Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Protein Preparation
2.3. Sample Separation and MS/MS Analysis
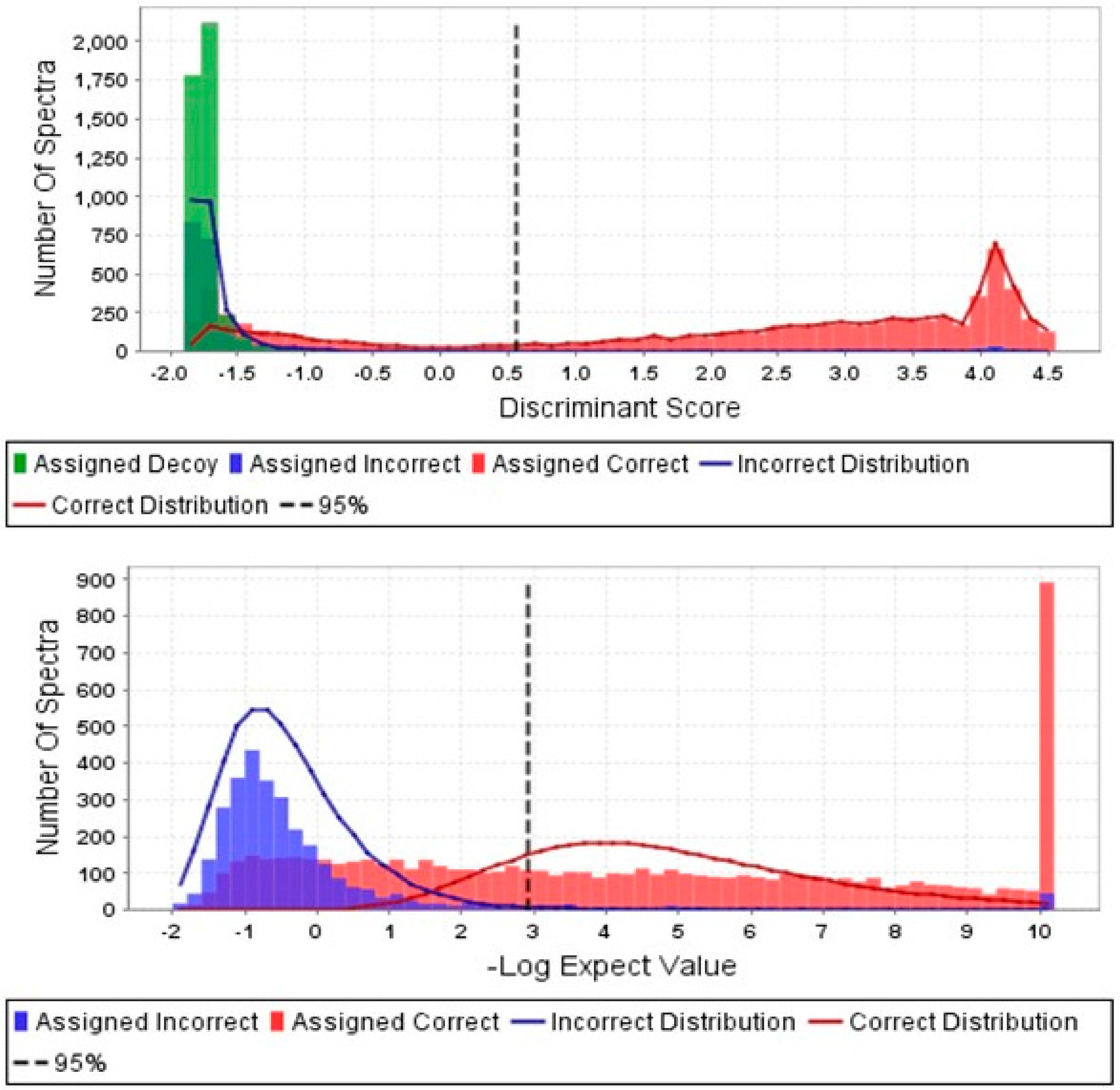
2.4. In Silico Analysis of Peptide Fragments
3. Results
3.1. Patient Characteristics
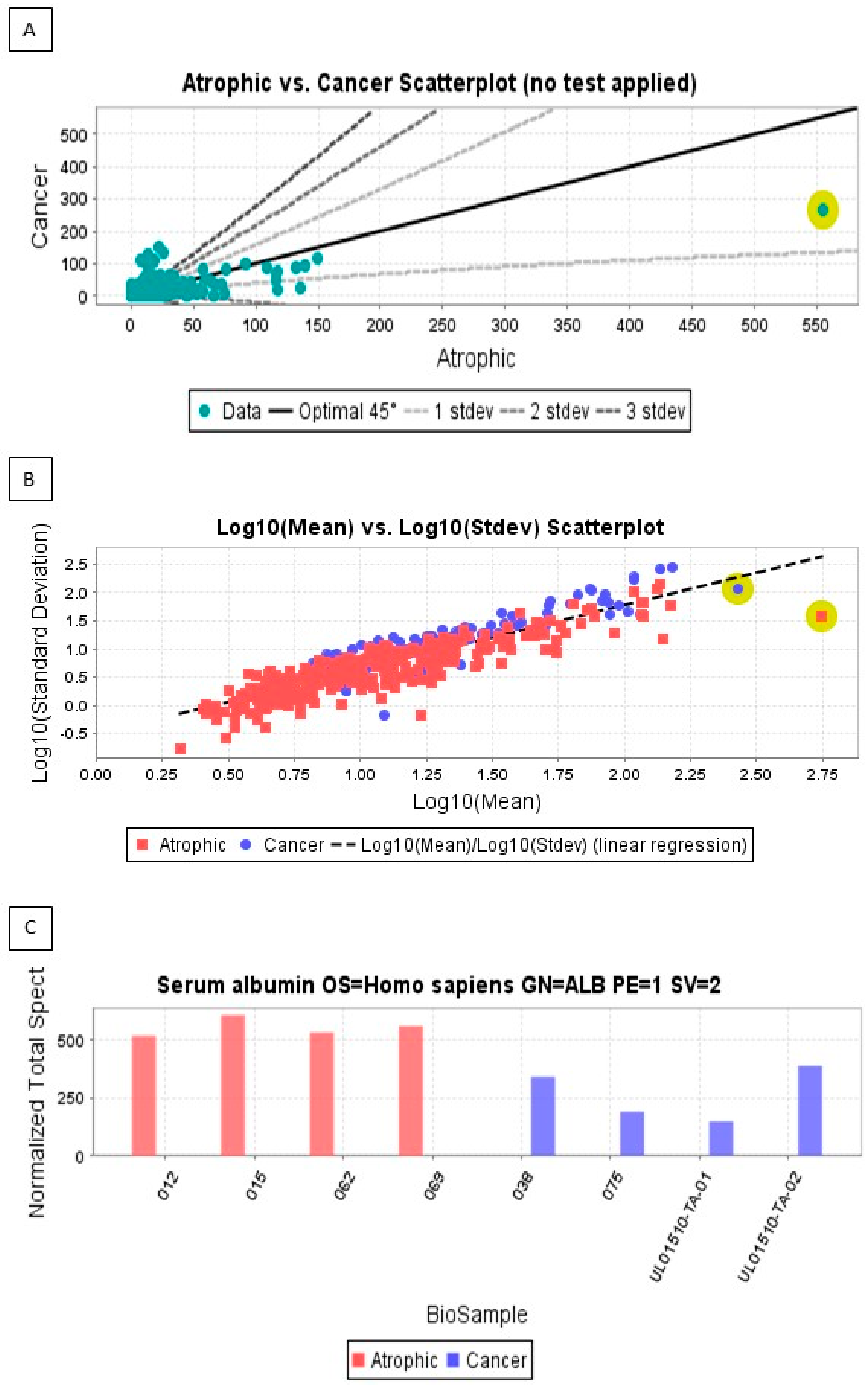
3.2. Proteins Expressed Only in Endometrial Cancer
| Patient † | Age (yr) | BMI (kg/m2) | Medical Conditions 1 | Sample Type 2 | Cancer Grade | Cancer Stage | LVSI 3 | Myometrial Invasion | Adjuvant Treatment | ER/PR Status 4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 012 | 55 | 19 | HTN | A | n/a | n/a | n/a | n/a | n/a | Nil |
| 015 | 65 | 22 | DM2 | A | n/a | n/a | n/a | n/a | n/a | Nil |
| 062 | 63 | 26 | HTN/DM2 | A | n/a | n/a | n/a | n/a | n/a | Nil |
| 069 | 57 | 24 | HTN | A | n/a | n/a | n/a | n/a | n/a | Nil |
| 038 | 88 | 24 | HTN | E | 1 | 1 | -ve | <50% | Nil, surgical follow-up for 3 years | ER + ve, PR − ve |
| 075 | 74 | 42 | DM2 | E | 1 | 1 | -ve | <50% | Nil, surgical follow up for 3 years | ER + ve, PR − ve |
| TA-01 | 61 | 38 | HTN | S | High grade | 1 | +ve | >50% | Chemo-radiation, surgical follow up for 3 years | ER − ve, PR − ve |
| TA-02 | 61 | 40 | DM2 | S | High grade | 1 | +ve | >50% | Chemo-radiation, surgical follow up for 3 years | ER − ve, PR − ve |
3.3. Pathway and Network Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coll-de la Rubia, E.; Martinez-Garcia, E.; Dittmar, G.; Gil-Moreno, A.; Cabrera, S.; Colas, E. Prognostic biomarkers in endometrial cancer: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 1900. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Ricceri, F.; Giraudo, M.T.; Fasanelli, F.; Milanese, D.; Sciannameo, V.; Fiorini, L.; Sacerdote, C. Diet and endometrial cancer: A focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer 2017, 17, 757. [Google Scholar] [CrossRef]
- Fiorelli, J.L.; Herzog, T.J.; Wright, J.D. Current treatment strategies for endometrial cancer. Expert Rev. Anticancer Ther. 2008, 8, 1149–1157. [Google Scholar] [CrossRef]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef]
- Faber, M.T.; Frederiksen, K.; Jensen, A.; Aarslev, P.B.; Kjaer, S.K. Time trends in the incidence of hysterectomy-corrected overall, type 1 and type 2 endometrial cancer in Denmark 1978–2014. Gynecol. Oncol. 2017, 146, 359–367. [Google Scholar] [CrossRef]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- von Gruenigen, V.E.; Gil, K.M.; Frasure, H.E.; Jenison, E.L.; Hopkins, M.P. The impact of obesity and age on quality of life in gynecologic surgery. Am. J. Obstet. Gynecol. 2005, 193, 1369–1375. [Google Scholar] [CrossRef]
- Aune, D.; Navarro Rosenblatt, D.A.; Chan, D.S.; Vingeliene, S.; Abar, L.; Vieira, A.R.; Greenwood, D.C.; Bandera, E.V.; Norat, T. Anthropometric factors and endometrial cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Ann. Oncol. 2015, 26, 1635–1648. [Google Scholar] [CrossRef]
- Sorosky, J.I. Endometrial cancer. Obstet. Gynecol. 2012, 120, 383–397. [Google Scholar] [CrossRef]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Blair, A.R.; Casas, C.M. Gynecologic cancers. Prim. Care 2009, 36, 115–130. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Maccarrone, M.; Konje, J.C. Identification of novel predictive biomarkers for endometrial malignancies: N-acylethanolamines. Front. Oncol. 2019, 9, 430. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Saccone, G.; Insabato, L.; Mollo, A.; De Placido, G.; Zullo, F. Immunohistochemical predictive markers of response to conservative treatment of endometrial hyperplasia and early endometrial cancer: A systematic review. Acta Obstet. Gynecol. Scand. 2019, 98, 1086–1099. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Chia, V.M. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas 2009, 63, 39–44. [Google Scholar] [CrossRef]
- Alkhas, A.; Hood, B.L.; Oliver, K.; Teng, P.N.; Oliver, J.; Mitchell, D.; Hamilton, C.A.; Maxwell, G.L.; Conrads, T.P. Standardization of a sample preparation and analytical workflow for proteomics of archival endometrial cancer tissue. J. Proteome Res. 2011, 10, 5264–5271. [Google Scholar] [CrossRef]
- Attarha, S.; Mints, M.; Andersson, S.; Souchelnytskyi, S. Endometrial cancer and application of proteomics. Exp. Oncol. 2011, 33, 174–177. [Google Scholar]
- Baak, J.P.; Path, F.R.; Hermsen, M.A.; Meijer, G.; Schmidt, J.; Janssen, E.A. Genomics and proteomics in cancer. Eur. J. Cancer 2003, 39, 1199–1215. [Google Scholar] [CrossRef]
- Dube, V.; Grigull, J.; DeSouza, L.V.; Ghanny, S.; Colgan, T.J.; Romaschin, A.D.; Siu, K.W. Verification of endometrial tissue biomarkers previously discovered using mass spectrometry-based proteomics by means of immunohistochemistry in a tissue microarray format. J. Proteome Res. 2007, 6, 2648–2655. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Tosner, J. Proteomics and biomarkers for detection of endometrial carcinoma. Ceska Gynekol. 2009, 74, 274–278. [Google Scholar]
- Li, Z.; Min, W.; Huang, C.; Bai, S.; Tang, M.; Zhao, X. Proteomics-based approach identified differentially expressed proteins with potential roles in endometrial carcinoma. Int. J. Gynecol. Cancer 2010, 20, 9–15. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.; Lesur, A.; Devis, L.; Cabrera, S.; Matias-Guiu, X.; Hirschfeld, M.; Asberger, J.; van Oostrum, J.; Casares de Cal, M.L.A.; Gomez-Tato, A.; et al. Targeted proteomics identifies proteomic signatures in liquid biopsies of the endometrium to diagnose endometrial cancer and assist in the prediction of the optimal surgical treatment. Clin. Cancer Res. 2017, 23, 6458–6467. [Google Scholar] [CrossRef]
- Mittal, P.; Klingler-Hoffmann, M.; Arentz, G.; Zhang, C.; Kaur, G.; Oehler, M.K.; Hoffmann, P. Proteomics of endometrial cancer diagnosis, treatment, and prognosis. Proteom. Clin. Appl. 2016, 10, 217–229. [Google Scholar] [CrossRef]
- Van Gorp, T.; Cadron, I.; Vergote, I. The utility of proteomics in gynecologic cancers. Curr. Opin. Obstet. Gynecol. 2011, 23, 3–7. [Google Scholar] [CrossRef]
- Yi, Z.; Jingting, C.; Yu, Z. Proteomics reveals protein profile changes in cyclooxygenase-2 inhibitor-treated endometrial cancer cells. Int. J. Gynecol. Cancer 2009, 19, 326–333. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.-H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinform. 2012, 13, S11. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Bari, M.; Mastrangelo, N.; Maccarrone, M.; Konje, J.C. Expression and function of the endocannabinoid modulating enzymes Fatty Acid Amide Hydrolase and N-Acylphosphatidylethanolamine-Specific Phospholipase D in endometrial carcinoma. Front. Oncol. 2019, 9, 1363. [Google Scholar] [CrossRef]
- Turapov, O.A.; Mukamolova, G.V.; Bottrill, A.R.; Pangburn, M.K. Digestion of native proteins for proteomics using a thermocycler. Anal. Chem. 2008, 80, 6093–6099. [Google Scholar] [CrossRef][Green Version]
- Speicher, K.D.; Kolbas, O.; Harper, S.; Speicher, D.W. Systematic analysis of peptide recoveries from in-gel digestions for protein identifications in proteome studies. J. Biomol. Tech. 2000, 11, 74–86. [Google Scholar]
- Moiseeva, T.N.; Bottrill, A.; Melino, G.; Barlev, N.A. DNA damage-induced ubiquitylation of proteasome controls its proteolytic activity. Oncotarget 2013, 4, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
- Zaino, R.J.; Kurman, R.J.; Diana, K.L.; Morrow, C.P. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer 1995, 75, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Kawaler, E.A.; Cui Zhou, D.; Gritsenko, M.A.; Huang, C.; Blumenberg, L.; Karpova, A.; Petyuk, V.A.; Savage, S.R.; Satpathy, S.; et al. Proteogenomic characterization of endometrial carcinoma. Cell 2020, 180, 729–748.e726. [Google Scholar] [CrossRef]
- Deb, B.; Sengupta, P.; Sambath, J.; Kumar, P. Bioinformatics Analysis of Global Proteomic and Phosphoproteomic Data Sets Revealed Activation of NEK2 and AURKA in Cancers. Biomolecules 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Y.; Akpinar, G.; Doger, E.; Kasap, M.; Guzel, N.; Karaosmanoglu, K.; Kopuk, S.Y.; Yucesoy, I. Proteomic analysis in endometrial cancer and endometrial hyperplasia tissues by 2D-DIGE technique. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101652. [Google Scholar] [CrossRef] [PubMed]
- Acland, M.; Arentz, G.; Mussared, M.; Whitehead, F.; Hoffmann, P.; Klingler-Hoffmann, M.; Oehler, M.K. Proteomic Analysis of Pre-Invasive Serous Lesions of the Endometrium and Fallopian Tube Reveals Their Metastatic Potential. Front. Oncol. 2020, 10, 523989. [Google Scholar] [CrossRef]
- Njoku, K.; Chiasserini, D.; Whetton, A.D.; Crosbie, E.J. Proteomic biomarkers for the detection of endometrial cancer. Cancers 2019, 11, 1572. [Google Scholar] [CrossRef]
- Liu, J.; Mei, J.; Li, S.; Wu, Z.; Zhang, Y. Establishment of a novel cell cycle-related prognostic signature predicting prognosis in patients with endometrial cancer. Cancer Cell Int. 2020, 20, 329. [Google Scholar] [CrossRef]
- Degez, M.; Caillon, H.; Chauvire-Drouard, A.; Leroy, M.; Lair, D.; Winer, N.; Thubert, T.; Dochez, V. Endometrial cancer: A systematic review of HE4, REM and REM-B. Clin. Chim. Acta 2020, 515, 27–36. [Google Scholar] [CrossRef]
- Gasiorowska, E.; Magnowska, M.; Izycka, N.; Warchol, W.; Nowak-Markwitz, E. The role of HE4 in differentiating benign and malignant endometrial pathology. Ginekol. Pol. 2016, 87, 260–264. [Google Scholar] [CrossRef]
- Galgano, M.T.; Hampton, G.M.; Frierson, H.F., Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod. Pathol. 2006, 19, 847–853. [Google Scholar] [CrossRef]
- Ponnambalam, S.; Jackson, A.P.; LeBeau, M.M.; Pravtcheva, D.; Ruddle, F.H.; Alibert, C.; Parham, P. Chromosomal location and some structural features of human clathrin light-chain genes (CLTA and CLTB). Genomics 1994, 24, 440–444. [Google Scholar] [CrossRef]
- Holzer, I.; Machado Weber, A.; Marshall, A.; Freis, A.; Jauckus, J.; Strowitzki, T.; Germeyer, A. GRN, NOTCH3, FN1, and PINK1 expression in eutopic endometrium-potential biomarkers in the detection of endometriosis—A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2723–2732. [Google Scholar] [CrossRef]
- Sosa, L.J.; Cáceres, A.; Dupraz, S.; Oksdath, M.; Quiroga, S.; Lorenzo, A. The physiological role of the amyloid precursor protein as an adhesion molecule in the developing nervous system. J. Neurochem. 2017, 143, 11–29. [Google Scholar] [CrossRef]
- Iuvone, T.; De Filippis, D.; Di Spiezio Sardo, A.; D’Amico, A.; Simonetti, S.; Sparice, S.; Esposito, G.; Bifulco, G.; Insabato, L.; Nappi, C.; et al. Selective CB2 up-regulation in women affected by endometrial inflammation. J. Cell Mol. Med. 2008, 12, 661–670. [Google Scholar] [CrossRef]
- Erikson, D.W.; Barragan, F.; Piltonen, T.T.; Chen, J.C.; Balayan, S.; Irwin, J.C.; Giudice, L.C. Stromal fibroblasts from perimenopausal endometrium exhibit a different transcriptome than those from the premenopausal endometrium. Biol. Reprod. 2017, 97, 387–399. [Google Scholar] [CrossRef]
- Lindsey-Boltz, L.A.; Wauson, E.M.; Graves, L.M.; Sancar, A. The human Rad9 checkpoint protein stimulates the carbamoyl phosphate synthetase activity of the multifunctional protein CAD. Nucleic Acids Res. 2004, 32, 4524–4530. [Google Scholar] [CrossRef][Green Version]
- Davis, A.M.; Mao, J.; Naz, B.; Kohl, J.A.; Rosenfeld, C.S. Comparative effects of estradiol, methyl-piperidino-pyrazole, raloxifene, and ICI 182 780 on gene expression in the murine uterus. J. Mol. Endocrinol. 2008, 41, 205–217. [Google Scholar] [CrossRef][Green Version]
- Herndon, C.N.; Aghajanova, L.; Balayan, S.; Erikson, D.; Barragan, F.; Goldfien, G.; Vo, K.C.; Hawkins, S.; Giudice, L.C. Global Transcriptome Abnormalities of the Eutopic Endometrium From Women With Adenomyosis. Reprod. Sci. 2016, 23, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Yoon, H.K.; Kim, Y.T.; Choi, Y.H.; Lee, W.K.; Jin, M. Tryptophanyl-tRNA Synthetase 1 Signals Activate TREM-1 via TLR2 and TLR4. Biomolecules 2020, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Oke, A.B.; Fung, J.C.; Vo, K.C.; Nezhat, C.; Giudice, L.C. Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PLoS Genet. 2020, 16, e1008601. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Pizarro, A.; Figueroa, P.; Brito, J.; Marín, J.C.; Munroe, D.J.; Croxatto, H.B. Endometrial gene expression reveals compromised progesterone signaling in women refractory to embryo implantation. Reprod. Biol. Endocrinol. 2014, 12, 92. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Zhang, X.; Wang, Y.; Yu, H.; Yin, X.; Li, J.; Du, P.; Plumas, J.; Chaperot, L.; et al. SCARB2/LIMP-2 Regulates IFN Production of Plasmacytoid Dendritic Cells by Mediating Endosomal Translocation of TLR9 and Nuclear Translocation of IRF7. J. Immunol. 2015, 194, 4737–4749. [Google Scholar] [CrossRef]
- García, P.; Rosa, L.; Vargas, S.; Weber, H.; Espinoza, J.A.; Suárez, F.; Romero-Calvo, I.; Elgueta, N.; Rivera, V.; Nervi, B.; et al. Hippo-YAP1 Is a Prognosis Marker and Potentially Targetable Pathway in Advanced Gallbladder Cancer. Cancers 2020, 12, 778. [Google Scholar] [CrossRef]
- Wan, L.C.; Maisonneuve, P.; Szilard, R.K.; Lambert, J.P.; Ng, T.F.; Manczyk, N.; Huang, H.; Laister, R.; Caudy, A.A.; Gingras, A.C.; et al. Proteomic analysis of the human KEOPS complex identifies C14ORF142 as a core subunit homologous to yeast Gon7. Nucleic Acids Res. 2017, 45, 805–817. [Google Scholar] [CrossRef]
- Kaplan, A.R.; Glazer, P.M. Pharmacological methods to transcriptionally modulate double-strand break DNA repair. Int. Rev. Cell Mol. Biol. 2020, 354, 187–213. [Google Scholar] [CrossRef]
- Staples, C.J.; Myers, K.N.; Beveridge, R.D.; Patil, A.A.; Howard, A.E.; Barone, G.; Lee, A.J.; Swanton, C.; Howell, M.; Maslen, S.; et al. Ccdc13 is a novel human centriolar satellite protein required for ciliogenesis and genome stability. J. Cell Sci. 2014, 127, 2910–2919. [Google Scholar] [CrossRef]
- Hodge, J.C.; Park, P.J.; Dreyfuss, J.M.; Assil-Kishawi, I.; Somasundaram, P.; Semere, L.G.; Quade, B.J.; Lynch, A.M.; Stewart, E.A.; Morton, C.C. Identifying the molecular signature of the interstitial deletion 7q subgroup of uterine leiomyomata using a paired analysis. Genes. Chromosomes Cancer 2009, 48, 865–885. [Google Scholar] [CrossRef]
- Yerushalmi, G.M.; Salmon-Divon, M.; Ophir, L.; Yung, Y.; Baum, M.; Coticchio, G.; Fadini, R.; Mignini-Renzini, M.; Dal Canto, M.; Machtinger, R.; et al. Characterization of the miRNA regulators of the human ovulatory cascade. Sci. Rep. 2018, 8, 15605. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.R.; Qiu, L.H.; Zhang, Z.Q.; Qin, Y.Y.; Cao, C.; Di, W. Genome-wide methylated DNA immunoprecipitation analysis of patients with polycystic ovary syndrome. PLoS ONE 2013, 8, e64801. [Google Scholar] [CrossRef] [PubMed]
- Muter, J.; Brighton, P.J.; Lucas, E.S.; Lacey, L.; Shmygol, A.; Quenby, S.; Blanks, A.M.; Brosens, J.J. Progesterone-Dependent Induction of Phospholipase C-Related Catalytically Inactive Protein 1 (PRIP-1) in Decidualizing Human Endometrial Stromal Cells. Endocrinology 2016, 157, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hardt, J.; Kim, J.J. Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol. Hum. Reprod. 2008, 14, 357–366. [Google Scholar] [CrossRef]
- Kumar, P.; Tathe, P.; Chaudhary, N.; Maddika, S. PPM1G forms a PPP-type phosphatase holoenzyme with B56δ that maintains adherens junction integrity. EMBO Rep. 2019, 20, e46965. [Google Scholar] [CrossRef]
- Bresch, A.M.; Yerich, N.; Wang, R.; Sperry, A.O. The PP1 regulator PPP1R2 coordinately regulates AURKA and PP1 to control centrosome phosphorylation and maintain central spindle architecture. BMC Mol. Cell Biol. 2020, 21, 84. [Google Scholar] [CrossRef]
- Holman, K.M.; Puppala, A.K.; Lee, J.W.; Lee, H.; Simonović, M. Insights into substrate promiscuity of human seryl-tRNA synthetase. Rna 2017, 23, 1685–1699. [Google Scholar] [CrossRef]
- Lübke, T.; Lobel, P.; Sleat, D.E. Proteomics of the lysosome. Biochim. Biophys. Acta 2009, 1793, 625–635. [Google Scholar] [CrossRef]
- Cai, H.; Zhu, X.X.; Li, Z.F.; Zhu, Y.P.; Lang, J.H. MicroRNA Dysregulation and Steroid Hormone Receptor Expression in Uterine Tissues of Rats with Endometriosis during the Implantation Window. Chin. Med. J. 2018, 131, 2193–2204. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, Y.; Wang, S.; Li, J.; Luan, J. Harnessing noncoding RNA-based macrophage polarization: Emerging therapeutic opportunities for fibrosis. Immun. Inflamm. Dis. 2020, 8, 793–806. [Google Scholar] [CrossRef]
- Hung, K.E.; Yu, K.H. Proteomic approaches to cancer biomarkers. Gastroenterology 2010, 138, 46–51.e41. [Google Scholar] [CrossRef] [PubMed]
- Makawita, S.; Diamandis, E.P. The bottleneck in the cancer biomarker pipeline and protein quantification through mass spectrometry-based approaches: Current strategies for candidate verification. Clin. Chem. 2010, 56, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Negishi, A.; Ono, M.; Handa, Y.; Kato, H.; Yamashita, K.; Honda, K.; Shitashige, M.; Satow, R.; Sakuma, T.; Kuwabara, H.; et al. Large-scale quantitative clinical proteomics by label-free liquid chromatography and mass spectrometry. Cancer Sci. 2009, 100, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Scherl, A.; Francois, P.; Converset, V.; Bento, M.; Burgess, J.A.; Sanchez, J.C.; Hochstrasser, D.F.; Schrenzel, J.; Corthals, G.L. Nonredundant mass spectrometry: A strategy to integrate mass spectrometry acquisition and analysis. Proteomics 2004, 4, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Dear, J.W. The application of mass-spectrometry-based protein biomarker discovery to theragnostics. Br. J. Clin. Pharmacol. 2010, 69, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Uyar, D.S.; Huang, Y.W.; Chesnik, M.A.; Doan, N.B.; Mirza, S.P. Comprehensive serum proteomic analysis in early endometrial cancer. J. Proteom. 2021, 234, 104099. [Google Scholar] [CrossRef]
- Song, Y.; Wang, M.; Tong, H.; Tan, Y.; Hu, X.; Wang, K.; Wan, X. Plasma exosomes from endometrial cancer patients contain LGALS3BP to promote endometrial cancer progression. Oncogene 2021, 40, 633–646. [Google Scholar] [CrossRef]
- Lei, D.; Hong, T.; Li, L.; Chen, L.; Luo, X.; Wu, Q.; Liu, Z. Isobaric tags for relative and absolute quantitation-based proteomics analysis of the effect of ginger oil on bisphenol A-induced breast cancer cell proliferation. Oncol. Lett. 2021, 21, 101. [Google Scholar] [CrossRef]
- Njoku, K.; Sutton, C.J.; Whetton, A.D.; Crosbie, E.J. Metabolomic biomarkers for detection, prognosis and identifying recurrence in endometrial cancer. Metabolites 2020, 10, 314. [Google Scholar] [CrossRef]
- Njoku, K.; Chiasserini, D.; Jones, E.R.; Barr, C.E.; O’Flynn, H.; Whetton, A.D.; Crosbie, E.J. Urinary biomarkers and their potential for the non-invasive detection of endometrial cancer. Front. Oncol. 2020, 10, 559016. [Google Scholar] [CrossRef]
- Liu, Z.; Hong, Z.; Qu, P. Proteomic analysis of human endometrial tissues reveals the roles of PI3K/AKT/mTOR pathway and tumor angiogenesis molecules in the pathogenesis of endometrial cancer. Biomed. Res. Int. 2020, 2020, 5273969. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, Q.T.; He, Q.Q. Identification of key transcription factors in endometrial cancer by systems bioinformatics analysis. J. Cell Biochem. 2019, 120, 15443–15454. [Google Scholar] [CrossRef] [PubMed]
- Kacirova, M.; Bober, P.; Alexovic, M.; Tomkova, Z.; Tkacikova, S.; Talian, I.; Mederova, L.; Beresova, D.; Toth, R.; Andrasina, I.; et al. Differential urinary proteomic analysis of endometrial cancer. Physiol. Res. 2019, 68, S483–S490. [Google Scholar] [CrossRef] [PubMed]
- Muinelo-Romay, L.; Casas-Arozamena, C.; Abal, M. Liquid biopsy in endometrial cancer: New opportunities for personalized oncology. Int. J. Mol. Sci. 2018, 19, 2311. [Google Scholar] [CrossRef]
- Ura, B.; Monasta, L.; Arrigoni, G.; Franchin, C.; Radillo, O.; Peterlunger, I.; Ricci, G.; Scrimin, F. A proteomic approach for the identification of biomarkers in endometrial cancer uterine aspirate. Oncotarget 2017, 8, 109536–109545. [Google Scholar] [CrossRef]
- Alonso-Alconada, L.; Santacana, M.; Garcia-Sanz, P.; Muinelo-Romay, L.; Colas, E.; Mirantes, C.; Monge, M.; Cueva, J.; Oliva, E.; Soslow, R.A.; et al. Annexin-A2 as predictor biomarker of recurrent disease in endometrial cancer. Int. J. Cancer 2015, 136, 1863–1873. [Google Scholar] [CrossRef]
- Tarney, C.M.; Wang, G.; Bateman, N.W.; Conrads, K.A.; Zhou, M.; Hood, B.L.; Loffredo, J.; Tian, C.; Darcy, K.M.; Hamilton, C.A.; et al. Biomarker panel for early detection of endometrial cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial. Am. J. Obstet. Gynecol. 2019, 221, 472.E1–472.E10. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bai, S.; Pan, X.; Zhou, R.; Wei, Y.; Zhao, X. Prognostic evaluation of epidermal fatty acid-binding protein and calcyphosine, two proteins implicated in endometrial cancer using a proteomic approach. Int. J. Cancer 2008, 123, 2377–2383. [Google Scholar] [CrossRef]
- Wang, N.; Li, L. Exploring the precursor ion exclusion feature of liquid chromatography-electrospray ionization quadrupole time-of-flight mass spectrometry for improving protein identification in shotgun proteome analysis. Anal. Chem. 2008, 80, 4696–4710. [Google Scholar] [CrossRef]
- Chen, H.S.; Rejtar, T.; Andreev, V.; Moskovets, E.; Karger, B.L. Enhanced characterization of complex proteomic samples using LC-MALDI MS/MS: Exclusion of redundant peptides from MS/MS analysis in replicate runs. Anal. Chem. 2005, 77, 7816–7825. [Google Scholar] [CrossRef]
- DeSouza, L.V.; Grigull, J.; Ghanny, S.; Dube, V.; Romaschin, A.D.; Colgan, T.J.; Siu, K.W. Endometrial carcinoma biomarker discovery and verification using differentially tagged clinical samples with multidimensional liquid chromatography and tandem mass spectrometry. Mol. Cell Proteom. 2007, 6, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Byrjalsen, I.; Mose Larsen, P.; Fey, S.J.; Nilas, L.; Larsen, M.R.; Christiansen, C. Two-dimensional gel analysis of human endometrial proteins: Characterization of proteins with increased expression in hyperplasia and adenocarcinoma. Mol. Hum. Reprod. 1999, 5, 748–756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maxwell, G.L.; Hood, B.L.; Day, R.; Chandran, U.; Kirchner, D.; Kolli, V.S.; Bateman, N.W.; Allard, J.; Miller, C.; Sun, M.; et al. Proteomic analysis of stage I endometrial cancer tissue: Identification of proteins associated with oxidative processes and inflammation. Gynecol. Oncol. 2011, 121, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Belkina, A.C.; Denis, G.V. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 2012, 12, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Bjune, J.I.; Haugen, C.; Gudbrandsen, O.; Nordbø, O.P.; Nielsen, H.J.; Våge, V.; Njølstad, P.R.; Sagen, J.V.; Dankel, S.N.; Mellgren, G. IRX5 regulates adipocyte amyloid precursor protein and mitochondrial respiration in obesity. Int. J. Obes. 2019, 43, 2151–2162. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Rimm, E.B.; Curhan, G.C.; Kraft, P.; Hunter, D.J.; Hu, F.B.; van Dam, R.M. Obesity susceptibility loci and uncontrolled eating, emotional eating and cognitive restraint behaviors in men and women. Obesity 2014, 22, E135–E141. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.V.; Gordillo, R.; Zhu, Y.; Ali, A.; Zhang, C.; Wang, X.; Shao, M.; Zhang, Z.; Iyengar, P.; et al. Adipocyte Xbp1s overexpression drives uridine production and reduces obesity. Mol. Metab. 2018, 11, 1–17. [Google Scholar] [CrossRef]
- Hooton, H.; Dubern, B.; Henegar, C.; Paternoster, L.; Nohr, E.A.; Alili, R.; Rousseau, F.; Pelloux, V.; Galan, P.; Hercberg, S.; et al. Association between CST3 rs2424577 polymorphism and corpulence related phenotypes during lifetime in populations of European ancestry. Obes. Facts. 2011, 4, 131–144. [Google Scholar] [CrossRef]
- Steinberg, G.R. Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle 2007, 6, 888–894. [Google Scholar] [CrossRef]
- Zanardini, R.; Benussi, L.; Fostinelli, S.; Saraceno, C.; Ciani, M.; Borroni, B.; Padovani, A.; Binetti, G.; Ghidoni, R. Serum C-Peptide, Visfatin, Resistin, and Ghrelin are Altered in Sporadic and GRN-Associated Frontotemporal Lobar Degeneration. J. Alzheimers Dis. 2018, 61, 1053–1060. [Google Scholar] [CrossRef]
- Urano, T.; Shiraki, M.; Sasaki, N.; Ouchi, Y.; Inoue, S. SLC25A24 as a novel susceptibility gene for low fat mass in humans and mice. J. Clin. Endocrinol. Metab. 2015, 100, E655–E663. [Google Scholar] [CrossRef] [PubMed]
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef] [PubMed]
- Boroń, D.; Zmarzły, N.; Wierzbik-Strońska, M.; Rosińczuk, J.; Mieszczański, P.; Grabarek, B.O. Recent Multiomics Approaches in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 1237. [Google Scholar] [CrossRef]
- Henry, J.L.; Coggin, D.L.; King, C.R. High-level expression of the ribosomal protein L19 in human breast tumors that overexpress erbB-2. Cancer Res. 1993, 53, 1403–1408. [Google Scholar]
- Barnard, G.F.; Staniunas, R.J.; Mori, M.; Puder, M.; Jessup, M.J.; Steele, G.D., Jr.; Chen, L.B. Gastric and hepatocellular carcinomas do not overexpress the same ribosomal protein messenger RNAs as colonic carcinoma. Cancer Res. 1993, 53, 4048–4052. [Google Scholar]
- Wang, H.; Zhao, L.N.; Li, K.Z.; Ling, R.; Li, X.J.; Wang, L. Overexpression of ribosomal protein L15 is associated with cell proliferation in gastric cancer. BMC Cancer 2006, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Nadano, D.; Hidaka, E.; Higuchi, K.; Kawakubo, M.; Sato, T.A.; Nakayama, J. Differential expression of ribosomal proteins in human normal and neoplastic colorectum. J. Histochem. Cytochem. 2003, 51, 567–574. [Google Scholar] [CrossRef]
- El Khoury, W.; Nasr, Z. Deregulation of ribosomal proteins in human cancers. Biosci. Rep. 2021, 41, BSR20211577. [Google Scholar] [CrossRef]
- Vaarala, M.H.; Porvari, K.S.; Kyllonen, A.P.; Mustonen, M.V.; Lukkarinen, O.; Vihko, P.T. Several genes encoding ribosomal proteins are over-expressed in prostate-cancer cell lines: Confirmation of L7a and L37 over-expression in prostate-cancer tissue samples. Int. J. Cancer 1998, 78, 27–32. [Google Scholar] [CrossRef]
- Al-Juboori, A.A.A.; Ghosh, A.; Jamaluddin, M.F.B.; Kumar, M.; Sahoo, S.S.; Syed, S.M.; Nahar, P.; Tanwar, P.S. Proteomic Analysis of Stromal and Epithelial Cell Communications in Human Endometrial Cancer Using a Unique 3D Co-Culture Model. Proteomics 2019, 19, e1800448. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Cannabinoid receptor expression in estrogen-dependent and estrogen-independent endometrial cancer. J. Recept. Signal Transduct. Res. 2018, 38, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Expression of the putative cannabinoid receptor GPR55 is increased in endometrial carcinoma. Histochem. Cell Biol. 2021, 156, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Pawałowska, M.; Lubin, J.; Markowska, J. Signalling pathways in endometrial cancer. Contemp. Oncol. 2014, 18, 143–148. [Google Scholar] [CrossRef]
- Moukarzel, L.A.; Ferrando, L.; Stylianou, A.; Lobaugh, S.; Wu, M.; Nobre, S.P.; Iasonos, A.; Zoppoli, G.; Giri, D.D.; Abu-Rustum, N.R.; et al. Impact of obesity and white adipose tissue inflammation on the omental microenvironment in endometrial cancer. Cancer 2022, 128, 3297–3309. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Zhang, Q.; Wei, S.; Shi, R.; Zhao, R.; An, L.; Grose, R.; Feng, D.; Wang, H. Single-cell sequencing reveals the heterogeneity and intratumoral crosstalk in human endometrial cancer. Cell Prolif. 2022, 55, e13249. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, C. Bioinformatics for Comparative Proteomics; Humana Press: New York, NY, USA, 2011; 387p. [Google Scholar]
- Zervou, S.; Yin, X.; Nabeebaccus, A.A.; O’Brien, B.A.; Cross, R.L.; McAndrew, D.J.; Atkinson, R.A.; Eykyn, T.R.; Mayr, M.; Neubauer, S.; et al. Proteomic and metabolomic changes driven by elevating myocardial creatine suggest novel metabolic feedback mechanisms. Amino Acids 2016, 48, 1969–1981. [Google Scholar] [CrossRef]

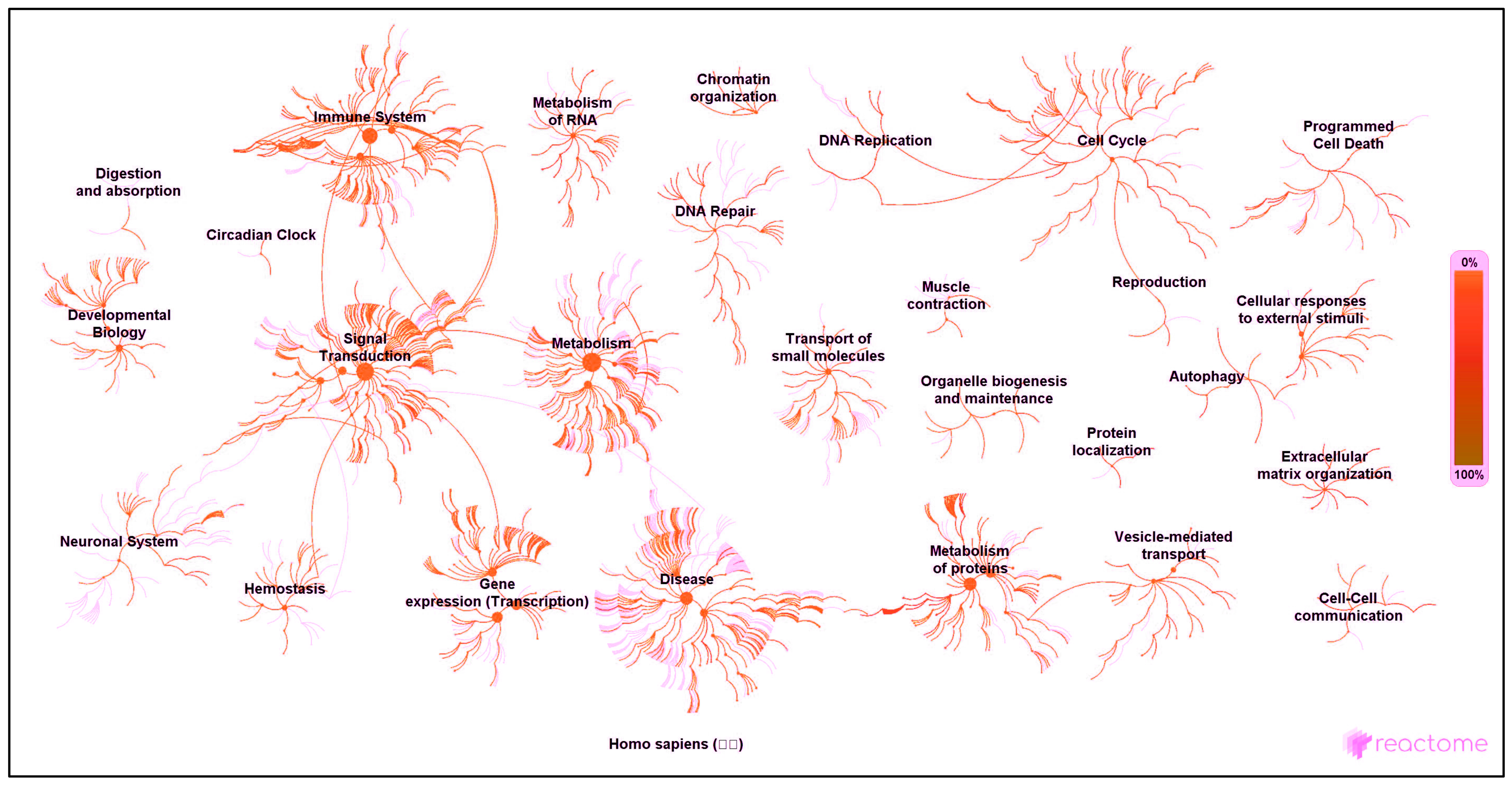

| Reactome® Network | Proteins Identified in Network * | PubMed ID (Where Available) ** |
|---|---|---|
| Autophagy | There are no proteins to report in this category. | |
| Cell cycle | There are no proteins to report in this category. | |
| Cell–cell communication | There are no proteins to report in this category. | |
| Cellular responses to external stimuli | There are no proteins to report in this category. | |
| Chromatin organisation | There are no proteins to report in this category. | |
| Circadian clock | There are no proteins to report in this category. | |
| Developmental biology | CLTB 2; GRN 2 | [44]; [45] |
| Digestion and absorption | There are no proteins to report in this category. | |
| Disease | APP B; HEXA B | [46]; [47] |
| DNA repair | There are no proteins to report in this category. | |
| DNA replication | There are no proteins to report in this category. | |
| Extracellular matrix organisation | APP B | [46] |
| Gene expression (transcription) | GRN 2 | [45] |
| Haemostasis | APP B; GRN 2 | [46]; [45] |
| Immune system | APP B; CST3 2; GRN 2; IGKV-I B; IGKV-II B; IGLV-III 1 | [46]; [48]; [45]; no ref.; no ref.; no ref. |
| Metabolism | CAD 2; COPE 1; HEXA B; QARS B; SIAE B | [49]; no ref.; [47]; [50]; [51] |
| Metabolism of protein | APP B; COPE 1; CST3 2; QARS B; WARS B | [46]; no ref.; [47]; [50]; [52] |
| Metabolism of RNA | QARS B | [50] |
| Muscle contraction | CAD 2; GRN 2; MYH8 2 | [49]; [45]; [53] |
| Neuronal system | There are no proteins to report in this category. | |
| Organelle biogenesis and maintenance | There are no proteins to report in this category. | |
| Programmed cell death | CAD 2 | [49] |
| Protein localisation | APP B | [46] |
| Reproduction | There are no proteins to report in this category. | |
| Signal transduction | APP B | [46] |
| Transport of small molecules | There are no proteins to report in this category. | |
| Vesicle-mediated transport | APP B; CLTB 2; COPE 1; GOLIM4 B; SCARB2 B | [46]; [44]; no ref.; [54]; [55] |
| Found by GO pathway analysis but missing from Reactome® network analysis | BOD1L1 2; C14orf142 1; C9orf142 2; CCDC13 2; CNPY4 B; DKFZp313H139 2; E7EPZ9-DECOY 2; FAM169A 2; HN1L B; J3QQ66-DECOY 2; PIGT 2; PLCL1 2; PMFBP1 2; PPM1G B; PPP1R2 B; Q8WZ42-12-DECOY 2; Q8WZ42-2-DECOY 2; SARS2 2; SCPEP1 2; SLC25A24 2; ZC3H4 2 | [56]; [57]; [58]; [59]; [60]; no ref.; no ref.; [61]; [54]; no ref.; [62]; [63]; [64]; [65]; [66]; no ref.; no ref.; [67]; [68]; [69]; [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, A.H.; Konje, J.C.; Ayakannu, T. Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach. Cancers 2023, 15, 4665. https://doi.org/10.3390/cancers15184665
Taylor AH, Konje JC, Ayakannu T. Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach. Cancers. 2023; 15(18):4665. https://doi.org/10.3390/cancers15184665
Chicago/Turabian StyleTaylor, Anthony H., Justin C. Konje, and Thangesweran Ayakannu. 2023. "Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach" Cancers 15, no. 18: 4665. https://doi.org/10.3390/cancers15184665
APA StyleTaylor, A. H., Konje, J. C., & Ayakannu, T. (2023). Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach. Cancers, 15(18), 4665. https://doi.org/10.3390/cancers15184665






