The Role of γδ T-Lymphocytes in Glioblastoma: Current Trends and Future Directions
Abstract
:Simple Summary
Abstract
1. Introduction
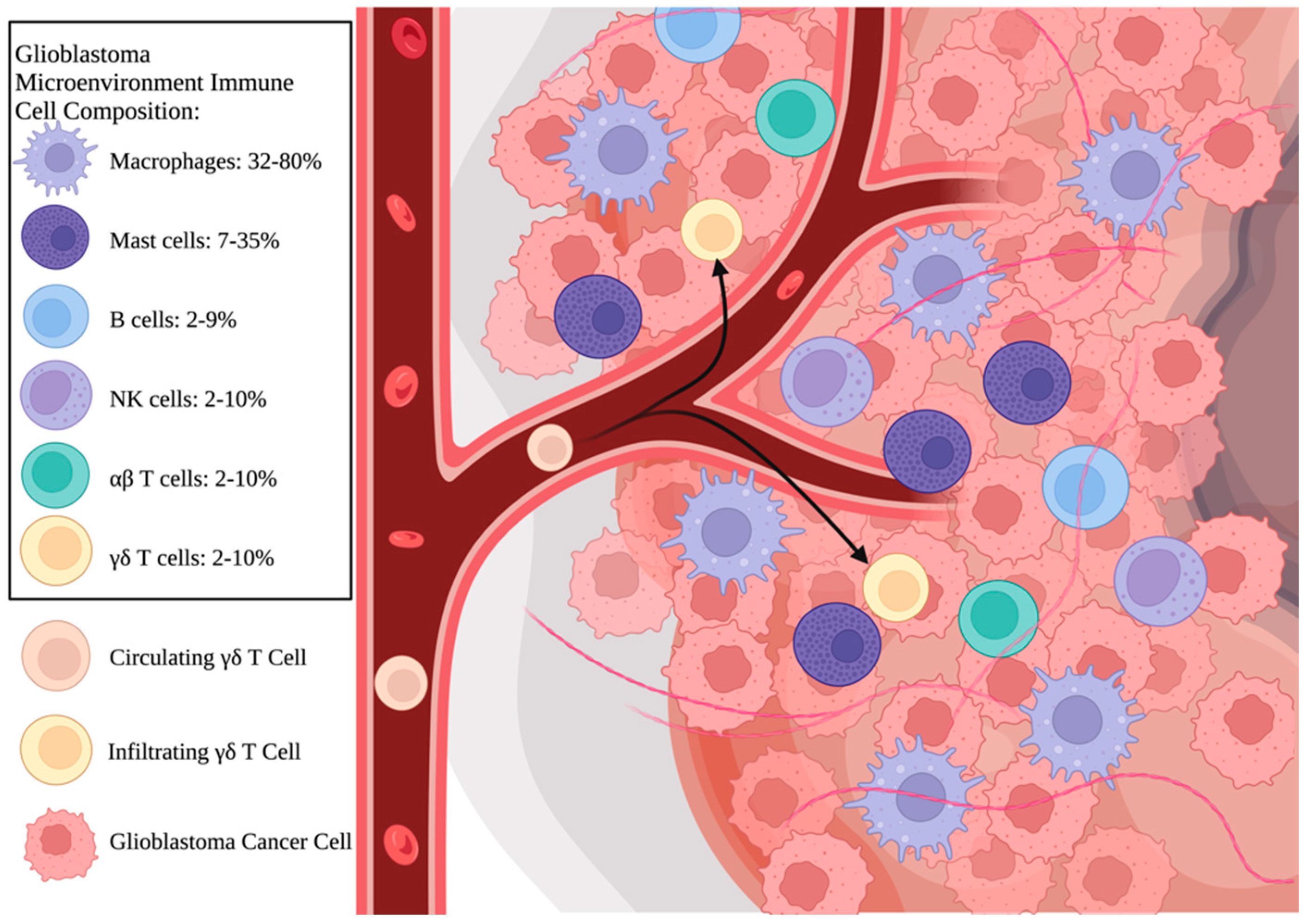
2. Introduction to γδ T Cells
3. Antitumor Activity of γδ T Cells in GBM
4. Phosphoantigen Stimulation and Combined Therapies
5. Challenges to Clinical Use
6. Protumor Activity of γδ T Cells
7. Effect of TME Hypoxia on γδ T Cells
8. Future Directions
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. Available online: https://www.nejm.org/ (accessed on 1 October 2023).
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma a randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Almeida, J.P.; Chaichana, K.L.; Rincon-Torroella, J.; Quinones-Hinojosa, A. The Value of Extent of Resection of Glioblastomas: Clinical Evidence and Current Approach. Curr. Neurol. Neurosci. Rep. 2015, 15, 517. [Google Scholar] [CrossRef]
- Pearson, J.R.D.; Cuzzubbo, S.; McArthur, S.; Durrant, L.G.; Adhikaree, J.; Tinsley, C.J.; Pockley, A.G.; McArdle, S.E.B. Immune Escape in Glioblastoma Multiforme and the Adaptation of Immunotherapies for Treatment. Front. Immunol. 2020, 11, 582106. [Google Scholar] [CrossRef]
- Scharping, N.E.; Delgoffe, G.M. Tumor microenvironment metabolism: A new checkpoint for anti-tumor immunity. Vaccines 2016, 4, 46. [Google Scholar] [CrossRef]
- Pombo Antunes, A.R.; Scheyltjens, I.; Duerinck, J.; Neyns, B.; Movahedi, K.; Van Ginderachter, J.A. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife 2020, 9, e52176. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef]
- Jarry, U.; Chauvin, C.; Joalland, N.; Léger, A.; Minault, S.; Robard, M.; Bonneville, M.; Oliver, L.; Vallette, F.M.; Vié, H.; et al. Stereotaxic administrations of allogeneic human Vγ9Vδ2 T cells efficiently control the development of human glioblastoma brain tumors. Oncoimmunology 2016, 5, e1168554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hambardzumyan, D. Immune microenvironment in glioblastoma subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chang, C.; Rashidi, A.; Miska, J.; Zhang, P.; Pituch, K.C.; Hou, D.; Xiao, T.; Fischietti, M.; Kang, S.J.; Appin, C.L.; et al. Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol. Res. 2019, 7, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lage, M.; Lynch, T.M.; Bi, Y.; Cocito, C.; Way, G.P.; Pal, S.; Haller, J.; Yan, R.E.; Ziober, A.; Nguyen, A.; et al. Immune landscapes associated with different glioblastoma molecular subtypes. Acta Neuropathol. Commun. 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Song, Z.; Zhang, T.; He, X.; Huang, K.; Zhang, Q.; Shen, J.; Pan, J. Identification of Immune Cell Infiltration and Immune-Related Genes in the Tumor Microenvironment of Glioblastomas. Front. Immunol. 2020, 11, 585034. [Google Scholar] [CrossRef]
- Lee, M.; Park, C.; Woo, J.; Kim, J.; Kho, I.; Nam, D.-H.; Park, W.-Y.; Kim, Y.-S.; Kong, D.-S.; Lee, H.W.; et al. Preferential Infiltration of Unique Vγ9Jγ2-Vδ2 T Cells Into Glioblastoma Multiforme. Front. Immunol. 2019, 10, 555. [Google Scholar] [CrossRef]
- Willcox, C.R.; Mohammed, F.; Willcox, B.E. The distinct MHC-unrestricted immunobiology of innate-like and adaptive-like human γδ T cell subsets—Nature’s CAR-T cells. Immunol. Rev. Vol. 2020, 298, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Kalyan, S.; Oberg, H.-H.; Wesch, D. Human Vδ2 versus non-Vδ2 γδ T cells in antitumor immunity. Oncoimmunology 2013, 2, e23304. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, X.; Yang, Y.; Qu, Y.; Zhu, X.; Ma, W.; Duan, J.; Xue, J.; Yang, H.; Huang, J.-W.; et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature 2023, 621, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Presti, E.L.; Toia, F.; Oieni, S.; Buccheri, S.; Turdo, A.; Mangiapane, L.R.; Campisi, G.; Caputo, V.; Todaro, M.; Stassi, G.; et al. Squamous cell tumors recruit γδ T cells producing either IL17 or IFNγ depending on the tumor stage. Cancer Immunol. Res. 2017, 5, 397–407. [Google Scholar] [CrossRef]
- de Lima, K.A.; Rustenhoven, J.; Da Mesquita, S.; Wall, M.; Salvador, A.F.; Smirnov, I.; Cebinelli, G.M.; Mamuladze, T.; Baker, W.; Papadopoulos, Z.; et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 2020, 21, 1421–1429. [Google Scholar] [CrossRef]
- de Vries, N.L.; van de Haar, J.; Veninga, V.; Chalabi, M.; Ijsselsteijn, M.E.; van der Ploeg, M.; Bulk, J.v.D.; Ruano, D.; Berg, J.G.v.D.; Haanen, J.B.; et al. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature 2023, 613, 743–750. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer immunotherapy with γδ T cells: Many paths ahead of us. Cell Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, C.; Joalland, N.; Perroteau, J.; Jarry, U.; Lafrance, L.; Willem, C.; Retière, C.; Oliver, L.; Gratas, C.; Gautreau-Rolland, L.; et al. NKG2D Controls Natural Reactivity of Vγ9Vδ2 T Lymphocytes against Mesenchymal Glioblastoma Cells. Clin. Cancer Res. 2019, 25, 7218–7228. [Google Scholar] [CrossRef] [PubMed]
- Chitadze, G.; Lettau, M.; Luecke, S.; Wang, T.; Janssen, O.; Fürst, D.; Mytilineos, J.; Wesch, D.; Oberg, H.-H.; Held-Feindt, J.; et al. NKG2D- and T-cell receptor-dependent lysis of malignant glioma cell lines by human γδ T cells: Modulation by temozolomide and A disintegrin and metalloproteases 10 and 17 inhibitors. Oncoimmunology 2016, 5, e1093276. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Schneider, H.; Silginer, M.; Steinle, A.; Pruschy, M.; Polić, B.; Weller, M.; Roth, P. NKG2D-Dependent Antitumor Effects of Chemotherapy and Radiotherapy against Glioblastoma. Clin. Cancer Res. 2018, 24, 882–895. [Google Scholar] [CrossRef]
- Flüh, C.; Chitadze, G.; Adamski, V.; Hattermann, K.; Synowitz, M.; Kabelitz, D.; Held-Feindt, J. NKG2D ligands in glioma stem-like cells: Expression in situ and in vitro. Histochem. Cell Biol. 2018, 149, 219–233. [Google Scholar] [CrossRef]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef]
- Bryant, N.L.; Suarez-Cuervo, C.; Gillespie, G.Y.; Markert, J.M.; Nabors, L.B.; Meleth, S.; Lopez, R.D.; Lamb, L.S., Jr. Characterization and immunotherapeutic potential of γδ T-cells in patients with glioblastoma. Neuro. Oncol. 2009, 11, 357–367. [Google Scholar] [CrossRef]
- Bryant, N.L.; Gillespie, G.Y.; Lopez, R.D.; Markert, J.M.; Cloud, G.A.; Langford, C.P.; Arnouk, H.; Su, Y.; Haines, H.L.; Suarez-Cuervo, C.; et al. Preclinical evaluation of ex vivo expanded/activated γδ T cells for immunotherapy of glioblastoma multiforme. J. Neurooncol. 2011, 101, 179–188. [Google Scholar] [CrossRef]
- Cimini, E.; Piacentini, P.; Sacchi, A.; Gioia, C.; Leone, S.; Lauro, G.; Martini, F.; Agrati, C. Zoledronic Acid Enhances Vδ2 T-Lymphocyte Antitumor Response to Human Glioma Cell Lines. Int. J. Immunopathol. Pharmacol. 2011, 24, 139–148. [Google Scholar] [CrossRef]
- Lamb, L.S., Jr.; Bowersock, J.; Dasgupta, A.; Gillespie, G.Y.; Su, Y.; Johnson, A.; Spencer, H.T. Engineered Drug Resistant γδ T Cells Kill Glioblastoma Cell Lines during a Chemotherapy Challenge: A Strategy for Combining Chemo- and Immunotherapy. PLoS ONE 2013, 8, e51805. [Google Scholar] [CrossRef]
- Nakazawa, T.; Nakamura, M.; Park, Y.S.; Motoyama, Y.; Hironaka, Y.; Nishimura, F.; Nakagawa, I.; Yamada, S.; Matsuda, R.; Tamura, K.; et al. Cytotoxic human peripheral blood-derived γδT cells kill glioblastoma cell lines: Implications for cell-based immunotherapy for patients with glioblastoma. J. Neuro-Oncology 2014, 116, 31–39. [Google Scholar] [CrossRef]
- Beck, B.H.; Kim, H.; O’brien, R.; Jadus, M.R.; Gillespie, G.Y.; Cloud, G.A.; Hoa, N.T.; Langford, C.P.; Lopez, R.D.; Harkins, L.E.; et al. Dynamics of Circulating γδ T Cell Activity in an Immunocompetent Mouse Model of High-Grade Glioma. PLoS ONE 2015, 10, e0122387. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Nakamura, M.; Matsuda, R.; Nishimura, F.; Park, Y.S.; Motoyama, Y.; Hironaka, Y.; Nakagawa, I.; Yokota, H.; Yamada, S.; et al. Antitumor effects of minodronate, a third-generation nitrogen-containing bisphosphonate, in synergy with γδT cells in human glioblastoma in vitro and in vivo. J. Neurooncol. 2016, 129, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Joalland, N.; Chauvin, C.; Oliver, L.; Vallette, F.M.; Pecqueur, C.; Jarry, U.; Scotet, E. IL-21 Increases the Reactivity of Allogeneic Human Vγ9Vδ2 T Cells Against Primary Glioblastoma Tumors. J. Immunother. 2018, 41, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, H.-J.; Kim, C.W.; Kim, H.C.; Jung, Y.; Lee, H.-S.; Lee, Y.; Ju, Y.S.; Oh, J.E.; Park, S.-H.; et al. Tumor hypoxia represses γδ T cell-mediated antitumor immunity against brain tumors. Nat. Immunol. 2021, 22, 336–346. [Google Scholar] [CrossRef]
- Rosso, D.A.; Rosato, M.; Iturrizaga, J.; González, N.; Shiromizu, C.M.; Keitelman, I.A.; Coronel, J.V.; Gómez, F.D.; Amaral, M.M.; Rabadan, A.T.; et al. Glioblastoma cells potentiate the induction of the Th1-like profile in phosphoantigen-stimulated γδ T lymphocytes. J. Neurooncol. 2021, 153, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S.; Pereboeva, L.; Youngblood, S.; Gillespie, G.Y.; Nabors, L.B.; Markert, J.M.; Dasgupta, A.; Langford, C.; Spencer, H.T. A combined treatment regimen of MGMT-modified γδ T cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci. Rep. 2021, 11, 21133. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Fleury-Cappellesso, S.; Gadelorge, M.; Dietrich, G.; Bourin, P.; Fournié, J.; Poupot, R. A regulatory cross-talk between Vγ9Vδ2 T lymphocytes and mesenchymal stem cells. Eur. J. Immunol. 2009, 39, 752–762. [Google Scholar] [CrossRef]
- Kazen, A.R.; Adams, E.J. Evolution of the V, D, and J gene segments used in the primate γδ T-cell receptor reveals a dichotomy of conservation and diversity. Proc. Natl. Acad. Sci. USA 2011, 108, E332–E340. [Google Scholar] [CrossRef]
- Silva-Santos, B.; Serre, K.; Norell, H. γδT cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Presti, E.L.; Dieli, F.; Meraviglia, S. Tumor-infiltrating γδ T lymphocytes: Pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front. Immunol. 2014, 5, 607. [Google Scholar] [CrossRef]
- Makkouk, A.; Yang, X.; Barca, T.; Lucas, A.; Turkoz, M.; Wong, J.T.S.; Nishimoto, K.P.; Brodey, M.M.; Tabrizizad, M.; Gundurao, S.R.Y.; et al. Off-the-shelf Vδ 1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, e003441. [Google Scholar] [CrossRef]
- Siegers, G.M.; Lamb, L.S. Cytotoxic and regulatory properties of circulating Vδ1+ γδ t cells: A new player on the cell therapy field? Mol. Ther. 2014, 22, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.J.; Tokuyama, H.; Mattarollo, S.R.; Hagi, T.; Suzuki, K.; Yokokawa, K.; Nieda, M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 2011, 105, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Wevers, E.; Schwartz, T.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; et al. Tumor-Infiltrating γδ T Lymphocytes Predict Clinical Outcome in Human Breast Cancer. J. Immunol. 2012, 189, 5029–5036. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. γδT17 Cells Promote the Accumulation and Expansion of Myeloid-Derived Suppressor Cells in Human Colorectal Cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Shah, S.U.; Shrikhande, S.V.; Goel, M.; Dikshit, R.P.; Chiplunkar, S.V. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 2016, 139, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Cui, X.; Xu, Z.; Zhao, Z.; Sui, D.; Ren, X.; Huang, Q.; Qin, J.; Hao, L.; Wang, Z.; Shen, L.; et al. Analysis of CD137l and IL-17 expression in tumor tissue as prognostic indicators for gliblastoma. Int. J. Biol. Sci. 2013, 9, 134–141. [Google Scholar] [CrossRef]
- Ribot, J.C.; Debarros, A.; Pang, D.J.; Neves, J.F.; Peperzak, V.; Roberts, S.J.; Girardi, M.; Borst, J.; Hayday, A.C.; Pennington, D.J.; et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat. Immunol. 2009, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-infiltrating IL-17-producing γδ T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef]
- Caccamo, N.; La Mendola, C.; Orlando, V.; Meraviglia, S.; Todaro, M.; Stassi, G.; Sireci, G.; Fournié, J.J.; Dieli, F. Differentiation, phenotype, and function of interleukin-17–producing human Vγ9Vδ2 T cells. Blood 2011, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Prud’Homme, G.J. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- van Beelen, A.J.; Zelinkova, Z.; Taanman-Kueter, E.W.; Muller, F.J.; Hommes, D.W.; Zaat, S.A.; Kapsenberg, M.L.; de Jong, E.C. Stimulation of the Intracellular Bacterial Sensor NOD2 Programs Dendritic Cells to Promote Interleukin-17 Production in Human Memory T Cells. Immunity 2007, 27, 660–669. [Google Scholar] [CrossRef]
- Zheng, Q.; Diao, S.; Wang, Q.; Zhu, C.; Sun, X.; Yin, B.; Zhang, X.; Meng, X.; Wang, B. IL-17A promotes cell migration and invasion of glioblastoma cells via activation of PI3K/AKT signalling pathway. J. Cell Mol. Med. 2019, 23, 357–369. [Google Scholar] [CrossRef]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009, 114, 1141–1149. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef]
- Gu, F.-M.; Gao, Q.; Shi, G.-M.; Zhang, X.; Wang, J.; Jiang, J.-H.; Wang, X.-Y.; Shi, Y.-H.; Ding, Z.-B.; Fan, J.; et al. Intratumoral IL-17+ Cells and Neutrophils show Strong Prognostic Significance in Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2012, 19, 2506–2514. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Colwell, N.; Larion, M.; Giles, A.J.; Seldomridge, A.N.; Sizdahkhani, S.; Gilbert, M.R.; Park, D.M. Hypoxia in the glioblastoma microenvironment: Shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017, 19, 887–896. [Google Scholar] [CrossRef]
- Beppu, T.; Kamada, K.; Yoshida, Y.; Arai, H.; Ogasawara, K.; Ogawa, A. Change of oxygen pressure in glioblastoma tissue under various conditions. J. Neuro-Oncology 2002, 58, 47–52. [Google Scholar] [CrossRef]
- Henze, A.-T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef]
- Ohta, A.; Diwanji, R.; Kini, R.; Subramanian, M.; Ohta, A.; Sitkovsky, M. In vivo T cell activation in lymphoid tissues is inhibited in the oxygen-poor microenvironment. Front. Immunol. 2011, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, S.K.; Chaukar, D.; Chiplunkar, S.V. Hypoxia regulates the differentiation and anti-tumor effector functions of γδT cells in oral cancer. Clin. Exp. Immunol. 2020, 201, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Siegers, G.M.; Dutta, I.; Lai, R.; Postovit, L.-M. Functional plasticity of Gamma delta T cells and breast tumor targets in hypoxia. Front. Immunol. 2018, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Xing, Y.; Sidhu, I.; Subudhi, I.; Mansfield, K.P.; Hsieh, B.; Biancur, D.E.; Larsen, S.B.; Cammer, M.; Li, D.; et al. Interleukin-17 governs hypoxic adaptation of injured epithelium. Science 2022, 377, 170. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]

| Authors, Year | Methods | Cell Lines | Questions Addressed | Results |
|---|---|---|---|---|
| Bryant et al., 2009 [28] | In vitro | U251MG, D54MG, U373MG, U87MG, normal human astrocyte cultures and primary GBM cultures from operative specimens | What is the innate role of γδ T cells in the immune response to GBM and how potent are they therapeutically against GBM cell lines and primary GBM cultures? |
|
| Bryant et al., 2011 [29] | In vivo | U251MG, U251ffluc | What is the immunotherapeutic potential of γδ T cells following in vivo injection? How effectively do γδ T cells migrate to and infiltrate GBM tumor xenografts in immunodeficient mice? |
|
| Cimini et al., 2011 [30] | In vitro | T70, U251, U373 | Does bisphosphonate treatment sensitize glioma cells to lysis by γδ T cells expanded in vitro? |
|
| Lamb et al., 2013 [31] | In vitro | U87, U373, SNB-19 | What are the effects of combining TMZ-mediated cytotoxicity and γδ T cell immunotherapy for GBM? |
|
| Nakazawa et al., 2014 [32] | In vitro | U87MG, U138MG, A172 | How potent is γδ T cell immunotherapy for GBM patients and how can the γδ T-cell-mediated killing of tumors be enhanced? |
|
| Beck et al., 2015 [33] | In vivo | GL261 | What are the properties of γδ T cell activity against high-grade glioma in fully immunocompetent mouse models? |
|
| Chitadze et al., 2016 [24] | In vitro | A172, T98G, U87MG, U251MG | What is the effect of metalloprotease inhibitors and TMZ on NKG2DL expression in human GBM cell lines? |
|
| Jarry et al., 2016 [8] | In vivo | U87MG, GBM-10 (primary GBM cell culture) | What is the efficacy of stereotactic γδ T cell injection immunotherapy in murine models of human GBM tumors and in primary GBM cells? |
|
| Nakazawa et al., 2016 [34] | In vitro and in vivo | U87MG, U138MG | What are the synergistic antitumor effects, if any, of minodronate on the γδ T-cell-mediated killing of GBM cells in vitro and in vivo? |
|
| Joalland et al., 2018 [35] | In vivo | GBM-1 (primary human GBM cell culture), U87MG | What effect does IL-21 sensitization have on the cytolytic activity of γδ T cells in GBM cells? |
|
| Lee et al., 2019 [13] | In vitro | Primary GBM cultures from operative specimens | What characterizes the identity of GBM-infiltrating γδ T cells? How do infiltrating γδ T cells interact with the GBM TME? |
|
| Chauvin et al., 2019 [23] | In vitro and in vivo | GBM-1, GBM-10, human primary GBM cultures from surgical specimens | What are the different immuno-reactivities of Vγ9Vδ2 T cells against various patient-derived human GBM cells? |
|
| Park et al., 2021 [36] | In vitro and in vivo | GL261, U87MG | How does tumor hypoxia affect γδ T cell antitumor activity? |
|
| Rosso et al., 2021 [37] | In vitro | U251, U373, human primary GBM cultures from surgical specimens | How do phosphoantigen-stimulated γδ T cells respond to human GBM cells in vitro? |
|
| Lamb et al., 2021 [38] | In vitro and in vivo | Patient-derived tumor xenografts | Do TMZ-resistant γδ T cells exhibit improved survival and efficacy against high-grade gliomas under therapeutic concentrations of TMZ? |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmedna, T.; Khela, H.; Weber-Levine, C.; Azad, T.D.; Jackson, C.M.; Gabrielson, K.; Bettegowda, C.; Rincon-Torroella, J. The Role of γδ T-Lymphocytes in Glioblastoma: Current Trends and Future Directions. Cancers 2023, 15, 5784. https://doi.org/10.3390/cancers15245784
Ahmedna T, Khela H, Weber-Levine C, Azad TD, Jackson CM, Gabrielson K, Bettegowda C, Rincon-Torroella J. The Role of γδ T-Lymphocytes in Glioblastoma: Current Trends and Future Directions. Cancers. 2023; 15(24):5784. https://doi.org/10.3390/cancers15245784
Chicago/Turabian StyleAhmedna, Taha, Harmon Khela, Carly Weber-Levine, Tej D. Azad, Christopher M. Jackson, Kathleen Gabrielson, Chetan Bettegowda, and Jordina Rincon-Torroella. 2023. "The Role of γδ T-Lymphocytes in Glioblastoma: Current Trends and Future Directions" Cancers 15, no. 24: 5784. https://doi.org/10.3390/cancers15245784
APA StyleAhmedna, T., Khela, H., Weber-Levine, C., Azad, T. D., Jackson, C. M., Gabrielson, K., Bettegowda, C., & Rincon-Torroella, J. (2023). The Role of γδ T-Lymphocytes in Glioblastoma: Current Trends and Future Directions. Cancers, 15(24), 5784. https://doi.org/10.3390/cancers15245784





