Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Vitamin D3 Cytotoxicity Assay
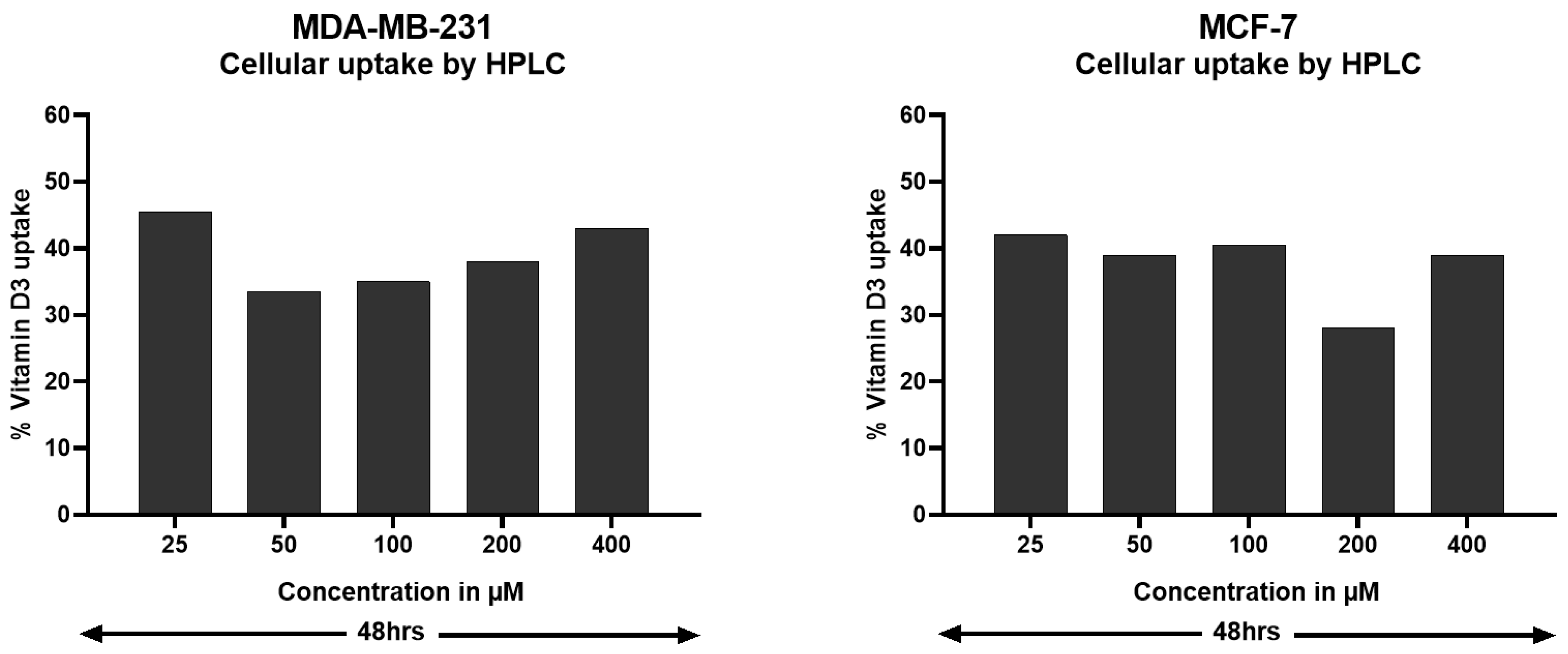
2.2.2. HPLC-Based Measurements of Vit-D3 Intracellular Uptake
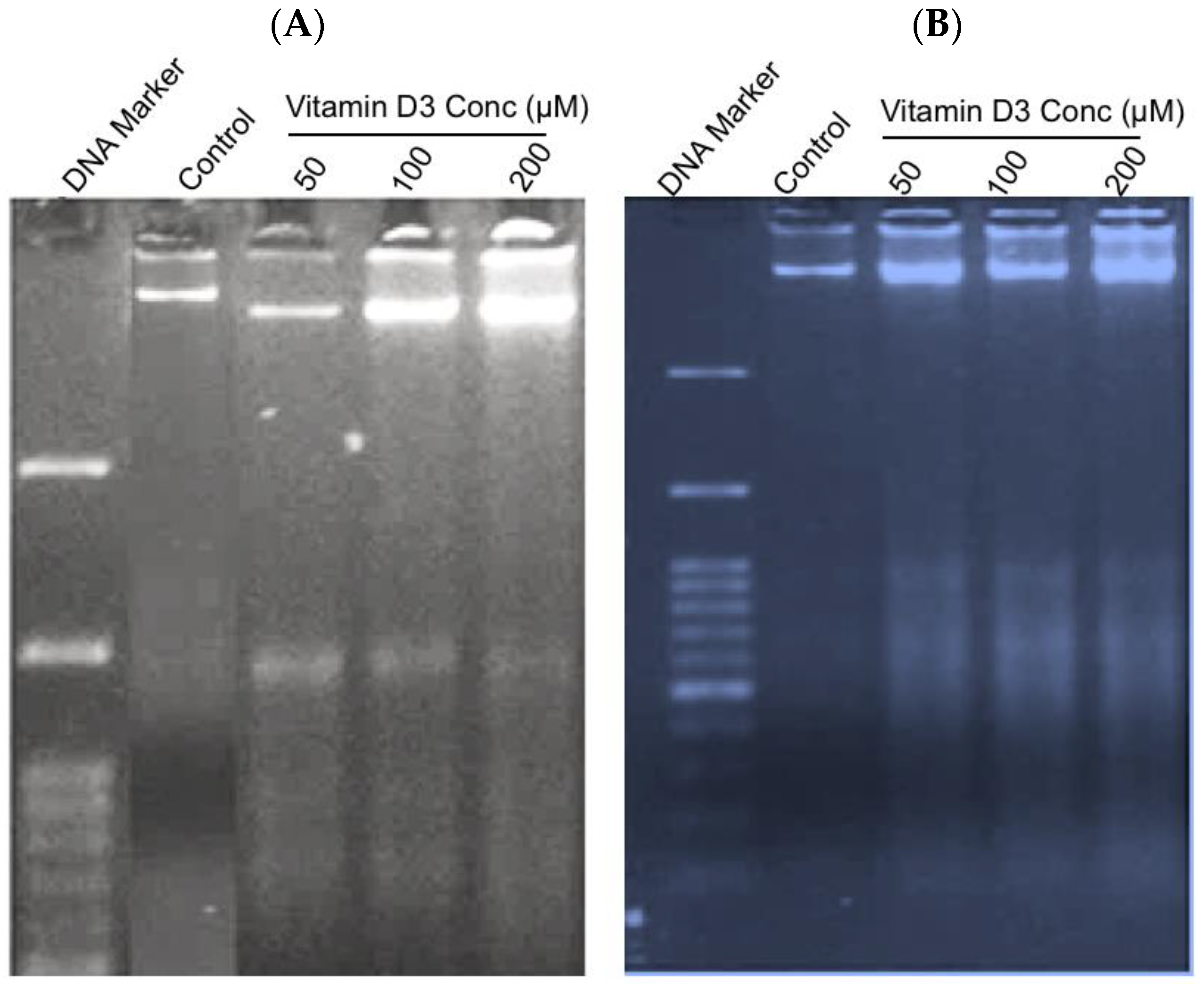
2.2.3. Measurement of DNA Integrity in Vit-D3-Treated Cells
2.2.4. Cell Death Assay with Acridine Orange and Ethidium Bromide (Et/Br) Staining
2.2.5. Cell Cycle Analysis
2.2.6. Caspase-3/7 Activity Assay
2.2.7. Wound Healing (Cell Migration) and Angiogenesis Assays
2.2.8. Western Blotting
2.2.9. Vit-D3 Effects in Swiss Albino Mice In Vivo
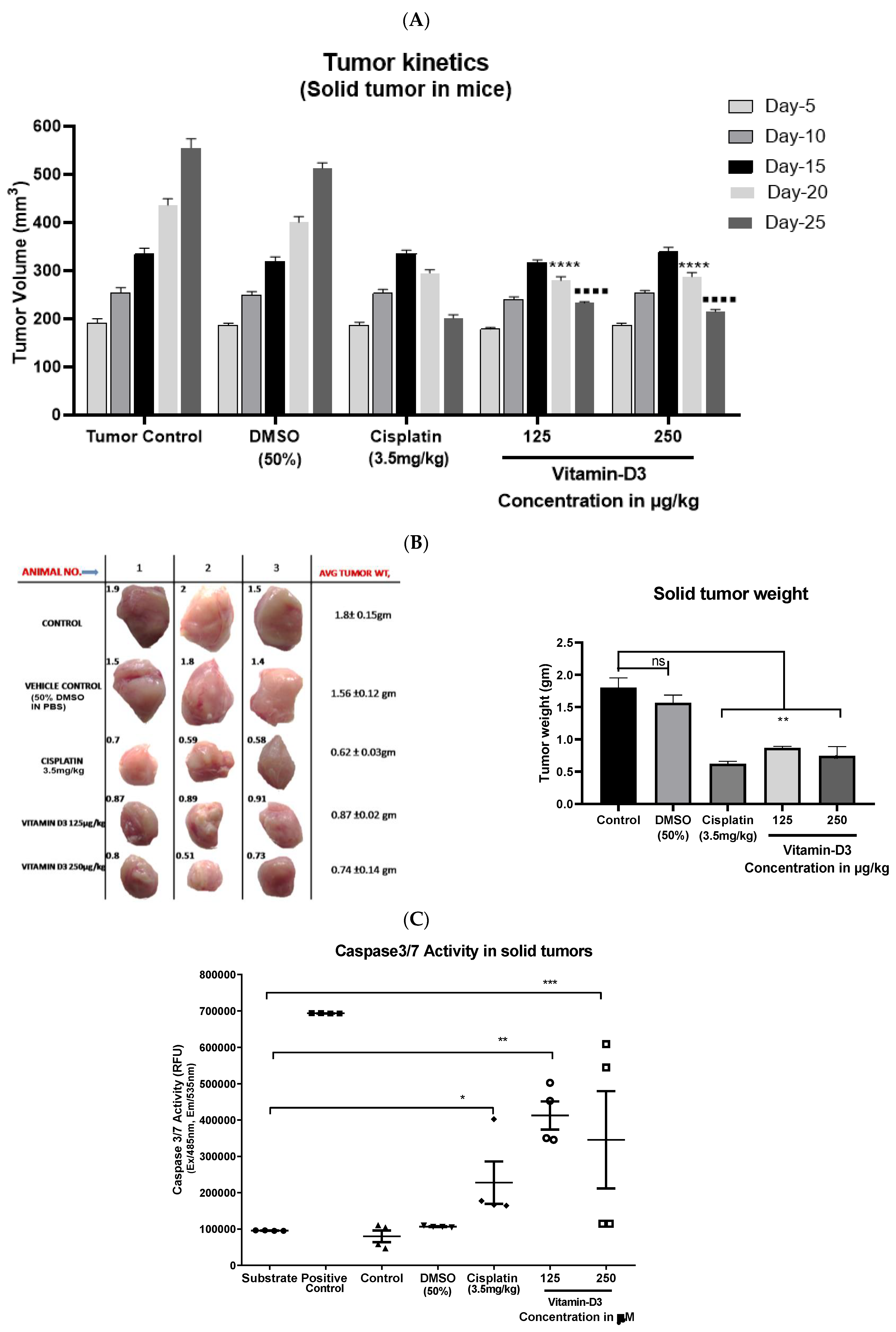
EAC Cell Growth in Mouse [Swiss Albino]
EAC Solid Tumors in Animal Model
2.2.10. Statistical Analysis
3. Results
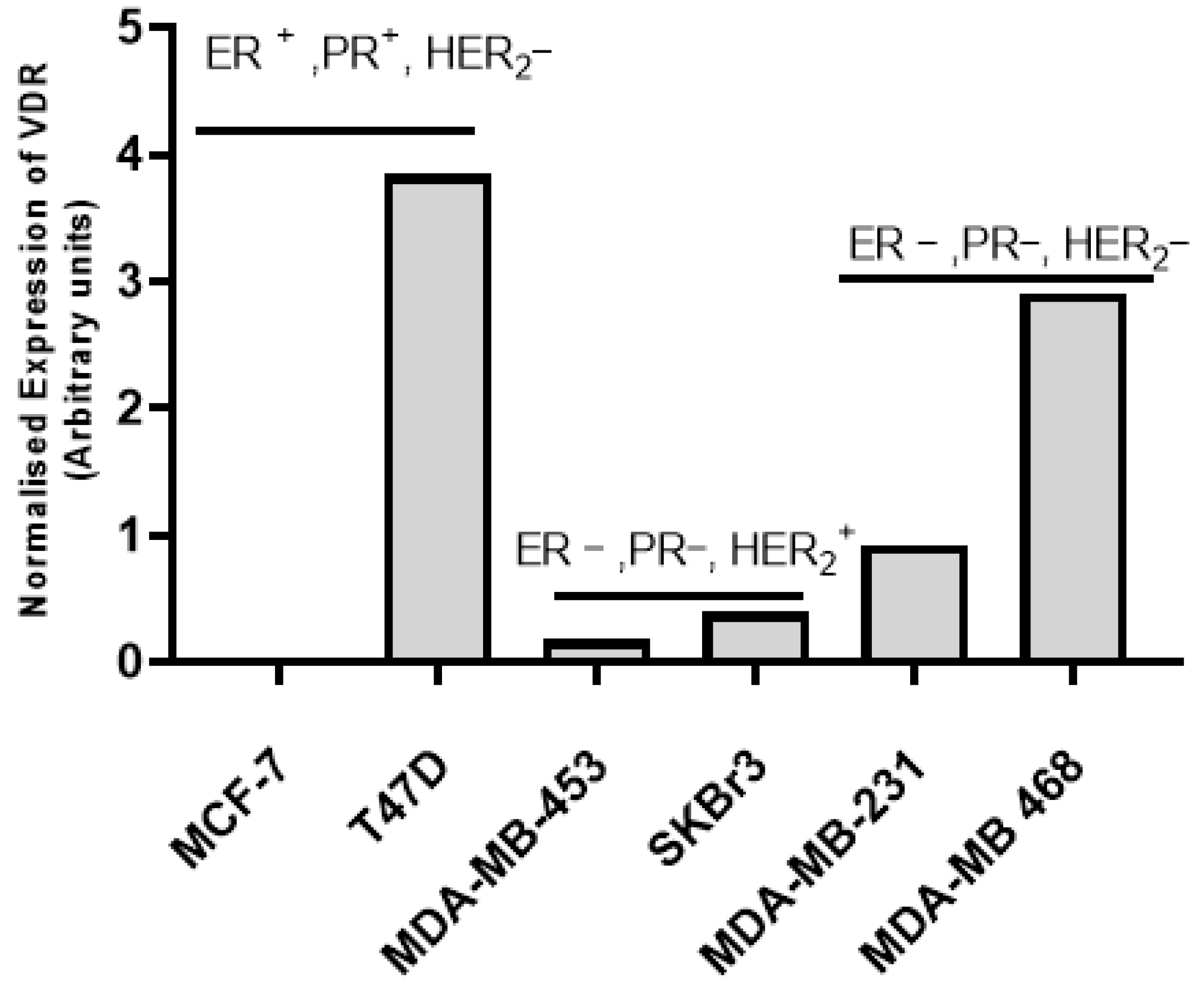
3.1. Vitamin D Receptor (VDR) Expression and Vitamin D3 Effects in BC Cell Lines
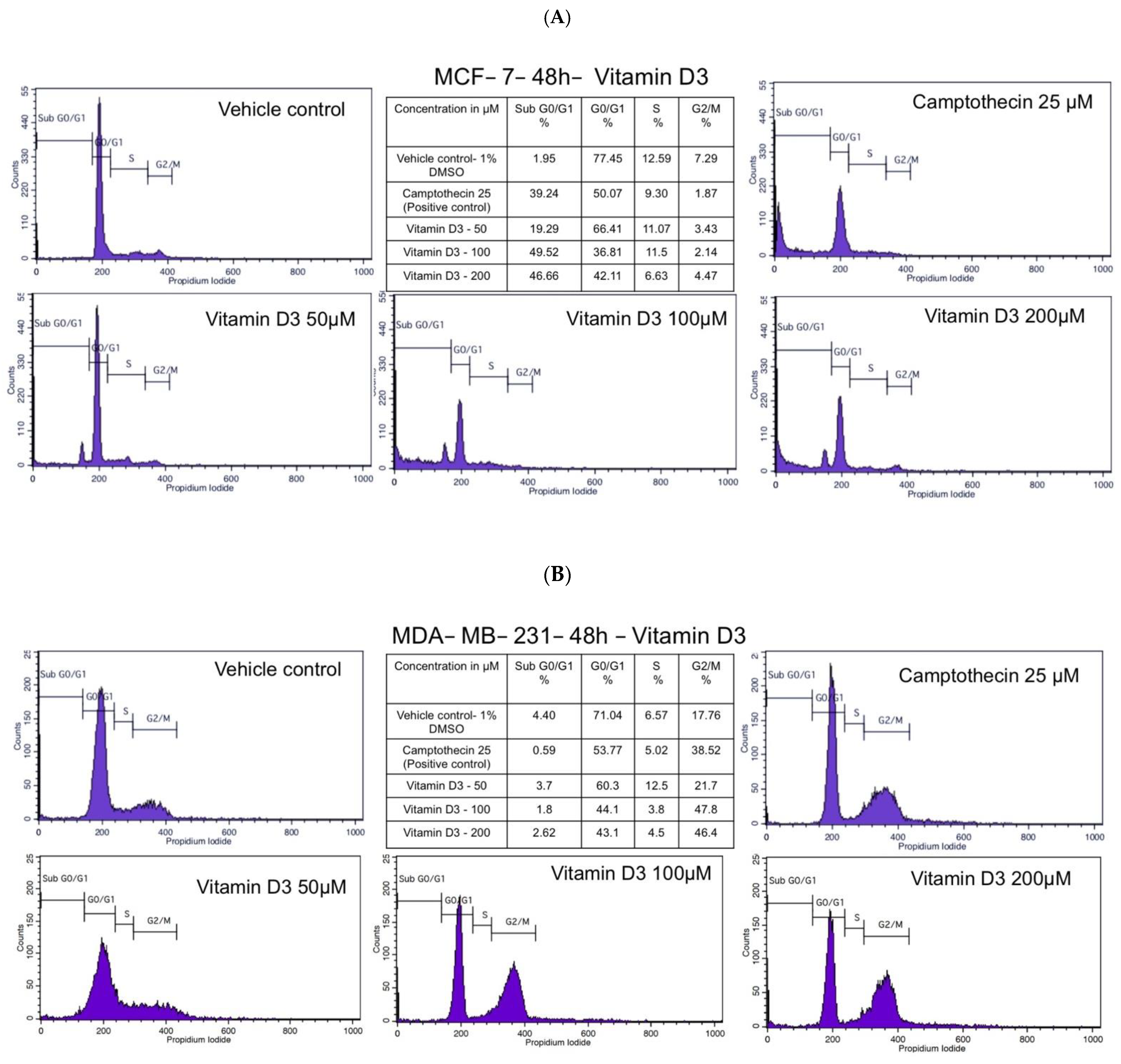
3.2. Vitamin D3 Inhibited MCF-7 and MDA-MB-231 BC Cell Cycle Progression
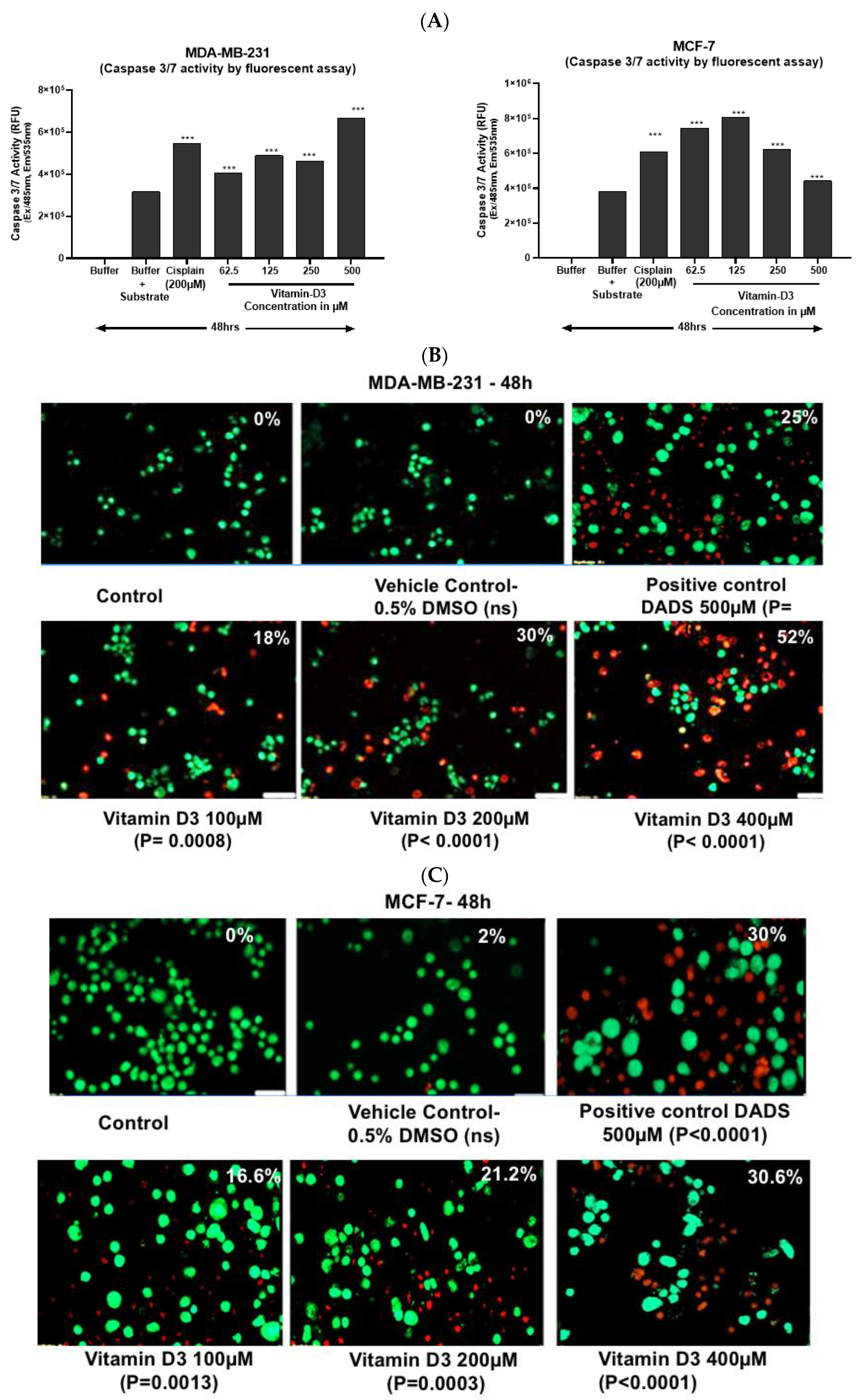
3.3. Vitamin D3-Induced Apoptosis Is Mediated by Caspase-3/7 Induction in MCF-7 and in MDA-MB-231 Cells
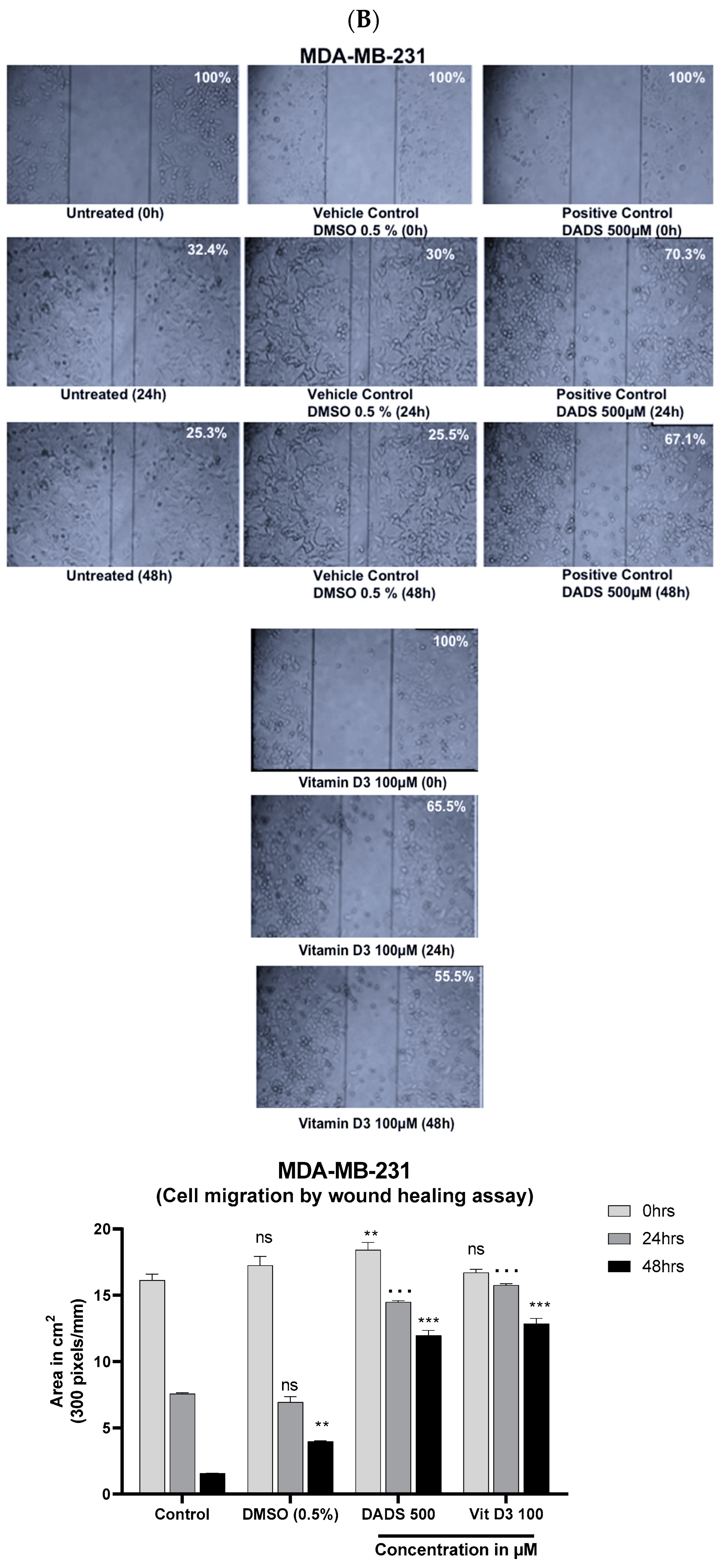
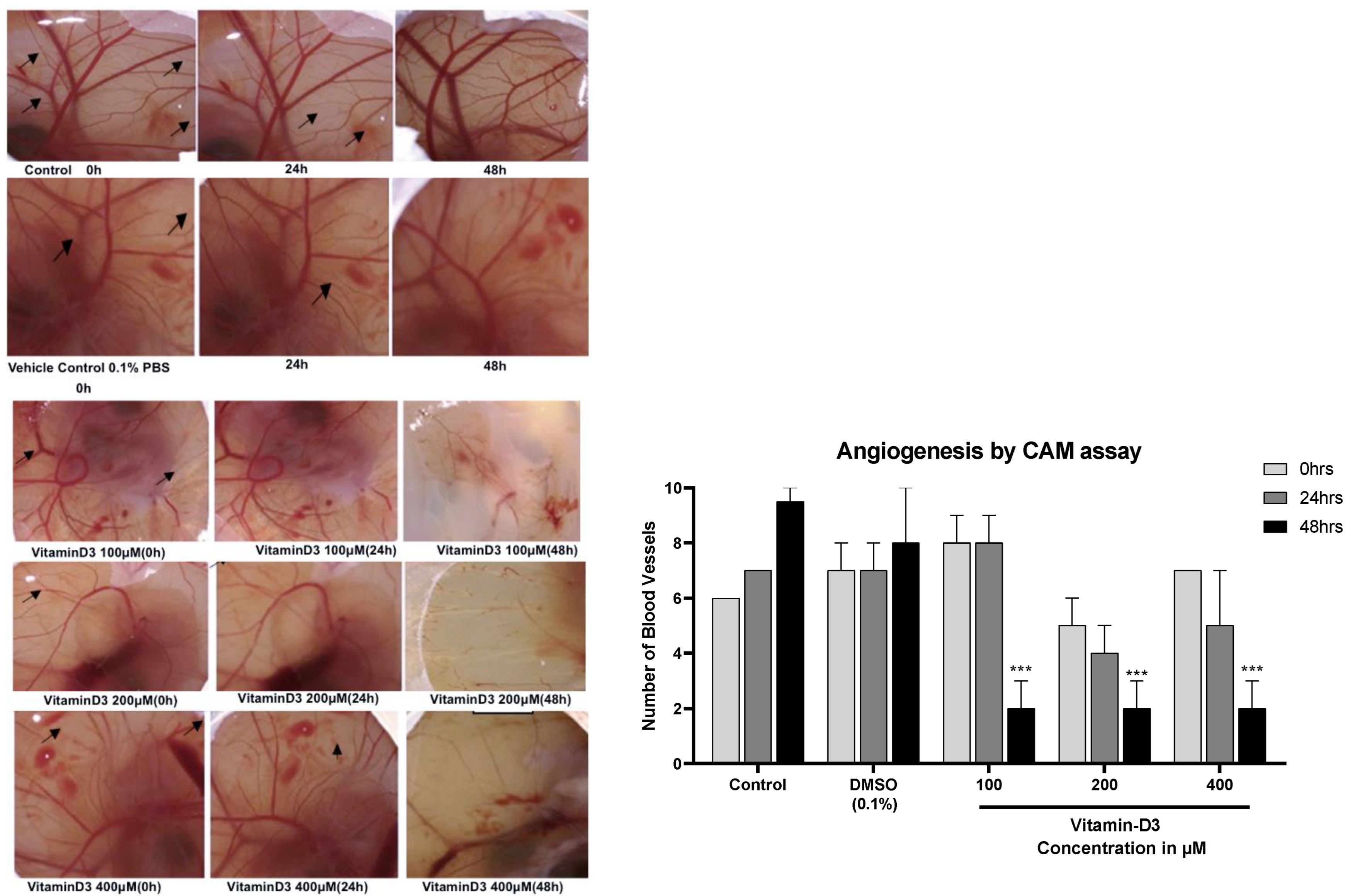
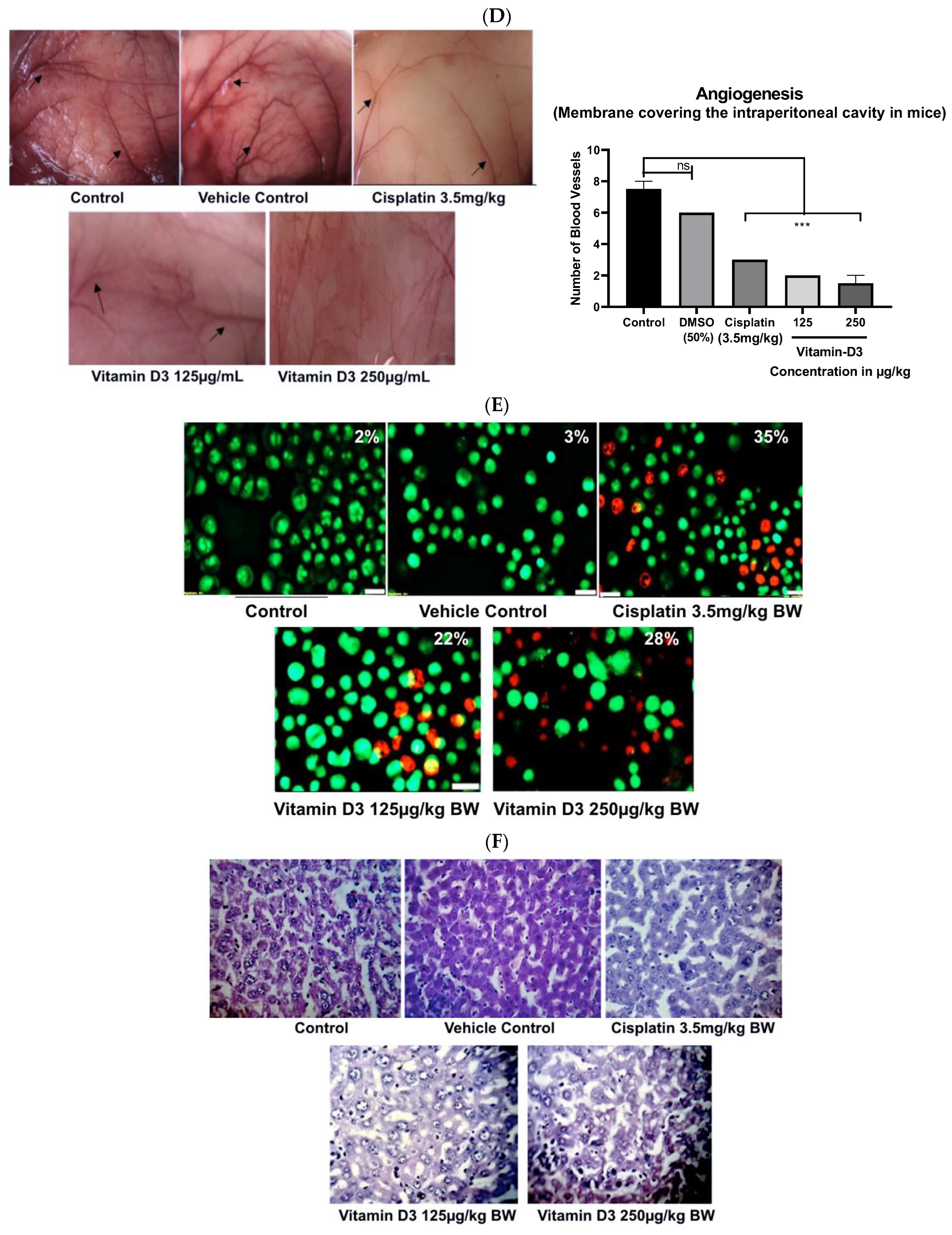
3.4. Vitamin D3 Reduced BC Cell Migration In Vitro and Angiogenesis Ex Vivo
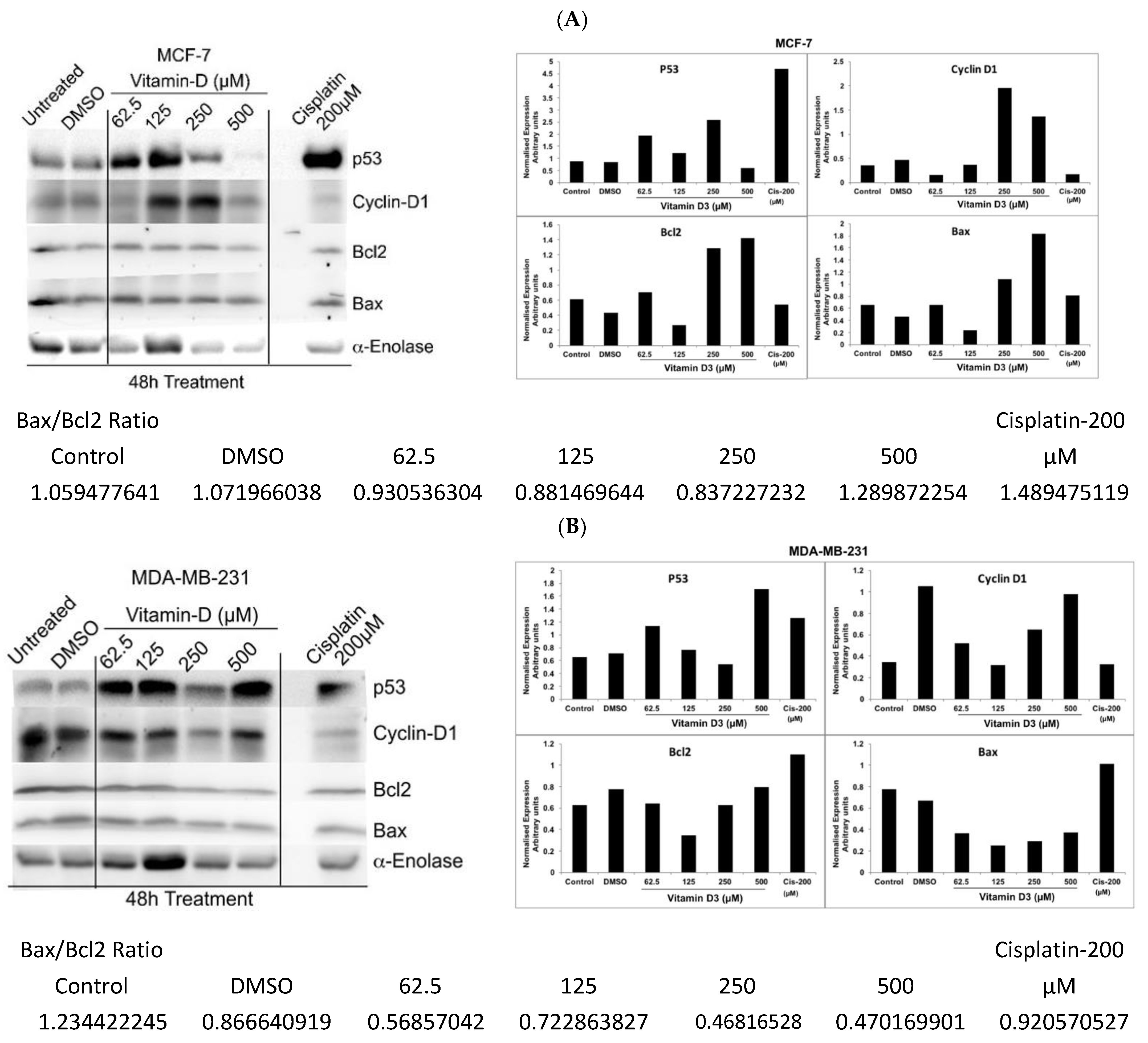
3.5. Analysis of Vitamin D3-Induced Effects on the Expression of p53, Cyclin-D, and Bcl-2
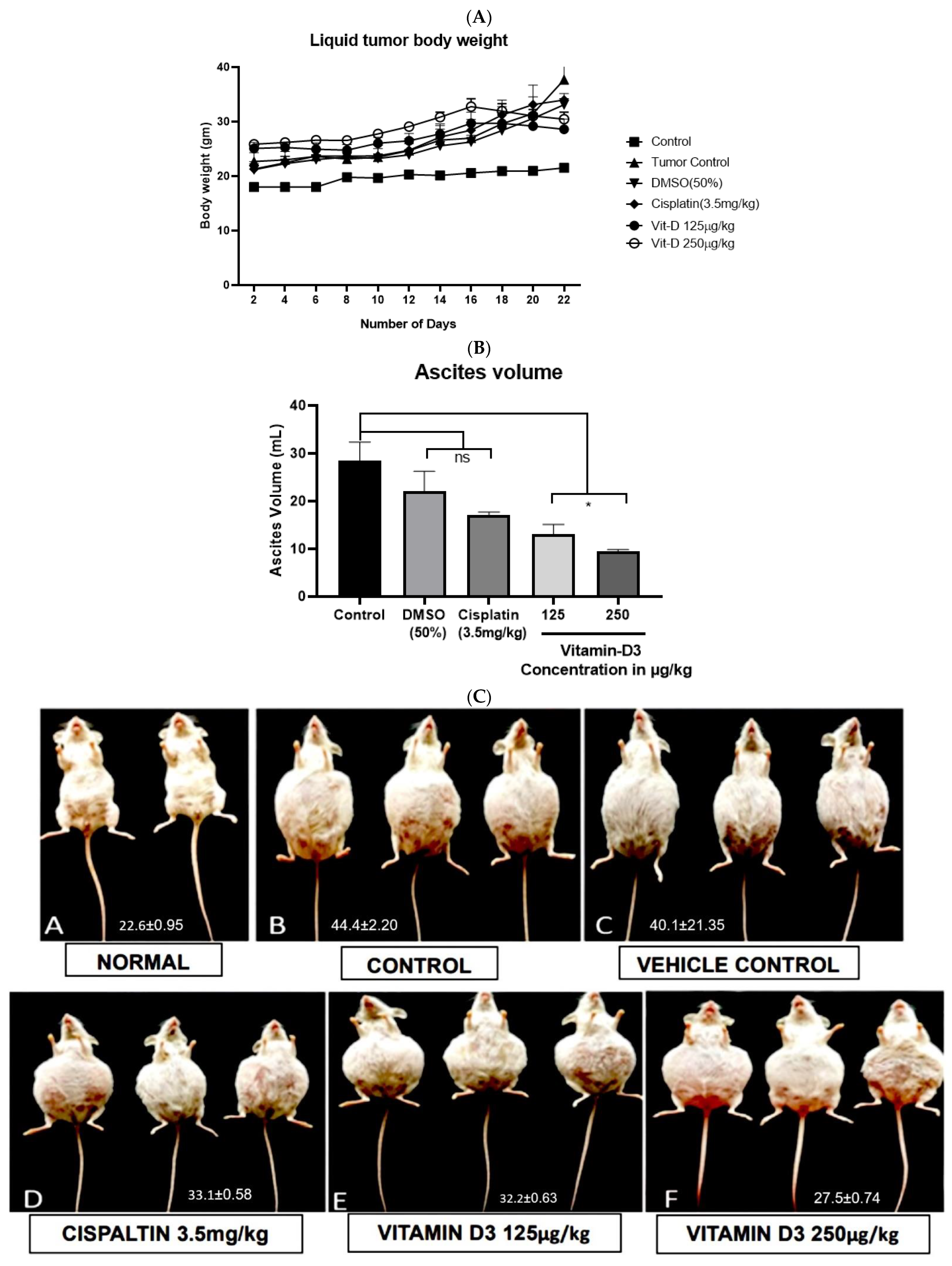
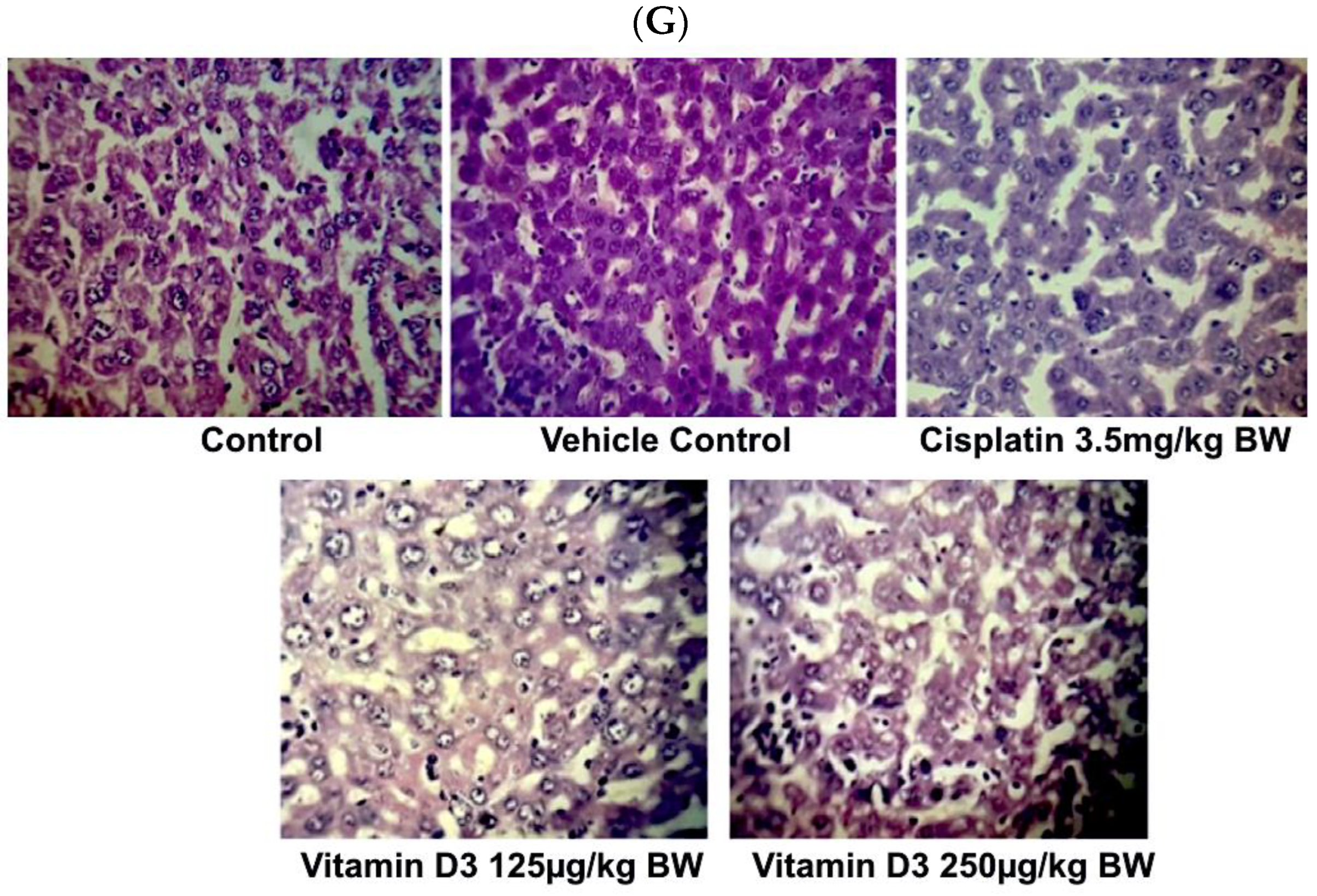
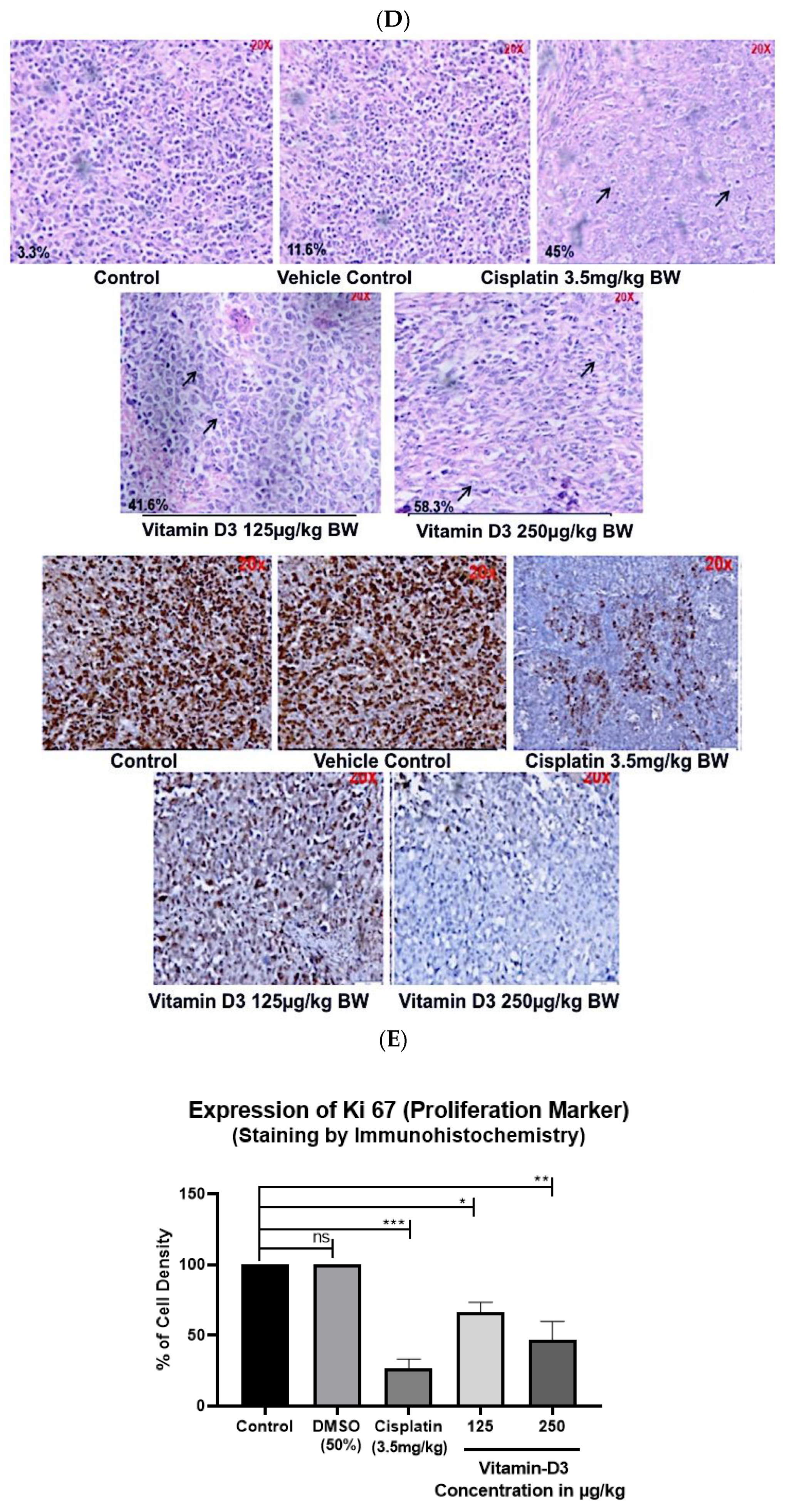
3.6. Vitamin D3 Effects on Tumor Growth In Vivo
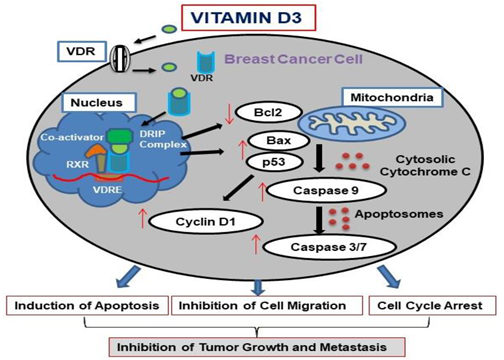
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Conte, L.; De Nunzio, G.; Lupo, R.; Mieli, M.; Lezzi, A.; Vitale, E.; Carriero, M.C.; Calabrò, A.; Carvello, M.; Rubbi, I.; et al. Breast Cancer Prevention: The Key Role of Population Screening, Breast Self-Examination (BSE) and Technological Tools. Survey of Italian Women. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2023, 1–15. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored. Am. Soc. Prev. Oncol. 2017, 26, 444–457. [Google Scholar] [CrossRef]
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer Targets Ther. 2016, 8, 93–107. [Google Scholar]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.E.; Pringle, J.H. Consideration of possible effects of vitamin D on established cancer, with reference to malignant melanoma. Pigment. Cell Melanoma Res. 2022, 35, 408–424. [Google Scholar] [CrossRef]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef]
- Rochel, N. Vitamin D and Its Receptor from a Structural Perspective. Nutrients 2022, 14, 2847. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef]
- Koivisto, O.; Hanel, A.; Carlberg, C. Key Vitamin D Target Genes with Functions in the Immune System. Nutrients 2020, 12, 1140. [Google Scholar] [CrossRef]
- Costa, P.L.F.; França, M.M.; Katayama, M.L.; Carneiro, E.T.; Martin, R.M.; Folgueira, M.A.K.; Latronico, A.C.; Ferraz-De-Souza, B. Transcriptomic Response to 1,25-Dihydroxyvitamin D in Human Fibroblasts with or without a Functional Vitamin D Receptor (VDR): Novel Target Genes and Insights into VDR Basal Transcriptional Activity. Cells 2019, 8, 318. [Google Scholar] [CrossRef]
- Campbell, M.J.; Trump, D.L. Vitamin D Receptor Signaling and Cancer. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1009–1038. [Google Scholar] [CrossRef]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef]
- Horas, K.; van Herck, U.; Maier, G.S.; Maus, U.; Harrasser, N.; Jakob, F.; Weissenberger, M.; Arnholdt, J.; Holzapfel, B.M.; Rudert, M. Does vitamin D deficiency predict tumour malignancy in patients with bone tumours? Data from a multi-center cohort analysis. J. Bone Oncol. 2020, 25, 100329. [Google Scholar] [CrossRef]
- Zemlin, C.; Altmayer, L.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Lang, M.; Scherer, L.-S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; et al. Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients 2023, 15, 1450. [Google Scholar] [CrossRef]
- Muszyński, T.; Polak, K.; Frątczak, A.; Miziołek, B.; Bergler-Czop, B.; Szczepanik, A. Vitamin D-The Nutritional Status of Post-Gastrectomy Gastric Cancer Patients-Systematic Review. Nutrients 2022, 14, 2712. [Google Scholar] [CrossRef]
- Taha, Z.; Eltom, S.E. The Role of Diet and Lifestyle in Women with Breast Cancer: An Update Review of Related Research in the Middle East. BioRes. Open Access 2018, 7, 73–80. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Zhang, J.; Huang, X.; Thabane, L.; Li, G. Effect of Vitamin D Supplementation on Risk of Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2021, 8, 655727. [Google Scholar] [CrossRef]
- Bhoora, S.; Punchoo, R. Policing Cancer: Vitamin D Arrests the Cell Cycle. Int. J. Mol. Sci. 2020, 21, 9296. [Google Scholar] [CrossRef] [PubMed]
- Bhoora, S.; Pather, Y.; Marais, S.; Punchoo, R. Cholecalciferol Inhibits Cell Growth and Induces Apoptosis in the CaSki Cell Line. Med. Sci. 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Reddy, D.B.; Padukudru, M.A.; Chitturi, C.M.K.; Vimalambike, M.G.; Madhunapantula, S.V. Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of Histone Deacetylases (HDAC). PLoS ONE 2017, 12, e0186208. [Google Scholar] [CrossRef]
- Shruthi, N.; Prashanthkumar, M.; Venugopalreddy, B.; Suma, M.; Subba, V. Analysis of the Cytotoxic Effects of Vitamin D3 on Colorectal, Breast and Cervical Carcinoma Cell Lines. Biochem. Anal. Biochem. 2017, 6, 1000318. Available online: https://www.omicsonline.org/open-access/analysis-of-the-cytotoxic-effects-of-vitamin-d3-on-colorectal-breast-andcervical-carcinoma-cell-lines-2161-1009-1000318.php?aid=88664 (accessed on 15 September 2023).
- Kuruburu, M.G.; Bovilla, V.R.; Leihang, Z.; Madhunapantula, S.V. Phytochemical-rich Fractions from Foxtail Millet (Setaria italica (L.) P. Beauv) Seeds Exhibited Antioxidant Activity and Reduced the Viability of Breast Cancer Cells In Vitro by Inducing DNA Fragmentation and Promoting Cell Cycle Arrest. Anti-Cancer Agents Med. Chem. 2022, 22, 2477–2493. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Gilad, L.A.; Bresler, T.; Gnainsky, J.; Smirnoff, P.; Schwartz, B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J. Endocrinol. 2005, 185, 577–592. [Google Scholar] [CrossRef]
- Kit, B.P.A. Bradford Protein Assay Kit. Available online: https://www.thermofisher.com/in/en/home/life-science/protein-biology/protein-assays-analysis/protein-assays/bradford-assays.html?gclid=Cj0KCQjwvL-oBhCxARIsAHkOiu1KJAzx5ihZ8-Ls6wvbXil0dhCu83TM6uNMPS6YmO5guDikQYr8sEIaAslHEALw_wcB&ef_id=Cj0KCQjwvLoBhCxARIsAHkOiu1KJAzx5ihZ8Ls6wvbXil0dhCu83TM6uNMPS6YmO5guDikQYr8sEIaAslHEALw_wcB:G:s&s_kwcid=AL!3652!3!555178769893!p!!g!!bradford%20reagent!10112486529!101044921906&cid=bid_pca_sbu_r01_co_cp1359_pjt0000_bid00000_0se_gaw_nt_pur_con (accessed on 15 September 2023).
- Kim, B. Western Blot Techniques. In Molecular Profiling: Methods and Protocols; Springer Science+Business Media LLC: Clifton, NJ, USA, 2017; Volume 1606, pp. 133–139. [Google Scholar]
- Worthley, E.G.; Schott, C.D. The toxicity of four concentrations of DMSO. Toxicol. Appl. Pharmacol. 1969, 15, 275–281. [Google Scholar] [CrossRef]
- Perše, M. Cisplatin Mouse Models: Treatment, Toxicity and Translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yasuda, K.; Takamatsu, M.; Abe, K.; Nakagawa, K.; Tsugawa, N.; Hirota, Y.; Tanaka, K.; Yamashita, S.; Ikushiro, S.; et al. Generation of 1,25-dihydroxyvitamin D3 in Cyp27b1 knockout mice by treatment with 25-hydroxyvitamin D3 rescued their rachitic phenotypes. J. Steroid Biochem. Mol. Biol. 2018, 185, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Koshizuka, K.; Koike, M.; Asou, H.; Cho, S.K.; Stephen, T.; Rude, R.K.; Binderup, L.; Uskokovic, M.; Koeffler, H.P. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res. Treat. 1999, 53, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwaya, K.; Moriya, T.; Yamasaki, T.; Tsuda, H.; Yamamoto, J.; Yamamoto, J.; Matsubara, O. A simple immunohistochemical panel comprising 2 conventional markers, Ki67 and p53, is a powerful tool for predicting patient outcome in luminal-type breast cancer. BMC Clin. Pathol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Duan, B.; Zhang, Q.; Ouyang, L.; Peng, W.; Qian, F.; Wang, Y.; Huang, S. Vitamin D-induced vitamin D receptor expression induces tamoxifen sensitivity in MCF-7 stem cells via suppression of Wnt/β-catenin signaling. Biosci. Rep. 2018, 38, BSR20180595. [Google Scholar] [CrossRef]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. Caspase activity assays. Methods Mol. Biol. 2008, 414, 137–150. [Google Scholar]
- Chen, W.; Park, S.; Patel, C.; Bai, Y.; Henary, K.; Raha, A.; Mohammadi, S.; You, L.; Geng, F. The migration of metastatic breast cancer cells is regulated by matrix stiffness via YAP signalling. Heliyon 2021, 7, e06252. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Gallucci, G.; Coppola, C.; Piscopo, G.; Cipresso, C.; Maurea, C.; Giudice, A.; Iaffaioli, R.V.; Arra, C.; Maurea, N. The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. Eur. J. Heart Fail. 2013, 15, 482–489. [Google Scholar] [CrossRef]
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef]
- Elebro, K.; Bendahl, P.O.; Jernström, H.; Borgquist, S. Androgen receptor expression and breast cancer mortality in a population-based prospective cohort. Breast Cancer Res. Treat. 2017, 165, 645–657. [Google Scholar] [CrossRef]
- Cordes, T.; Diesing, D.; Becker, S.; Diedrich, K.; Reichrath, J.; Friedrich, M. Modulation of MAPK ERK1 and ERK2 in VDR-positive and -negative breast cancer cell lines. Anticancer Res. 2006, 26, 2749–2753. [Google Scholar] [PubMed]
- Filip-Psurska, B.; Psurski, M.; Anisiewicz, A.; Libako, P.; Zbrojewicz, E.; Maciejewska, M.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Vitamin D Compounds PRI-2191 and PRI-2205 Enhance Anastrozole Activity in Human Breast Cancer Models. Int. J. Mol. Sci. 2021, 22, 2781. [Google Scholar] [CrossRef] [PubMed]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. BCR 2019, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Al-Azhri, J.; Zhang, Y.; Bshara, W.; Zirpoli, G.; McCann, S.E.; Khoury, T.; Morrison, C.D.; Edge, S.B.; Ambrosone, C.B.; Yao, S.; et al. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 97–103. [Google Scholar]
- Berger, U.; McClelland, R.A.; Wilson, P.; Greene, G.L.; Haussler, M.R.; Pike, J.W.; Colston, K.; Easton, D.; Coombes, R.C. Immunocytochemical determination of estrogen receptor, progesterone receptor, and 1,25-dihydroxyvitamin D3 receptor in breast cancer and relationship to prognosis. Cancer Res. 1991, 51, 239–244. [Google Scholar]
- Ditsch, N.; Toth, B.; Mayr, D.; Lenhard, M.; Gallwas, J.; Weissenbacher, T.; Dannecker, C.; Friese, K.; Jeschke, U. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 121–129. [Google Scholar] [CrossRef]
- Zinser, G.M.; Suckow, M.; Welsh, J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J. Steroid Biochem. Mol. Biol. 2005, 97, 153–164. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar]
- Nirgude, S.; Mahadeva, R.; Koroth, J.; Kumar, S.; Kumar, K.S.S.; Gopalakrishnan, V.; Karki, S.S.; Choudhary, B. ST09, A Novel Curcumin Derivative, Blocks Cell Migration by Inhibiting Matrix Metalloproteases in Breast Cancer Cells and Inhibits Tumor Progression in EAC Mouse Tumor Models. Molecules 2020, 25, 4499. [Google Scholar]
- Bikle, D.D. Vitamin D and cancer: The promise not yet fulfilled. Endocrine 2014, 46, 29–38. [Google Scholar]
- Shah, S.; Islam, M.N.; Dakshanamurthy, S.; Rizvi, I.; Rao, M.; Herrell, R.; Zinser, G.; Valrance, M.; Aranda, A.; Moras, D.; et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006, 21, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Sea, A.; Feldman, S.; Strawbridge, R.; Hoopes, P.J.; Demidenko, E.; Binderup, L.; A Gewirtz, D. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 2350–2356. [Google Scholar]
- Krishnan, A.V.; Swami, S.; Feldman, D. Vitamin D and breast cancer: Inhibition of estrogen synthesis and signaling. J. Steroid Biochem. Mol. Biol. 2010, 121, 343–348. [Google Scholar] [CrossRef]
- Mathiasen, I.S.; Lademann, U.; Jäättelä, M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999, 59, 4848–4856. [Google Scholar]
- López de Andrés, J.; Griñán-Lisón, C.; Jiménez, G.; Marchal, J.A. Cancer stem cell secretome in the tumor microenvironment: A key point for an effective personalized cancer treatment. J. Hematol. Oncol. 2020, 13, 136. [Google Scholar]
- Lu, X.; Chen, Z.; Mylarapu, N.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Epithelial Proliferation, Migration and Vitamin D Metabolizing and Catabolizing Enzymes. Sci. Rep. 2017, 7, 16951. [Google Scholar] [CrossRef] [PubMed]
- Graziano, S.; Johnston, R.; Deng, O.; Zhang, J.; Gonzalo, S. Vitamin D/vitamin D receptor axis regulates DNA repair during oncogene-induced senescence. Oncogene 2016, 35, 5362–5376. [Google Scholar] [CrossRef]



| Cell-Lines | 24 h | 48 h | 72 h |
|---|---|---|---|
| MDA-MB-468 | 228.1 ± 52.41 | 169.7 ± 40.32 | 115.6 ± 35.54 |
| MDA-MB-231 | 166.0 ± 59.04 | 230.2 ± 45.88 | 327.6 ± 22.521 |
| MCF-7 | 168.6 ± 37.47 | 185.3 ± 38.04 | 297.1 ± 7.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeresh, P.K.M.; Basavaraju, C.G.; Dallavalasa, S.; Anantharaju, P.G.; Natraj, S.M.; Sukocheva, O.A.; Madhunapantula, S.V. Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis. Cancers 2023, 15, 4833. https://doi.org/10.3390/cancers15194833
Veeresh PKM, Basavaraju CG, Dallavalasa S, Anantharaju PG, Natraj SM, Sukocheva OA, Madhunapantula SV. Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis. Cancers. 2023; 15(19):4833. https://doi.org/10.3390/cancers15194833
Chicago/Turabian StyleVeeresh, Prashanth Kumar M., Chaithanya G. Basavaraju, Siva Dallavalasa, Preethi G. Anantharaju, Suma M. Natraj, Olga A. Sukocheva, and SubbaRao V. Madhunapantula. 2023. "Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis" Cancers 15, no. 19: 4833. https://doi.org/10.3390/cancers15194833
APA StyleVeeresh, P. K. M., Basavaraju, C. G., Dallavalasa, S., Anantharaju, P. G., Natraj, S. M., Sukocheva, O. A., & Madhunapantula, S. V. (2023). Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis. Cancers, 15(19), 4833. https://doi.org/10.3390/cancers15194833








