Exploring a Novel Technique to Tackle the Shortage of Devices for Hepatic Arterial Infusion Chemotherapy: Early Results of an Alternate Approach for Percutaneous Arterial Port Catheter Placement
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Method
2.1. Study Population
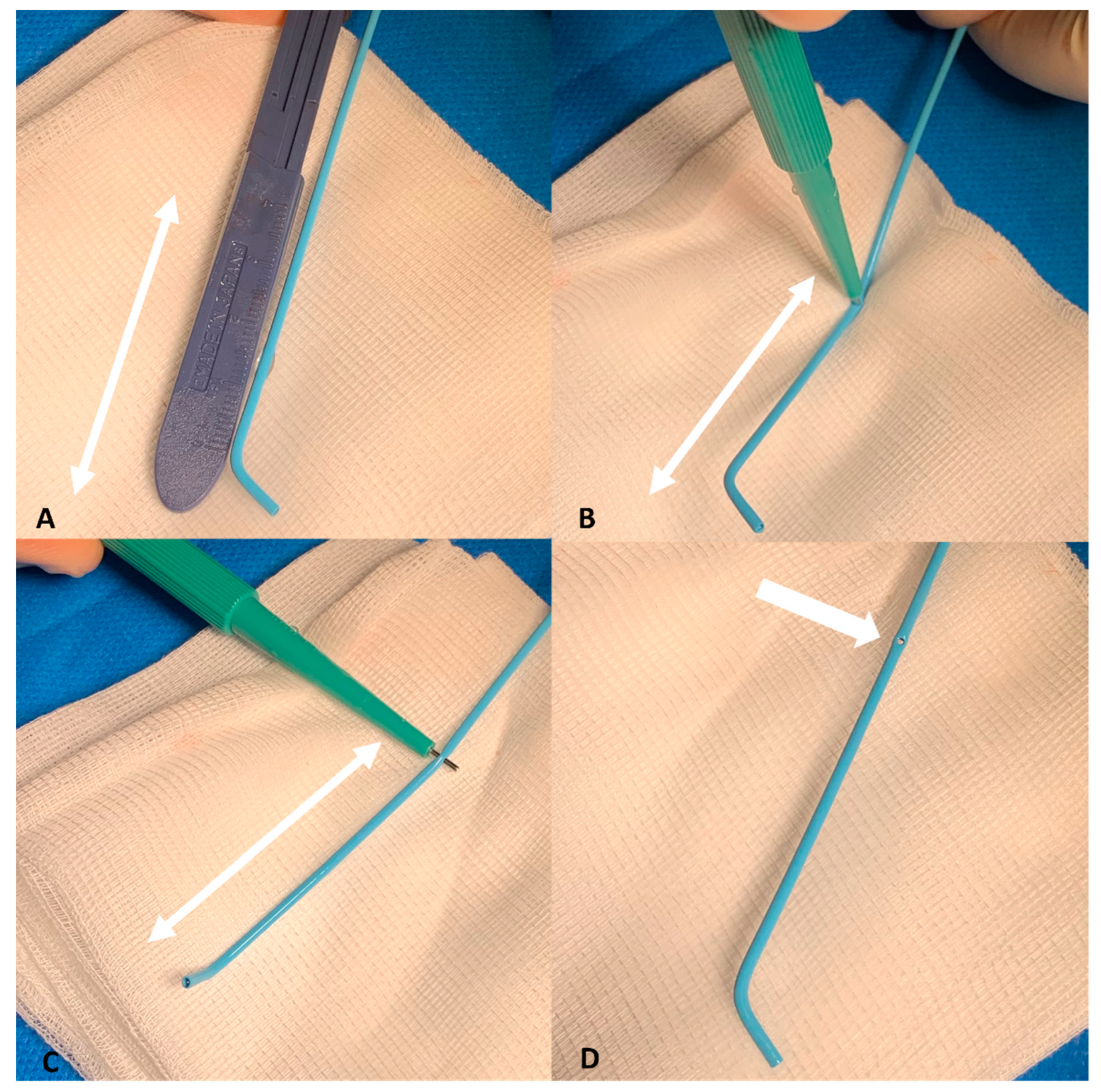
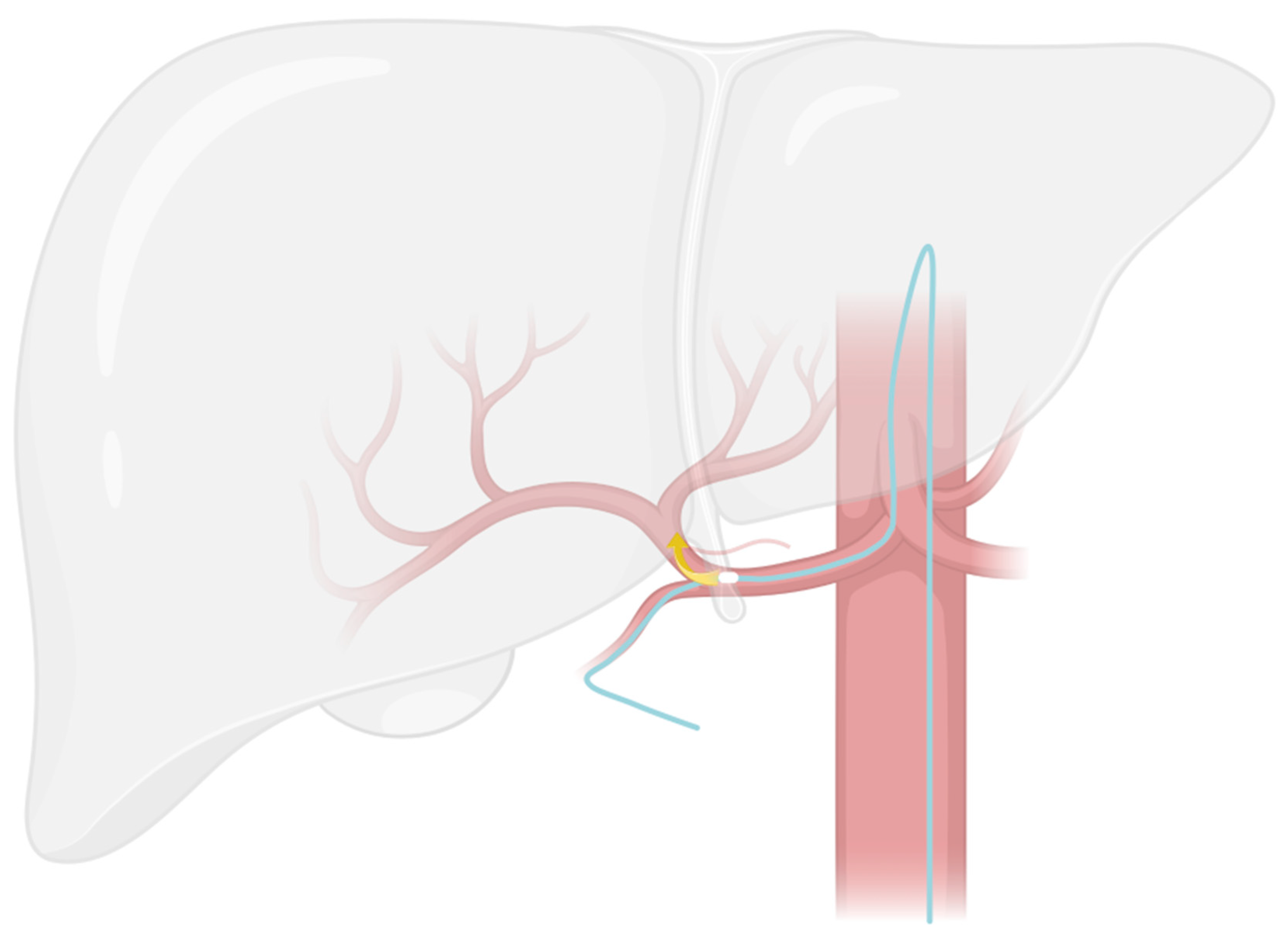
2.2. Modified Placement Technique
- The search for the optimal injection point.
- The skeletonization of the hepatic artery.
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
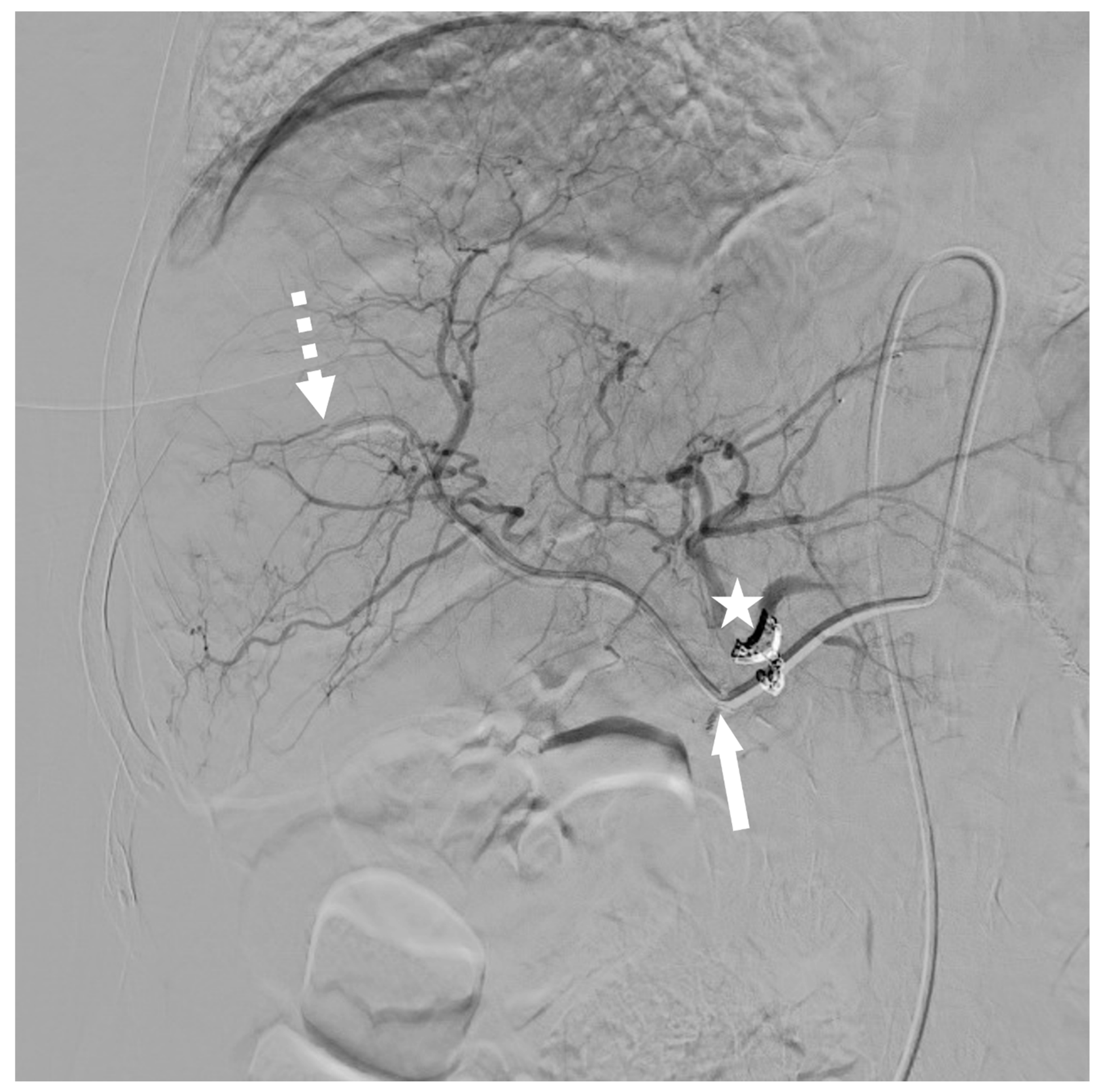
3.2. Modified Implantation Technique
3.3. Technical Outcomes
3.4. Oncological Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHA | common hepatic artery |
| CLRM | colorectal liver metastases |
| CT | computed tomography |
| DAP | dose area product |
| DSA | digital subtraction angiography |
| Fr | French |
| GDA | gastroduodenal artery |
| HAIC | hepatic arterial infusion chemotherapy |
| IRB | institutional review board |
| LGA | left gastric artery |
| LHA | left hepatic artery |
| NBCA | n-butyl cyanoacrylate |
| OS | overall survival |
| PFS | progression-free survival |
| PHA | proper hepatic artery |
| PS | performance status |
| RECIST | response evaluation criteria in solid tumors |
| RGA | right gastric artery |
| RHA | right hepatic artery |
| TNM | tumor node metastasis |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.; Pua, U. Review of Intra-Arterial Therapies for Colorectal Cancer Liver Metastasis. Cancers 2021, 13, 1371. [Google Scholar] [CrossRef]
- Napier, K.J.; Lidsky, M.E.; James, O.G.; Wildman-Tobriner, B. Hepatic Arterial Infusion Pumps: What the Radiologist Needs to Know. RadioGraphics 2021, 41, 895–908. [Google Scholar] [CrossRef]
- Ammori, J.B.; Kemeny, N.E. Regional hepatic chemotherapies in treatment of colorectal cancer metastases to the liver. Semin. Oncol. 2010, 37, 139–148. [Google Scholar] [CrossRef]
- Goéré, D.; Pignon, J.-P.; Gelli, M.; Elias, D.; Benhaim, L.; Deschamps, F.; Caramella, C.; Boige, V.; Ducreux, M.; de Baere, T.; et al. Postoperative hepatic arterial chemotherapy in high-risk patients as adjuvant treatment after resection of colorectal liver metastases—A randomized phase II/III trial—PACHA-01 (NCT02494973). BMC Cancer 2018, 18, 787. [Google Scholar] [CrossRef]
- de Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef]
- De Baere, T.; Tselikas, L.; Delpla, A.; Roux, C.; Varin, E.; Kobe, A.; Yevich, S.; Deschamps, F. Thermal ablation in the management of oligometastatic colorectal cancer. Int. J. Hyperth. 2022, 39, 627–632. [Google Scholar] [CrossRef]
- Pernot, S.; Pellerin, O.; Mineur, L.; Monterymard, C.; Smith, D.; Lapuyade, B.; Gallois, C.; Khemissa Akouz, F.; De Baere, T.; Tougeron, D.; et al. Phase III randomized trial comparing systemic versus intra-arterial oxaliplatin, combined with LV5FU2 +/− irinotecan and a targeted therapy, in the first-line treatment of metastatic colorectal cancer restricted to the liver (OSCAR): PRODIGE 49. Dig. Liver Dis. 2022, 54, 324–330. [Google Scholar] [CrossRef]
- de Baere, T.; Tselikas, L.; Boige, V.; Ducreux, M.; Malka, D.; Goéré, D.; Benahim, E.; Deschamps, F. Intra-arterial therapies for colorectal cancer liver metastases (radioembolization excluded). Bull. Cancer 2017, 104, 402–406. [Google Scholar] [CrossRef]
- Deschamps, F.; Rao, P.; Teriitehau, C.; Hakime, A.; Malka, D.; Boige, V.; Ducreux, M.; Elias, D.; Goere, D.; de Baere, T. Percutaneous femoral implantation of an arterial port catheter for intraarterial chemotherapy: Feasibility and predictive factors of long-term functionality. J. Vasc. Interv. Radiol. 2010, 21, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, O.; Mvouama, S.; Pellegrinelli, J.; Guillen, K.; Manfredi, S.; Ghiringhelli, F.; Falvo, N.; Midulla, M.; Loffroy, R. Percutaneous Implantation of a Microcatheter-Port System for Hepatic Arterial Infusion Chemotherapy of Unresectable Liver Tumors: Technical Feasibility, Functionality, and Complications. Diagnostics 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Nissan, A.; Picon, A.I.; Kemeny, N.; Dudrick, P.; Ben-Porat, L.; Espat, J.; Stojadinovic, A.; Cohen, A.M.; Fong, Y.; et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: An institutional experience of 544 consecutive cases. J. Am. Coll. Surg. 2005, 201, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Huberman, M.S. Comparison of systemic chemotherapy with hepatic arterial infusion in metastatic colorectal carcinoma. Semin. Oncol. 1983, 10, 238–248. [Google Scholar]
- Tanaka, T.; Arai, Y.; Inaba, Y.; Matsueda, K.; Aramaki, T.; Takeuchi, Y.; Kichikawa, K. Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J. Vasc. Interv. Radiol. 2003, 14, 63–68. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Kundu, S.; Sacks, D.; Wallace, M.J.; Wojak, J.C.; Rose, S.C.; Clark, T.W.I.; d’Othee, B.J.; Itkin, M.; Jones, R.S.; et al. Practice Guideline for Adult Antibiotic Prophylaxis during Vascular and Interventional Radiology Procedures. J. Vasc. Interv. Radiol. 2010, 21, 1611–1630. [Google Scholar] [CrossRef]
- Arai, Y.; Takeuchi, Y.; Inaba, Y.; Yamaura, H.; Sato, Y.; Aramaki, T.; Matsueda, K.; Seki, H. Percutaneous catheter placement for hepatic arterial infusion chemotherapy. Tech. Vasc. Interv. Radiol. 2007, 10, 30–37. [Google Scholar] [CrossRef]
- Michels, N.A. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am. J. Surg. 1966, 112, 337–347. [Google Scholar] [CrossRef]
- Kobe, A.; Deschamps, F.; Meyblum, L.; Varin, E.; Delpla, A.; Hakime, A.; Teriitehau, C.; Roux, C.; Boileve, A.; Gelli, M.; et al. Coil Embolization of Variant Hepatic Arteries During Percutaneous Arterial Port Catheter Placement for Intraarterial Chemotherapy: Analysis of Intrahepatic Perfusion Redistribution and Treatment Efficacy. Cardiovasc. Intervent. Radiol. 2023, 46, 69–79. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Barat, M.; Jannot, A.-S.; Dohan, A.; Soyer, P. How to report and compare quantitative variables in a radiology article. Diagn. Interv. Imaging 2022, 103, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, O.; Geschwind, J.-F. Traitement intra-artériel des métastases hépatiques de cancer colorectal. J. Radiol. 2011, 92, 835–841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barnett, K.T.; Malafa, M.P. Complications of hepatic artery infusion. Int. J. Gastrointest. Cancer 2001, 30, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Nakamura, T.; Yamazaki, T.; Iida, S.; Kato, T.; Nishimura, T. Catheter-tip fixation of a percutaneously implanted port-catheter system to prevent dislocation. Eur. Radiol. 2002, 12, 443–449. [Google Scholar] [CrossRef]
- Matsumoto, T.; Yamagami, T.; Yoshimatsu, R.; Morishita, H.; Kitamura, N.; Sato, O.; Hasebe, T. Hepatic Arterial Infusion Chemotherapy by the Fixed-Catheter-Tip Method: Retrospective Comparison of Percutaneous Left Subclavian and Femoral Port-Catheter System Implantation. Am. J. Roentgenol. 2014, 202, 211–215. [Google Scholar] [CrossRef]
- Farouil, G.; Deschamps, F.; Barah, A.; Auperin, A.; Goere, D.; Elias, D.; de Baere, T. Interventional revisions of malfunctions affecting surgically implanted port-catheters for hepatic artery infusion. Surg. Oncol. 2013, 22, 48–54. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Sadot, E.; Kemeny, N.E.; Gönen, M.; Leal, J.N.; Allen, P.J.; Cercek, A.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R.; et al. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J. Clin. Oncol. 2017, 35, 1938–1944. [Google Scholar] [CrossRef]
- Lévi, F.A.; Boige, V.; Hebbar, M.; Smith, D.; Lepère, C.; Focan, C.; Karaboué, A.; Guimbaud, R.; Carvalho, C.; Tumolo, S.; et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann. Oncol. 2016, 27, 267–274. [Google Scholar] [CrossRef]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, N.; Brennan, M.F.; Blumgart, L.H.; D’Angelica, M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef]

| Variable | N (%) or Median (Range) | |
|---|---|---|
| Gender | Male | 8 (57.1) |
| Female | 6 (42.9) | |
| Age | 60 (53–81) | |
| PS * | 0 | 8 (57.1) |
| 1 | 6 (42.9) | |
| Time lapse from diagnosis to hepatic intra-arterial catheter implantation (months) | 32.2 (0.7–161.7) | |
| Localization of primitive tumor | Rectum | 3 (21.4) |
| Rectosigmoid hinge | 1 (7.1) | |
| Sigmoid | 6 (42.9) | |
| Left colon | 1 (7.1) | |
| Right colon | 1 (7.1) | |
| Cecum | 2 (14.3) | |
| Histological type | Adenocarcinoma | 14 (100) |
| MSS/MSI Status | MSS | 14 (100) |
| MSI | 0 (0) | |
| RAS Status | Wild type | 6 (42.9) |
| Mutated | 8 (57.1) | |
| BRAF Status | Wild type | 14 (100) |
| Mutated | 0 (0) | |
| Number of metastatic sites (liver included) * | 1 | 6 (42.9) |
| 2 | 8 (57.1) | |
| Extrahepatic metastatic site * | Extraregional node | 2 (14.3) |
| Lung | 6 (42.9) | |
| Kidney | 1 (7.1) | |
| Hepatic burden * | 0–25% | 10 (71.4) |
| 25–50% | 3 (21.4) | |
| 50–75% | 1 (7.1) | |
| 75–100% | 0 (0) | |
| Previous surgical treatment of primitive tumor | 10 (71.4) | |
| Previous surgical or radiological treatment of liver metastases | Yes | 9 (64.3) |
| Surgical | 2 (14.3) | |
| Interventional Radiology | 3 (21.4) | |
| Both | 4 (28.6) | |
| Number of systemic therapy lines before HAIC | 0 | 1 (7.1) |
| 1 | 2 (14.3) | |
| 2 | 6 (42.9) | |
| 3 | 0 (0) | |
| 4 | 4 (28.6) | |
| >4 | 1 (7.1) | |
| Hepatic arterial anatomy | Modal | 8 (57.1) |
| Variant | 6 (42.9) | |
| Type of anatomical variants | LHA | 4 (28.6) |
| RHA | 2 (14.3) | |
| GDA rising from left branch of PHA | 2 (14.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedra, A.; Boeken, T.; Di Gaeta, A.; Querub, C.; Al Ahmar, M.; Déan, C.; Sapoval, M.; Pellerin, O. Exploring a Novel Technique to Tackle the Shortage of Devices for Hepatic Arterial Infusion Chemotherapy: Early Results of an Alternate Approach for Percutaneous Arterial Port Catheter Placement. Cancers 2023, 15, 4730. https://doi.org/10.3390/cancers15194730
Kedra A, Boeken T, Di Gaeta A, Querub C, Al Ahmar M, Déan C, Sapoval M, Pellerin O. Exploring a Novel Technique to Tackle the Shortage of Devices for Hepatic Arterial Infusion Chemotherapy: Early Results of an Alternate Approach for Percutaneous Arterial Port Catheter Placement. Cancers. 2023; 15(19):4730. https://doi.org/10.3390/cancers15194730
Chicago/Turabian StyleKedra, Alice, Tom Boeken, Alessandro Di Gaeta, Charles Querub, Marc Al Ahmar, Carole Déan, Marc Sapoval, and Olivier Pellerin. 2023. "Exploring a Novel Technique to Tackle the Shortage of Devices for Hepatic Arterial Infusion Chemotherapy: Early Results of an Alternate Approach for Percutaneous Arterial Port Catheter Placement" Cancers 15, no. 19: 4730. https://doi.org/10.3390/cancers15194730
APA StyleKedra, A., Boeken, T., Di Gaeta, A., Querub, C., Al Ahmar, M., Déan, C., Sapoval, M., & Pellerin, O. (2023). Exploring a Novel Technique to Tackle the Shortage of Devices for Hepatic Arterial Infusion Chemotherapy: Early Results of an Alternate Approach for Percutaneous Arterial Port Catheter Placement. Cancers, 15(19), 4730. https://doi.org/10.3390/cancers15194730






