Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
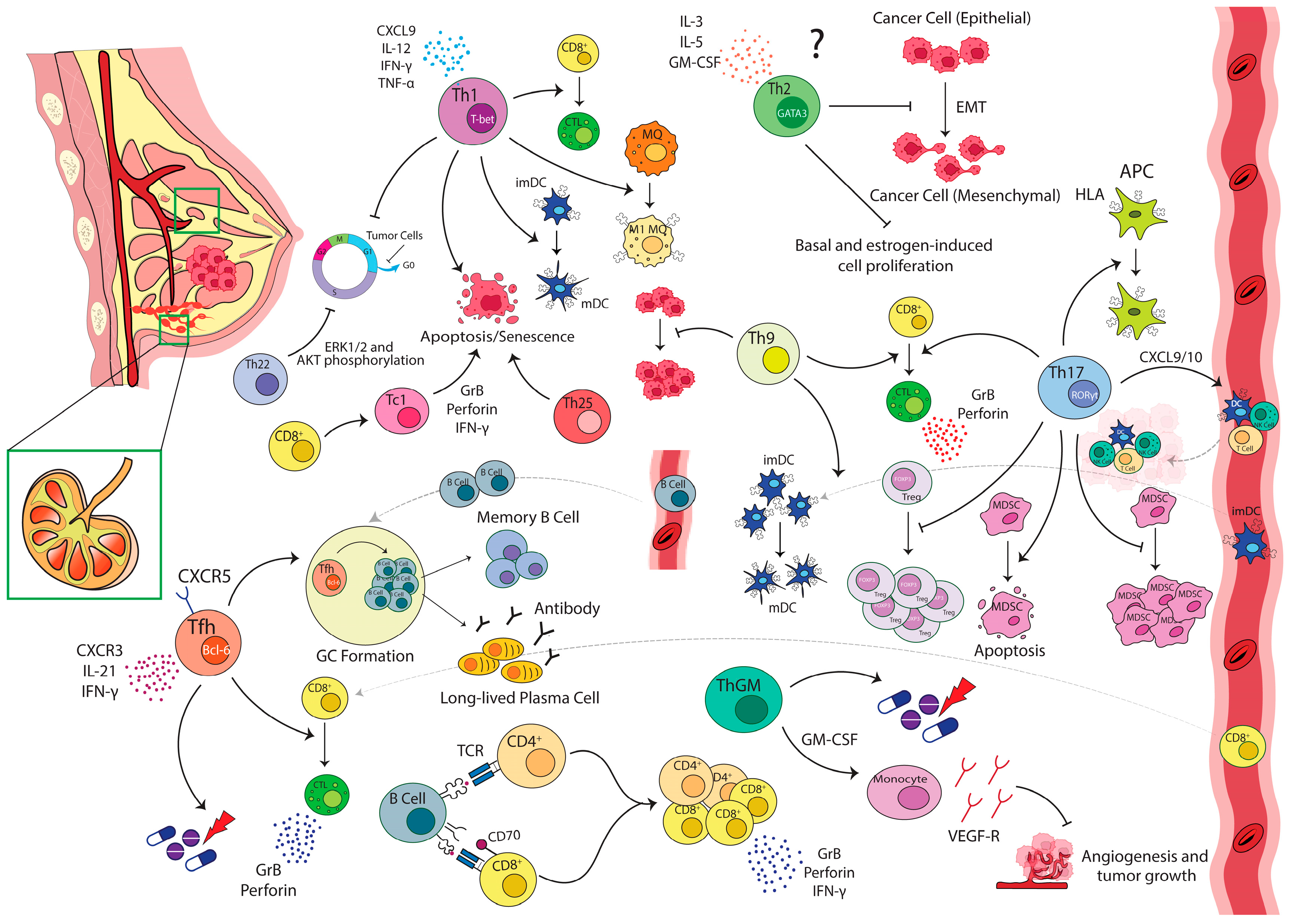
2. Th1/Th2 Paradigm in Breast Carcinoma
3. Th17 in Breast Carcinoma
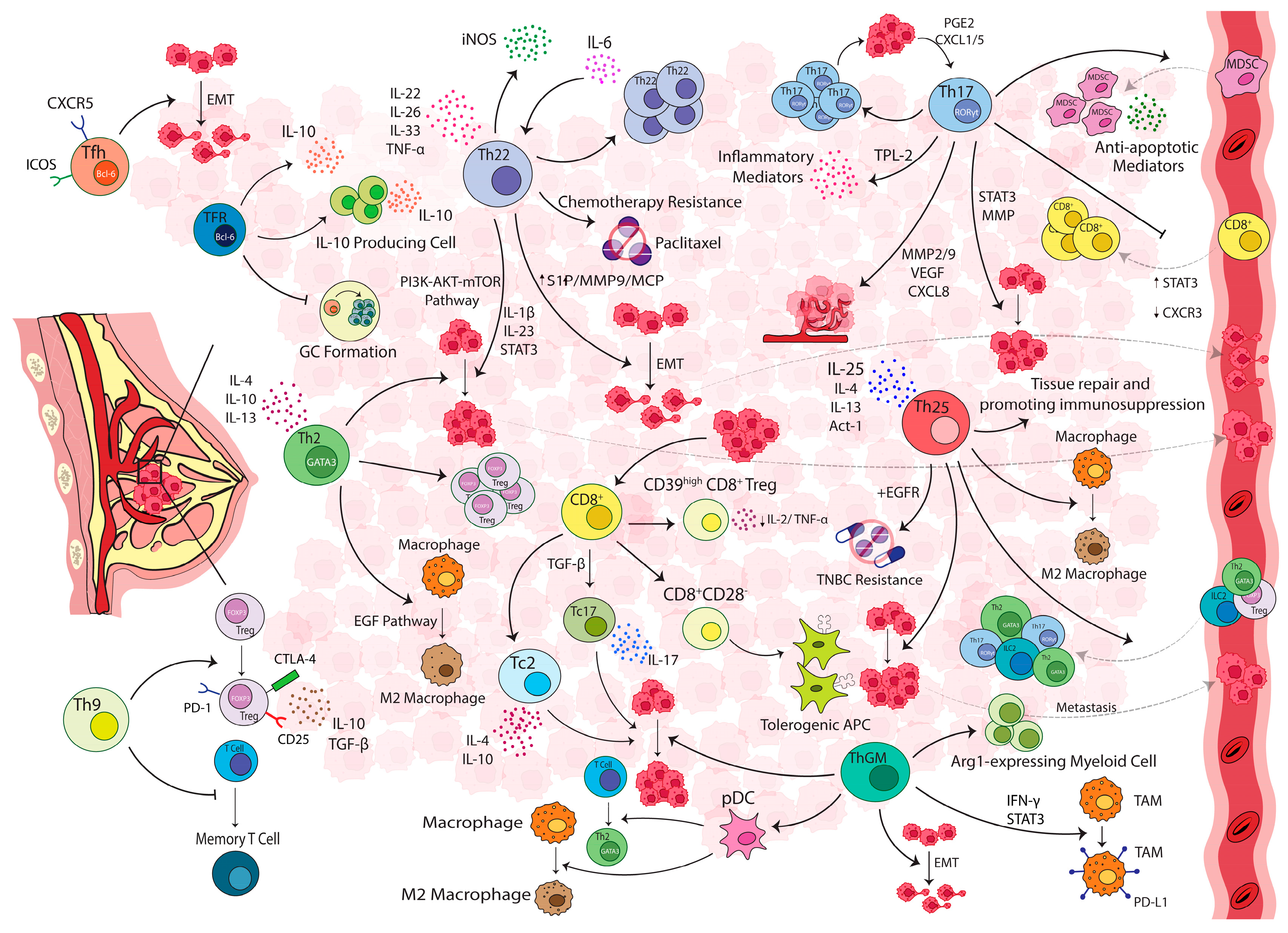
4. IL-25-Producing T-Cells
5. IL-22-Producing T-Cells
6. IL-9-Producing T-Cells
7. GM-CSF-Producing T-Cells
8. Helper and Regulatory Follicular T-Cells in Breast Carcinoma
9. Cytotoxic T-Cells and Their Effector Subsets
10. Memory T-Cells
11. Immune Suppression in Breast Carcinoma: A Role for Regulatory T-Cells
12. T-Cell and B-Cell Crosstalk
13. Conclusions
Funding
Authors Contribution
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med. Press) 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Rody, A.; Holtrich, U.; Pusztai, L.; Liedtke, C.; Gaetje, R.; Ruckhaeberle, E.; Solbach, C.; Hanker, L.; Ahr, A.; Metzler, D.; et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. BCR 2009, 11, R15. [Google Scholar] [CrossRef]
- Hanker, L.C.; Rody, A.; Holtrich, U.; Pusztai, L.; Ruckhaeberle, E.; Liedtke, C.; Ahr, A.; Heinrich, T.M.; Sanger, N.; Becker, S.; et al. Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res. Treat. 2013, 137, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Bohm, D.; von Torne, C.; Steiner, E.; Puhl, A.; Pilch, H.; Lehr, H.A.; Hengstler, J.G.; Kolbl, H.; Gehrmann, M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008, 68, 5405–5413. [Google Scholar] [CrossRef]
- Huang, E.; Cheng, S.H.; Dressman, H.; Pittman, J.; Tsou, M.H.; Horng, C.F.; Bild, A.; Iversen, E.S.; Liao, M.; Chen, C.M.; et al. Gene expression predictors of breast cancer outcomes. Lancet 2003, 361, 1590–1596. [Google Scholar] [CrossRef]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2010, 29, 1093–1102. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Coussens, L.M. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. BCR 2007, 9, 212. [Google Scholar] [CrossRef]
- Rahir, G.; Moser, M. Tumor microenvironment and lymphocyte infiltration. Cancer Immunol. Immunother. CII 2012, 61, 751–759. [Google Scholar] [CrossRef]
- Ahmadvand, S.; Faghih, Z.; Montazer, M.; Safaei, A.; Mokhtari, M.; Jafari, P.; Talei, A.R.; Tahmasebi, S.; Ghaderi, A. Importance of CD45RO+ tumor-infiltrating lymphocytes in post-operative survival of breast cancer patients. Cell. Oncol. 2019, 42, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast 2017, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Staaf, J.; Ringnér, M.; Vallon-Christersson, J.; Jönsson, G.; Bendahl, P.O.; Holm, K.; Arason, A.; Gunnarsson, H.; Hegardt, C.; Agnarsson, B.A.; et al. Identification of subtypes in human epidermal growth factor receptor 2—Positive breast cancer reveals a gene signature prognostic of outcome. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Georgiannos, S.N.; Renaut, A.; Goode, A.W.; Sheaff, M. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery 2003, 134, 827–834. [Google Scholar] [CrossRef]
- Wong, P.Y.; Staren, E.D.; Tereshkova, N.; Braun, D.P. Functional analysis of tumor-infiltrating leukocytes in breast cancer patients. J. Surg. Res. 1998, 76, 95–103. [Google Scholar] [CrossRef]
- Helal, T.E.; Ibrahim, E.A.; Alloub, A.I. Immunohistochemical analysis of tumor-infiltrating lymphocytes in breast carcinoma: Relation to prognostic variables. Indian J. Pathol. Microbiol. 2013, 56, 89–93. [Google Scholar] [CrossRef]
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2796–2801. [Google Scholar] [CrossRef]
- Reome, J.B.; Hylind, J.C.; Dutton, R.W.; Dobrzanski, M.J. Type 1 and type 2 tumor infiltrating effector cell subpopulations in progressive breast cancer. Clin. Immunol. 2004, 111, 69–81. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Faghih, Z.; Deihimi, S.; Talei, A.; Ghaderi, A.; Erfani, N. Analysis of T cell receptor repertoire based on Vβ chain in patients with breast cancer. Cancer Biomark. 2018, 22, 733–745. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Faghih, Z.; Erfani, N.; Haghshenas, M.R.; Safaei, A.; Talei, A.R.; Ghaderi, A. Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol. Lett. 2014, 158, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Faghih, Z.; Rezaeifard, S.; Safaei, A.; Ghaderi, A.; Erfani, N. IL-17 and IL-4 producing CD8+ T cells in tumor draining lymph nodes of breast cancer patients: Positive association with tumor progression. Iran. J. Immunol. IJI 2013, 10, 193–204. [Google Scholar] [PubMed]
- Knutson, K.L.; Disis, M.L. Augmenting T helper cell immunity in cancer. Curr. Drug Targets. Immune Endocr. Metab. Disord. 2005, 5, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Luo, Y.; Liu, J.; Wang, X.; Feng, R.; Huang, J.; Du, H.; Li, Q.; Tan, J.; et al. Pharmaceutical targeting Th2-mediated immunity enhances immunotherapy response in breast cancer. J. Transl. Med. 2022, 20, 615. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, R.; Gisterek, I.; Halon, A.; Lacko, A.; Szewczyk, K.; Staszek, U.; Pudelko, M.; Szynglarewicz, B.; Szelachowska, J.; Zolnierek, A.; et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009, 29, 2445–2451. [Google Scholar]
- Ehi, K.; Ishigami, S.; Masamoto, I.; Uenosono, Y.; Natsugoe, S.; Arigami, T.; Arima, H.; Kijima, Y.; Yoshinaka, H.; Yanagita, S.; et al. Analysis of T-helper type 1 and 2 cells and T-cytotoxic type 1 and 2 cells of sentinel lymph nodes in breast cancer. Oncol. Rep. 2008, 19, 601–607. [Google Scholar] [CrossRef][Green Version]
- Matsuura, K.; Yamaguchi, Y.; Ueno, H.; Osaki, A.; Arihiro, K.; Toge, T. Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 2006, 106, 1227–1236. [Google Scholar] [CrossRef]
- Caras, I.; Grigorescu, A.; Stavaru, C.; Radu, D.L.; Mogos, I.; Szegli, G.; Salageanu, A. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol. Immunother. CII 2004, 53, 1146–1152. [Google Scholar] [CrossRef]
- Fracol, M.; Datta, J.; Lowenfeld, L.; Xu, S.; Zhang, P.J.; Fisher, C.S.; Czerniecki, B.J. Loss of Anti-HER-3 CD4+ T-Helper Type 1 Immunity Occurs in Breast Tumorigenesis and is Negatively Associated with Outcomes. Ann. Surg. Oncol. 2017, 24, 407–417. [Google Scholar] [CrossRef]
- Eftekhari, R.; Esmaeili, R.; Mirzaei, R.; Bidad, K.; de Lima, S.; Ajami, M.; Shirzad, H.; Hadjati, J.; Majidzadeh-A, K. Study of the tumor microenvironment during breast cancer progression. Cancer Cell Int. 2017, 17, 123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finak, G.; Bertos, N.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Manjili, M.H.; Najarian, K.; Wang, X.Y. Signatures of tumor-immune interactions as biomarkers for breast cancer prognosis. Future Oncol. 2012, 8, 703–711. [Google Scholar] [CrossRef]
- Einav, U.; Tabach, Y.; Getz, G.; Yitzhaky, A.; Ozbek, U.; Amariglio, N.; Izraeli, S.; Rechavi, G.; Domany, E. Gene expression analysis reveals a strong signature of an interferon-induced pathway in childhood lymphoblastic leukemia as well as in breast and ovarian cancer. Oncogene 2005, 24, 6367–6375. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Sherman, L.A. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010, 70, 8368–8377. [Google Scholar] [CrossRef] [PubMed]
- Nocera, N.F.; Lee, M.C.; De La Cruz, L.M.; Rosemblit, C.; Czerniecki, B.J. Restoring Lost Anti-HER-2 Th1 Immunity in Breast Cancer: A Crucial Role for Th1 Cytokines in Therapy and Prevention. Front. Pharmacol. 2016, 7, 356. [Google Scholar] [CrossRef]
- Namjoshi, P.; Showalter, L.; Czerniecki, B.J.; Koski, G.K. T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget 2016, 5, 6006. [Google Scholar] [CrossRef][Green Version]
- Braumüller, H.; Wieder, T.; Brenner, E.; Aßmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef]
- Datta, J.; Xu, S.; Rosemblit, C.; Smith, J.B.; Cintolo, J.A.; Powell, D.J., Jr.; Czerniecki, B.J. CD4(+) T-Helper Type 1 Cytokines and Trastuzumab Facilitate CD8(+) T-cell Targeting of HER2/neu-Expressing Cancers. Cancer Immunol. Res. 2015, 3, 455–463. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Ge, S.; Chen, C.; Li, S.; Wu, X.; Feng, X.; Wang, Y.; Cai, D. Saikosaponin A Inhibits Breast Cancer by Regulating Th1/Th2 Balance. Front. Pharmacol. 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Park, J.M.; Terabe, M.; Donaldson, D.D.; Forni, G.; Berzofsky, J.A. Natural immunosurveillance against spontaneous, autochthonous breast cancers revealed and enhanced by blockade of IL-13-mediated negative regulation. Cancer Immunol. Immunother. CII 2008, 57, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Wang, S.H.; Wu, C.C.; Su, Y.A.; Chiang, C.Y.; Lai, C.H.; Wang, T.H.; Cheng, T.L.; Kuo, J.Y.; Hsu, T.C.; et al. IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1. Int. J. Mol. Sci. 2021, 22, 12008. [Google Scholar] [CrossRef] [PubMed]
- Aspord, C.; Pedroza-Gonzalez, A.; Gallegos, M.; Tindle, S.; Burton, E.C.; Su, D.; Marches, F.; Banchereau, J.; Palucka, A.K. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 2007, 204, 1037–1047. [Google Scholar] [CrossRef]
- Todaro, M.; Lombardo, Y.; Francipane, M.G.; Alea, M.P.; Cammareri, P.; Iovino, F.; Di Stefano, A.B.; Di Bernardo, C.; Agrusa, A.; Condorelli, G.; et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008, 15, 762–772. [Google Scholar] [CrossRef]
- Pedroza-Gonzalez, A.; Xu, K.; Wu, T.C.; Aspord, C.; Tindle, S.; Marches, F.; Gallegos, M.; Burton, E.C.; Savino, D.; Hori, T.; et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J. Exp. Med. 2011, 208, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Boieri, M.; Malishkevich, A.; Guennoun, R.; Marchese, E.; Kroon, S.; Trerice, K.E.; Awad, M.; Park, J.H.; Iyer, S.; Kreuzer, J.; et al. CD4+ T helper 2 cells suppress breast cancer by inducing terminal differentiation. J. Exp. Med. 2022, 219, e20201963. [Google Scholar] [CrossRef]
- Gooch, J.L.; Christy, B.; Yee, D. STAT6 mediates interleukin-4 growth inhibition in human breast cancer cells. Neoplasia 2002, 4, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Toi, M. Interleukin-4 and breast cancer. Breast Cancer 2000, 7, 181–186. [Google Scholar] [CrossRef]
- Haricharan, S.; Li, Y. STAT signaling in mammary gland differentiation, cell survival and tumorigenesis. Mol. Cell. Endocrinol. 2014, 382, 560–569. [Google Scholar] [CrossRef]
- Bożek, A.; Jarzab, J.; Mielnik, M.; Bogacz, A.; Kozlowska, R.; Mangold, D. Can atopy have a protective effect against cancer? PLoS ONE 2020, 15, e0226950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Diepgen, T.L. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy 2005, 60, 1098–1111. [Google Scholar] [CrossRef]
- Dong, C. Defining the T(H)17 cell lineage. Nat. Rev. Immunol. 2021, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Benevides, L.; da Fonseca, D.M.; Donate, P.B.; Tiezzi, D.G.; De Carvalho, D.D.; de Andrade, J.M.; Martins, G.A.; Silva, J.S. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer Res. 2015, 75, 3788–3799. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.H.; Chen, H.Y.; Guo, H.R.; Su, I.J.; Chen, H.H. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013, 63, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Benevides, L.; Cardoso, C.R.; Tiezzi, D.G.; Marana, H.R.; Andrade, J.M.; Silva, J.S. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur. J. Immunol. 2013, 43, 1518–1528. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, W. Th17 cells: Positive or negative role in tumor? Cancer Immunol. Immunother. CII 2010, 59, 979–987. [Google Scholar] [CrossRef]
- Wilke, C.M.; Kryczek, I.; Wei, S.; Zhao, E.; Wu, K.; Wang, G.; Zou, W. Th17 cells in cancer: Help or hindrance? Carcinogenesis 2011, 32, 643–649. [Google Scholar] [CrossRef]
- Wang, D.; Yu, W.; Lian, J.; Wu, Q.; Liu, S.; Yang, L.; Li, F.; Huang, L.; Chen, X.; Zhang, Z.; et al. Th17 cells inhibit CD8(+) T cell migration by systematically downregulating CXCR3 expression via IL-17A/STAT3 in advanced-stage colorectal cancer patients. J. Hematol. Oncol. 2020, 13, 68. [Google Scholar] [CrossRef]
- Shibabaw, T.; Teferi, B.; Ayelign, B. The role of Th-17 cells and IL-17 in the metastatic spread of breast cancer: As a means of prognosis and therapeutic target. Front. Immunol. 2023, 14, 1094823. [Google Scholar] [CrossRef]
- Welte, T.; Zhang, X.H. Interleukin-17 Could Promote Breast Cancer Progression at Several Stages of the Disease. Mediat. Inflamm. 2015, 2015, 804347. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Khanal, P.; Lim, S.C.; Yun, H.J.; Ahn, S.G.; Ki, S.H.; Choi, H.S. Interleukin-17 induces AP-1 activity and cellular transformation via upregulation of tumor progression locus 2 activity. Carcinogenesis 2013, 34, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Shen, R.; Kong, B.; Chen, W.; Liang, J.; Tang, G.; Zhang, B. Th17 cells regulate the production of CXCL1 in breast cancer. Int. Immunopharmacol. 2018, 56, 320–329. [Google Scholar] [CrossRef]
- Fabre, J.A.S.; Giustinniani, J.; Garbar, C.; Merrouche, Y.; Antonicelli, F.; Bensussan, A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int. J. Mol. Sci. 2018, 19, 3880. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ye, J.; Hsueh, E.C.; Zhang, Y.; Hoft, D.F.; Peng, G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 2010, 184, 1630–1641. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Bae, S.; Kong, J.M.; Choi, J.; Jang, M.; Choi, J.; Hong, J.M.; Hwang, Y.I.; Kang, J.S.; et al. Direct Interaction of CD40 on Tumor Cells with CD40L on T Cells Increases the Proliferation of Tumor Cells by Enhancing TGF-beta Production and Th17 Differentiation. PLoS ONE 2015, 10, e0125742. [Google Scholar] [CrossRef]
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011, 1, 430–441. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Hu, J.; Tang, L.; Zhao, S.; Shan, B. Expression of Th17 cells in breast cancer tissue and its association with clinical parameters. Cell Biochem. Biophys. 2012, 62, 153–159. [Google Scholar] [CrossRef]
- Faucheux, L.; Grandclaudon, M.; Perrot-Dockès, M.; Sirven, P.; Berger, F.; Hamy, A.S.; Fourchotte, V.; Vincent-Salomon, A.; Mechta-Grigoriou, F.; Reyal, F.; et al. A multivariate Th17 metagene for prognostic stratification in T cell non-inflamed triple negative breast cancer. Oncoimmunology 2019, 8, e1624130. [Google Scholar] [CrossRef]
- Wang, J.; Cai, D.; Ma, B.; Wu, G.; Wu, J. Skewing the balance of regulatory T-cells and T-helper 17 cells in breast cancer patients. J. Int. Med. Res. 2011, 39, 691–701. [Google Scholar] [CrossRef]
- Horlock, C.; Stott, B.; Dyson, P.J.; Morishita, M.; Coombes, R.C.; Savage, P.; Stebbing, J. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br. J. Cancer 2009, 100, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, N.Y. Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol. Lett. 2013, 149, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020, 217, e20190456. [Google Scholar] [CrossRef]
- Ma, M.; Huang, W.; Kong, D. IL-17 inhibits the accumulation of myeloid-derived suppressor cells in breast cancer via activating STAT3. Int. Immunopharmacol. 2018, 59, 148–156. [Google Scholar] [CrossRef]
- Tato, C.M.; Laurence, A.; O’Shea, J.J. Helper T cell differentiation enters a new era: Le Roi est mort; vive le Roi! J. Exp. Med. 2006, 203, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Vock, C.; Hauber, H.P.; Wegmann, M. The other T helper cells in asthma pathogenesis. J. Allergy 2010, 2010, 519298. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, N.; Xiao, F.; Shi, X.; Zhu, B.; Rui, K.; Tian, J.; Lu, L. New insights into the function of Interleukin-25 in disease pathogenesis. Biomark. Res. 2023, 11, 36. [Google Scholar] [CrossRef]
- de Sousa, J.R.; Quaresma, J.A.S. The role of T helper 25 cells in the immune response to Mycobacterium leprae. J. Am. Acad. Dermatol. 2018, 78, 1009–1011. [Google Scholar] [CrossRef]
- Gowhari Shabgah, A.; Amir, A.; Gardanova, Z.R.; Olegovna Zekiy, A.; Thangavelu, L.; Ebrahimi Nik, M.; Ahmadi, M.; Gholizadeh Navashenaq, J. Interleukin-25: New perspective and state-of-the-art in cancer prognosis and treatment approaches. Cancer Med. 2021, 10, 5191–5202. [Google Scholar] [CrossRef]
- Mombelli, S.; Cochaud, S.; Merrouche, Y.; Garbar, C.; Antonicelli, F.; Laprevotte, E.; Alberici, G.; Bonnefoy, N.; Eliaou, J.F.; Bastid, J.; et al. IL-17A and its homologs IL-25/IL-17E recruit the c-RAF/S6 kinase pathway and the generation of pro-oncogenic LMW-E in breast cancer cells. Sci. Rep. 2015, 5, 11874. [Google Scholar] [CrossRef]
- Merrouche, Y.; Fabre, J.; Cure, H.; Garbar, C.; Fuselier, C.; Bastid, J.; Antonicelli, F.; Al-Daccak, R.; Bensussan, A.; Giustiniani, J. IL-17E synergizes with EGF and confers in vitro resistance to EGFR-targeted therapies in TNBC cells. Oncotarget 2016, 7, 53350–53361. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, X.; Pan, H.; Han, W. Estrogen Receptor Downregulates Expression of PD-1/PD-L1 and Infiltration of CD8(+) T Cells by Inhibiting IL-17 Signaling Transduction in Breast Cancer. Front. Oncol. 2020, 10, 582863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, J.; Du, X.; Cheng, H.; Wang, X.; Dong, C. IL-25 blockade inhibits metastasis in breast cancer. Protein Cell 2017, 8, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Yang, C.Y.; Jeng, Y.M.; Chen, C.L.; Wu, H.H.; Chang, Y.C.; Ma, C.; Kuo, W.H.; Chang, K.J.; Shew, J.Y.; et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway. Oncogene 2014, 33, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Sinaeian, M.; Shokrollahi Barough, M.; Pak, F.; Semnani, V.; Kokhaei, P.; Momtazi-Borojeni, A.A. Evaluation of Interleukin 25 and Interleukin 25 Receptor Expression in Peripheral Blood Mononuclear Cells of Breast Cancer Patients and Normal Subjects. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2020, 40, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Jian, F.Y.; Chen, Y.H.; Chien, S.C.; Hsieh, M.C.; Hsiao, P.W.; Lee, W.H.; Kuo, Y.H.; Yang, N.S. Induction of IL-25 secretion from tumour-associated fibroblasts suppresses mammary tumour metastasis. Nat. Commun. 2016, 7, 11311. [Google Scholar] [CrossRef]
- Furuta, S.; Jeng, Y.M.; Zhou, L.; Huang, L.; Kuhn, I.; Bissell, M.J.; Lee, W.H. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci. Transl. Med. 2011, 3, 78ra31. [Google Scholar] [CrossRef]
- Gelaleti, G.B.; Borin, T.F.; Maschio-Signorini, L.B.; Moschetta, M.G.; Jardim-Perassi, B.V.; Calvinho, G.B.; Facchini, M.C.; Viloria-Petit, A.M.; de Campos Zuccari, D.A.P. Efficacy of melatonin, IL-25 and siIL-17B in tumorigenesis-associated properties of breast cancer cell lines. Life Sci. 2017, 183, 98–109. [Google Scholar] [CrossRef]
- Younesi, V.; Nejatollahi, F. Induction of anti-proliferative and apoptotic effects by anti-IL-25 receptor single chain antibodies in breast cancer cells. Int. Immunopharmacol. 2014, 23, 624–632. [Google Scholar] [CrossRef]
- Tian, T.; Yu, S.; Ma, D. Th22 and related cytokines in inflammatory and autoimmune diseases. Expert. Opin. Ther. Targets 2013, 17, 113–125. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef]
- Doulabi, H.; Masoumi, E.; Rastin, M.; Foolady Azarnaminy, A.; Esmaeili, S.A.; Mahmoudi, M. The role of Th22 cells, from tissue repair to cancer progression. Cytokine 2022, 149, 155749. [Google Scholar] [CrossRef]
- Cui, G. T(H)9, T(H)17, and T(H)22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front. Oncol. 2019, 9, 1002. [Google Scholar] [CrossRef]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. CR 2019, 38, 195. [Google Scholar] [CrossRef] [PubMed]
- Salmanpour, A.; Rezaeifard, S.; Kiani, R.; Tahmasebi, S.; Faghih, Z.; Erfani, N. IFNγ-IL-17-IL-22+CD4+ subset and IL-22-producing cells in tumor draining lymph nodes of patients with breast cancer. Breast Dis. 2022, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahid, A.G.; Abdullah, H.N. Expression of Serum IL-22, IL-23, and TLR9 as Tumor Markers in Untreated Breast Cancer Patients. 2020. Available online: http://impactfactor.org/PDF/IJDDT/10/IJDDT,Vol10,Issue3,Article30.pdf (accessed on 18 August 2023).
- Zhao, J.; Liu, H.; Zhang, X.; Zhang, W.; Liu, L.; Yu, Y.; Ren, S.; Yang, Q.; Liu, B.; Li, J.; et al. Tumor Cells Interleukin-22 Expression Associates with Elevated Tumor-Associated Macrophages Infiltrating and Poor Prognosis in Patients with Breast Cancer. Cancer Biother. Radiopharm. 2021, 36, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Gao, J.; Shao, S.; Cui, Y.; Yin, S.; Pan, B. IL-22 promotes tumor growth of breast cancer cells in mice. Aging 2020, 12, 13354–13364. [Google Scholar] [CrossRef]
- Rasé, V.J.; Hayward, R.; Haughian, J.M.; Pullen, N.A. T(h)17, T(h)22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma. Int. J. Mol. Sci. 2022, 23, 10299. [Google Scholar] [CrossRef]
- Katara, G.K.; Kulshrestha, A.; Schneiderman, S.; Riehl, V.; Ibrahim, S.; Beaman, K.D. Interleukin-22 promotes development of malignant lesions in a mouse model of spontaneous breast cancer. Mol. Oncol. 2020, 14, 211–224. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, B.; Kim, J.E.; Park, S.O.; Kim, S.M.; Chang, E.J. Interleukin-22 Mediates the Chemotactic Migration of Breast Cancer Cells and Macrophage Infiltration of the Bone Microenvironment by Potentiating S1P/SIPR Signaling. Cells 2020, 9, 131. [Google Scholar] [CrossRef]
- Rui, J.; Chunming, Z.; Binbin, G.; Na, S.; Shengxi, W.; Wei, S. IL-22 promotes the progression of breast cancer through regulating HOXB-AS5. Oncotarget 2017, 8, 103601–103612. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.; May, P.; Gottschlich, A.; Markota, A.; Wenk, D.; Gerlach, I.; Voigt, S.; Stathopoulos, G.T.; Arendt, K.A.M.; Heise, C.; et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc. Natl. Acad. Sci. USA 2017, 114, 12994–12999. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Gaertner, F.C.; Erl, W.; Janssen, K.P.; Blechert, B.; Holzmann, B.; Weighardt, H.; Essler, M. IL-22-mediated tumor growth reduction correlates with inhibition of ERK1/2 and AKT phosphorylation and induction of cell cycle arrest in the G2-M phase. J. Immunol. 2006, 177, 8266–8272. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, J.; Cai, Z.; Li, B.; Sun, L.; Shen, Y.; Wang, S.; Wang, Z.; Wang, Z.; Wang, Y.; et al. Th9 Cell Differentiation and Its Dual Effects in Tumor Development. Front. Immunol. 2020, 11, 1026. [Google Scholar] [CrossRef]
- Fischer, M.; Bijman, M.; Molin, D.; Cormont, F.; Uyttenhove, C.; van Snick, J.; Sundstrom, C.; Enblad, G.; Nilsson, G. Increased serum levels of interleukin-9 correlate to negative prognostic factors in Hodgkin’s lymphoma. Leukemia 2003, 17, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Elyaman, W.; Bradshaw, E.M.; Uyttenhove, C.; Dardalhon, V.; Awasthi, A.; Imitola, J.; Bettelli, E.; Oukka, M.; van Snick, J.; Renauld, J.C.; et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 12885–12890. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Bai, Q.; Qin, C.; Mohamud, A.O.; Zhu, Z.; Ball, T.W.; Ruth, C.M.; Newcomer, D.R.; Herrick, E.J.; et al. IL-9 inhibits HTB-72 melanoma cell growth through upregulation of p21 and TRAIL. J. Surg. Oncol. 2015, 111, 969–974. [Google Scholar] [CrossRef]
- Schlapbach, C.; Gehad, A.; Yang, C.; Watanabe, R.; Guenova, E.; Teague, J.E.; Campbell, L.; Yawalkar, N.; Kupper, T.S.; Clark, R.A. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci. Transl. Med. 2014, 6, 219ra218. [Google Scholar] [CrossRef]
- Lu, Y.; Hong, B.; Li, H.; Zheng, Y.; Zhang, M.; Wang, S.; Qian, J.; Yi, Q. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 2265–2270. [Google Scholar] [CrossRef]
- Carlsson, A.; Wingren, C.; Kristensson, M.; Rose, C.; Fernö, M.; Olsson, H.; Jernström, H.; Ek, S.; Gustavsson, E.; Ingvar, C.; et al. Molecular serum portraits in patients with primary breast cancer predict the development of distant metastases. Proc. Natl. Acad. Sci. USA 2011, 108, 14252–14257. [Google Scholar] [CrossRef]
- You, F.P.; Zhang, J.; Cui, T.; Zhu, R.; Lv, C.Q.; Tang, H.T.; Sun, D.W. Th9 cells promote antitumor immunity via IL-9 and IL-21 and demonstrate atypical cytokine expression in breast cancer. Int. Immunopharmacol. 2017, 52, 163–167. [Google Scholar] [CrossRef]
- Ding, P.; Zhu, R.; Cai, B.; Zhang, J.; Bu, Q.; Sun, D.W. IL-9-producing CD8(+) T cells represent a distinctive subset with different transcriptional characteristics from conventional CD8(+) T cells, and partially infiltrate breast tumors. Int. J. Biochem. Cell Biol. 2019, 115, 105576. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Dominguez, A.L.; Cohen, P.A.; Gendler, S.J. Inhibition of adaptive immunity by IL-9 can be disrupted to achieve rapid T cell sensitization and rejection of progressive tumor challenges. Cancer Res. 2014, 74, 6845–6855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Roberts, A.I.; Liu, C.; Ren, G.; Xu, G.; Zhang, L.; Devadas, S.; Shi, Y. A novel subset of helper T cells promotes immune responses by secreting GM-CSF. Cell Death Differ. 2013, 20, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, A.; Usui, T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediat. Inflamm. 2015, 2015, 568543. [Google Scholar] [CrossRef]
- Robinson, R.T. T Cell Production of GM-CSF Protects the Host during Experimental Tuberculosis. mBio 2017, 8, e02087-17. [Google Scholar] [CrossRef]
- Ariafar, A.; Zareinejad, M.; Soltani, M.; Vahidi, Y.; Faghih, Z. GM-CSF-producing lymphocytes in tumor-draining lymph nodes of patients with bladder cancer. Eur. Cytokine Netw. 2021, 32, 1–7. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Chaubey, N.; Ghosh, S.S. Overexpression of granulocyte macrophage colony stimulating factor in breast cancer cells leads towards drug sensitization. Appl. Biochem. Biotechnol. 2015, 175, 1948–1959. [Google Scholar] [CrossRef]
- Eubank, T.D.; Roberts, R.; Galloway, M.; Wang, Y.; Cohn, D.E.; Marsh, C.B. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity 2004, 21, 831–842. [Google Scholar] [CrossRef]
- Razmkhah, M.; Karimzadeh, P.; Eghbali, F.; Rezaeifard, S.; Faghih, Z. Serum level of colony stimulating factors, Granulocyte, Monocyte and Granulocyte-Monocyte, in peripheral blood of patients with breast cancer. J. Sabzevar Univ. Med. Sci. 2019, 25, 809–817. [Google Scholar]
- Al-Rashed, F.; Thomas, R.; Al-Roub, A.; Al-Mulla, F.; Ahmad, R. LPS Induces GM-CSF Production by Breast Cancer MDA-MB-231 Cells via Long-Chain Acyl-CoA Synthetase 1. Molecules 2020, 25, 4709. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Rashed, F.; Akhter, N.; Al-Mulla, F.; Ahmad, R. ACSL1 Regulates TNFα-Induced GM-CSF Production by Breast Cancer MDA-MB-231 Cells. Biomolecules 2019, 9, 555. [Google Scholar] [CrossRef]
- Yonemitsu, K.; Pan, C.; Fujiwara, Y.; Miyasato, Y.; Shiota, T.; Yano, H.; Hosaka, S.; Tamada, K.; Yamamoto, Y.; Komohara, Y. GM-CSF derived from the inflammatory microenvironment potentially enhanced PD-L1 expression on tumor-associated macrophages in human breast cancer. Sci. Rep. 2022, 12, 12007. [Google Scholar] [CrossRef] [PubMed]
- Ghirelli, C.; Reyal, F.; Jeanmougin, M.; Zollinger, R.; Sirven, P.; Michea, P.; Caux, C.; Bendriss-Vermare, N.; Donnadieu, M.H.; Caly, M.; et al. Breast Cancer Cell-Derived GM-CSF Licenses Regulatory Th2 Induction by Plasmacytoid Predendritic Cells in Aggressive Disease Subtypes. Cancer Res. 2015, 75, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xu, Y.; Fox, G.C.; Xiang, J.; Kwakwa, K.A.; Davis, J.L.; Belle, J.I.; Lee, W.C.; Wong, W.H.; Fontana, F.; et al. Breast cancer-derived GM-CSF regulates arginase 1 in myeloid cells to promote an immunosuppressive microenvironment. J. Clin. Investig. 2021, 131, e145296. [Google Scholar] [CrossRef]
- Cho, H.; Seo, Y.; Loke, K.M.; Kim, S.W.; Oh, S.M.; Kim, J.H.; Soh, J.; Kim, H.S.; Lee, H.; Kim, J.; et al. Cancer-Stimulated CAFs Enhance Monocyte Differentiation and Protumoral TAM Activation via IL6 and GM-CSF Secretion. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5407–5421. [Google Scholar] [CrossRef]
- Espinoza-Sánchez, N.A.; Vadillo, E.; Balandrán, J.C.; Monroy-García, A.; Pelayo, R.; Fuentes-Pananá, E.M. Evidence of lateral transmission of aggressive features between different types of breast cancer cells. Int. J. Oncol. 2017, 51, 1482–1496. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Chen, J.; Chen, J.; Chen, F.; He, C.; Huang, D.; Wu, W.; Lin, L.; Huang, W.; et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014, 25, 605–620. [Google Scholar] [CrossRef]
- Chen, G.; Gupta, R.; Petrik, S.; Laiko, M.; Leatherman, J.M.; Asquith, J.M.; Daphtary, M.M.; Garrett-Mayer, E.; Davidson, N.E.; Hirt, K.; et al. A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol. Res. 2014, 2, 949–961. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Tangye, S.G.; Mackay, C.R. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 2008, 26, 741–766. [Google Scholar] [CrossRef]
- Liu, X.; Nurieva, R.I.; Dong, C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol. Rev. 2013, 252, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Melo, N.; Baumjohann, D. T follicular helper cells in cancer. Trends Cancer 2023, 9, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Noël, G.; Fontsa, M.L.; Garaud, S.; De Silva, P.; de Wind, A.; Van den Eynden, G.G.; Salgado, R.; Boisson, A.; Locy, H.; Thomas, N.; et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Investig. 2021, 131, e139905. [Google Scholar] [CrossRef]
- Razis, E.; Kalogeras, K.T.; Kotoula, V.; Eleftheraki, A.G.; Nikitas, N.; Kronenwett, R.; Timotheadou, E.; Christodoulou, C.; Pectasides, D.; Gogas, H.; et al. Improved outcome of high-risk early HER2 positive breast cancer with high CXCL13-CXCR5 messenger RNA expression. Clin. Breast Cancer 2012, 12, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Panse, J.; Friedrichs, K.; Marx, A.; Hildebrandt, Y.; Luetkens, T.; Barrels, K.; Horn, C.; Stahl, T.; Cao, Y.; Milde-Langosch, K.; et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br. J. Cancer 2008, 99, 930–938. [Google Scholar] [CrossRef]
- Biswas, S.; Sengupta, S.; Roy Chowdhury, S.; Jana, S.; Mandal, G.; Mandal, P.K.; Saha, N.; Malhotra, V.; Gupta, A.; Kuprash, D.V.; et al. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res. Treat. 2014, 143, 265–276. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Migliori, E.; Buisseret, L.; de Wind, A.; Brohée, S.; Garaud, S.; Noël, G.; Dang Chi, V.L.; Lodewyckx, J.N.; Naveaux, C.; et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2017, 2, e91487. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, J.; Qiao, G.; Wang, X.; Xu, Y. Tim-3 identifies exhausted follicular helper T cells in breast cancer patients. Immunobiology 2016, 221, 986–993. [Google Scholar] [CrossRef]
- Linterman, M.A.; Pierson, W.; Lee, S.K.; Kallies, A.; Kawamoto, S.; Rayner, T.F.; Srivastava, M.; Divekar, D.P.; Beaton, L.; Hogan, J.J.; et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011, 17, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, Y.; Xu, Y. Follicular regulatory T cells infiltrated the ovarian carcinoma and resulted in CD8 T cell dysfunction dependent on IL-10 pathway. Int. Immunopharmacol. 2019, 68, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cha, Z.; Gu, H.; Zang, Y.; Wang, Z.; Li, J.; Huang, W.; Qin, A.; Zhu, L.; Tu, X.; Cheng, N.; et al. The prevalence and function of CD4(+)CXCR5(+)Foxp3(+) follicular regulatory T cells in diffuse large B cell lymphoma. Int. Immunopharmacol. 2018, 61, 132–139. [Google Scholar] [CrossRef]
- Brady, M.T.; Hilchey, S.P.; Hyrien, O.; Spence, S.A.; Bernstein, S.H. Mesenchymal stromal cells support the viability and differentiation of follicular lymphoma-infiltrating follicular helper T-cells. PLoS ONE 2014, 9, e97597. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, A.; Liu, G.; Wu, F.; Li, Z. T follicular regulatory cells suppress Tfh-mediated B cell help and synergistically increase IL-10-producing B cells in breast carcinoma. Immunol. Res. 2019, 67, 416–423. [Google Scholar] [CrossRef]
- Xie, M.M.; Dent, A.L. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front. Immunol. 2018, 9, 1536. [Google Scholar] [CrossRef]
- Leong, P.P.; Mohammad, R.; Ibrahim, N.; Ithnin, H.; Abdullah, M.; Davis, W.C.; Seow, H.F. Phenotyping of lymphocytes expressing regulatory and effector markers in infiltrating ductal carcinoma of the breast. Immunol. Lett. 2006, 102, 229–236. [Google Scholar] [CrossRef]
- Sun, Y.P.; Ke, Y.L.; Li, X. Prognostic value of CD8(+) tumor-infiltrating T cells in patients with breast cancer: A systematic review and meta-analysis. Oncol. Lett. 2023, 25, 39. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Feng, Z.; Huang, M.; Wei, L.; Yan, Z.; Long, M.; Hu, Q.; Wang, J.; Liu, S.; et al. CD8(+) T cell immunity blocks the metastasis of carcinogen-exposed breast cancer. Sci. Adv. 2021, 7, eabd8936. [Google Scholar] [CrossRef]
- Egelston, C.A.; Avalos, C.; Tu, T.Y.; Simons, D.L.; Jimenez, G.; Jung, J.Y.; Melstrom, L.; Margolin, K.; Yim, J.H.; Kruper, L.; et al. Human breast tumor-infiltrating CD8(+) T cells retain polyfunctionality despite PD-1 expression. Nat. Commun. 2018, 9, 4297. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Li, L.; Zhou, M.; Jiang, W.; Niu, H.; Yang, K. Distribution and prognostic value of tumorinfiltrating T cells in breast cancer. Mol. Med. Rep. 2018, 18, 4247–4258. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Baker, K.; Lachapelle, J.; Zlobec, I.; Bismar, T.A.; Terracciano, L.; Foulkes, W.D. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 2011, 58, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, T.; Siegal, G.P.; Wei, S. The CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. Hum. Pathol. 2017, 69, 110–117. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Nam, J.S.; Terabe, M.; Kang, M.J.; Chae, H.; Voong, N.; Yang, Y.; Laurence, A.; Michalowska, A.; Mamura, M.; Lonning, S.; et al. TGF-β subverts the immune system into directly promoting tumor growth through IL-17. Cancer Res. 2008, 68, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lin, J.; Qiao, G.; Xu, Y.; Zou, H. Differential regulation and function of tumor-infiltrating T cells in different stages of breast cancer patients. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 7907–7913. [Google Scholar] [CrossRef]
- Schule, J.M.; Bergkvist, L.; Hakansson, L.; Gustafsson, B.; Hakansson, A. CD28 expression in sentinel node biopsies from breast cancer patients in comparison with CD3-zeta chain expression. J. Transl. Med. 2004, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8(+) T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef]
- Shariati, S.; Ghods, A.; Zohouri, M.; Rasolmali, R.; Talei, A.R.; Mehdipour, F.; Ghaderi, A. Significance of TIM-3 expression by CD4(+) and CD8(+) T lymphocytes in tumor-draining lymph nodes from patients with breast cancer. Mol. Immunol. 2020, 128, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yi, Z.; Wang, L.; Li, Z.; Niu, B.; Ren, G. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int. Immunopharmacol. 2020, 78, 106113. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Canale, F.P.; Ramello, M.C.; Nunez, N.; Araujo Furlan, C.L.; Bossio, S.N.; Gorosito Serran, M.; Tosello Boari, J.; Del Castillo, A.; Ledesma, M.; Sedlik, C.; et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells. Cancer Res. 2018, 78, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Tallón de Lara, P.; Castañón, H.; Vermeer, M.; Núñez, N.; Silina, K.; Sobottka, B.; Urdinez, J.; Cecconi, V.; Yagita, H.; Movahedian Attar, F.; et al. CD39(+)PD-1(+)CD8(+) T cells mediate metastatic dormancy in breast cancer. Nat. Commun. 2021, 12, 769. [Google Scholar] [CrossRef]
- Cortesini, R.; LeMaoult, J.; Ciubotariu, R.; Cortesini, N.S.F. CD8+ CD28− T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol. Rev. 2001, 182, 201–206. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef]
- Coventry, B.J.; Weightman, M.J.; Bradley, J.; Skinner, J.M. Immune profiling in human breast cancer using high-sensitivity detection and analysis techniques. JRSM Open 2015, 6, 2054270415603909. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.-N.; Duvillier, H. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228. [Google Scholar] [CrossRef]
- Schnellhardt, S.; Erber, R.; Büttner-Herold, M.; Rosahl, M.C.; Ott, O.J.; Strnad, V.; Beckmann, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Tumour-Infiltrating Inflammatory Cells in Early Breast Cancer: An Underrated Prognostic and Predictive Factor? Int. J. Mol. Sci. 2020, 21, 8238. [Google Scholar] [CrossRef] [PubMed]
- Yajima, R.; Yajima, T.; Fujii, T.; Yanagita, Y.; Fujisawa, T.; Miyamoto, T.; Tsutsumi, S.; Iijima, M.; Kuwano, H. Tumor-infiltrating CD45RO(+) memory cells are associated with a favorable prognosis breast cancer. Breast. Cancer 2016, 23, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Li, X.; Shi, P. CD45RO positive expression correlates with lower histological grade, less lymph node metastasis and prolonged overall survival in surgical patients with breast cancer. Int. J. Clin. Exp. Med. 2017, 10, 16336–16343. [Google Scholar]
- Naylor, K.; Li, G.; Vallejo, A.N.; Lee, W.-W.; Koetz, K.; Bryl, E.; Witkowski, J.; Fulbright, J.; Weyand, C.M.; Goronzy, J.J. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005, 174, 7446–7452. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.; Mathieu, M.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 2015, 26, 1698–1704. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Goldstein, L.J.; Sparano, J.A.; Demaria, S.; Badve, S.S. Tumor infiltrating lymphocytes (TILs) improve prognosis in patients with triple negative breast cancer (TNBC). Oncoimmunology 2015, 4, e985930. [Google Scholar] [CrossRef]
- Cejalvo, J.M.; Pascual, T.; Fernández-Martínez, A.; Brasó-Maristany, F.; Gomis, R.R.; Perou, C.M.; Muñoz, M.; Prat, A. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat. Rev. 2018, 67, 63–70. [Google Scholar] [CrossRef]
- Bardou, V.-J.; Arpino, G.; Elledge, R.M.; Osborne, C.K.; Clark, G.M. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003, 21, 1973–1979. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Pelto-Huikko, M.; Holli, K.; Isola, J. Estrogen receptor β is coexpressed with ERα and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am. J. Pathol. 2000, 156, 29–35. [Google Scholar] [CrossRef]
- Triulzi, T.; Forte, L.; Regondi, V.; Di Modica, M.; Ghirelli, C.; Carcangiu, M.L.; Sfondrini, L.; Balsari, A.; Tagliabue, E. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology 2019, 8, e1512942. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.C.; Masopust, D. Understanding Subset Diversity in T Cell Memory. Immunity 2018, 48, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Milne, K.; Derocher, H.; Webb, J.R.; Nelson, B.H.; Watson, P.H. CD103 and Intratumoral Immune Response in Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 6290–6297. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.Y.; Jeon, S.H.; Nam, H.; Jung, J.H.; Jeon, M.; Kim, E.S.; Bae, S.J.; Ahn, J.; Yoo, T.K.; et al. CD39(+) tissue-resident memory CD8(+) T cells with a clonal overlap across compartments mediate antitumor immunity in breast cancer. Sci. Immunol. 2022, 7, eabn8390. [Google Scholar] [CrossRef]
- Vahidi, Y.; Faghih, Z.; Talei, A.R.; Doroudchi, M.; Ghaderi, A. Memory CD4(+) T cell subsets in tumor draining lymph nodes of breast cancer patients: A focus on T stem cell memory cells. Cell. Oncol. 2018, 41, 1–11. [Google Scholar] [CrossRef]
- Vahidi, Y.; Bagheri, M.; Ghaderi, A.; Faghih, Z. CD8-positive memory T cells in tumor-draining lymph nodes of patients with breast cancer. BMC Cancer 2020, 20, 257. [Google Scholar] [CrossRef]
- Legat, A.; Speiser, D.E.; Pircher, H.; Zehn, D.; Fuertes Marraco, S.A. Inhibitory Receptor Expression Depends More Dominantly on Differentiation and Activation than “Exhaustion” of Human CD8 T Cells. Front. Immunol. 2013, 4, 455. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Nouri, N.; Nowels, K.; Johnson, D.; Holmes, S.; Lee, P.P. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005, 2, e284. [Google Scholar] [CrossRef]
- Razmkhah, M.; Abedi, N.; Hosseini, A.; Imani, M.T.; Talei, A.R.; Ghaderi, A. Induction of T regulatory subsets from naive CD4+ T cells after exposure to breast cancer adipose derived stem cells. Iran. J. Immunol. IJI 2015, 12, 1–15. [Google Scholar]
- Razmkhah, M.; Jaberipour, M.; Erfani, N.; Habibagahi, M.; Talei, A.R.; Ghaderi, A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: Do they protect breast cancer cells from the immune response? Cell. Immunol. 2011, 266, 116–122. [Google Scholar] [CrossRef]
- Jafarinia, M.; Mehdipour, F.; Hosseini, S.V.; Ghahramani, L.; Hosseinzadeh, M.; Ghaderi, A. Determination of a CD4(+)CD25(-)FoxP3(+) T cells subset in tumor-draining lymph nodes of colorectal cancer secreting IL-2 and IFN-γ. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 14659–14666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, N.; Aierken, N.; Xie, C.; Ye, R.; Qian, X.; Hu, Z.; Zhang, J.; Lin, Y. Prognostic value of tumor-infiltrating Foxp3+ regulatory T cells in patients with breast cancer: A meta-analysis. J. Cancer 2017, 8, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Zhang, Z.; Lai, Y.; Chen, Z.; Huang, J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: A systematic review and meta-analysis. BMC Cancer 2016, 16, 687. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Lee, A.H.; Ellis, I.O.; Green, A.R. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res. Treat. 2011, 127, 99–108. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, Z.; Cai, Z.; Wang, M.; He, J. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: A meta-analysis. BMC Cancer 2015, 15, 727. [Google Scholar] [CrossRef]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef]
- Niakan, A.; Faghih, Z.; Talei, A.R.; Ghaderi, A. Cytokine profile of CD4(+)CD25(-)FoxP3(+) T cells in tumor-draining lymph nodes from patients with breast cancer. Mol. Immunol. 2019, 116, 90–97. [Google Scholar] [CrossRef]
- Bos, P.D.; Plitas, G.; Rudra, D.; Lee, S.Y.; Rudensky, A.Y. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 2013, 210, 2435–2466. [Google Scholar] [CrossRef]
- Chen, L.; Huang, T.G.; Meseck, M.; Mandeli, J.; Fallon, J.; Woo, S.L. Rejection of metastatic 4T1 breast cancer by attenuation of Treg cells in combination with immune stimulation. Mol. Ther. J. Am. Soc. Gene Ther. 2007, 15, 2194–2202. [Google Scholar] [CrossRef]
- Ladoire, S.; Arnould, L.; Mignot, G.; Coudert, B.; Rebe, C.; Chalmin, F.; Vincent, J.; Bruchard, M.; Chauffert, B.; Martin, F.; et al. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2011, 125, 65–72. [Google Scholar] [CrossRef]
- Ladoire, S.; Mignot, G.; Dalban, C.; Chevriaux, A.; Arnould, L.; Rebe, C.; Apetoh, L.; Boidot, R.; Penault-Llorca, F.; Fumoleau, P.; et al. FOXP3 expression in cancer cells and anthracyclines efficacy in patients with primary breast cancer treated with adjuvant chemotherapy in the phase III UNICANCER-PACS 01 trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2552–2561. [Google Scholar] [CrossRef]
- Syed Khaja, A.S.; Toor, S.M.; El Salhat, H.; Faour, I.; Ul Haq, N.; Ali, B.R.; Elkord, E. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget 2017, 8, 33159–33171. [Google Scholar] [CrossRef]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef]
- Li, Y.Q.; Liu, F.F.; Zhang, X.M.; Guo, X.J.; Ren, M.J.; Fu, L. Tumor secretion of CCL22 activates intratumoral Treg infiltration and is independent prognostic predictor of breast cancer. PLoS ONE 2013, 8, e76379. [Google Scholar] [CrossRef]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Freier, C.P.; Kuhn, C.; Endres, S.; Mayr, D.; Friese, K.; Jeschke, U.; Anz, D. FOXP3+ Cells Recruited by CCL22 into Breast Cancer Correlates with Less Tumor Nodal Infiltration. Anticancer Res. 2016, 36, 3139–3145. [Google Scholar] [PubMed]
- Kuehnemuth, B.; Piseddu, I.; Wiedemann, G.M.; Lauseker, M.; Kuhn, C.; Hofmann, S.; Schmoeckel, E.; Endres, S.; Mayr, D.; Jeschke, U.; et al. CCL1 is a major regulatory T cell attracting factor in human breast cancer. BMC Cancer 2018, 18, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zohouri, M.; Mehdipour, F.; Razmkhah, M.; Faghih, Z.; Ghaderi, A. CD4(+)CD25(-)FoxP3(+) T cells: A distinct subset or a heterogeneous population? Int. Rev. Immunol. 2021, 40, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef]
- Li, M.; Quintana, A.; Alberts, E.; Hung, M.S.; Boulat, V.; Ripoll, M.M.; Grigoriadis, A. B Cells in Breast Cancer Pathology. Cancers 2023, 15, 1517. [Google Scholar] [CrossRef]
- Linnebacher, M.; Maletzki, C. Tumor-infiltrating B cells: The ignored players in tumor immunology. Oncoimmunology 2012, 1, 1186–1188. [Google Scholar] [CrossRef]
- Mehdipour, F.; Razmkhah, M.; Hosseini, A.; Bagheri, M.; Safaei, A.; Talei, A.R.; Ghaderi, A. Increased B Regulatory Phenotype in Non-Metastatic Lymph Nodes of Node-Positive Breast Cancer Patients. Scand. J. Immunol. 2016, 83, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Masunaga, A. Tumor-infiltrating B cells and T cells correlate with postoperative prognosis in triple-negative carcinoma of the breast. BMC Cancer 2021, 21, 286. [Google Scholar] [CrossRef]
- Garaud, S.; Buisseret, L.; Solinas, C.; Gu-Trantien, C.; de Wind, A.; Van den Eynden, G.; Naveaux, C.; Lodewyckx, J.N.; Boisson, A.; Duvillier, H.; et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight 2019, 5, e129641. [Google Scholar] [CrossRef]
- Deola, S.; Panelli, M.C.; Maric, D.; Selleri, S.; Dmitrieva, N.I.; Voss, C.Y.; Klein, H.; Stroncek, D.; Wang, E.; Marincola, F.M. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J. Immunol. 2008, 180, 1362–1372. [Google Scholar] [CrossRef]
- Mehdipour, F.; Razmkhah, M.; Faghih, Z.; Bagheri, M.; Talei, A.R.; Ghaderi, A. The significance of cytokine-producing B cells in breast tumor-draining lymph nodes. Cell. Oncol. 2019, 42, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Kessel, A.; Haj, T.; Peri, R.; Snir, A.; Melamed, D.; Sabo, E.; Toubi, E. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun. Rev. 2012, 11, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Zhang, Y.H.; Guo, X.H.; Zhang, H.Y.; Zhang, Y. The double-edge role of B cells in mediating antitumor T-cell immunity: Pharmacological strategies for cancer immunotherapy. Int. Immunopharmacol. 2016, 36, 73–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zareinejad, M.; Mehdipour, F.; Roshan-Zamir, M.; Faghih, Z.; Ghaderi, A. Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis. Cancers 2023, 15, 4771. https://doi.org/10.3390/cancers15194771
Zareinejad M, Mehdipour F, Roshan-Zamir M, Faghih Z, Ghaderi A. Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis. Cancers. 2023; 15(19):4771. https://doi.org/10.3390/cancers15194771
Chicago/Turabian StyleZareinejad, Mohammadrasul, Fereshteh Mehdipour, Mina Roshan-Zamir, Zahra Faghih, and Abbas Ghaderi. 2023. "Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis" Cancers 15, no. 19: 4771. https://doi.org/10.3390/cancers15194771
APA StyleZareinejad, M., Mehdipour, F., Roshan-Zamir, M., Faghih, Z., & Ghaderi, A. (2023). Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis. Cancers, 15(19), 4771. https://doi.org/10.3390/cancers15194771





