MRI-Guided Radiation Therapy for Prostate Cancer: The Next Frontier in Ultrahypofractionation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Limitations of CT-Based Treatment Planning and Delivery
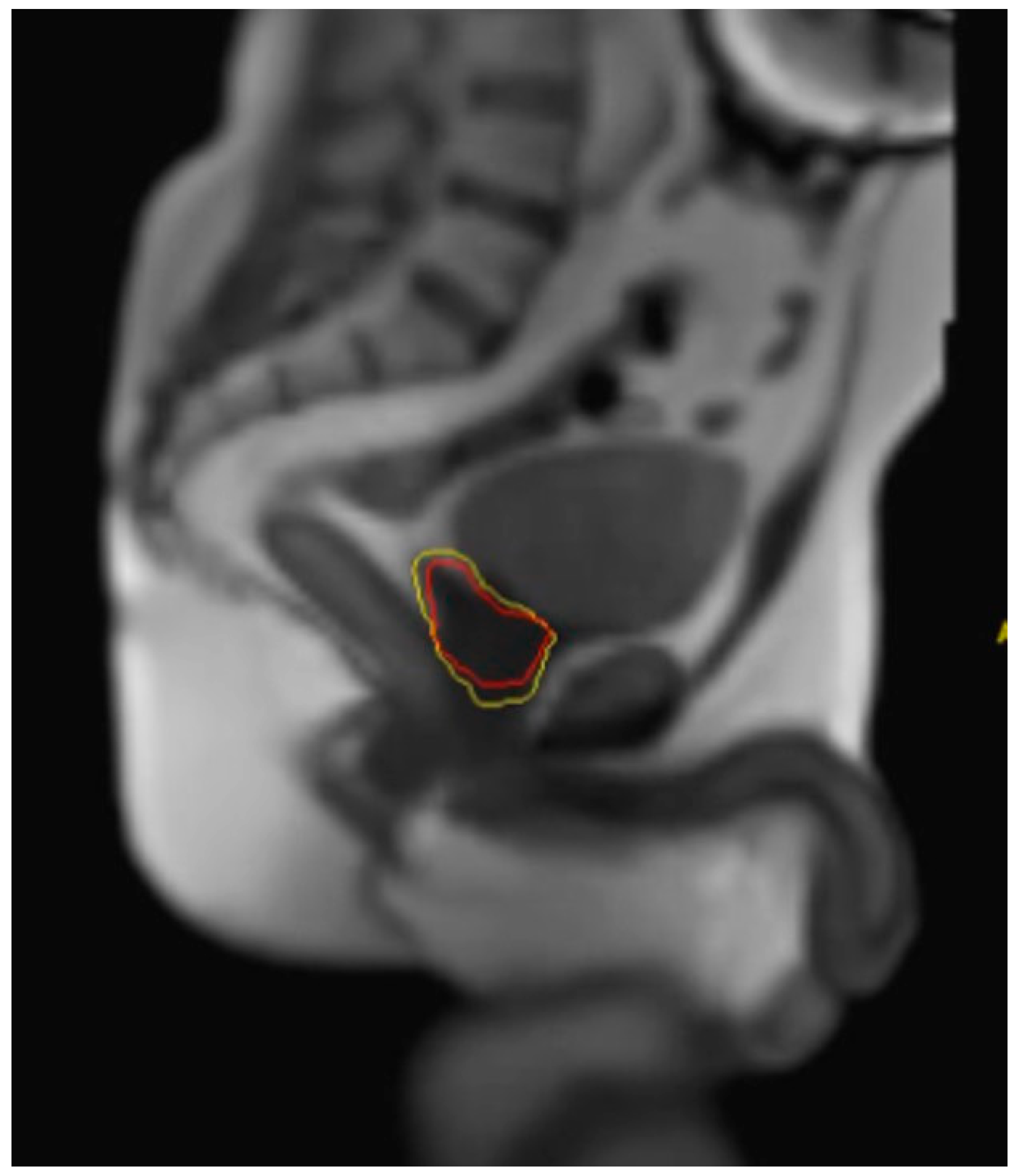
3. Advantages of MRIgRT
4. Limitations of MRIgRT
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Key Statistics for Prostate Cancer|Prostate Cancer Facts. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 19 July 2023).
- NCCN Clinical Practice Guidelines in Oncology Prostate Cancer Version 2.2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 19 July 2023).
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; Reiter, R.E.; Rettig, M.; Calais, J.; Nickols, N.G.; et al. A Systematic Review and Meta-Analysis of Local Salvage Therapies after Radiotherapy for Prostate Cancer (MASTER). Eur. Urol. 2021, 80, 280–292. [Google Scholar] [CrossRef]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-Dose versus Control-Dose Conformal Radiotherapy for Prostate Cancer: Long-Term Results from the MRC RT01 Randomised Controlled Trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar] [CrossRef]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.-P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients with Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e180039. [Google Scholar] [CrossRef]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus Hypofractionated High-Dose Intensity-Modulated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the Randomised, Non-Inferiority, Phase 3 CHHiP Trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Dignam, J.J.; Amin, M.B.; Bruner, D.W.; Low, D.; Swanson, G.P.; Shah, A.B.; D’Souza, D.P.; Michalski, J.M.; Dayes, I.S.; et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients with Low-Risk Prostate Cancer. J. Clin. Oncol. 2016, 34, 2325–2332. [Google Scholar] [CrossRef]
- Catton, C.N.; Lukka, H.; Gu, C.-S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.-P.; Ahmed, S.; Cheung, P.; et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-Hypofractionated versus Conventionally Fractionated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the HYPO-RT-PC Randomised, Non-Inferiority, Phase 3 Trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Fransson, P.; Nilsson, P.; Gunnlaugsson, A.; Beckman, L.; Tavelin, B.; Norman, D.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; et al. Ultra-Hypofractionated versus Conventionally Fractionated Radiotherapy for Prostate Cancer (HYPO-RT-PC): Patient-Reported Quality-of-Life Outcomes of a Randomised, Controlled, Non-Inferiority, Phase 3 Trial. Lancet Oncol. 2021, 22, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Ostler, P.; van der Voet, H.; Chu, W.; Loblaw, A.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; Staffurth, J.; et al. Intensity-Modulated Radiotherapy versus Stereotactic Body Radiotherapy for Prostate Cancer (PACE-B): 2-Year Toxicity Results from an Open-Label, Randomised, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2022, 23, 1308–1320. [Google Scholar] [CrossRef]
- Brand, D.H.; Tree, A.C.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-Modulated Fractionated Radiotherapy versus Stereotactic Body Radiotherapy for Prostate Cancer (PACE-B): Acute Toxicity Findings from an International, Randomised, Open-Label, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Dang, A.; Katz, A.J.; Mantz, C.A.; Collins, S.P.; Aghdam, N.; Chu, F.-I.; Kaplan, I.D.; Appelbaum, L.; Fuller, D.B.; et al. Long-Term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw. Open 2019, 2, e188006. [Google Scholar] [CrossRef] [PubMed]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.W.; Eppinga, W.S.C.; Tijssen, R.H.N.; Kerkmeijer, L.G.W.; et al. Adaptive Radiotherapy: The Elekta Unity MR-Linac Concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance-Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Rigo, M.; Figlia, V.; Nicosia, L.; Mazzola, R.; Giaj Levra, N.; Ricchetti, F.; Trapani, G.; Attinà, G.; Vitale, C.; et al. 1.5T MR-Guided Daily-Adaptive SBRT for Prostate Cancer: Preliminary Report of Toxicity and Quality of Life of the First 100 Patients. J. Pers. Med. 2022, 12, 1982. [Google Scholar] [CrossRef]
- Zellars, R.C.; Roberson, P.L.; Strawderman, M.; Zhang, D.; Sandler, H.M.; Ten Haken, R.K.; Osher, D.; McLaughlin, P.W. Prostate Position Late in the Course of External Beam Therapy: Patterns and Predictors. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 655–660. [Google Scholar] [CrossRef]
- Nederveen, A.J.; van der Heide, U.A.; Dehnad, H.; van Moorselaar, R.J.A.; Hofman, P.; Lagendijk, J.J.W. Measurements and Clinical Consequences of Prostate Motion during a Radiotherapy Fraction. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 206–214. [Google Scholar] [CrossRef]
- Nejad-Davarani, S.P.; Sevak, P.; Moncion, M.; Garbarino, K.; Weiss, S.; Kim, J.; Schultz, L.; Elshaikh, M.A.; Renisch, S.; Glide-Hurst, C. Geometric and Dosimetric Impact of Anatomical Changes for MR-Only Radiation Therapy for the Prostate. J. Appl. Clin. Med. Phys. 2019, 20, 10–17. [Google Scholar] [CrossRef]
- Snoj, Z.; Gill, A.B.; Rundo, L.; Sushentsev, N.; Barrett, T. Three-Dimensional MRI Evaluation of the Effect of Bladder Volume on Prostate Translocation and Distortion. Radiol. Oncol. 2020, 54, 48–56. [Google Scholar] [CrossRef]
- O’Neill, A.G.M.; Jain, S.; Hounsell, A.R.; O’Sullivan, J.M. Fiducial Marker Guided Prostate Radiotherapy: A Review. Br. J. Radiol. 2016, 89, 20160296. [Google Scholar] [CrossRef]
- Holmes, O.E.; Gratton, J.; Szanto, J.; Vandervoort, E.; Doody, J.; Henderson, E.; Morgan, S.C.; O’Sullivan, J.; Malone, S. Reducing Errors in Prostate Tracking with an Improved Fiducial Implantation Protocol for CyberKnife Based Stereotactic Body Radiotherapy (SBRT). J. Radiosurg. SBRT 2018, 5, 217–227. [Google Scholar]
- Poggi, M.M.; Gant, D.A.; Sewchand, W.; Warlick, W.B. Marker Seed Migration in Prostate Localization. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, V.; Liu, M.; Kristensen, S.; Gelowitz, G.; Berthelet, E. Effect of Bladder Filling on Doses to Prostate and Organs at Risk: A Treatment Planning Study. J. Appl. Clin. Med. Phys. 2006, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Brand, V.J.; Milder, M.T.W.; Christianen, M.E.M.C.; Hoogeman, M.S.; Incrocci, L. Seminal Vesicle Inter- and Intra-Fraction Motion during Radiotherapy for Prostate Cancer: A Review. Radiother. Oncol. 2022, 169, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Topkan, E.; Efe, E.; Yavuz, M.; Arslan, G.; Yavuz, A. The Effect of Concurrent Androgen Deprivation and 3D Conformal Radiotherapy on Prostate Volume and Clinical Organ Doses during Treatment for Prostate Cancer. Br. J. Radiol. 2009, 82, 1019–1026. [Google Scholar] [CrossRef]
- Hötker, A.M.; Mazaheri, Y.; Zheng, J.; Moskowitz, C.S.; Berkowitz, J.; Lantos, J.E.; Pei, X.; Zelefsky, M.J.; Hricak, H.; Akin, O. Prostate Cancer: Assessing the Effects of Androgen-Deprivation Therapy Using Quantitative Diffusion-Weighted and Dynamic Contrast-Enhanced MRI. Eur. Radiol. 2015, 25, 2665–2672. [Google Scholar] [CrossRef]
- Alexander, S.E.; McNair, H.A.; Oelfke, U.; Huddart, R.; Murray, J.; Pathmanathan, A.; Patel, P.; Sritharan, K.; van As, N.; Tree, A.C. Prostate Volume Changes during Extreme and Moderately Hypofractionated Magnetic Resonance Image-Guided Radiotherapy. Clin. Oncol. 2022, 34, e383–e391. [Google Scholar] [CrossRef]
- Vanhanen, A.; Reinikainen, P.; Kapanen, M. Radiation-Induced Prostate Swelling during SBRT of the Prostate. Acta Oncol. 2022, 61, 698–704. [Google Scholar] [CrossRef]
- Ma, T.M.; Neylon, J.; Casado, M.; Sharma, S.; Sheng, K.; Low, D.; Yang, Y.; Steinberg, M.L.; Lamb, J.; Cao, M.; et al. Dosimetric Impact of Interfraction Prostate and Seminal Vesicle Volume Changes and Rotation: A Post-Hoc Analysis of a Phase III Randomized Trial of MRI-Guided versus CT-Guided Stereotactic Body Radiotherapy. Radiother. Oncol. 2022, 167, 203–210. [Google Scholar] [CrossRef]
- Tree, A.C.; Satchwell, L.; Alexander, E.; Blasiak-Wal, I.; deSouza, N.M.; Gao, A.; Greenlay, E.; McNair, H.; Parker, C.; Talbot, J.; et al. Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Nodules in Localized Prostate Cancer: 5-Year Efficacy and Toxicity in the DELINEATE Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 305–316. [Google Scholar] [CrossRef]
- Kishan, A.U.; Ma, T.M.; Lamb, J.M.; Casado, M.; Wilhalme, H.; Low, D.A.; Sheng, K.; Sharma, S.; Nickols, N.G.; Pham, J.; et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023, 9, 365–373. [Google Scholar] [CrossRef]
- Hannan, R.; Tumati, V.; Xie, X.-J.; Cho, L.C.; Kavanagh, B.D.; Brindle, J.; Raben, D.; Nanda, A.; Cooley, S.; Kim, D.W.N.; et al. Stereotactic Body Radiation Therapy for Low and Intermediate Risk Prostate Cancer-Results from a Multi-Institutional Clinical Trial. Eur. J. Cancer 2016, 59, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.M.; Ballas, L.K.; Wilhalme, H.; Sachdeva, A.; Chong, N.; Sharma, S.; Yang, T.; Basehart, V.; Reiter, R.E.; Saigal, C.; et al. Quality-of-Life Outcomes and Toxicity Profile Among Patients with Localized Prostate Cancer After Radical Prostatectomy Treated with Stereotactic Body Radiation: The SCIMITAR Multicenter Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Rigo, M.; Figlia, V.; Giaj-Levra, N.; Mazzola, R.; Nicosia, L.; Ricchetti, F.; Trapani, G.; De Simone, A.; Gurrera, D.; et al. 1.5 T MR-Guided Daily Adaptive Stereotactic Body Radiotherapy for Prostate Re-Irradiation: A Preliminary Report of Toxicity and Clinical Outcomes. Front. Oncol. 2022, 12, 1507. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, L.; Romano, A.; Chiloiro, G.; Corradini, S.; De Luca, V.; Verusio, V.; D’Aviero, A.; Castelluccia, A.; Alitto, A.R.; Catucci, F.; et al. Magnetic Resonance Guided SBRT Reirradiation in Locally Recurrent Prostate Cancer: A Multicentric Retrospective Analysis. Radiat. Oncol. 2023, 18, 84. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kiljunen, T.; Kangasmaki, A.; Kairemo, K.; von Eyben, R.; Joensuu, T. Radiotherapy Boost for the Dominant Intraprostatic Cancer Lesion-A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2016, 14, 189–197. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Draulans, C.; van der Heide, U.A.; Haustermans, K.; Pos, F.J.; van der Voort van Zyp, J.; De Boer, H.; Groen, V.H.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; et al. Primary Endpoint Analysis of the Multicentre Phase II Hypo-FLAME Trial for Intermediate and High Risk Prostate Cancer. Radiother. Oncol. 2020, 147, 92–98. [Google Scholar] [CrossRef]
- Leeman, J.E.; Cagney, D.N.; Mak, R.H.; Huynh, M.A.; Tanguturi, S.K.; Singer, L.; Catalano, P.; Martin, N.E.; D’Amico, A.V.; Mouw, K.W.; et al. Magnetic Resonance-Guided Prostate Stereotactic Body Radiation Therapy with Daily Online Plan Adaptation: Results of a Prospective Phase 1 Trial and Supplemental Cohort. Adv. Radiat. Oncol. 2022, 7, 100934. [Google Scholar] [CrossRef]
- Bruynzeel, A.M.E.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Piet, A.H.M.; Meijnen, P.; Bakker van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094. [Google Scholar] [CrossRef]
- Pham, J.; Savjani, R.R.; Gao, Y.; Cao, M.; Hu, P.; Sheng, K.; Low, D.A.; Steinberg, M.; Kishan, A.U.; Yang, Y. Evaluation of T2-Weighted MRI for Visualization and Sparing of Urethra with MR-Guided Radiation Therapy (MRgRT) On-Board MRI. Cancers 2021, 13, 3564. [Google Scholar] [CrossRef]
- Xu, D.; Ma, T.M.; Savjani, R.; Pham, J.; Cao, M.; Yang, Y.; Kishan, A.U.; Scalzo, F.; Sheng, K. Fully Automated Segmentation of Prostatic Urethra for MR-Guided Radiation Therapy. Med. Phys. 2023, 50, 354–364. [Google Scholar] [CrossRef]
- Pham, J.; Savjani, R.R.; Yoon, S.M.; Yang, T.; Gao, Y.; Cao, M.; Hu, P.; Sheng, K.; Low, D.A.; Steinberg, M.; et al. Urethral Interfractional Geometric and Dosimetric Variations of Prostate Cancer Patients: A Study Using an Onboard MRI. Front. Oncol. 2022, 12, 916254. [Google Scholar] [CrossRef]
- Spratt, D.E.; Lee, J.Y.; Dess, R.T.; Narayana, V.; Evans, C.; Liss, A.; Winfield, R.; Schipper, M.J.; Lawrence, T.S.; McLaughlin, P.W. Vessel-Sparing Radiotherapy for Localized Prostate Cancer to Preserve Erectile Function: A Single-Arm Phase 2 Trial. Eur. Urol. 2017, 72, 617–624. [Google Scholar] [CrossRef]
- Verkooijen, H.M. EREctile Function Preservation for Prostate Cancer Radiation Therapy (ERECT). A Prospective Phase II Trial; NL73192.041.20; Clinicaltrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- Nikitas, J.; Smith, L.M.; Gao, Y.; Ma, T.M.; Sachdeva, A.; Yoon, S.M.; Jiang, T.; Low, D.A.; Ballas, L.K.; Steinberg, M.L.; et al. The Role of Adaptive Planning in Margin-Reduced, MRI-Guided Stereotactic Body Radiotherapy to the Prostate Bed Following Radical Prostatectomy: Post-Hoc Analysis of a Phase II Clinical Trial. Radiother. Oncol. 2023, 183, 109631. [Google Scholar] [CrossRef]
- Paudel, M.R.; Kim, A.; Sarfehnia, A.; Ahmad, S.B.; Beachey, D.J.; Sahgal, A.; Keller, B.M. Experimental Evaluation of a GPU-Based Monte Carlo Dose Calculation Algorithm in the Monaco Treatment Planning System. J. Appl. Clin. Med. Phys. 2016, 17, 230–241. [Google Scholar] [CrossRef]
- Dunlop, A.; Mitchell, A.; Tree, A.; Barnes, H.; Bower, L.; Chick, J.; Goodwin, E.; Herbert, T.; Lawes, R.; McNair, H.; et al. Daily Adaptive Radiotherapy for Patients with Prostate Cancer Using a High Field MR-Linac: Initial Clinical Experiences and Assessment of Delivered Doses Compared to a C-Arm Linac. Clin. Transl. Radiat. Oncol. 2020, 23, 35–42. [Google Scholar] [CrossRef]
- Keall, P.J.; Glide-Hurst, C.K.; Cao, M.; Lee, P.; Murray, B.; Raaymakers, B.W.; Tree, A.; van der Heide, U.A. ICRU REPORT 97: MRI-Guided Radiation Therapy Using MRI-Linear Accelerators. J. ICRU 2022, 22, 1–100. [Google Scholar] [CrossRef]
- Keesman, R.; van der Bijl, E.; Janssen, T.M.; Vijlbrief, T.; Pos, F.J.; van der Heide, U.A. Clinical Workflow for Treating Patients with a Metallic Hip Prosthesis Using Magnetic Resonance Imaging-Guided Radiotherapy. Phys. Imaging Radiat. Oncol. 2020, 15, 85–90. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, J.; Cheung, K.Y.; Huang, C.-Y.; Poon, D.M.C.; Yu, S.K. Magnetic Resonance-Guided Radiation Therapy of Patients with Cardiovascular Implantable Electronic Device on a 1.5 T Magnetic Resonance-Linac. Pract. Radiat. Oncol. 2022, 12, e56–e61. [Google Scholar] [CrossRef]
- Gach, H.M.; Green, O.L.; Cuculich, P.S.; Wittland, E.J.; Marko, A.; Luchtefeld, M.E.; Entwistle, J.M.; Yang, D.; Wilber, D.J.; Mutic, S.; et al. Lessons Learned From the First Human Low-Field MRI Guided Radiation Therapy of the Heart in the Presence of an Implantable Cardiac Defibrillator. Pract. Radiat. Oncol. 2019, 9, 274–279. [Google Scholar] [CrossRef]
- Tocco, B.R.; Kishan, A.U.; Ma, T.M.; Kerkmeijer, L.G.W.; Tree, A.C. MR-Guided Radiotherapy for Prostate Cancer. Front. Oncol. 2020, 10, 616291. [Google Scholar] [CrossRef]
- Pollack, A.; Karrison, T.G.; Balogh, A.G.; Gomella, L.G.; Low, D.A.; Bruner, D.W.; Wefel, J.S.; Martin, A.-G.; Michalski, J.M.; Angyalfi, S.J.; et al. The Addition of Androgen Deprivation Therapy and Pelvic Lymph Node Treatment to Prostate Bed Salvage Radiotherapy (NRG Oncology/RTOG 0534 SPPORT): An International, Multicentre, Randomised Phase 3 Trial. Lancet 2022, 399, 1886–1901. [Google Scholar] [CrossRef]
- Barnes, H.; Alexander, S.; Bower, L.; Ehlers, J.; Gani, C.; Herbert, T.; Lawes, R.; Møller, P.K.; Morgan, T.; Nowee, M.E.; et al. Development and Results of a Patient-Reported Treatment Experience Questionnaire on a 1.5 T MR-Linac. Clin. Transl. Radiat. Oncol. 2021, 30, 31–37. [Google Scholar] [CrossRef]
- Güngör, G.; Serbez, İ.; Temur, B.; Gür, G.; Kayalılar, N.; Mustafayev, T.Z.; Korkmaz, L.; Aydın, G.; Yapıcı, B.; Atalar, B.; et al. Time Analysis of Online Adaptive Magnetic Resonance–Guided Radiation Therapy Workflow According to Anatomical Sites. Pract. Radiat. Oncol. 2021, 11, e11–e21. [Google Scholar] [CrossRef]
- Wahlstedt, I.; Andratschke, N.; Behrens, C.P.; Ehrbar, S.; Gabryś, H.S.; Schüler, H.G.; Guckenberger, M.; Smith, A.G.; Tanadini-Lang, S.; Tascón-Vidarte, J.D.; et al. Gating Has a Negligible Impact on Dose Delivered in MRI-Guided Online Adaptive Radiotherapy of Prostate Cancer. Radiother. Oncol. 2022, 170, 205–212. [Google Scholar] [CrossRef]
- Johnstone, E.; Wyatt, J.J.; Henry, A.M.; Short, S.C.; Sebag-Montefiore, D.; Murray, L.; Kelly, C.G.; McCallum, H.M.; Speight, R. Systematic Review of Synthetic Computed Tomography Generation Methodologies for Use in Magnetic Resonance Imaging-Only Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 199–217. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, D.; O’Connor, L.; Chalup, S.; Welsh, J.S.; Dowling, J.; Greer, P.B. Bulk Anatomical Density Based Dose Calculation for Patient-Specific Quality Assurance of MRI-Only Prostate Radiotherapy. Front. Oncol. 2019, 9, 997. [Google Scholar] [CrossRef]
- Karotki, A.; Mah, K.; Meijer, G.; Meltsner, M. Comparison of Bulk Electron Density and Voxel-Based Electron Density Treatment Planning. J. Appl. Clin. Med. Phys. 2011, 12, 97–104. [Google Scholar] [CrossRef]
- Korsholm, M.E.; Waring, L.W.; Edmund, J.M. A Criterion for the Reliable Use of MRI-Only Radiotherapy. Radiat. Oncol. 2014, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.A.; Sun, J.; Pichler, P.; Rivest-Hénault, D.; Ghose, S.; Richardson, H.; Wratten, C.; Martin, J.; Arm, J.; Best, L.; et al. Automatic Substitute Computed Tomography Generation and Contouring for Magnetic Resonance Imaging (MRI)-Alone External Beam Radiation Therapy From Standard MRI Sequences. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Edmund, J.M.; Kjer, H.M.; Van Leemput, K.; Hansen, R.H.; Andersen, J.A.L.; Andreasen, D. A Voxel-Based Investigation for MRI-Only Radiotherapy of the Brain Using Ultra Short Echo Times. Phys. Med. Biol. 2014, 59, 7501–7519. [Google Scholar] [CrossRef] [PubMed]
- Chourak, H.; Barateau, A.; Tahri, S.; Cadin, C.; Lafond, C.; Nunes, J.-C.; Boue-Rafle, A.; Perazzi, M.; Greer, P.B.; Dowling, J.; et al. Quality Assurance for MRI-Only Radiation Therapy: A Voxel-Wise Population-Based Methodology for Image and Dose Assessment of Synthetic CT Generation Methods. Front. Oncol. 2022, 12, 968689. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Dunlop, A.; Barnes, H.; Herbert, T.; Lawes, R.; Mohajer, J.; Tree, A.C.; McNair, H.A. Bladder Filling in Patients Undergoing Prostate Radiotherapy on a MR-Linac: The Dosimetric Impact. Tech. Innov. Patient Support. Radiat. Oncol. 2022, 21, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Otazo, R.; Lambin, P.; Pignol, J.-P.; Ladd, M.E.; Schlemmer, H.-P.; Baumann, M.; Hricak, H. MRI-Guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology. Radiology 2021, 298, 248. [Google Scholar] [CrossRef]
- Adair Smith, G.; Dunlop, A.; Alexander, S.E.; Barnes, H.; Casey, F.; Chick, J.; Gunapala, R.; Herbert, T.; Lawes, R.; Mason, S.A.; et al. Evaluation of Therapeutic Radiographer Contouring for Magnetic Resonance Image Guided Online Adaptive Prostate Radiotherapy. Radiother. Oncol. 2023, 180, 109457. [Google Scholar] [CrossRef]
- Cusumano, D.; Boldrini, L.; Dhont, J.; Fiorino, C.; Green, O.; Güngör, G.; Jornet, N.; Klüter, S.; Landry, G.; Mattiucci, G.C.; et al. Artificial Intelligence in Magnetic Resonance Guided Radiotherapy: Medical and Physical Considerations on State of Art and Future Perspectives. Phys. Med. 2021, 85, 175–191. [Google Scholar] [CrossRef]
- Nachbar, M.; Lo Russo, M.; Gani, C.; Boeke, S.; Wegener, D.; Paulsen, F.; Zips, D.; Roque, T.; Paragios, N.; Thorwarth, D. Automatic AI-Based Contouring of Prostate MRI for Online Adaptive Radiotherapy. Z. Med. Phys. 2023, in press. [Google Scholar] [CrossRef]
- Schumacher, L.-E.D.; Dal Pra, A.; Hoffe, S.E.; Mellon, E.A. Toxicity Reduction Required for MRI-Guided Radiotherapy to Be Cost-Effective in the Treatment of Localized Prostate Cancer. Br. J. Radiol. 2020, 93, 20200028. [Google Scholar] [CrossRef]
- Hehakaya, C.; Sharma, A.M.; van der Voort Van Zijp, J.R.N.; Grobbee, D.E.; Verkooijen, H.M.; Izaguirre, E.W.; Moors, E.H.M. Implementation of Magnetic Resonance Imaging-Guided Radiation Therapy in Routine Care: Opportunities and Challenges in the United States. Adv. Radiat. Oncol. 2022, 7, 100953. [Google Scholar] [CrossRef]
- Persson, E.; Svanberg, N.; Scherman, J.; Jamtheim Gustafsson, C.; Fridhammar, A.; Hjalte, F.; Bäck, S.; Nilsson, P.; Gunnlaugsson, A.; Olsson, L.E. MRI-Only Radiotherapy from an Economic Perspective: Can New Techniques in Prostate Cancer Treatment Be Cost Saving? Clin. Transl. Radiat. Oncol. 2023, 38, 183–187. [Google Scholar] [CrossRef]
- Harris, E.; Fontanarosa, D.; Baldock, C. In the Future, Ultrasound Guidance in Radiotherapy Will Become a Clinical Standard. Phys. Eng. Sci. Med. 2021, 44, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Stephans, K.; Godley, A.; Kolar, M.; Magnelli, A.; Tendulkar, R.; Mian, O.; Majkszak, D.; Xia, P. Transperineal Ultrasound Is a Good Alternative for Intra-Fraction Motion Monitoring for Prostate Stereotactic Body Radiotherapy. J. Appl. Clin. Med. Phys. 2023, e14021. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benitez, C.M.; Steinberg, M.L.; Cao, M.; Qi, X.S.; Lamb, J.M.; Kishan, A.U.; Valle, L.F. MRI-Guided Radiation Therapy for Prostate Cancer: The Next Frontier in Ultrahypofractionation. Cancers 2023, 15, 4657. https://doi.org/10.3390/cancers15184657
Benitez CM, Steinberg ML, Cao M, Qi XS, Lamb JM, Kishan AU, Valle LF. MRI-Guided Radiation Therapy for Prostate Cancer: The Next Frontier in Ultrahypofractionation. Cancers. 2023; 15(18):4657. https://doi.org/10.3390/cancers15184657
Chicago/Turabian StyleBenitez, Cecil M., Michael L. Steinberg, Minsong Cao, X. Sharon Qi, James M. Lamb, Amar U. Kishan, and Luca F. Valle. 2023. "MRI-Guided Radiation Therapy for Prostate Cancer: The Next Frontier in Ultrahypofractionation" Cancers 15, no. 18: 4657. https://doi.org/10.3390/cancers15184657
APA StyleBenitez, C. M., Steinberg, M. L., Cao, M., Qi, X. S., Lamb, J. M., Kishan, A. U., & Valle, L. F. (2023). MRI-Guided Radiation Therapy for Prostate Cancer: The Next Frontier in Ultrahypofractionation. Cancers, 15(18), 4657. https://doi.org/10.3390/cancers15184657







