- Mishra, V.; Nayak, P.; Singh, M.; Tambuwala, M.M.; Aljabali, A.A.; Chellappan, D.K.; Dua, K. Pharmaceutical Aspects of Green Synthesized Silver Nanoparticles: A Boon to Cancer Treatment. Anticancer Agents Med. Chem. 2021, 21, 1490–1509. [Google Scholar] [CrossRef]
- Arora, N.; Shome, R.; Ghosh, S.S. Deciphering Therapeutic Potential of PEGylated Recombinant PTEN-Silver Nanoclusters Ensemble on 3D Spheroids. Mol. Biol. Rep. 2019, 46, 5103–5112. [Google Scholar] [CrossRef]
- Kaur, M.; Sudhakar, K.; Mishra, V. Fabrication and Biomedical Potential of Nanogels: An Overview. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 287–296. [Google Scholar] [CrossRef]
- Saraogi, G.K.; Tholiya, S.; Mishra, Y.; Mishra, V.; Albutti, A.; Nayak, P.; Tambuwala, M.M. Formulation Development and Evaluation of Pravastatin-Loaded Nanogel for Hyperlipidemia Management. Gels 2022, 8, 81. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, X.; Li, D.; Wang, X.; Sun, M.; He, L.; Tang, R. TPGS-Grafted and Acid-Responsive Soy Protein Nanogels for Efficient Intracellular Drug Release, Accumulation, Penetration in 3D Tumor Spheroids of Drug-Resistant Cancer Cells. Mater. Sci. Eng. C 2019, 102, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D.; Gupta, S. Cancer treatment with nanodiamonds. Front. Biosci. 2017, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Sharma, A.; Toma, M.; Samaniego, S.; Gallud, A.; Wang, E.; Pal, K.; Mukhopadhyay, D.; Fadeel, B. Tumor Selective Uptake of Drug-Nanodiamond Complexes Improves Therapeutic Outcome in Pancreatic Cancer. Nanomedicine 2019, 18, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Choi, J.H.; Dai, Z.; Wang, J.; Kim, D.; Tang, X.; Zhu, L. Improving Tumor Specificity and Anticancer Activity of Dasatinib by Dual-Targeted Polymeric Micelles. ACS Appl. Mater. Interfaces 2017, 9, 36642–36654. [Google Scholar] [CrossRef]
- Varani, M.; Bentivoglio, V.; Serafinelli, M.; Lauri, C.; Signore, A.A. Designed radiolabelled Poly (Lactic-co-Glycolic Acid) nanoparticle for theragnostic applications. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, S433. [Google Scholar]
- Varani, M.; Galli, F.; Capriotti, G.; Mattei, M.; Cicconi, R.; Campagna, G.; Panzuto, F.; Signore, A. Theranostic Designed Near-Infrared Fluorescent Poly (Lactic-Co-Glycolic Acid) Nanoparticles and Preliminary Studies with Functionalized VEGF-Nanoparticles. J. Clin. Med. 2020, 9, 1750. [Google Scholar] [CrossRef]
- Varani, M.; Galli, F.; Bentivoglio, V.; Signore, A. Particles and nanoparticles in nuclear medicine: Basic principles and instrumentation. In Nuclear Medicine and Molecular Imaging; Signore, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 202–211. [Google Scholar]
- Varani, M.; Campagna, G.; Bentivoglio, V.; Serafinelli, M.; Martini, M.L.; Galli, F.; Signore, A. Synthesis and Biodistribution of 99mTc-Labeled PLGA Nanoparticles by Microfluidic Technique. Pharmaceutics 2021, 13, 1769. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Nayak, P.; Varani, M.; Lauri, C.; Signore, A. Methods for Radiolabeling Nanoparticles (Part 3): Therapeutic Use. Biomolecules 2023, 13, 1241. [Google Scholar] [CrossRef]
- Le, T.T.D.; Pham, T.H.; Nguyen, T.N.; Ngo, T.H.G.; Hoang, T.M.N.; Le, Q.H. Evaluation of Anti-HER2 ScFv-Conjugated PLGA–PEG Nanoparticles on 3D Tumor Spheroids of BT474 and HCT116 Cancer Cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025004. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Chen, X.; Zhao, Y. Nanozymes: Versatile platforms for cancer diagnosis and therapy. Nano-Micro Lett. 2022, 14, 95. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Mansur, A.A.; da Silveira, I.B.; Pires, T.F.; Victória, H.F.; Krambrock, K.; Leite, M.F.; Mansur, H.S. Nanozymes with Peroxidase-like Activity for Ferroptosis-Driven Biocatalytic Nanotherapeutics of Glioblastoma Cancer: 2D and 3D Spheroids Models. Pharmaceutics 2023, 15, 1702. [Google Scholar] [CrossRef]
| Culture Models |  PDX Animal Model |  2D Model |  3D Model |
|---|---|---|---|
| Ethical and regulation issues | Yes | No | No |
| Model complexity | Very complex | Complex | Intermediate |
| Physiological relevance | High | Intermediate | More than 2D |
| Reproducibility | Unsuited | High | Less than 2D |
| Data provider | Difficulty in exploit | Easy exploit | Easy exploit |
| Drug screening | Less effective | Highly effective | Effective |
| Controlled microenvironment | No | Yes | Yes |
| Mimicking the original tumors | Intermediate | Less | Intermediate |
| Preservation of tumor morphology | Intermediate | Less | Less |
| Success rate of model generation | Less | Less | Intermediate |
| Maintenance | High | Less | Intermediate |
| Cost | High | Less | Intermediate |
| 3D Tumor Models | Formulation Technique | Benefits | Associated Risks | References |
|---|---|---|---|---|
Scaffold-based systems  |
|
|
| [7,8] |
Scaffold-free systems |
|
|
| [9,10] |
| Techniques | Advantages | Disadvantages | References |
|---|---|---|---|
Hanging drop technique (HDT) |
|
| [26,27] |
Spinner flask |
|
| [28,29] |
Magnetic levitation and magnetic bio-printing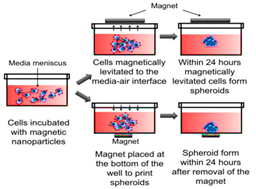 |
|
| [28,29] |
Liquid overlay technique (LOT) |
|
| [30] |
Microfluidics |
|
| [31] |
| Radionuclide | Conjugation | Targeted Tumor | Result | Ref. |
|---|---|---|---|---|
| 224Ra and 212Pb | 224Ra/212Pb-TCMC-TP-3 and 212Pb-TCMC-TP-3 | Osteosarcoma | An 11.4-fold reduction in spheroid viability has been shown in treatment with 1 kBq/mL of 224Ra/212Pb-TCMC-TP-3 for 24 h compared with unconjugated 224Ra/212Pb. | [54] |
| 223Ra | 223Ra-hydroxyapatite (HAp) 3DS model | Prostate cancer cells | It generated high levels of apoptosis by inhibiting cell growth irrespective of cell type. | [55] |
| 90Y | Cetuximab (C225) | Head and neck squamous cell cancer (HNSCC) | Unconjugated C225 treatment did not affect spheroid development or cell viability. | [56] |
| 213Bi | HER-2/neu antigen | Breast cancer | Effective in treating early-stage HER-2/neu--expressing micrometastases. | [57] |
| 177Lu | DOTATATE peptide | Neuroendocrine tumors | 177LuDOTATATE inhibited the growth of BON and NCIH727 spheroids but did not affect NCIH460 spheroids. | [58] |
| 212Pb | Monoclonal antibody (mAb) 376.96 | Pancreatic ductal adenocarcinoma | PDAC3 cell clonogenic survival was decreased by 212Pb-376.96. | [59] |
| 131I | ICF01012 MEK inhibitors (MEKi) | Melanoma cells | MEKi combined therapy may be beneficial in treating advanced pigmented BRAF-mutant melanoma. | [60] |
| 225Ac | Polymersomes | Glioblastoma | Effectively inhibit tumor spheroid growth | [61] |
| 131I | Meta-iodobenzylguanidine (MIBG) | Neuroblastoma | In vivo, 13II-MIBG may spare smaller micrometastases. | [62] |
| 125I | Deoxyuridine (IUdR) | Glioblastoma | Nuclear incorporation of [125I]IUdR decreased significantly as spheroid size increased. | [63] |
| Drug/Bioactive | Nanocarriers | Target | Ligand | MCTS | In Vivo Study | Drug Resistance | Ref. |
|---|---|---|---|---|---|---|---|
| Oregon Green PTX | Liposomes/micelles | Integrin | iRGD peptide | Lung cancer | Negative | Negative | [101] |
| PTX + CUR + Rhodamine | PEG-phosphatidyl | Tf receptors | Tf | Ovarian cancer | Positive | Positive | [103] |
| DOX + CUR | Micelles | GLUT1 | GLUT1-scFv | Brain cancer U87MG | Negative | Positive | [104] |
| DOX | Chitosan NPs | Sialic acid groups | CPBA | Brain cancer SH-SY5Y | Positive | Negative | [105] |
| DOX | Liposomes | Tf receptor | TAT | Brain cancer C6 | Positive | Negative | [106] |
| DOX | PLGA NPs | Tf receptors | Tf | Lung cancer A549 | Positive | Negative | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. https://doi.org/10.3390/cancers15194846
Nayak P, Bentivoglio V, Varani M, Signore A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers. 2023; 15(19):4846. https://doi.org/10.3390/cancers15194846
Chicago/Turabian StyleNayak, Pallavi, Valeria Bentivoglio, Michela Varani, and Alberto Signore. 2023. "Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates" Cancers 15, no. 19: 4846. https://doi.org/10.3390/cancers15194846
APA StyleNayak, P., Bentivoglio, V., Varani, M., & Signore, A. (2023). Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers, 15(19), 4846. https://doi.org/10.3390/cancers15194846








