Complications of Central Venous Access Devices Used in Palliative Care Settings for Terminally Ill Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Quality Assessment of Selected Studies
2.3. Statistical Analysis
3. Results
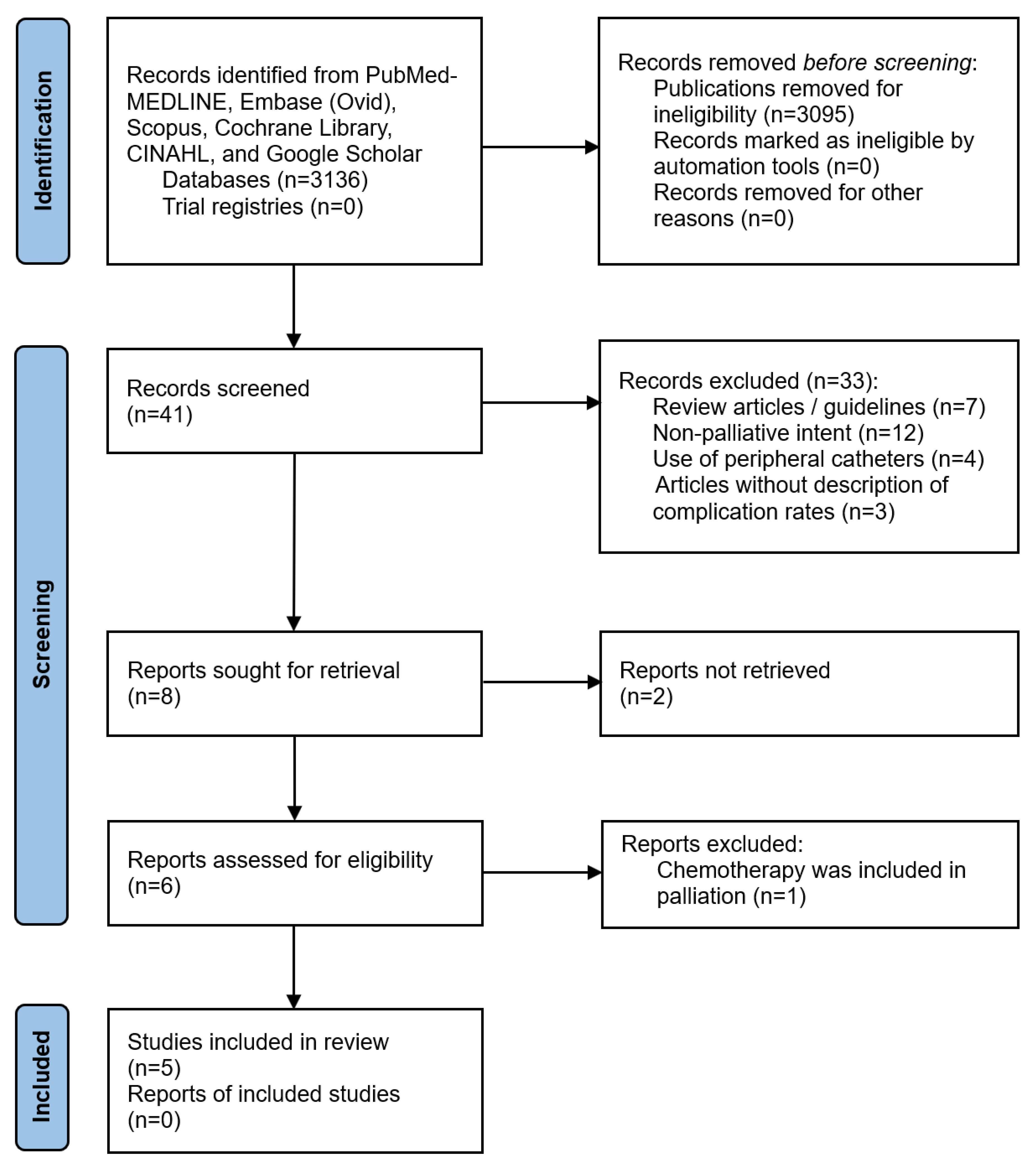
3.1. Search Strategy Results
3.2. Baseline Patient Characteristics
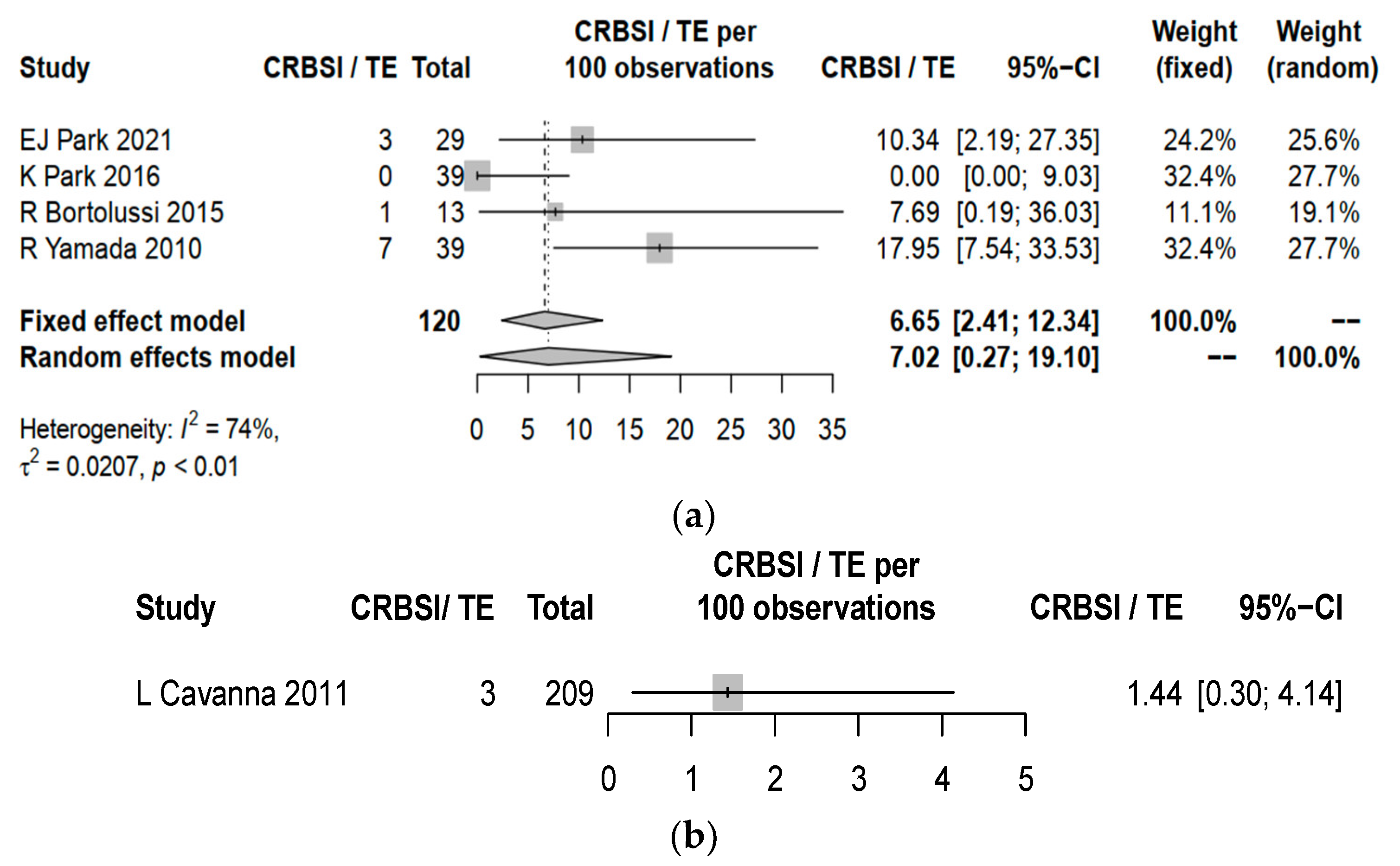
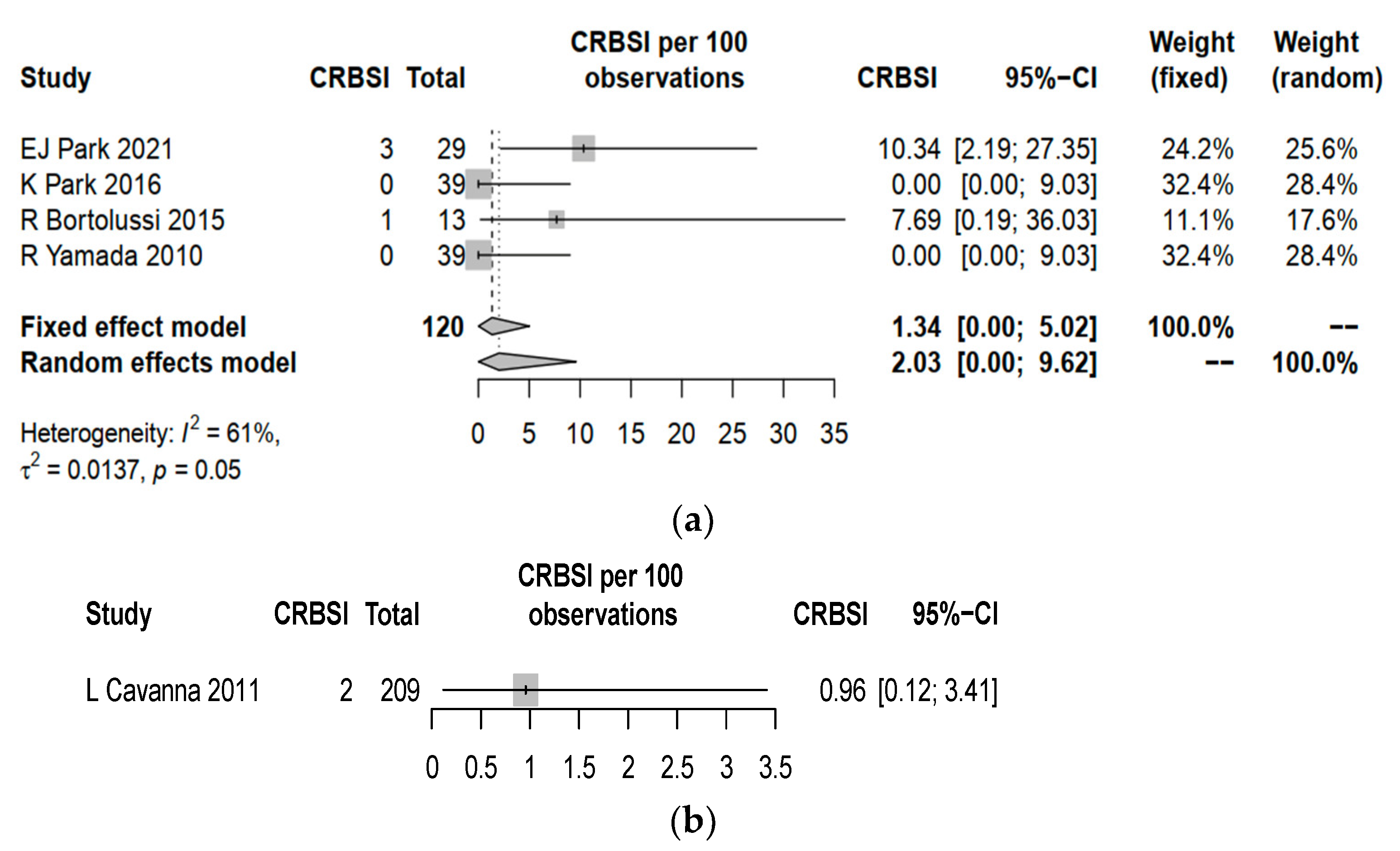
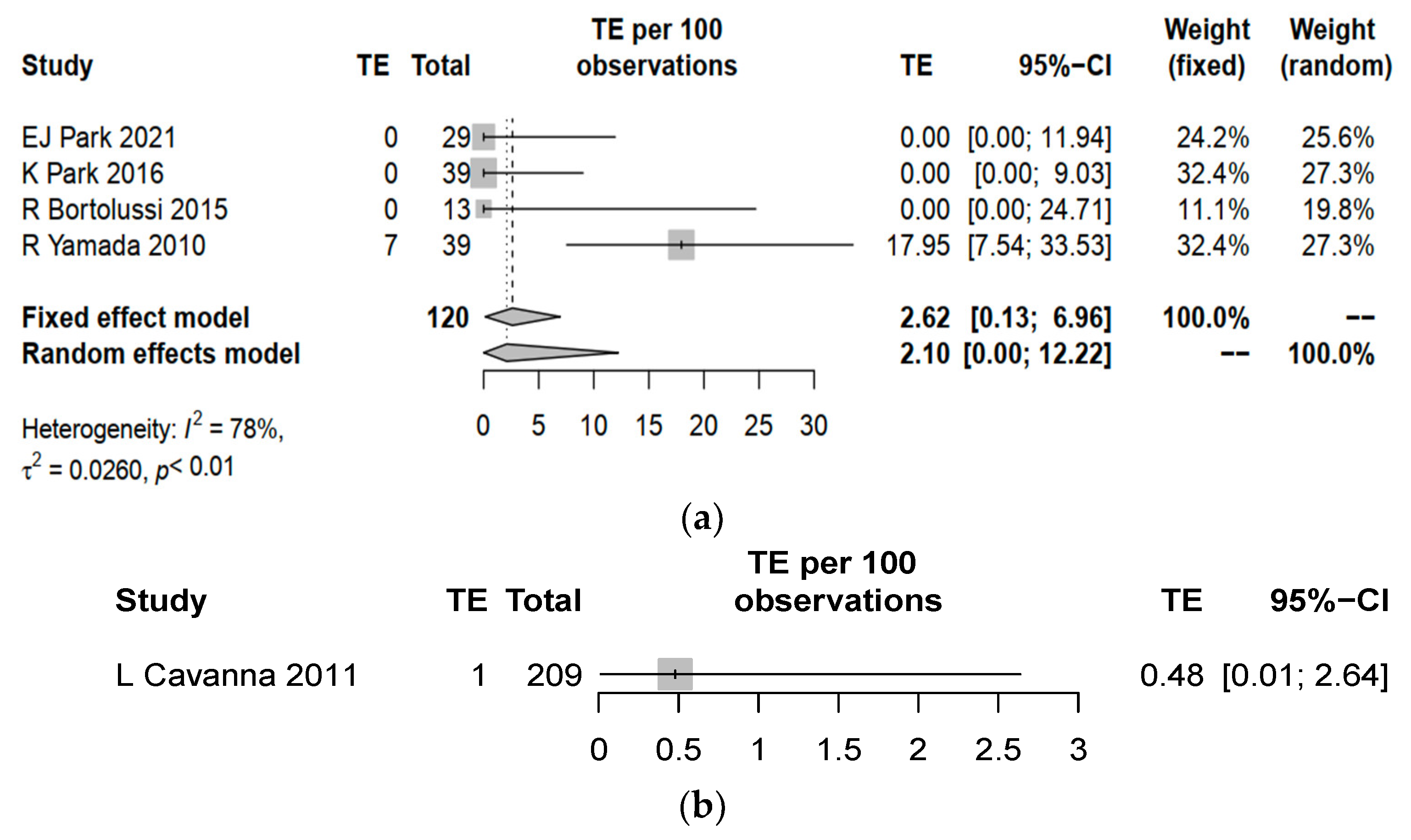
3.3. Complication Rates between Central Line and PICC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ener, R.; Meglathery, S.; Styler, M. Extravasation of systemic hemato-oncological therapies. Ann. Oncol. 2004, 15, 858–862. [Google Scholar] [CrossRef]
- Bishop, L.; Dougherty, L.; Bodenham, A.; Mansi, J.; Crowe, P.; Kibbler, C.; Shannon, M.; Treleaven, J. Guidelines on the insertion and management of central venous access devices in adults. Int. J. Lab. Hematol. 2007, 29, 261–278. [Google Scholar] [CrossRef]
- Johansson, E.; Hammarskjöld, F.; Lundberg, D.; Arnlind, M.H. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: A systematic review of the literature. Acta. Oncol. 2013, 52, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.; Wright, E.; Ream, E. The experiences of patients and nurses with a nurse-led peripherally inserted central venous catheter line service. Eur. J. Oncol. Nurs. 2000, 4, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Wu, O.; Kasthuri, R.; Moss, J.G. Centrally inserted external catheters and totally implantable ports for the delivery of chemotherapy: A systematic review and meta-analysis of device-related complications. Cardiovasc. Intervent. Radiol. 2014, 37, 990–1008. [Google Scholar] [CrossRef]
- Taxbro, K.; Hammarskjöld, F.; Thelin, B.; Lewin, F.; Hagman, H.; Hanberger, H.; Berg, S. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: An open-label, randomised, two-centre trial. Br. J. Anaesth. 2019, 122, 734–741. [Google Scholar] [CrossRef]
- Gallieni, M.; Pittiruti, M.; Biffi, R. Vascular access in oncology patients. CA. Cancer J. Clin. 2008, 58, 323–346. [Google Scholar] [CrossRef]
- Moss, J.G.; Wu, O.; Bodenham, A.R.; Agarwal, R.; Menne, T.F.; Jones, B.J.; Heggie, R.; Hill, S.; Dixon-Hughes, J.; Soulis, E.; et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): A randomised controlled trial. Lancet 2021, 398, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Monfardini, L.; Sozzi, G.; Armano, G.; Butera, D.; Scarpelli, E.; Barresi, G.; Benegiamo, A.; Berretta, R. Peripherally Inserted Central Venous Catheters (PICC) versus totally implantable venous access device (PORT) for chemotherapy administration: A meta-analysis on gyanecological cancer patients. Acta Biomed. 2021, 92, e2021257. [Google Scholar]
- He, E.; Ye, K.; Zheng, H. Clinical effect and safety of venous access ports and peripherally inserted central catheters in patients receiving tumor chemotherapy: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 9105–9113. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.; Metharom, P. Cancer-associated thrombosis: An overview of mechanisms, risk factors, and treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- van Rooden, C.J.; Tesselaar, M.E.; Osanto, S.; Rosendall, F.R.; Huisman, M.V. Deep vein thrombosis associated with central venous catheters-a review. J. Thromb. Haemost. 2005, 3, 2409–2419. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013, 119, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. 3rd Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef] [PubMed]
- Mielke, D.; Wittig, A.; Teichgräber, U. Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support. Care. Cancer 2020, 28, 4753–4760. [Google Scholar] [CrossRef]
- Schedin, A.; Goordrose-Flores, C.; Bonn, S.; Björkhem-Bergman, L. Catheter-related bloodstream infections in palliative care patients receiving parenteral nutrition by medical home care. BMJ Support. Palliat. Care 2020, 002331. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Lo, A.-C.; Tsai, C.-H.; Lee, P.-Y.; Sun, S.; Chang, T.-H.; Chen, C.-C.; Chang, Y.-S.; Chen, J.-R. Higher complication risk of totally implantable venous access port systems in patients with advanced cancer-a single institution retrospective analysis. Palliat. Med. 2013, 27, 185–191. [Google Scholar] [CrossRef]
- Park, E.J.; Park, K.; Kim, J.-J.; Oh, S.-B.; Jung, K.S.; Oh, S.Y.; Hong, Y.J.; Kim, J.H.; Jang, J.Y.; Jeon, U.B. Safety, efficacy, and patient satisfaction with initial peripherally inserted central catheters compared with usual intravenous access in terminally ill cancer patients: A randomized phase II study. Cancer Res. Treat. 2021, 53, 881–888. [Google Scholar] [CrossRef]
- Park, K.; Jun, H.J.; Oh, S.Y. Safety, efficacy, and patient-perceived satisfaction of peripherally inserted central catheters in terminally ill cancer patients: A prospective multicenter observational study. Support. Care Cancer 2016, 24, 4987–4992. [Google Scholar] [CrossRef]
- Bortolussi, R.; Zotti, P.; Conte, E.; Marson, R.; Polesel, J.; Colussi, A.; Piazza, D.; Tabaro, G.; Spazzapan, S. Quality of life, pain perception, and distress correlated to ultrasound-guided peripherally inserted central venous catheters in palliative care patients in a home or hospice setting. J. Pain Symptom Manag. 2015, 50, 118–123. [Google Scholar] [CrossRef]
- Yamada, R.; Morita, T.; Yashiro, E.; Otani, H.; Amano, K.; Inoue, S. Patient-reported usefulness of peripherally inserted central venous catheters in terminally ill cancer patients. J. Pain Symptom Manag. 2010, 40, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, L.; Cordani, M.R.; Biasini, C.; Di Nunzio, C.; Monfredo, M.; Stroppa, E.; Muroni, M.; Ambroggi, M.; Muroni, L.; Di Cicilia, R.; et al. Ultrasound-guided central venous catheterization for home parenteral nutrition and hydratation in advanced incurable cancer patients: Results of a prospective observational study. World J. Oncol. 2011, 2, 238–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taxbro, K.; Hammarskjöld, F.; Juhlin, D.; Hagman, H.; Bernfort, R.; Berg, S. Cost analysis comparison between peripherally interested central catheters and implanted chest ports in patients with cancer–A health economic evaluation of the PICCPORT trial. Acta Anaesthesiol. Scand. 2020, 64, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yeow, M.; Soh, S.; Yap, R.; Tay, D.; Low, Y.F.; Goh, S.S.N.; Yeo, C.S.; Lo, Z.J. A systematic review and network meta-analysis of randomized controlled trials on choice of central venous access device for delivery of chemotherapy. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 1184–1191.e8. [Google Scholar] [CrossRef]
- Kim, H.J.; Yun, J.; Kim, H.J.; Kim, K.H.; Kim, S.H.; Lee, S.-C.; Bae, S.B.; Kim, C.K.; Lee, N.S.; Lee, K.T.; et al. Safety and effectiveness of central venous catheterization in patients with cancer: Prospective observational study. J. Korean Med. Sci. 2010, 25, 1748–1753. [Google Scholar] [CrossRef]
- Oppelt, P.; Betbadal, A.; Nayak, L. Approach to chemotherapy-associated thrombosis. Vasc. Med. 2015, 20, 153–161. [Google Scholar] [CrossRef]
- Cool, R.M.; Herrington, J.D.; Wong, L. Recurrent peripheral arterial thrombosis induced by cisplatin and etoposide. Pharmacotherapy 2022, 22, 1200–1204. [Google Scholar] [CrossRef]
- Hamza, M.S.; Mousa, S.A. Cancer-associated thrombosis: Risk factors, molecular mechanisms, future management. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620954282. [Google Scholar] [CrossRef]
- Walser, E.M. Venous access ports: Indications, implantation technique, follow-up, and complications. Cardiovasc. Intervent. Radiol. 2012, 35, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Akelma, H.; Salık, F.; Biçak, M.; Erbatur, M.E. Local Anesthesia for Port Catheter Placement in Oncology Patients: An Alternative to Landmark Technique Using Ultrasound-Guided Superficial Cervical Plexus Block-A Prospective Randomized Study. J. Oncol. 2019, 2019, 2585478. [Google Scholar] [CrossRef] [PubMed]
- Hipskind, J.E.; Ahmed, A.A. Cervical Plexus Block; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Song, X.; Chen, S.; Dai, Y.; Sun, Y.; Lin, X.; He, J.; Xu, R. A novel incision technique of a totally implanted venous access port in the upper arm for patients with breast cancer. World J. Surg. Oncol. 2023, 21, 162. [Google Scholar] [CrossRef] [PubMed]
| Study | EJ Park, 2021 [20] | K Park, 2016 [21] | R Bortolussi, 2015 [22] | R Yamada, 2010 [23] | L Cavanna, 2011 [24] |
|---|---|---|---|---|---|
| Selection | |||||
| 1. Representativeness of the exposed cohort |  |  |  |  |  |
| 2. Selection of the non-exposed cohort |  |  | |||
| 3. Ascertainment of exposure |  |  |  |  |  |
| 4. Demonstration that outcome of interest was not present at start of study |  |  |  |  |  |
| Comparability | |||||
| 1. Comparability of cohorts on the basis of the design or analysis |  |  |  |  |  |
| Outcome | |||||
| 1. Assessment of outcome |  |  |  |  |  |
| 2. Was follow-up long enough for outcomes to occur |  |  |  |  |  |
| 3. Adequacy of follow up of cohorts |  |  |  |  |  |
| Score | 9 | 7 | 9 | 7 | 7 |
 can be given for comparability.
can be given for comparability.| Characteristics | Number of Patients (%) Total (n = 327) |
|---|---|
| Mean age (range) | 68.4 (22–89) |
| Sex | |
| Male | 180 (55.0) |
| Female | 134 (41.0) |
| Undetermined | 13 (4.0) |
| Type of primary cancer | |
| Gastrointestinal | 156 (47.7) |
| Hepatobiliary and pancreatic | 41 (12.5) |
| Genitourinary | 46 (14.1) |
| Other solid tumors | 64 (19.6) |
| Hematological | 7 (2.1) |
| Undetermined | 13 (4.0) |
| Indication of CVAD | |
| Total parenteral nutrition | 277 (84.7) |
| Intravenous fluid replacement | 247 (75.5) |
| Intravenous medication | 45 (13.8) |
| Blood product transfusion | 12 (3.67) |
| Undetermined | 13 (4.0) |
| Type of CVAD | |
| PICC | 120 (36.7) |
| Central line | 207 (63.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.C.-H.; Choi, H.C.-W.; Lee, V.H.-F. Complications of Central Venous Access Devices Used in Palliative Care Settings for Terminally Ill Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4712. https://doi.org/10.3390/cancers15194712
Wong CC-H, Choi HC-W, Lee VH-F. Complications of Central Venous Access Devices Used in Palliative Care Settings for Terminally Ill Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(19):4712. https://doi.org/10.3390/cancers15194712
Chicago/Turabian StyleWong, Clement Chun-Him, Horace Cheuk-Wai Choi, and Victor Ho-Fun Lee. 2023. "Complications of Central Venous Access Devices Used in Palliative Care Settings for Terminally Ill Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 15, no. 19: 4712. https://doi.org/10.3390/cancers15194712
APA StyleWong, C. C.-H., Choi, H. C.-W., & Lee, V. H.-F. (2023). Complications of Central Venous Access Devices Used in Palliative Care Settings for Terminally Ill Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 15(19), 4712. https://doi.org/10.3390/cancers15194712






