Can Exercise Enhance the Efficacy of Checkpoint Inhibition by Modulating Anti-Tumor Immunity?
Abstract
Simple Summary
Abstract
1. Introduction
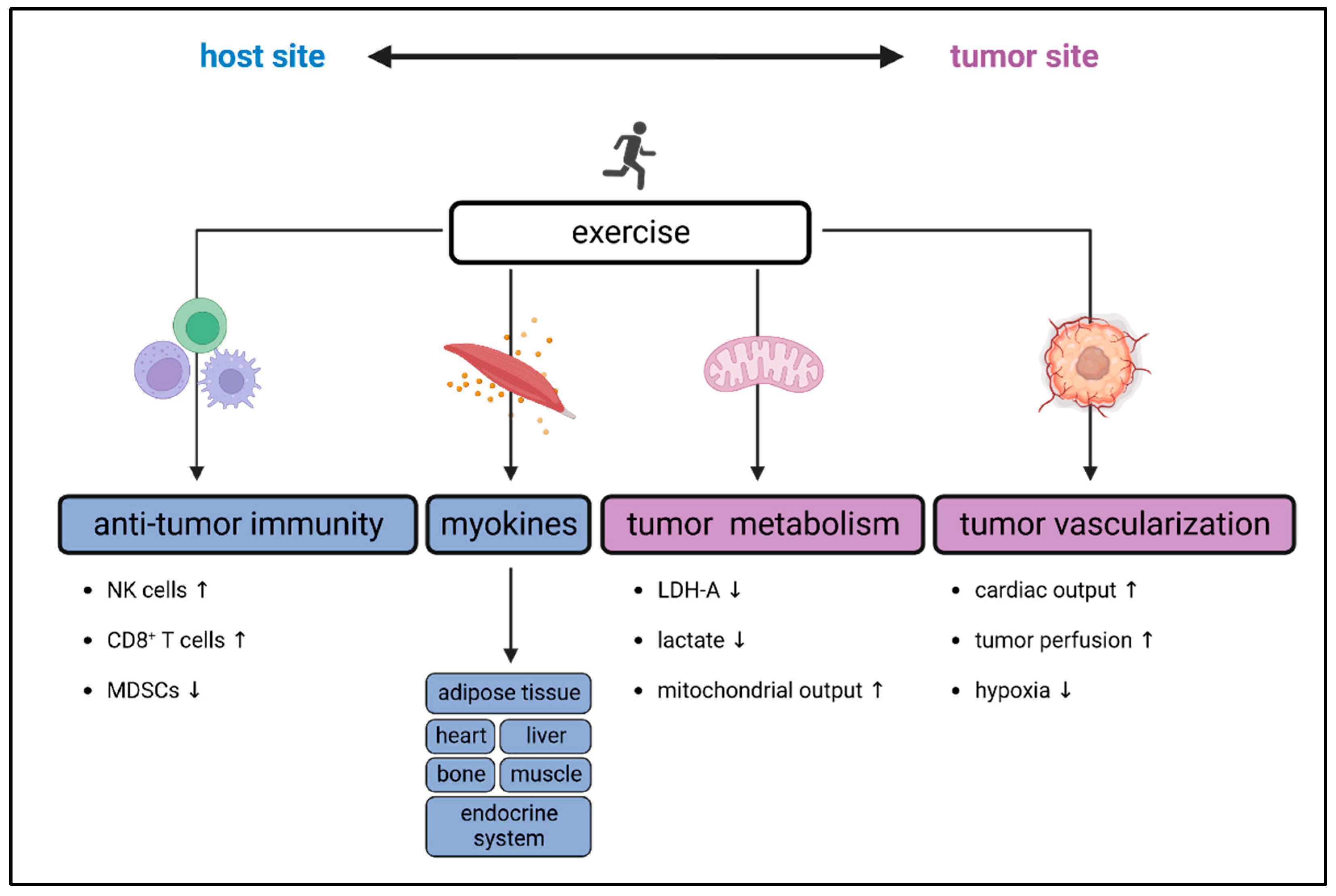
2. Exercise-Induced Effects on the Immune System
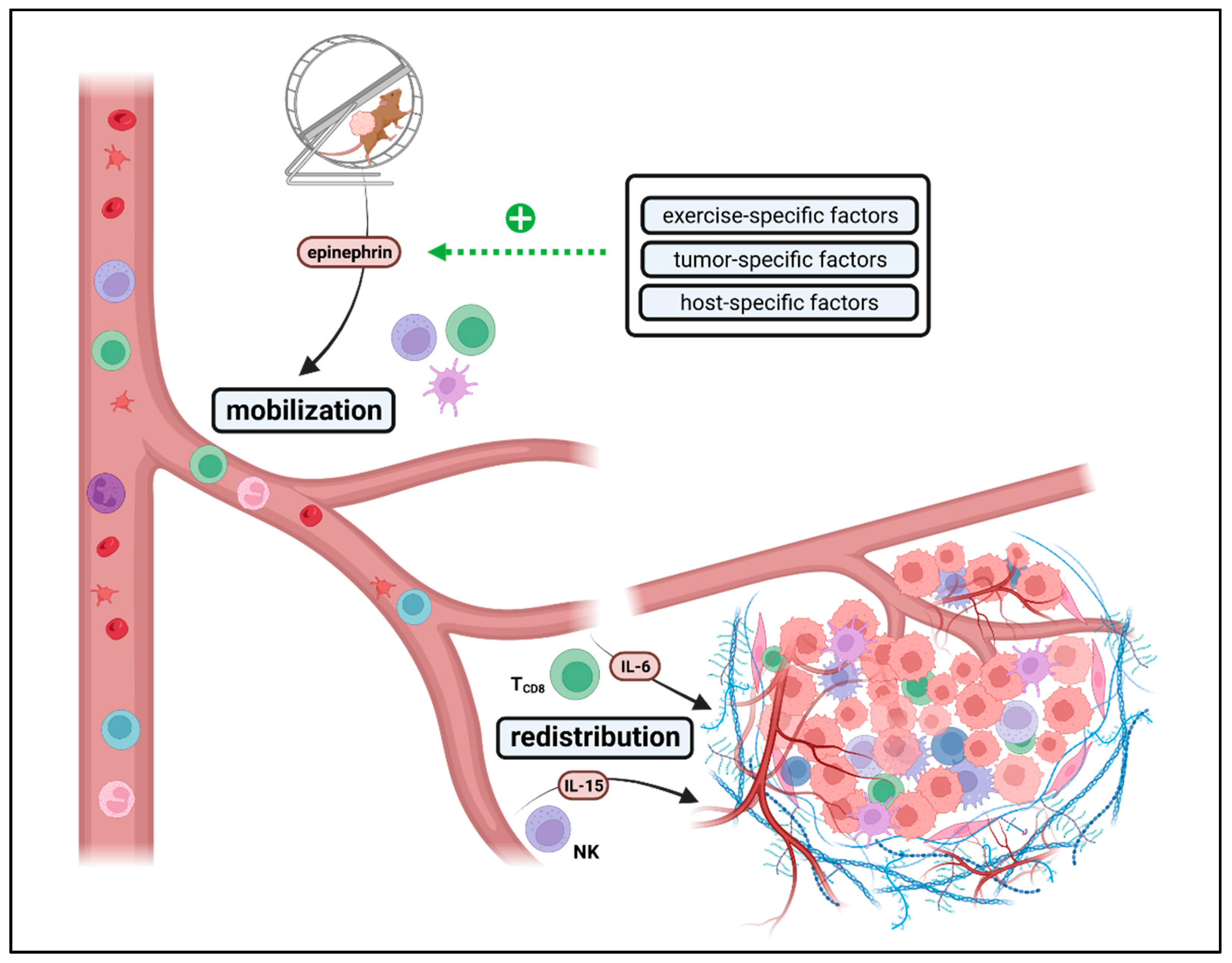
2.1. Exercise-Induced Immune Cell Mobilization and Redistribution
2.2. Exercise-Derived Immunomodulatory Myokines
2.3. Exercise-Induced Alterations in Immune Cell Metabolism
2.4. Exercise-Mediated Effects on Immunosenescence
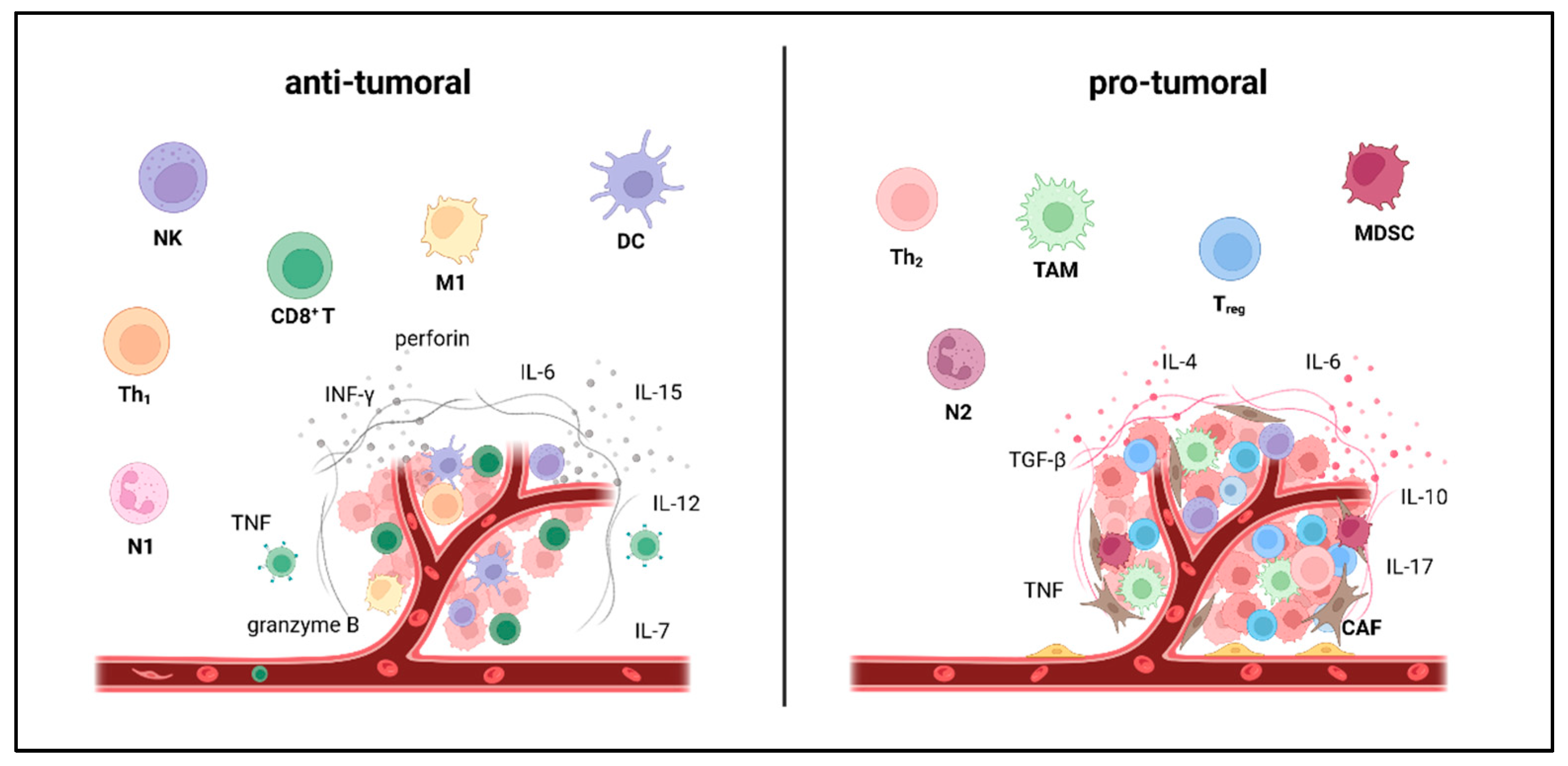
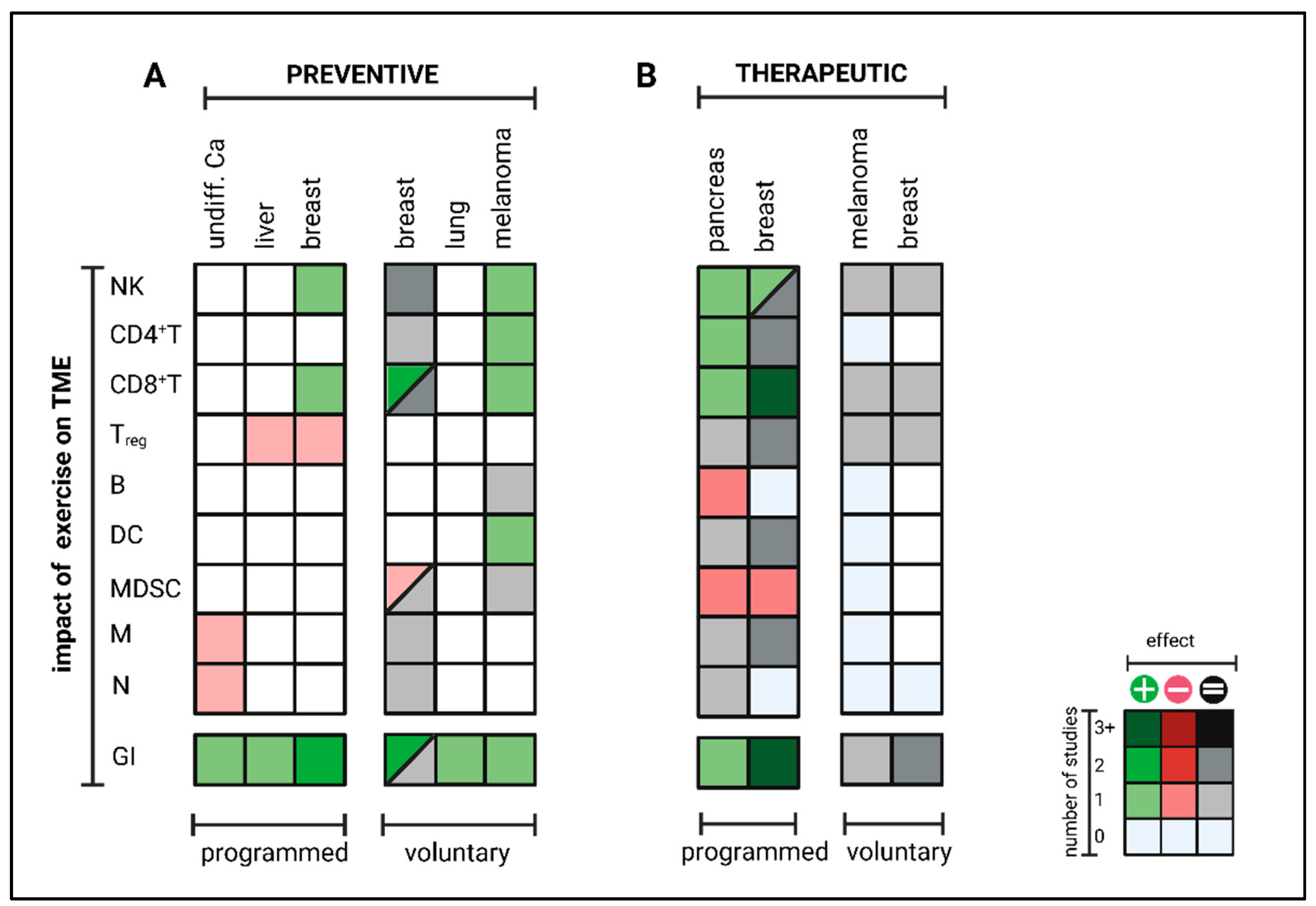
3. Exercise-Induced Impact on the TME and Tumor Immunity
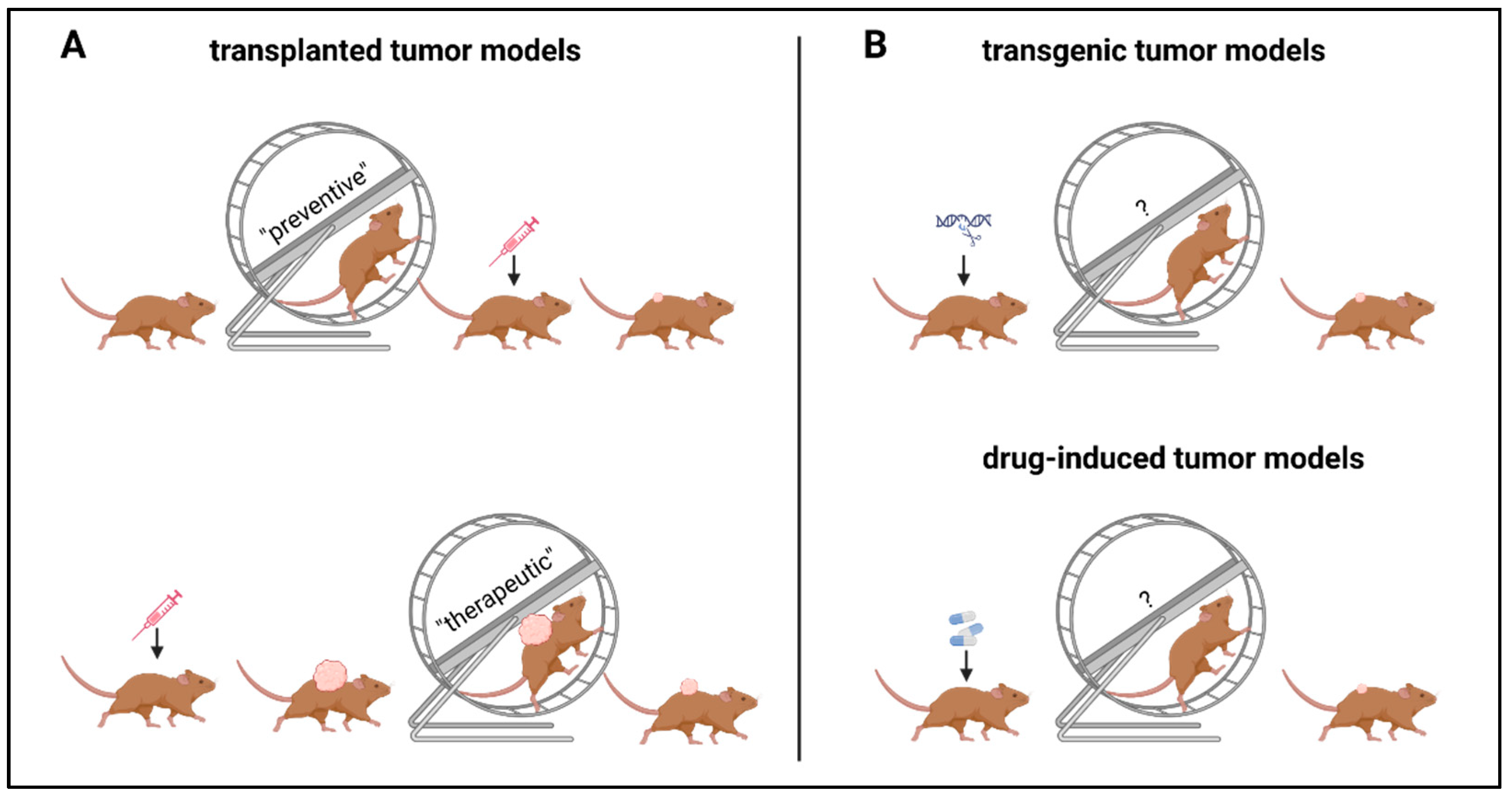
3.1. Preclinical Data
3.1.1. Preventive Setting
3.1.2. Therapeutic Setting
| Entity | Study | Cell line | Start | End | Exercise | Program | Distance or Duration | Intensity | Frequency | Duration (Pre Injection) | Duration (Post Injection) | Tumor Growth | Tumor Microenvironment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NK | TCD4 | TCD8 | Treg | B | DC | N | M | MDSC | |||||||||||||
| undiff. carcinoma | [144] | Ehrlich | d0 | d14 | swim | yes | 1 h/d | 50% max. cap. | 5 d/week | 4 weeks | 2 weeks | ↓ | ↓ | ↓ | |||||||
| liver | [145] | Hepa1-6 | d0 | d42 | swim | yes | 5–8 min/d | variable | 5 d/week | 3 weeks | 6 weeks | ↓ | ↓ | ||||||||
| breast | [146] | 4T1 | d0 | 1 cm³ | run | yes | 250 m/d | to 12 m/min | 5 d/week | 8 weeks | no | ↓ | ↑ | ↓ | |||||||
| breast | [147] | 4T1 | d0 | d22 | run | yes | 600–900 m/d | 10–15 m/min | 5 d/week | 20 days | no | ↓ | ↑ | ||||||||
| breast | [147] | 4T1 | d0 | d22 | run | yes | 360 m/d | 6 m/min | 5 d/week | 20 days | no | ↔ | |||||||||
| breast | [148] | 4T1.2 | d0 | d35 | run | no | 2.5–8.7 km/d | variable | daily | 8 weeks | 5 weeks | ↔ | ↔ | ↔ | |||||||
| breast | [149] | 4T1 | d0 | d28 | run | no | variable | variable | daily | 6 weeks | 4 weeks | ↔ | ↓ | ||||||||
| breast | [150] | E0771 | d0 | d14 | run | no | variable | variable | daily | 5 weeks | 2 weeks | ↔ | ↔ | ↔ | |||||||
| breast | [83] | I3TC | d0 | d32 | run | no | variable | variable | daily | 2 weeks | 8 weeks | ↓ | ↔ | ↔ | ↑ | ↔ | ↔ | ||||
| lung | [150,151] | LLC | d0 | d14 | run | no | variable | variable | daily | 4–5 weeks | 2 weeks | ↓ | ↑ | ↔ | ↑ | ↑ | |||||
| melanoma | [150,151] | B16 | d0 | d14 | run | no | 4.1 km/d | variable | daily | 4–5 weeks | 2 weeks | ↓ | ↑↑↑ | ↑ | ↑ | ↔ | ↑ | ↔ | |||
| melanoma | [151] | B16 | d0 | d14 | run | no | 4.1 km/d | variable | daily | 4 weeks | no | ↓ | |||||||||
| Entity | Study | Cell line | Start | End | Exercise | Program | Distance or Duration | Intensity | Frequency | Duration (Pre Injection) | Duration (Post Injection) | Tumor Growth | Tumor Microenvironment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NK | TCD4 | TCD8 | Treg | B | DC | N | M | MDSC | |||||||||||||
| pancreas | [152] | KPC 4662 | d0 | d21 | run | yes | 270 m/d | 9 m/min | 5 d/week | no | 3 weeks | ↓ | ↑ | ↑ | ↑↑↑ | ↔ | ↓ | ↔/↓ | ↓ | ↔/↓ | ↓ |
| breast | [154] | 4T1 | d0 | d30 | run | yes | 540 m/d | 18 m/min | 5 d/week | no | 3 weeks | ↓ | ↑ | ↑ | ↓ | ||||||
| breast | [153] | E0771 | d0 | d14 | run | yes | 30–45 min/d | 60% Vmax | daily | no | ~d10 | ↓ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | |||
| breast | [153] | EMT6 | d0 | d14 | run | yes | 30–45 min/d | 60% Vmax | daily | no | ~d10 | ↓ | ↑ | ||||||||
| breast | [153] | MCa-M3C | d0 | d14 | run | yes | 30–45 min/d | 60% Vmax | daily | no | ~d20 | ↓ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | |||
| breast | [155] | E0771 | d0 | d21 | run | no | 8 km/d | variable | daily | no | 3 weeks | ↔ | ↔ | ↔ | ↔ | ||||||
| melanoma | [151] | B16 | d0 | d14 | run | no | 4.1 km/d | variable | daily | no | 2 weeks | ↔ | |||||||||
| melanoma | [155] | B16 | d0 | d17 | run | no | 9 km/d | variable | daily | no | 17 days | ↔ | ↔ | ↔ | ↔ | ||||||
3.2. Transfer from the Preclinic into the Clinic
- (i)
- Exercise-specific factors: EIL is mainly mediated by epinephrin [151,152]. Results of Wang et al. indicate that a minimum of exercise intensity and volume is needed for beneficial effects on intra-tumoral immune cell composition since effects were lost at small daily running distances at low velocities [147]. Taking the physiological correlation between exercise intensity, heart rate, cardiac output, and catecholamine release, this seems to be intuitive. For transfer from mouse to man, further studies must answer what exercise frequency, intensity, time, and type (FITT) is needed for measurable immunomodulatory effects. In this context, it should be considered that preclinical mouse experiments only cover aerobic exercise interventions (swimming, running) but no resistance training, leisure activities or other sports activities. However, during, e.g., resistance training, heart rate and engagement of β-adrenergic signaling are usually lower than during aerobic exercise. Especially resistance training is often beneficial for cancer patients by preventing and reversing tumor cachexia. Thus, more studies are needed to elucidate whether other training modalities, such as resistance training, could have a similar impact on anti-tumor immunity as aerobic exercise training. In terms of dose dependency, the role of exercise intensity and volume must be further analyzed. While all studies conducting programmed training reported beneficial effects on anti-tumor immunity, voluntary exercise outcomes differed widely. Since several studies could not report any impact on anti-tumor immunity by voluntary running, high exercise volume might blunt exercise-induced immunomodulatory effects. Notably, mice with unlimited access to running wheels run up to 9 km daily, often for hours, daily, for weeks and upon total exhaustion. This setting can hardly be applied to humans, especially not for cancer patients. Thus, in contrast to programmed exercise, the transfer of voluntary exercise interventions from rodents to patients is limited due to high volume. Nevertheless, it is striking that during voluntary running, some groups reported beneficial effects on anti-tumor immunity in a preventive setting but not at all in a therapeutic setting. This difference between the preventive and therapeutic settings cannot be observed for programmed exercise but has been consistently confirmed for voluntary running, even within the same tumor model and identical exercise setting [151]. An explanation for these results could be that in a therapeutic setting, exercise-induced effects are more dose-sensitive than in a preventive setting due to the different biology of tumorigenesis vs. tumor outgrowth. While tumor initiation (preventive setting) usually does not impact the host’s immunocompetency, manifest tumor growth (therapeutic setting) often leads to systemic immune alterations, possibly modulating the threshold for EIL and EIR [157].
- (ii)
- Host-specific factors: Besides exercise-specific factors, endogenous host immune status seems to be a key factor for provoking exercise-induced leukocytosis and modulating exercise-induced immune cell redistribution. Several studies have shown that exercise plus additional immunogenic stimuli such as metabolic restriction, targeted therapy or radiation could enhance anti-tumor immunity synergistically, even if exercise alone had no beneficial impact [148,158,159]. This indicates that the threshold for exercise-induced immunomodulatory effects might be lowered by co-medication, radiation or diet, all known to support antitumor immunity by different mechanisms such as elevated antigen presentation or altered metabolism. Furthermore, strain-intrinsic differences in immunity should be considered when comparing and transferring results of preclinical exercise intervention studies. The most commonly used strains for exercise intervention studies are either C57BL/6 or BALB/c mice that significantly differ in structural and functional parameters of their immune system [160].
- (iii)
- Tumor-specific factors: Exercise-induced leukocytosis is followed by myokine-mediated redistribution of immune cells towards the TME. In some cancer entities, effects are mainly dependent on CD8+ T cells. In others, it is mainly mediated by NK cells, which might relate to mutational burden and MHC class-I expression. In contrast to CD8+ T cells, NK cells preferentially recognize cells with low MHC class-I levels [161,162]. While Pedersen et al. described IL-6-induced CD8+ T cell enhancement as a key regulator of TME reprograming in melanoma [151], Kurz et al. claim IL-15-induced NK cell redistribution as a main factor of exercise-induced enhancement of anti-tumor immunity in pancreatic ductal adenocarcinoma [152]. Whether NK or T cells arethe main mediators, correlates with antigen presentation, a tumor intrinsic feature, rather than with the exercise program. This indicates that cancer biology (high- vs. low immunogenic) might also influence EIL and EIR.
3.3. Clinical Data
4. Exercise-Induced Impact on Checkpoint Inhibition
4.1. Preclinical Data
4.2. Clinical Data
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T Cell Anergy, Exhaustion, Senescence, and Stemness in the Tumor Microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An Overview of the Immune System. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of Response, Resistance, and Toxicity to Immune Checkpoint Blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-Infiltrating Regulatory T Cells as Targets of Cancer Immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte Heterogeneity and Functions in Cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T Cell Help in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T Cell States in Human Cancer: Insights from Single-Cell Analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8+ T Cell Differentiation and Dysfunction in Cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Barnestein, R.; Galland, L.; Kalfeist, L.; Ghiringhelli, F.; Ladoire, S.; Limagne, E. Immunosuppressive Tumor Microenvironment Modulation by Chemotherapies and Targeted Therapies to Enhance Immunotherapy Effectiveness. Oncoimmunology 2022, 11, 2120676. [Google Scholar] [CrossRef]
- Boustani, J.; Lecoester, B.; Baude, J.; Latour, C.; Adotevi, O.; Mirjolet, C.; Truc, G. Anti-PD-1/Anti-PD-L1 Drugs and Radiation Therapy: Combinations and Optimization Strategies. Cancers 2021, 13, 4893. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Martinez, A.; Stahl, J.M.; Logan, S.J.; Perricone, A.J.; Ferris, M.J.; Buchwald, Z.S.; Chowdhary, M.; Delman, K.A.; Monson, D.K.; et al. Increase in PD-L1 Expression after Pre-Operative Radiotherapy for Soft Tissue Sarcoma. Oncoimmunology 2018, 7, e1442168. [Google Scholar] [CrossRef] [PubMed]
- Offringa, R.; Kötzner, L.; Huck, B.; Urbahns, K. The Expanding Role for Small Molecules in Immuno-Oncology. Nat. Rev. Drug Discov. 2022, 21, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Warner, A.B.; Jones, L.W.; Dewhirst, M.W. Exercise as Adjunct Therapy in Cancer. Semin. Radiat. Oncol. 2019, 29, 16–24. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, H.; He, A.; Li, Y.; He, C.; Xia, Y. Exercise in Cancer Prevention and Anticancer Therapy: Efficacy, Molecular Mechanisms and Clinical Information. Cancer Lett. 2022, 544, 215814. [Google Scholar] [CrossRef]
- Holmen Olofsson, G.; Jensen, A.W.P.; Idorn, M.; Thor Straten, P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. Int. J. Mol. Sci. 2020, 21, E3816. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Wheatley-Guy, C.M.; Rosenthal, A.C.; Gastineau, D.A.; Katsanis, E.; Johnson, B.D.; Simpson, R.J. Exercise and the Immune System: Taking Steps to Improve Responses to Cancer Immunotherapy. J. Immunother. Cancer 2021, 9, e001872. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Li, G.; Xiao, J. Exercise Regulates the Immune System. Adv. Exp. Med. Biol. 2020, 1228, 395–408. [Google Scholar] [CrossRef]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Adhesion Molecules, Catecholamines and Leucocyte Redistribution during and Following Exercise. Sports Med. 2003, 33, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Benschop, R.J.; Nijkamp, F.P.; Ballieux, R.E.; Heijnen, C.J. The Effects of Beta-Adrenoceptor Stimulation on Adhesion of Human Natural Killer Cells to Cultured Endothelium. Br. J. Pharmacol. 1994, 113, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Lechtermann, A.; Fobker, M.; Völker, K.; Mooren, F.C. Exercise-Induced Redistribution of T Lymphocytes is Regulated by Adrenergic Mechanisms. Brain. Behav. Immun. 2008, 22, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Born, J. Selective Mobilization of Cytotoxic Leukocytes by Epinephrine. J. Immunol. 2010, 184, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.M.; Brenner, I.K.; Moldoveanu, A.I.; Vasiliou, P.; Shek, P.; Shephard, R.J. Effects of Three Different Types of Exercise on Blood Leukocyte Count during and Following Exercise. Sao Paulo Med. J. Rev. Paul. Med. 2003, 121, 9–14. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Johnson, R.; Lebeck, L.; Davis, J.M.; Nehlsen-Cannarella, S.L. Effects of Brief, Heavy Exertion on Circulating Lymphocyte Subpopulations and Proliferative Response. Med. Sci. Sports Exerc. 1992, 24, 1339–1345. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position Statement. Part one: Immune Function and Exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Turner, J.E.; Spielmann, G.; Wadley, A.J.; Aldred, S.; Simpson, R.J.; Campbell, J.P. Exercise-Induced B Cell Mobilisation: Preliminary Evidence for an Influx of Immature Cells into the Bloodstream. Physiol. Behav. 2016, 164, 376–382. [Google Scholar] [CrossRef]
- Krüger, K.; Alack, K.; Ringseis, R.; Mink, L.; Pfeifer, E.; Schinle, M.; Gindler, K.; Kimmelmann, L.; Walscheid, R.; Muders, K.; et al. Apoptosis of T-Cell Subsets after Acute High-Intensity Interval Exercise. Med. Sci. Sports Exerc. 2016, 48, 2021–2029. [Google Scholar] [CrossRef]
- Clifford, T.; Wood, M.J.; Stocks, P.; Howatson, G.; Stevenson, E.J.; Hilkens, C.M.U. T-Regulatory Cells Exhibit a Biphasic Response to Prolonged Endurance Exercise in Humans. Eur. J. Appl. Physiol. 2017, 117, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Riddell, N.E.; Burns, V.E.; Turner, M.; van Zanten, J.J.C.S.V.; Drayson, M.T.; Bosch, J.A. Acute Exercise Mobilises CD8+ T Lymphocytes Exhibiting an Effector-Memory Phenotype. Brain. Behav. Immun. 2009, 23, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Szlezak, A.M.; Szlezak, S.L.; Keane, J.; Tajouri, L.; Minahan, C. Establishing a Dose-Response Relationship between Acute Resistance-Exercise and the Immune System: Protocol for a Systematic Review. Immunol. Lett. 2016, 180, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Halson, S.L.; Khan, Q.; Drysdale, P.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. Effects of Acute exhaustive Exercise and Chronic Exercise Training on type 1 and type 2 T lymphocytes. Exerc. Immunol. Rev. 2004, 10, 91–106. [Google Scholar]
- Bigley, A.B.; Rezvani, K.; Pistillo, M.; Reed, J.; Agha, N.; Kunz, H.; O’Connor, D.P.; Sekine, T.; Bollard, C.M.; Simpson, R.J. Acute exercise Preferentially Redeploys NK-Cells with a Highly-Differentiated Phenotype and Augments Cytotoxicity against Lymphoma and Multiple Myeloma Target Cells. Part II: Impact of Latent Cytomegalovirus Infection and Catecholamine Sensitivity. Brain. Behav. Immun. 2015, 49, 59–65. [Google Scholar] [CrossRef]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute Exercise Preferentially Redeploys NK-Cells with a Highly-Differentiated Phenotype and Augments Cytotoxicity against Lymphoma and Multiple Myeloma Target Cells. Brain. Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef]
- Alack, K.; Weiss, A.; Krüger, K.; Höret, M.; Schermuly, R.; Frech, T.; Eggert, M.; Mooren, F.-C. Profiling of Human Lymphocytes Reveals a Specific Network of Protein Kinases Modulated by Endurance Training Status. Sci. Rep. 2020, 10, 888. [Google Scholar] [CrossRef]
- Liu, R.; Fan, W.; Krüger, K.; Xiao, Y.U.; Pilat, C.; Seimetz, M.; Ringseis, R.; Baumgart-Vogt, E.; Eder, K.; Weissmann, N.; et al. Exercise Affects T-Cell Function by Modifying Intracellular Calcium Homeostasis. Med. Sci. Sports Exerc. 2017, 49, 29–39. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Szlezak, A.M.; Tajouri, L.; Keane, J.; Szlezak, S.L.; Minahan, C. Isometric Thumb Exertion Induces B Cell and T Cell Lymphocytosis in Trained and Untrained Males: Physical Aptitude Determines Response Profiles. Int. J. Kinesiol. Sports Sci. 2016, 4, 55–66. [Google Scholar] [CrossRef][Green Version]
- Mukaimoto, T.; Ohno, M. Effects of Circuit Low-Intensity Resistance Exercise with Slow Movement on Oxygen Consumption during and after Exercise. J. Sports Sci. 2012, 30, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the Immune System after Exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Callegari, I.O.M.; Rocha, G.Z.; Oliveira, A.G. Physical Exercise, Health, and Disease Treatment: The Role of Macrophages. Front. Physiol. 2023, 14, 1061353. [Google Scholar] [CrossRef]
- Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise Training Inhibits Inflammation in Adipose Tissue via both Suppression of Macrophage Infiltration and Acceleration of Phenotypic Switching from M1 to M2 Macrophages in High-Fat-Diet-Induced Obese Mice. Exerc. Immunol. Rev. 2010, 16, 105–118. [Google Scholar]
- Murugathasan, M.; Jafari, A.; Amandeep, A.; Hassan, S.A.; Chihata, M.; Abdul-Sater, A.A. Moderate Exercise Induces Trained Immunity in Macrophages. Am. J. Physiol.-Cell Physiol. 2023, 325, C429–C442. [Google Scholar] [CrossRef]
- Kawanishi, M.; Kami, K.; Nishimura, Y.; Minami, K.; Senba, E.; Umemoto, Y.; Kinoshita, T.; Tajima, F. Exercise-Induced Increase in M2 Macrophages Accelerates Wound Healing in Young Mice. Physiol. Rep. 2022, 10, e15447. [Google Scholar] [CrossRef]
- Sugiura, H.; Nishida, H.; Sugiura, H.; Mirbod, S.M. Immunomodulatory Action of Chronic Exercise on Macrophage and Lymphocyte Cytokine Production in Mice: Chronic Exercise and Immune Function. Acta Physiol. Scand. 2002, 174, 247–256. [Google Scholar] [CrossRef]
- Kizaki, T.; Takemasa, T.; Sakurai, T.; Izawa, T.; Hanawa, T.; Kamiya, S.; Haga, S.; Imaizumi, K.; Ohno, H. Adaptation of Macrophages to Exercise Training Improves Innate Immunity. Biochem. Biophys. Res. Commun. 2008, 372, 152–156. [Google Scholar] [CrossRef]
- Abdalla, D.R.; Aleixo, A.A.R.; Murta, E.F.C.; Michelin, M.A. Innate Immune Response Adaptation in Mice Subjected to Administration of DMBA and Physical Activity. Oncol. Lett. 2014, 7, 886–890. [Google Scholar] [CrossRef]
- Ginhoux, F.; Schultze, J.L.; Murray, P.J.; Ochando, J.; Biswas, S.K. New Insights into the Multidimensional Concept of Macrophage Ontogeny, Activation and Function. Nat. Immunol. 2016, 17, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscle as a Secretory Organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.D.d.J.; Silva, D.D.S.; Pereira, E.V.M.; Pereira, D.D.; de Sousa Fernandes, M.S.; Santos, D.F.C.; Oliveira, D.P.M.; Vieira-Souza, L.M.; Aidar, F.J.; de Souza, R.F. Changes in Cytokines Concentration Following Long-Distance Running: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 838069. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF Activity and T Cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Leggatt, G.; Waterhouse, N.; Frazer, I.H. Interferon-γ Derived from Cytotoxic Lymphocytes Directly Enhances Their Motility and Cytotoxicity. Cell Death Dis. 2017, 8, e2836. [Google Scholar] [CrossRef]
- Brincks, E.L.; Woodland, D.L. Novel Roles for IL-15 in T Cell Survival. F1000 Biol. Rep. 2010, 2, 67. [Google Scholar] [CrossRef]
- Lum, J.J.; Schnepple, D.J.; Nie, Z.; Sanchez-Dardon, J.; Mbisa, G.L.; Mihowich, J.; Hawley, N.; Narayan, S.; Kim, J.E.; Lynch, D.H.; et al. Differential Effects of Interleukin-7 and Interleukin-15 on NK Cell Anti-Human Immunodeficiency Virus Activity. J. Virol. 2004, 78, 6033–6042. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Lesjean-Pottier, S.; Vieira, P.; Sawa, S.; Eberl, G.; Vosshenrich, C.A.J.; Di Santo, J.P. IL-7 and IL-15 Independently Program the Differentiation of Intestinal CD3−NKp46+ Cell Subsets from Id2-Dependent Precursors. J. Exp. Med. 2010, 207, 273–280. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that Interleukin-6 is Produced in Human Skeletal Muscle during Prolonged Running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Starkie, R.L.; Rolland, J.; Angus, D.J.; Anderson, M.J.; Febbraio, M.A. Circulating Monocytes are not the Source of Elevations in Plasma IL-6 and TNF-Alpha Levels after Prolonged Running. Am. J. Physiol. Cell Physiol. 2001, 280, C769–C774. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 Myokine Signaling in Skeletal Muscle: A Double-edged Sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Daou, H.N. Exercise as an Anti-Inflammatory Therapy for Cancer Cachexia: A Focus on Interleukin-6 Regulation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 318, R296–R310. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. CANCER IMMUNOLOGY. The “Cancer Immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-Y.; Choi, Y.S.; Yeom, C.H.; Kwak, S.M.; Yoon, H.M.; Kim, D.G.; Koh, S.-J.; Park, J.; Lee, M.A.; Lee, Y.J.; et al. Interleukin-6 but Not Tumour Necrosis Factor-Alpha Predicts Survival in Patients with Advanced Cancer. Support. Care Cancer 2013, 21, 3071–3077. [Google Scholar] [CrossRef]
- Hoene, M.; Runge, H.; Häring, H.U.; Schleicher, E.D.; Weigert, C. Interleukin-6 Promotes Myogenic Differentiation of Mouse Skeletal Muscle Cells: Role of the STAT3 Pathway. Am. J. Physiol. Cell Physiol. 2013, 304, C128–C136. [Google Scholar] [CrossRef]
- Rosa-Neto, J.C.; Lira, F.S.; Little, J.P.; Landells, G.; Islam, H.; Chazaud, B.; Pyne, D.B.; Teixeira, A.M.; Batatinha, H.; Moura Antunes, B.; et al. Immunometabolism-fit: How Exercise and Training can Modify T Cell and Macrophage Metabolism in Health and Disease. Exerc. Immunol. Rev. 2022, 28, 29–46. [Google Scholar]
- Nieman, D.C.; Pence, B.D. Exercise Immunology: Future Directions. J. Sport Health Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef]
- Wasinski, F.; Gregnani, M.F.; Ornellas, F.H.; Bacurau, A.V.N.; Câmara, N.O.; Araujo, R.C.; Bacurau, R.F. Lymphocyte Glucose and Glutamine Metabolism as Targets of the Anti-Inflammatory and Immunomodulatory Effects of Exercise. Mediators Inflamm. 2014, 2014, 326803. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Horning, M.A.; Lehman, S.L.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Pyruvate Shuttling during Rest and Exercise before and after Endurance Training in Men. J. Appl. Physiol. 2004, 97, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Veliça, P.; Barbieri, L.; Gameiro, P.A.; Bargiela, D.; Gojkovic, M.; Mijwel, S.; Reitzner, S.M.; Wulliman, D.; Ahlstedt, E.; et al. Cytotoxic T-Cells Mediate Exercise-Induced Reductions in Tumor Growth. eLife 2020, 9, e59996. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Bacurau, A.V.N.; Pereira, G.B.; Araújo, R.C.; Almeida, S.S.; Moraes, M.R.; Uchida, M.C.; Costa Rosa, L.F.B.P.; Navalta, J.; Prestes, J.; et al. Moderate Exercise Increases the Metabolism and Immune Function of Lymphocytes in Rats. Eur. J. Appl. Physiol. 2013, 113, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, H.; Zhang, D.; Ma, Y.; Zhu, B. Metabolic Plasticity and Regulation of T Cell Exhaustion. Immunology 2022, 167, 482–494. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T Cell Metabolism in Infection and Cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Gasparini, S.O.; Martins, G.L.; Costa, L.K.F.; Araujo, C.C.; Lugaresi, R.; Kopfler, M.; Lancha, A.H. Crosstalk Between Skeletal Muscle and Immune System: Which Roles Do IL-6 and Glutamine Play? Front. Physiol. 2020, 11, 582258. [Google Scholar] [CrossRef]
- Freyssenet, D.; Berthon, P.; Denis, C. Mitochondrial Biogenesis in Skeletal Muscle in Response to Endurance Exercises. Arch. Physiol. Biochem. 1996, 104, 129–141. [Google Scholar] [CrossRef]
- Buss, L.A.; Hock, B.; Merry, T.L.; Ang, A.D.; Robinson, B.A.; Currie, M.J.; Dachs, G.U. Effect of Immune Modulation on the Skeletal Muscle Mitochondrial Exercise Response: An Exploratory Study in Mice with Cancer. PLoS ONE 2021, 16, e0258831. [Google Scholar] [CrossRef]
- Alley, J.R.; Valentine, R.J.; Kohut, M.L. Mitochondrial Mass of Naïve T Cells Is Associated with Aerobic Fitness and Energy Expenditure of Active and Inactive Adults. Med. Sci. Sports Exerc. 2022, 54, 1288–1299. [Google Scholar] [CrossRef]
- Boulé, N.G.; Weisnagel, S.J.; Lakka, T.A.; Tremblay, A.; Bergman, R.N.; Rankinen, T.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Rao, D.C.; et al. Effects of Exercise Training on Glucose Homeostasis. Diabetes Care 2005, 28, 108–114. [Google Scholar] [CrossRef]
- Suh, S.-H.; Paik, I.-Y.; Jacobs, K. Regulation of Blood Glucose Homeostasis during Prolonged Exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’Acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef] [PubMed]
- Péronnet, F.; Aguilaniu, B. Lactic Acid Buffering, Nonmetabolic CO2 and Exercise Hyperventilation: A Critical Reappraisal. Respir. Physiol. Neurobiol. 2006, 150, 4–18. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Aksoylar, H.I.; Horng, T. Control of Macrophage Metabolism and Activation by mTOR and Akt Signaling. Semin. Immunol. 2015, 27, 286–296. [Google Scholar] [CrossRef]
- Yang, Z.; Kahn, B.B.; Shi, H.; Xue, B. Macrophage α1 AMP-Activated Protein Kinase (α1AMPK) Antagonizes Fatty Acid-Induced Inflammation through SIRT1. J. Biol. Chem. 2010, 285, 19051–19059. [Google Scholar] [CrossRef]
- Zeng, H.; Chi, H. mTOR and Lymphocyte Metabolism. Curr. Opin. Immunol. 2013, 25, 347–355. [Google Scholar] [CrossRef]
- Watson, K.; Baar, K. mTOR and the Health Benefits of Exercise. Semin. Cell Dev. Biol. 2014, 36, 130–139. [Google Scholar] [CrossRef]
- Linke, M.; Fritsch, S.D.; Sukhbaatar, N.; Hengstschläger, M.; Weichhart, T. mTORC1 and mTORC2 as Regulators of Cell Metabolism in Immunity. FEBS Lett. 2017, 591, 3089–3103. [Google Scholar] [CrossRef]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Förster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1α Is Essential for Myeloid Cell-Mediated Inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1α–Dependent Glycolytic Pathway Orchestrates a Metabolic Checkpoint for the Differentiation of TH17 and Treg Cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Cho, S.H.; Raybuck, A.L.; Blagih, J.; Kemboi, E.; Haase, V.H.; Jones, R.G.; Boothby, M.R. Hypoxia-Inducible Factors in CD4 + T Cells Promote Metabolism, Switch Cytokine Secretion, and T Cell Help in Humoral Immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 8975–8984. [Google Scholar] [CrossRef] [PubMed]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 Promotes Alternative Activation of Macrophages to Limit Endotoxemia and Obesity-Associated Resistance to Insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles can Account for the Exercise-Induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Cabral-Santos, C.; Lima Junior, E.A.; Fernandes, I.M.D.C.; Pinto, R.Z.; Rosa-Neto, J.C.; Bishop, N.C.; Lira, F.S. Interleukin-10 Responses from Acute Exercise in Healthy Subjects: A Systematic Review. J. Cell. Physiol. 2019, 234, 9956–9965. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-Dependent Regulation of the Tumour Microenvironment. Nat. Rev. Cancer 2017, 17, 620–632. [Google Scholar] [CrossRef]
- de Araújo, A.L.; Silva, L.C.R.; Fernandes, J.R.; Benard, G. Preventing or Reversing Immunosenescence: Can Exercise Be an Immunotherapy? Immunotherapy 2013, 5, 879–893. [Google Scholar] [CrossRef]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Karandikar, N.J.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Crotty, L.E.; Casazza, J.P.; Kuruppu, J.; Migueles, S.A.; Connors, M.; et al. Expression of CD57 Defines Replicative Senescence and Antigen-Induced Apoptotic Death of CD8+ T Cells. Blood 2003, 101, 2711–2720. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.D.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef]
- Pawelec, G. Immunosenescence: Impact in the Young as well as the Old? Mech. Ageing Dev. 1999, 108, 1–7. [Google Scholar] [CrossRef]
- Alves, A.S.; Bueno, V. Immunosenescence: Participation of T Lymphocytes and Myeloid-Derived Suppressor Cells in Aging-Related Immune Response Changes. Einstein 2019, 17, eRB4733. [Google Scholar] [CrossRef]
- Kohut, M.L.; Arntson, B.A.; Lee, W.; Rozeboom, K.; Yoon, K.-J.; Cunnick, J.E.; McElhaney, J. Moderate Exercise Improves Antibody Response to Influenza Immunization in Older Adults. Vaccine 2004, 22, 2298–2306. [Google Scholar] [CrossRef]
- de Araújo, A.L.; Silva, L.C.R.; Fernandes, J.R.; Matias, M.d.S.T.; Boas, L.S.; Machado, C.M.; Garcez-Leme, L.E.; Benard, G. Elderly Men with Moderate and Intense Training Lifestyle Present Sustained Higher Antibody Responses to Influenza Vaccine. Age Dordr. Neth. 2015, 37, 105. [Google Scholar] [CrossRef]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major Features of Immunesenescence, Including Reduced Thymic Output, are Ameliorated by High Levels of Physical Activity in Adulthood. Aging Cell 2018, 17, e12750. [Google Scholar] [CrossRef]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can Physical Activity Ameliorate Immunosenescence and Thereby Reduce Age-Related Multi-Morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef]
- Donovan, T.; Bain, A.L.; Tu, W.; Pyne, D.B.; Rao, S. Influence of Exercise on Exhausted and Senescent T Cells: A Systematic Review. Front. Physiol. 2021, 12, 668327. [Google Scholar] [CrossRef]
- Eschke, R.-C.K.-R.; Lampit, A.; Schenk, A.; Javelle, F.; Steindorf, K.; Diel, P.; Bloch, W.; Zimmer, P. Impact of Physical Exercise on Growth and Progression of Cancer in Rodents—A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 35. [Google Scholar] [CrossRef]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 165–205. ISBN 978-0-470-65071-4. [Google Scholar]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. CMLS 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia and Chemotherapeutic Response by Exercise. J. Natl. Cancer Inst. 2015, 107, djv040. [Google Scholar] [CrossRef]
- McCullough, D.J.; Stabley, J.N.; Siemann, D.W.; Behnke, B.J. Modulation of Blood Flow, Hypoxia, and Vascular Function in Orthotopic Prostate Tumors during Exercise. J. Natl. Cancer Inst. 2014, 106, dju036. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, E.J.; Schroeder, T.; Herndon, J.E.; et al. Effect of Aerobic Exercise on Tumor Physiology in an Animal Model of Human Breast Cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor Vessel Normalization after Aerobic Exercise Enhances Chemotherapeutic Efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Tsukada, K.; Xu, L.; Miyazaki, J.; Kozin, S.V.; Tyrrell, J.A.; Sessa, W.C.; Gerweck, L.E.; Jain, R.K.; Fukumura, D. Perivascular Nitric Oxide Gradients Normalize Tumor Vasculature. Nat. Med. 2008, 14, 255–257. [Google Scholar] [CrossRef]
- Aveseh, M.; Nikooie, R.; Aminaie, M. Exercise-Induced Changes in Tumour LDH-B and MCT1 Expression are Modulated by Oestrogen-Related Receptor Alpha in Breast Cancer-Bearing BALB/c Mice. J. Physiol. 2015, 593, 2635–2648. [Google Scholar] [CrossRef]
- Bacuau, R.F.P.; Belmonte, M.A.; Seelaender, M.C.L.; Costa Rosa, L.F.B.P. Effect of a Moderate Intensity Exercise Training Protocol on the Metabolism of Macrophages and Lymphocytes of Tumour-Bearing rats. Cell Biochem. Funct. 2000, 18, 249–258. [Google Scholar] [CrossRef]
- Heuser, C.; Renner, K.; Kreutz, M.; Gattinoni, L. Targeting Lactate Metabolism for Cancer Immunotherapy—A Matter of Precision. Semin. Cancer Biol. 2023, 88, 32–45. [Google Scholar] [CrossRef]
- Gardiner, C.M. NK Cell Metabolism. J. Leukoc. Biol. 2019, 105, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.D.; O’Sullivan, D.; Klein Geltink, R.I.; Curtis, J.D.; Chang, C.-H.; Sanin, D.E.; Qiu, J.; Kretz, O.; Braas, D.; van der Windt, G.J.W.; et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 2016, 166, 63–76. [Google Scholar] [CrossRef]
- Schrörs, B.; Boegel, S.; Albrecht, C.; Bukur, T.; Bukur, V.; Holtsträter, C.; Ritzel, C.; Manninen, K.; Tadmor, A.D.; Vormehr, M.; et al. Multi-Omics Characterization of the 4T1 Murine Mammary Gland Tumor Model. Front. Oncol. 2020, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 Breast Tumor Model. Curr. Protoc. Immunol. 2000, 39. [Google Scholar] [CrossRef]
- Le Naour, A.; Rossary, A.; Vasson, M.-P. EO771, Is It a Well-Characterized Cell Line for Mouse Mammary Cancer Model? Limit and Uncertainty. Cancer Med. 2020, 9, 8074–8085. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ruiz, A.; Fiuza-Luces, C.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Gálvez, B.G.; Martínez-Martínez, E.; Martín-Acosta, P.; Coronado, M.J.; Franco-Luzón, L.; González-Murillo, Á.; et al. Benefits of Exercise and Immunotherapy in a Murine Model of Human Non-Small-Cell Lung Carcinoma. Exerc. Immunol. Rev. 2020, 26, 100–115. [Google Scholar]
- Spiliopoulou, P.; Gavriatopoulou, M.; Kastritis, E.; Dimopoulos, M.A.; Terzis, G. Exercise-Induced Changes in Tumor Growth via Tumor Immunity. Sports 2021, 9, 46. [Google Scholar] [CrossRef]
- Emery, A.; Moore, S.; Turner, J.E.; Campbell, J.P. Reframing How Physical Activity Reduces the Incidence of Clinically-Diagnosed Cancers: Appraising Exercise-Induced Immuno-Modulation as An Integral Mechanism. Front. Oncol. 2022, 12, 788113. [Google Scholar] [CrossRef]
- Shaver, A.L.; Sharma, S.; Nikita, N.; Lefler, D.S.; Basu-Mallick, A.; Johnson, J.M.; Butryn, M.; Lu-Yao, G. The Effects of Physical Activity on Cancer Patients Undergoing Treatment with Immune Checkpoint Inhibitors: A Scoping Review. Cancers 2021, 13, 6364. [Google Scholar] [CrossRef]
- Zheng, A.; Zhang, L.; Yang, J.; Yin, X.; Zhang, T.; Wu, X.; Ma, X. Physical Activity Prevents Tumor Metastasis through Modulation of Immune Function. Front. Pharmacol. 2022, 13, 1034129. [Google Scholar] [CrossRef] [PubMed]
- Handford, J.; Chen, M.; Rai, R.; Moss, C.L.; Enting, D.; Peat, N.; Karagiannis, S.N.; Van Hemelrijck, M.; Russell, B. Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review. Cancers 2022, 14, 5039. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.W.M.; Gomes-Filho, A.; Ferreira, A.J.; Rodrigues, C.E.M.; Dias-Peixoto, M.F.; Russo, R.C.; Teixeira, M.M.; Cassali, G.D.; Ferreira, E.; Santos, I.C.; et al. Swim Training Suppresses Tumor Growth in Mice. J. Appl. Physiol. 2009, 107, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-B.; Zhang, B.-H.; Zhang, K.-Z.; Meng, X.-T.; Jia, Q.-A.; Zhang, Q.-B.; Bu, Y.; Zhu, X.-D.; Ma, D.-N.; Ye, B.-G.; et al. Moderate Swimming Suppressed the Growth and Metastasis of the Transplanted Liver Cancer in Mice Model: With Reference to Nervous System. Oncogene 2016, 35, 4122–4131. [Google Scholar] [CrossRef]
- Hagar, A.; Wang, Z.; Koyama, S.; Serrano, J.A.; Melo, L.; Vargas, S.; Carpenter, R.; Foley, J. Endurance Training Slows Breast Tumor Growth in Mice by Suppressing Treg Cells Recruitment to Tumors. BMC Cancer 2019, 19, 536. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic Inhibition of Daidzein and Regular Exercise on Breast Cancer in Bearing-4T1 Mice by Regulating NK Cells and Apoptosis Pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef]
- Turbitt, W.J.; Xu, Y.; Sosnoski, D.M.; Collins, S.D.; Meng, H.; Mastro, A.M.; Rogers, C.J. Physical Activity Plus Energy Restriction Prevents 4T1.2 Mammary Tumor Progression, MDSC Accumulation, and an Immunosuppressive Tumor Microenvironment. Cancer Prev. Res. 2019, 12, 493–506. [Google Scholar] [CrossRef]
- Garritson, J.; Krynski, L.; Haverbeck, L.; Haughian, J.M.; Pullen, N.A.; Hayward, R. Physical Activity Delays Accumulation of Immunosuppressive Myeloid-Derived Suppressor Cells. PLoS ONE 2020, 15, e0234548. [Google Scholar] [CrossRef]
- Bay, M.L.; Unterrainer, N.; Stagaard, R.; Pedersen, K.S.; Schauer, T.; Staffeldt, M.M.; Christensen, J.F.; Hojman, P.; Pedersen, B.K.; Gehl, J. Voluntary Wheel Running can Lead to Modulation of Immune Checkpoint Molecule Expression. Acta Oncol. 2020, 59, 1447–1454. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S.; et al. Exercise-Induced Engagement of the IL-15/IL-15Rα Axis Promotes Anti-Tumor Immunity in Pancreatic Cancer. Cancer Cell 2022, 40, 720–737.e5. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.A.; Williams, T.; Hock, B.; Ang, A.D.; Robinson, B.A.; Currie, M.J.; Dachs, G.U. Effects of Exercise and Anti-PD-1 on the Tumour Microenvironment. Immunol. Lett. 2021, 239, 60–71. [Google Scholar] [CrossRef]
- Buss, L.A.; Ang, A.D.; Hock, B.; Robinson, B.A.; Currie, M.J.; Dachs, G.U. Effect of Post-Implant Exercise on Tumour Growth Rate, Perfusion and Hypoxia in Mice. PLoS ONE 2020, 15, e0229290. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Liu, X.-F.; Zhu, X.-D.; Feng, L.-H.; Li, X.-L.; Xu, B.; Li, K.-S.; Xiao, N.; Lei, M.; Sun, H.-C.; Tang, Z.-Y. Physical Activity Improves Outcomes of Combined Lenvatinib Plus Anti-PD-1 Therapy in Unresectable Hepatocellular Carcinoma: A Retrospective Study and Mouse Model. Exp. Hematol. Oncol. 2022, 11, 20. [Google Scholar] [CrossRef]
- Dufresne, S.; Guéritat, J.; Chiavassa, S.; Noblet, C.; Assi, M.; Rioux-Leclercq, N.; Rannou-Bekono, F.; Lefeuvre-Orfila, L.; Paris, F.; Rébillard, A. Exercise Training Improves Radiotherapy Efficiency in a Murine Model of Prostate Cancer. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 4984–4996. [Google Scholar] [CrossRef]
- Trunova, G.V.; Makarova, O.V.; Diatroptov, M.E.; Bogdanova, I.M.; Mikchailova, L.P.; Abdulaeva, S.O. Morphofunctional Characteristic of the Immune System in BALB/c and C57Bl/6 Mice. Bull. Exp. Biol. Med. 2011, 151, 99–102. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA Class-I Molecules in Human Natural Killer Cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Huang, A.; Parker, N.H.; Bruera, E.; Lee, R.E.; Simpson, R.; O’Connor, D.P.; Petzel, M.Q.B.; Fontillas, R.C.; Schadler, K.; Xiao, L.; et al. Home-Based Exercise Prehabilitation during Preoperative Treatment for Pancreatic Cancer Is Associated with Improvement in Physical Function and Quality of Life. Integr. Cancer Ther. 2019, 18, 1534735419894061. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Huang, A.; Parker, N.H.; Wang, X.; Petzel, M.Q.B.; Fogelman, D.; Schadler, K.L.; Bruera, E.; Fleming, J.B.; Lee, J.E.; Katz, M.H.G. Home-Based Exercise during Preoperative Therapy for Pancreatic Cancer. Langenbecks Arch. Surg. 2017, 402, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Foucher, E.D.; Ghigo, C.; Chouaib, S.; Galon, J.; Iovanna, J.; Olive, D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front. Immunol. 2018, 9, 1044. [Google Scholar] [CrossRef]
- Djurhuus, S.S.; Simonsen, C.; Toft, B.G.; Thomsen, S.N.; Wielsøe, S.; Røder, M.A.; Hasselager, T.; Østergren, P.B.; Jakobsen, H.; Pedersen, B.K.; et al. Exercise Training to Increase Tumour Natural Killer-Cell Infiltration in Men with Localised Prostate Cancer: A Randomised Controlled Trial. BJU Int. 2023, 131, 116–124. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Charles, C.; Bardet, A.; Ibrahimi, N.; Aromatario, O.; Cambon, L.; Imbert, A.; Pons, M.; Raynard, B.; Sauveplane, D.; Pouchepadass, C.; et al. Delivering Adapted Physical Activity by Videoconference to Patients with Fatigue under Immune Checkpoint Inhibitors: Lessons Learned from the PACTIMe-FEAS Feasibility Study. J. Telemed. Telecare 2021, 1357633X2110217. [Google Scholar] [CrossRef]
- Hyatt, A.; Gough, K.; Murnane, A.; Au-Yeung, G.; Dawson, T.; Pearson, E.; Dhillon, H.; Sandhu, S.; Williams, N.; Paton, E.; et al. i-Move, A Personalised Exercise Intervention for Patients with Advanced Melanoma Receiving Immunotherapy: A Randomised Feasibility Trial Protocol. BMJ Open 2020, 10, e036059. [Google Scholar] [CrossRef]
- Lacey, J.; Lomax, A.J.; McNeil, C.; Marthick, M.; Levy, D.; Kao, S.; Nielsen, T.; Dhillon, H.M. A Supportive Care Intervention for People with Metastatic Melanoma Being Treated with Immunotherapy: A Pilot Study Assessing Feasibility, Perceived Benefit, and Acceptability. Support. Care Cancer 2019, 27, 1497–1507. [Google Scholar] [CrossRef]
- Gouez, M.; Pérol, O.; Pérol, M.; Caux, C.; Ménétrier-Caux, C.; Villard, M.; Walzer, T.; Delrieu, L.; Saintigny, P.; Marijnen, P.; et al. Effect of Acute Aerobic Exercise before Immunotherapy and Chemotherapy Infusion in Patients with Metastatic Non-Small-Cell Lung Cancer: Protocol for the ERICA Feasibility Trial. BMJ Open 2022, 12, e056819. [Google Scholar] [CrossRef]
- Holmen Olofsson, G.; Mikkelsen, M.K.; Ragle, A.-M.; Christiansen, A.B.; Olsen, A.P.; Heide-Ottosen, L.; Horsted, C.B.; Pedersen, C.M.S.; Engell-Noerregaard, L.; Lorentzen, T.; et al. High Intensity Aerobic Exercise Training and Immune Cell Mobilization in Patients with Lung Cancer (HI AIM)-a Randomized Controlled Trial. BMC Cancer 2022, 22, 246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brummer, C.; Pukrop, T.; Wiskemann, J.; Bruss, C.; Ugele, I.; Renner, K. Can Exercise Enhance the Efficacy of Checkpoint Inhibition by Modulating Anti-Tumor Immunity? Cancers 2023, 15, 4668. https://doi.org/10.3390/cancers15184668
Brummer C, Pukrop T, Wiskemann J, Bruss C, Ugele I, Renner K. Can Exercise Enhance the Efficacy of Checkpoint Inhibition by Modulating Anti-Tumor Immunity? Cancers. 2023; 15(18):4668. https://doi.org/10.3390/cancers15184668
Chicago/Turabian StyleBrummer, Christina, Tobias Pukrop, Joachim Wiskemann, Christina Bruss, Ines Ugele, and Kathrin Renner. 2023. "Can Exercise Enhance the Efficacy of Checkpoint Inhibition by Modulating Anti-Tumor Immunity?" Cancers 15, no. 18: 4668. https://doi.org/10.3390/cancers15184668
APA StyleBrummer, C., Pukrop, T., Wiskemann, J., Bruss, C., Ugele, I., & Renner, K. (2023). Can Exercise Enhance the Efficacy of Checkpoint Inhibition by Modulating Anti-Tumor Immunity? Cancers, 15(18), 4668. https://doi.org/10.3390/cancers15184668






