Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment and Disease Evaluation
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatments
3.2. Responses to Chemo-Immunotherapy HITS
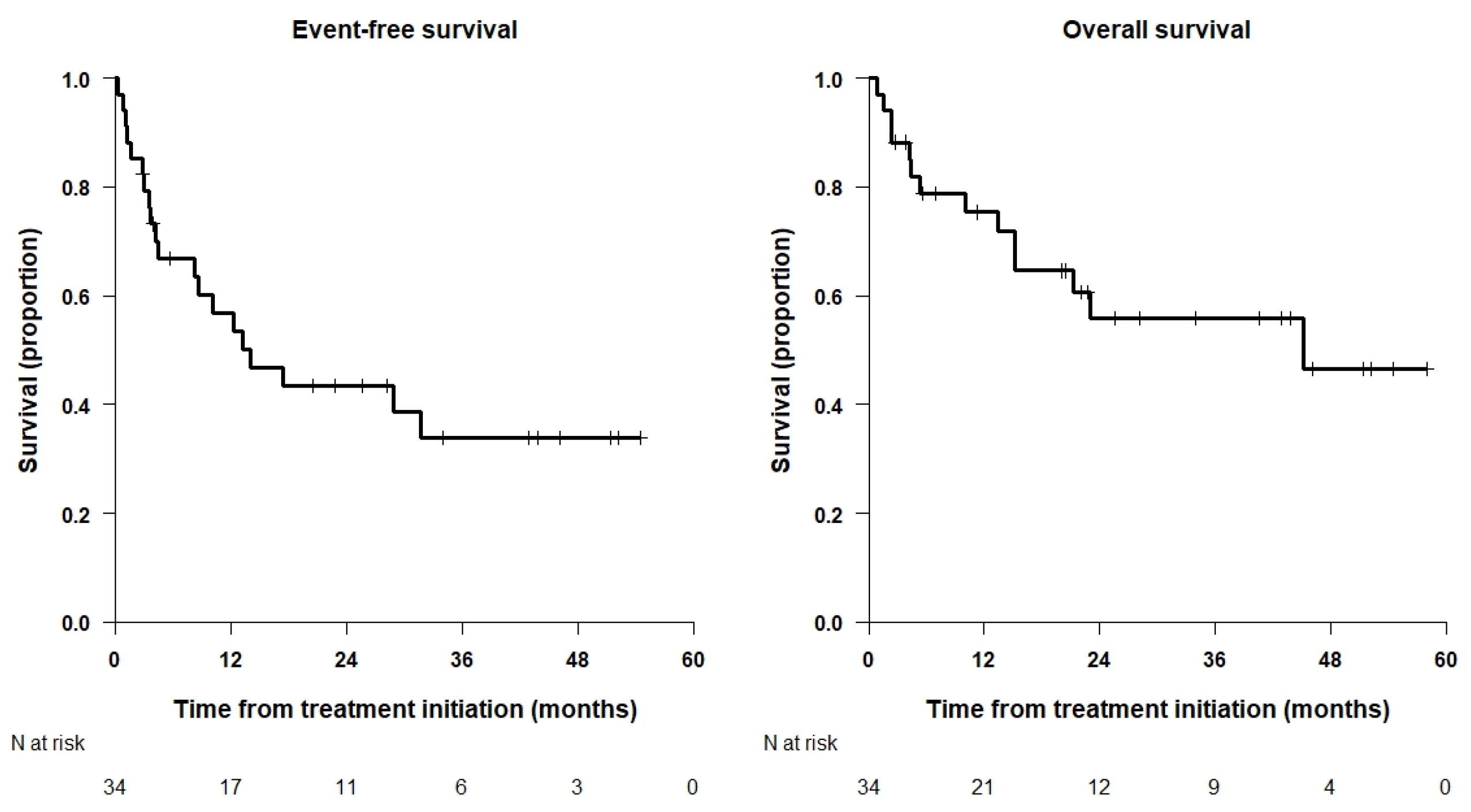
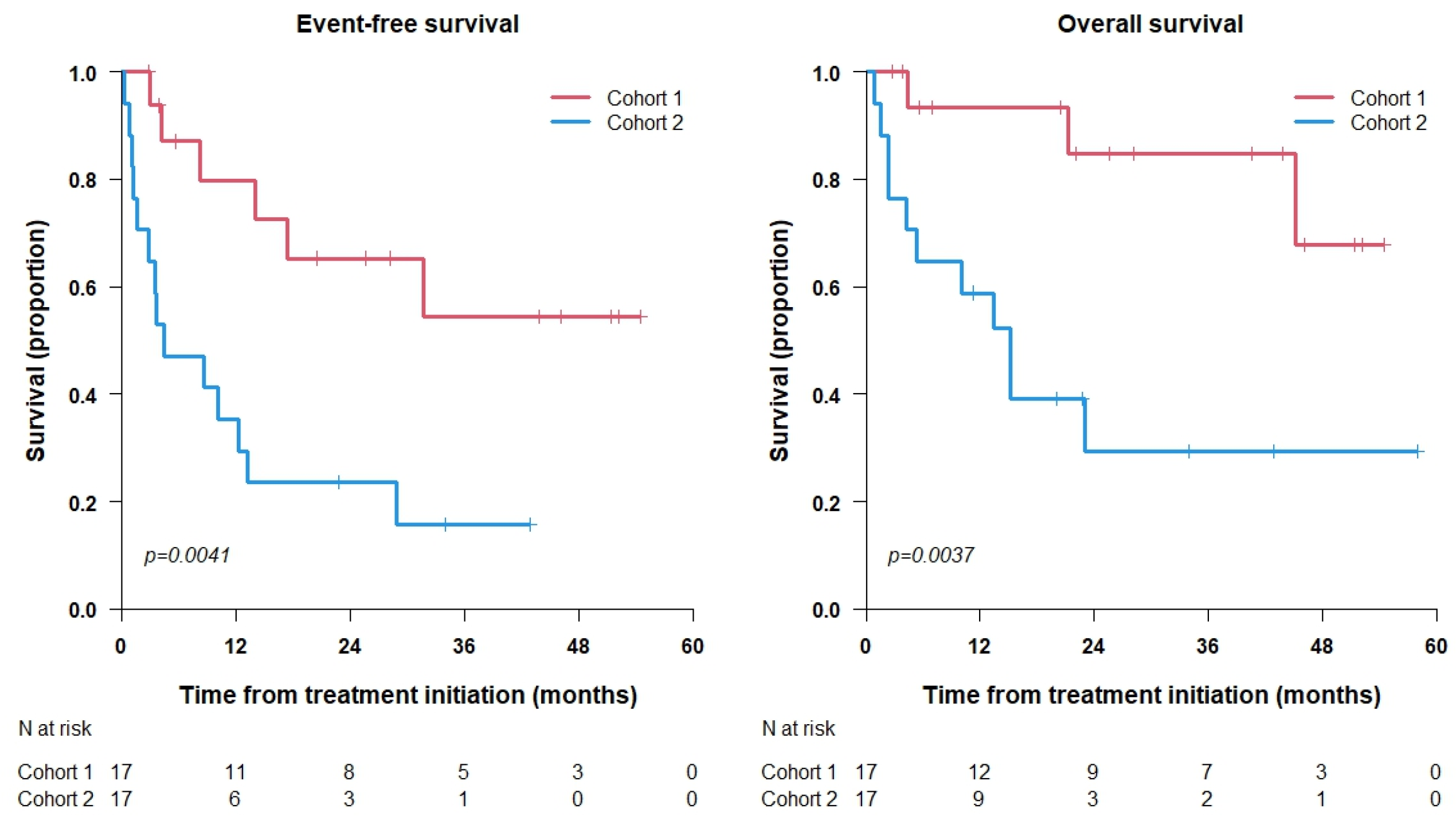
3.3. Survival Analysis
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Desai, A.V.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; London, W.B.; Tenney, S.C.; Diccianni, M.; Hank, J.A.; Parisi, M.T.; Shulkin, B.L.; et al. Outcomes Following GD2-Directed Postconsolidation Therapy for Neuroblastoma After Cessation of Random Assignment on ANBL0032: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2022, 40, 4107–4118. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M.; Danks, M.K. New therapeutic targets for the treatment of high-risk neuroblastoma. J. Cell. Biochem. 2009, 107, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Yanik, G.A.; Parisi, M.T.; Shulkin, B.L.; Naranjo, A.; Kreissman, S.G.; London, W.B.; Villablanca, J.G.; Maris, J.M.; Park, J.R.; Cohn, S.L.; et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the Children’s Oncology Group. J. Nucl. Med. 2013, 54, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Lambert, B.; Potschger, U.; Castellani, M.R.; Lewington, V.; Bar-Sever, Z.; Oudoux, A.; Śliwińska, A.; Taborska, K.; Biassoni, L.; et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: The SIOPEN/HR-NBL1 and COG-A3973 trials. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.; Naranjo, A.; Hibbitts, E.; Kreissman, S.G.; Granger, M.M.; Irwin, M.S.; Bagatell, R.; London, W.B.; Greengard, E.G.; Park, J.R.; et al. Predictors of differential response to induction therapy in high-risk neuroblastoma: A report from the Children’s Oncology Group (COG). Eur. J. Cancer 2019, 112, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Scott, J.R.; Stewart, C.F.; London, W.B.; Naranjo, A.; Santana, V.M.; Shaw, P.J.; Cohn, S.L.; Matthay, K.K. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: A Children’s Oncology Group study. J. Clin. Oncol. 2011, 29, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.J.; Doral, M.Y.; DuBois, S.G.; Villablanca, J.G.; Yanik, G.A.; Matthay, K.K. Different outcomes for relapsed versus refractory neuroblastoma after therapy with 131I-metaiodobenzylguanidine (131I-MIBG). Eur. J. Cancer 2015, 51, 2465–2472. [Google Scholar] [CrossRef]
- Moreno, L.; Rubie, H.; Varo, A.; Le Deley, M.C.; Amoroso, L.; Chevance, A.; Garaventa, A.; Gambart, M.; Bautista, F.; Valteau-Couanet, D.; et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr. Blood Cancer 2017, 64, 25–31. [Google Scholar] [CrossRef]
- Yanik, G.A.; Villablanca, J.G.; Maris, J.M.; Weiss, B.; Groshen, S.; Marachelian, A.; Park, J.R.; Tsao-Wei, D.; Hawkins, R.; Shulkin, B.L.; et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A New Approaches to Neuroblastoma Therapy (NANT) phase II study. Biol. Blood Marrow Transplant. 2015, 21, 673–681. [Google Scholar] [CrossRef]
- DuBois, S.G.; Matthay, K.K. 131I-metaiodobenzylguanidine therapy in children with advanced neuroblastoma. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 53–65. [Google Scholar]
- London, W.B.; Frantz, C.N.; Campbell, L.A.; Seeger, R.C.; Brumback, B.A.; Cohn, S.L.; Matthay, K.K.; Castleberry, R.P.; Diller, L. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: A Children’s Oncology Group study. J. Clin. Oncol. 2010, 28, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, L.; Erminio, G.; Makin, G.; Pearson, A.D.J.; Brock, P.; Valteau-Couanet, D.; Castel, V.; Pasquet, M.; Laureys, G.; Thomas, C.; et al. Topotecan-Vincristine-Doxorubicin in Stage 4 High-Risk Neuroblastoma Patients Failing to Achieve a Complete Metastatic Response to Rapid COJEC: A SIOPEN Study. Cancer Res. Treat. 2018, 50, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bagatell, R.; London, W.B.; Wagner, L.M.; Voss, S.D.; Stewart, C.F.; Maris, J.M.; Kretschmar, C.; Cohn, S.L. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: A Children’s Oncology Group study. J. Clin. Oncol. 2011, 29, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Kramer, K.; Modak, S.; Cheung, N.K.V. Irinotecan Plus Temozolomide for Relapsed or Refractory Neuroblastoma. J. Clin. Oncol. 2006, 24, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Rubie, H.; Chisholm, J.; Defachelles, A.S.; Morland, B.; Munzer, C.; Valteau-Couanet, D.; Mosseri, V.; Bergeron, C.; Weston, C.; Coze, C.; et al. Phase II Study of Temozolomide in Relapsed or Refractory High-Risk Neuroblastoma: A Joint Société Franc¸aise des Cancers de l’Enfant and United Kingdom Children Cancer Study Group–New Agents Group Study. J. Clin. Oncol. 2006, 24, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Kramer, K.; Modak, S.; Cheung, N.K.V. Five-Day Courses of Irinotecan as Palliative Therapy for Patients with Neuroblastoma. Cancer 2005, 103, 858–862. [Google Scholar] [CrossRef]
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.E.; Diccianni, M.B.; Sondel, P.M. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957. [Google Scholar] [CrossRef]
- Mody, R.; Yu, A.L.; Naranjo, A.; Zhang, F.F.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.E.; Diccianni, M.B.; Hank, J.A.; et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 2160–2169. [Google Scholar] [CrossRef]
- Mora, J.; Chan, G.C.; Morgenstern, D.A.; Amoroso, L.; Nysom, K.; Faber, J.; Wingerter, A.; Bear, M.; Rubio San Simon, A.; Tornøe, K.; et al. 891P –naxitamab treatment for relapsed or refractory high-risk neuroblastoma: Outcomes from the first prespecified analyses of the pivotal 201 trial. Ann. Oncol. 2022, 33 (Suppl. 7), S956. [Google Scholar] [CrossRef]
- Olgun, N.; Cecen, E.; Ince, D.; Kizmazoglu, D.; Baysal, B.; Onal, A.; Ozdogan, O.; Guleryuz, H.; Cetingoz, R.; Demiral, A.; et al. Dinutuximab beta plus conventional chemotherapy for relapsed/refractory high-risk neuroblastoma: A single-center experience. Front. Oncol. 2022, 12, 1041443. [Google Scholar] [CrossRef]
- Wieczorek, A.; Zaniewska-Tekieli, A.; Ehlert, K.; Pawinska-Wasikowska, K.; Balwierz, W.; Lode, H. Dinutuximab beta combined with chemotherapy in patients with relapsed or refractory neuroblastoma. Front. Oncol. 2023, 13, 1082771. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Kramer, K.; LaQuaglia, M.P.; Modak, S.; Yataghene, K.; Cheung, N.K.V. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J. Clin. Oncol. 2004, 22, 4888–4892. [Google Scholar] [CrossRef] [PubMed]
- Furman, W.L.; Mccarville, B.; Shulkin, B.L.; Davidoff, A.; Krasin, M.; Hsu, C.W.; Wu, J.; Brennan, R.; Bishop, M.W.; Federico, S.M.; et al. Improved Outcome in Children with Newly Diagnosed High-Risk Neuroblastoma Treated With Chemoimmunotherapy: Updated Results of a Phase II Study Using Hu14.18K322A. J. Clin. Oncol. 2021, 40, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Kushner, B.H.; Flores, M.A.; Santa-María, V.; Garraus, M.; Basu, E.M.; Roberts, S.S.; Castañeda, A.; Gorostegui, M.; Cheung, N.K.V.; et al. Naxitamab-based chemoimmunotherapy for resistant high-risk neuroblastoma: Preliminary results of HITS pilot/phase II study. J. Clin. Oncol. 2019, 37 (Suppl. 15), 10025. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement from the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Chan, G.C.; Morgenstern, D.A.; Nysom, K.; Bear, M.K.; Tornøe, K.; Kushner, B.H. Outpatient administration of naxitamab in combination with granulocyte-macrophage colony-stimulating factor in patients with refractory and/or relapsed high-risk neuroblastoma: Management of adverse events. Cancer Rep. 2022, 6, e1627. [Google Scholar] [CrossRef]
- Mora, J.; Cruz, O.; Lavarino, C.; Rios, J.; Vancells, M.; Parareda, A.; Salvador, H.; Suñol, M.; Carrasco, R.; Guillen, A.; et al. Results of induction chemotherapy in children older than 18 months with stage-4 neuroblastoma treated with an adaptive-to-response modified N7 protocol (mN7). Clin. Transl. Oncol. 2015, 17, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Meier, P. Non parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Mora, J.; Castañeda, A.; Gorostegui, M.; Varo, A.; Perez-Jaume, S.; Simao, M.; Muñoz, J.P.; Garraus, M.; Larrosa, C.; Salvador, N.; et al. Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use-Updated Outcome Report. Cancers 2023, 28, 2535. [Google Scholar] [CrossRef]
- Troschke-Meurer, S.; Zumpe, M.; Meißner, L.; Siebert, N.; Grabarczyk, P.; Forkel, H.; Maletzki, C.; Bekeschus, S.; Lode, H.N. Chemotherapeutics Used for High-Risk Neuroblastoma Therapy Improve the Efficacy of Anti-GD2 Antibody Dinutuximab Beta in Preclinical Spheroid Models. Cancers 2023, 15, 904. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Edeline, V.; Lumbroso, J.; Tanguy, M.L.; Asselain, B.; Zucker, J.M.; Valteau-Couanet, D.; Hartmann, O.; Michon, J. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J. Clin. Oncol. 2003, 21, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, H.M.; Cohn, S.L.; Shore, R.M.; Bardo, D.M.; Haut, P.R.; Olszewski, M.; Schmoldt, J.; Liu, D.; Rademaker, A.W.; Kletzel, M. Scintigraphic response by 123Imetaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J. Clin. Oncol. 2004, 22, 3909–3915. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Simon, T.; Hero, B.; Schicha, H.; Berthold, F. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: Results of the German Neuroblastoma Trial NB97. Eur. J. Cancer 2008, 44, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Heller, G.; Kushner, B.H.; Burch, L.; O’Reilly, R.J. Stage IV neuroblastoma more than 1 year of age at diagnosis: Major response to chemotherapy and survival durations correlated strongly with dose intensity. Prog. Clin. Biol. Res. 1991, 366, 567–573. [Google Scholar] [PubMed]
- Cheung, N.-K.V.; Heller, G. Chemotherapy Dose Intensity Correlates Strongly with Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J. Clin. Oncol. 1991, 9, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- London, W.B.; Bagatell, R.; Weigel, B.J.; Fox, E.; Guo, D.; Van Ryn, C.; Naranjo, A.; Park, J.R. Historical time-to-progression (TTP) and progression-free survival (PFS) in relapsed/refractory neuroblastoma modern-era (2002-14) patients from Children’s Oncology Group (COG) early-phase trials. Cancer 2017, 123, 4914–4923. [Google Scholar] [CrossRef]
- Kushner, B.H.; Modak, S.; Kramer, K.; LaQuaglia, M.P.; Yataghene, K.; Basu, E.M.; Roberts, S.S.; Cheung, N.K. Striking dichotomy in outcome of MYCN-amplified neuroblastoma in the contemporary era. Cancer 2014, 12, 2050–2059. [Google Scholar] [CrossRef]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef]
- Castleberry, R.P.; Cantor, A.B.; Green, A.A.; Joshi, V.; Berkow, R.L.; Buchanan, G.R.; Leventhal, B.; Mahoney, D.H.; Smith, E.I.; Hayes, F.A. Phase II investigational window using carboplatin, iproplatin, ifosfamide, and epirubicin in children with untreated disseminated neuroblastoma: A Pediatric Oncology Group study. J. Clin. Oncol. 1994, 12, 1616–1620. [Google Scholar] [CrossRef]
- McWilliams, N.B.; Hayes, F.A.; Green, A.A.; Smith, E.I.; Nitschke, R.; Altshuler, G.A.; Shuster, J.J.; Castleberry, R.P.; Vietti, T.J. Cyclophosphamide/doxorubicin vs. cisplatin/teniposide in the treatment of children older than 12 months of age with disseminated neuroblastoma: A Pediatric Oncology Group randomized phase II study. Med. Pediatr. Oncol. 1995, 24, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, C.R.; Blanc Vincent, M.P.; Bergeron, C.; Fervers, B.; Philip, T. Induction chemotherapy in metastatic neuroblastoma—Does dose influence response? A critical review of published data standards, options and recommendations (SOR) project of the National Federation of French Cancer Centres (FNCLCC). Eur. J. Cancer 2000, 36, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; LaQuaglia, M.P.; Bonilla, M.A.; Lindsley, K.; Rosenfield, N.; Yeh, S.; Eddy, J.; Gerald, W.L.; Heller, G.; Cheung, N.K. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. J. Clin. Oncol. 1994, 12, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Valteau-Couanet, D.; Michon, J.; Boneu, A.; Rodary, C.; Perel, Y.; Bergeron, C.; Rubie, H.; Coze, C.; Plantaz, D.; Bernard, F.; et al. Results of Induction Chemotherapy in Children Older Than 1 Year with a Stage 4 Neuroblastoma Treated with the NB 97 French Society of Pediatric Oncology (SFOP) Protocol. J. Clin. Oncol. 2005, 23, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.D.J.; Pinkerton, C.R.; Lewis, I.J.; Imeson, J.; Ellershaw, C.; Machin, D.; for the European Neuroblastoma Study Group and the Children’s Cancer and Leukaemia Group (CCLG; formerly United Kingdom Children’s Cancer Study Group). High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008, 9, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Minard-Colin, V.; Aupérin, A.; Pillon, M.; Burke, G.A.A.; Barkauskas, D.A.; Wheatley, K.; Delgado, R.F.; Alexander, S.; Uyttebroeck, A.; Gross, T.G.; et al. European Intergroup for Childhood Non-Hodgkin Lymphoma; Children’s Oncology Group. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin’s Lymphoma in Children. N. Engl. J. Med. 2020, 382, 2207–2219. [Google Scholar] [CrossRef]
- Goldsmith, K.C.; Park, J.R.; Kayser, K.; Malvar, J.; Chi, Y.Y.; Groshen, S.G.; Villablanca, J.G.; Krytska, K.; Lai, L.M.; Acharya, P.T.; et al. Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: Phase 1 trial results. Nat. Med. 2023, 29, 1092–1102. [Google Scholar] [CrossRef]
- Corbacioglu, S. Irinotecan and temozolomide combined with dasatinib and rapamycin for patients with relapsed or refractory neuroblastoma: Results of the prospective randomized RIST trial. In Proceedings of the Advances in Neuroblastoma (ANR) Conference, Amsterdam, The Netherlands, 14–18 May 2023. [Google Scholar]
| n = 34 | n | |
|---|---|---|
| Race: | 34 | |
| Asian | 15 (44.1%) | |
| Caucasian | 19 (55.9%) | |
| Gender: | 34 | |
| Female | 12 (35.3%) | |
| Male | 22 (64.7%) | |
| MYCN: | 34 | |
| No amplification | 28 (82.4%) | |
| Amplified | 6 (17.6%) | |
| Prior IT: | 34 | |
| No | 15 (44.1%) | |
| Yes | 19 (55.9%) | |
| Prior hu3F8: | 34 | |
| No | 28 (82.4%) | |
| Yes | 6 (17.6%) | |
| Prior to another Ab: | 34 | |
| No | 28 (82.4%) | |
| Yes | 6 (17.6%) | |
| Age at diagnosis (years) | 3.7 [0.7; 27.6] | 34 |
| Age at treatment initiation (years) | 4.9 [1.8; 33.9] | 34 |
| Time from diagnosis to treatment initiation (years) | 1.1 [0.2; 6.4] | 34 |
| Cohort: | 34 | |
| 1 | 17 (50.0%) | |
| 2 | 17 (50.0%) | |
| Follow-up time for alive patients (months) | 26.9 [2.8; 57.9] | 20 |
| Whole Cohort | Cohort #1 | Cohort #2 | |
|---|---|---|---|
| Early HITS | Late HITS | ||
| n = 34 | n = 17 | n = 17 | |
| Post 2nd cycle | n = 30 | n = 17 | n = 13 |
| CR | 7 | 5 | 2 |
| PR | 3 | 2 | 1 |
| SD | 18 | 10 | 8 |
| PD | 2 | 0 | 2 |
| Best response | n = 34 | n = 17 | n = 17 |
| CR | 10 | 8 | 2 |
| PR | 1 | 0 | 1 |
| SD | 18 | 9 | 9 |
| PD | 5 | 0 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, J.P.; Larrosa, C.; Chamorro, S.; Perez-Jaume, S.; Simao, M.; Sanchez-Sierra, N.; Varo, A.; Gorostegui, M.; Castañeda, A.; Garraus, M.; et al. Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes. Cancers 2023, 15, 4837. https://doi.org/10.3390/cancers15194837
Muñoz JP, Larrosa C, Chamorro S, Perez-Jaume S, Simao M, Sanchez-Sierra N, Varo A, Gorostegui M, Castañeda A, Garraus M, et al. Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes. Cancers. 2023; 15(19):4837. https://doi.org/10.3390/cancers15194837
Chicago/Turabian StyleMuñoz, Juan Pablo, Cristina Larrosa, Saray Chamorro, Sara Perez-Jaume, Margarida Simao, Nazaret Sanchez-Sierra, Amalia Varo, Maite Gorostegui, Alicia Castañeda, Moira Garraus, and et al. 2023. "Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes" Cancers 15, no. 19: 4837. https://doi.org/10.3390/cancers15194837
APA StyleMuñoz, J. P., Larrosa, C., Chamorro, S., Perez-Jaume, S., Simao, M., Sanchez-Sierra, N., Varo, A., Gorostegui, M., Castañeda, A., Garraus, M., Lopez-Miralles, S., & Mora, J. (2023). Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes. Cancers, 15(19), 4837. https://doi.org/10.3390/cancers15194837







