Dynamic Risk Stratification Integrated with ATA Risk System for Predicting Long-Term Outcome in Papillary Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Clinical–Pathological and Epidemiological Features of PTC Patients
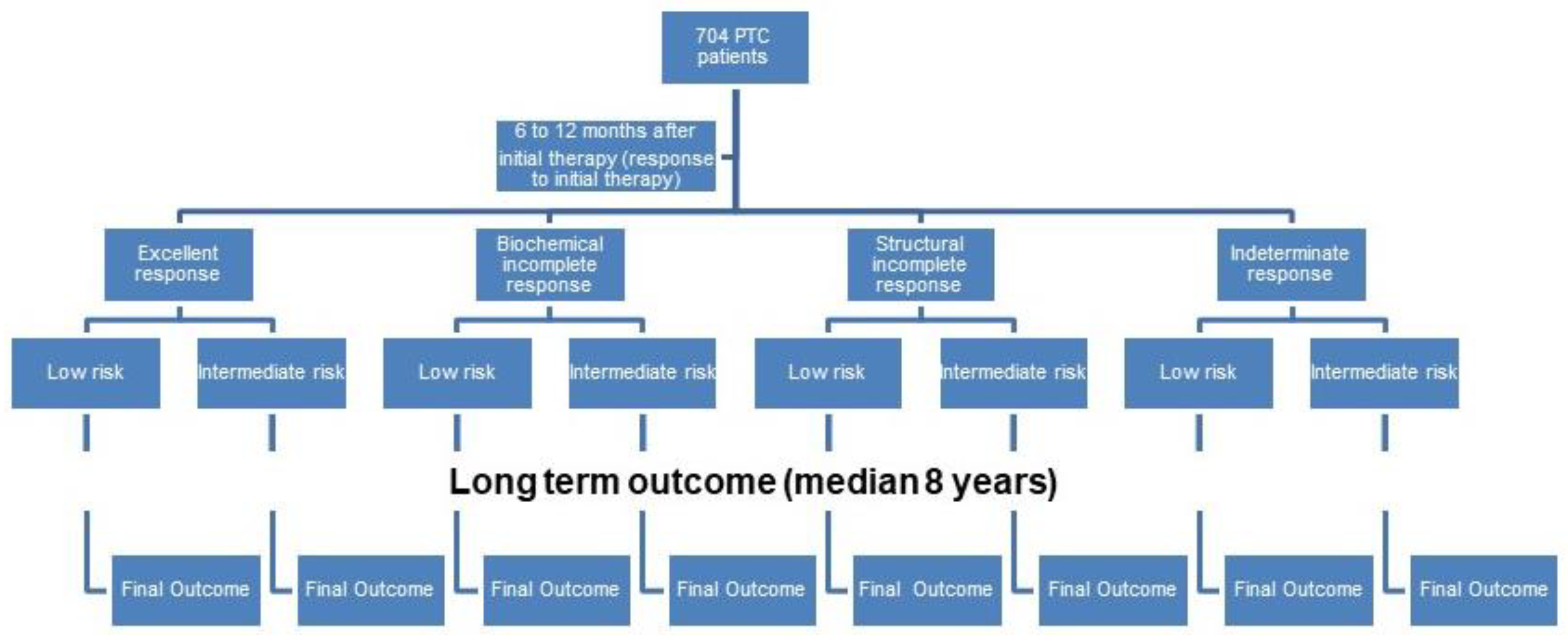
3.2. First Evaluation after Initial Treatment
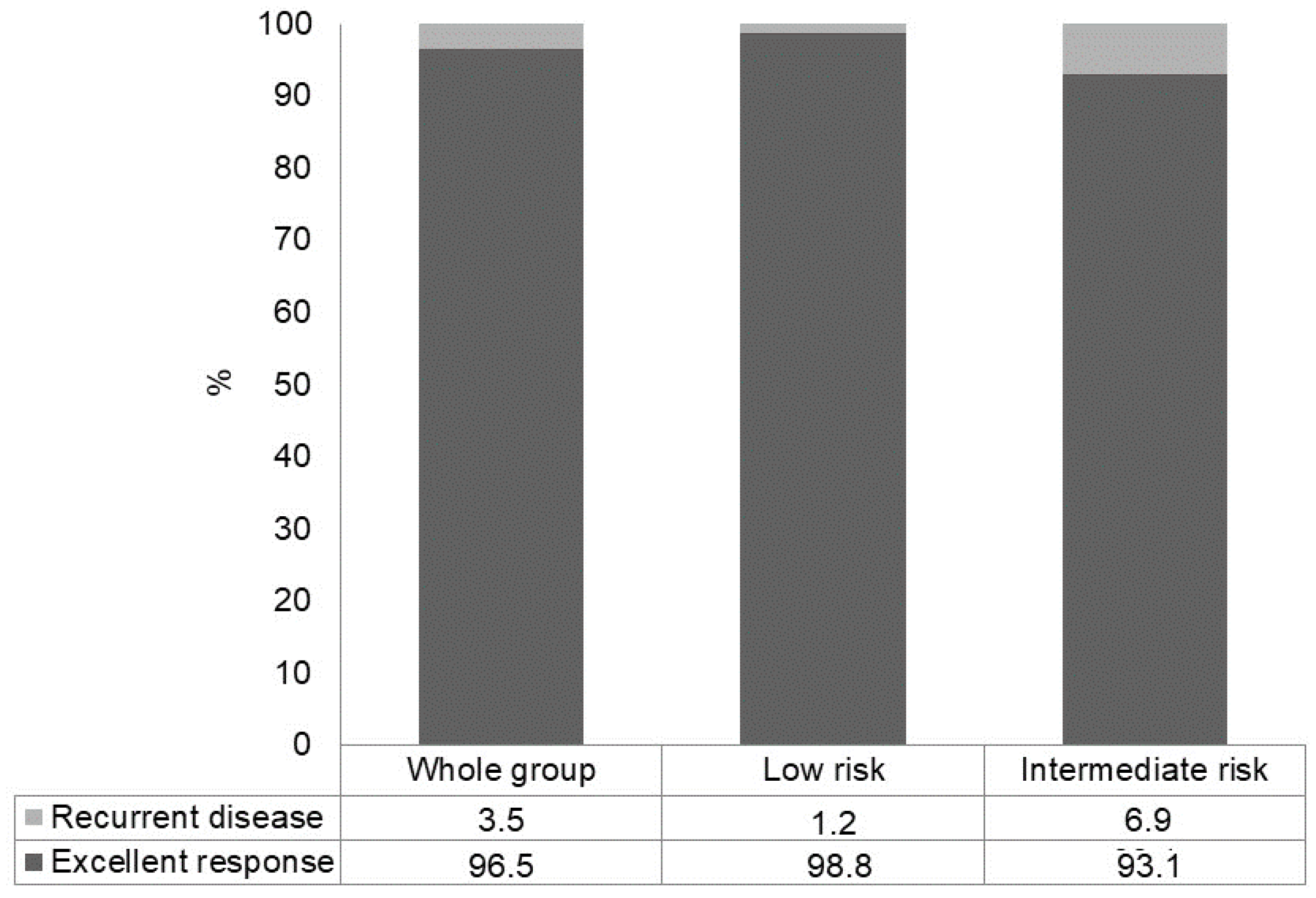
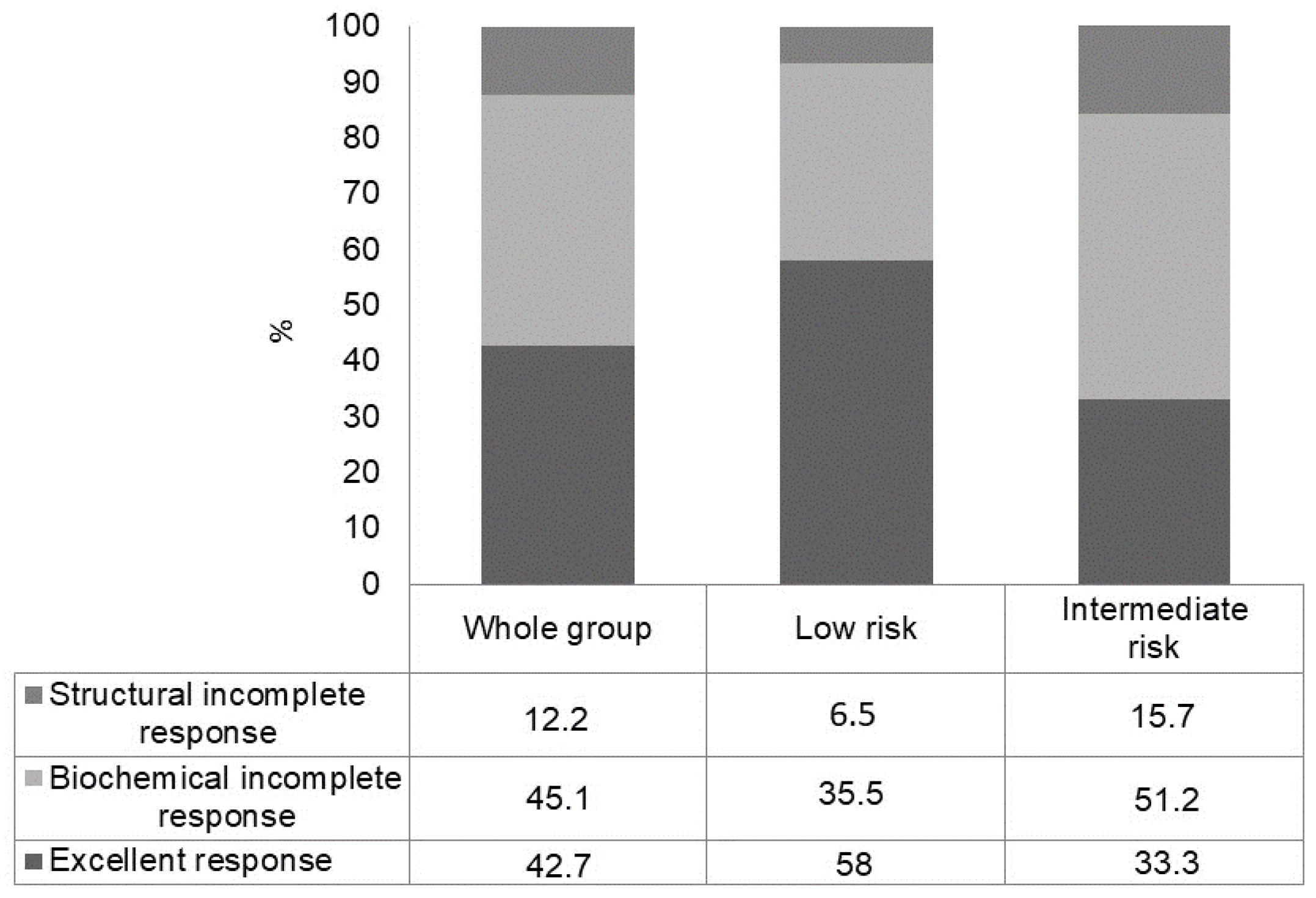
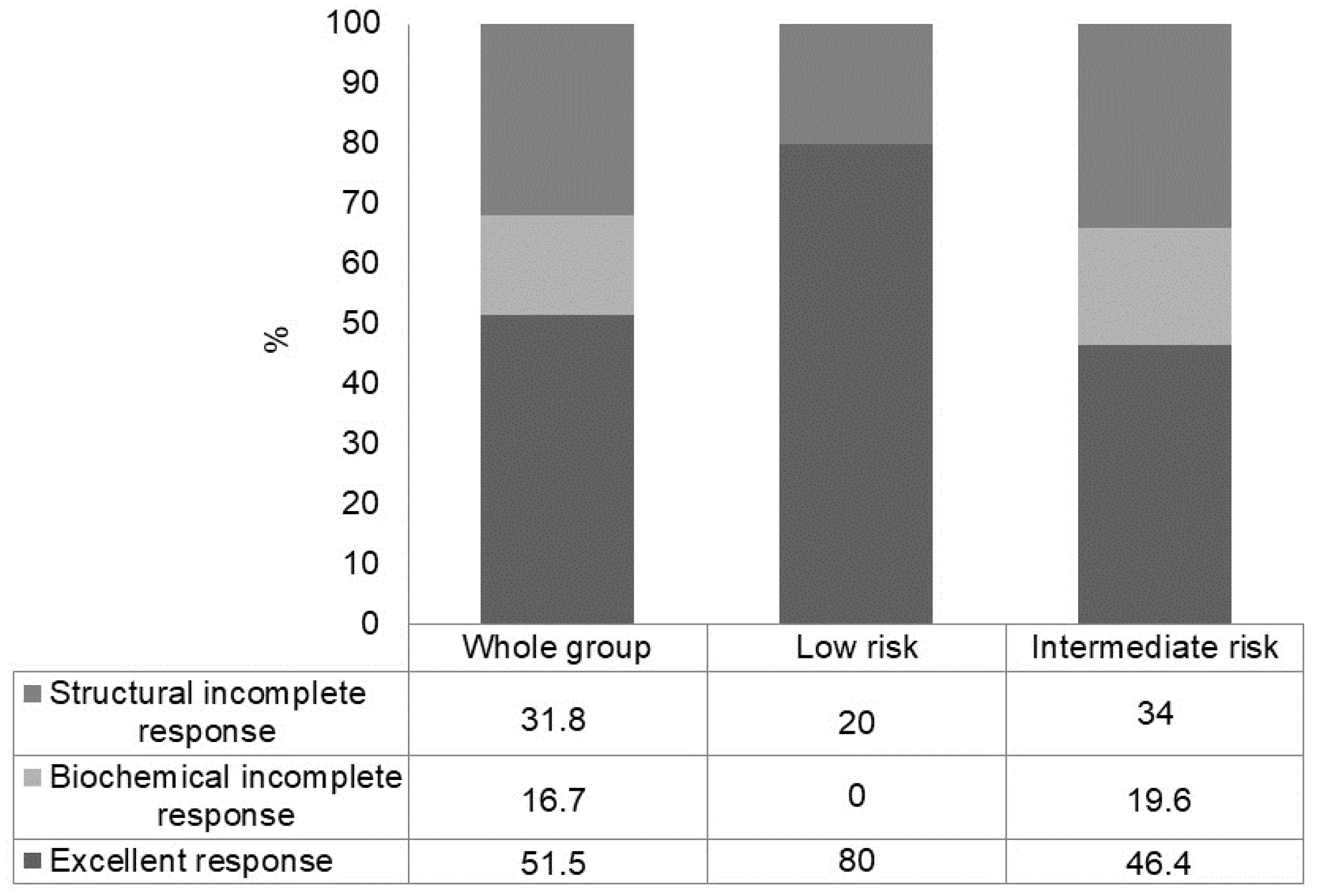
3.3. Long-Term Outcome in the Whole Group According to the Initial ATA Risk Class
3.4. Predictors of Long-Term Outcome
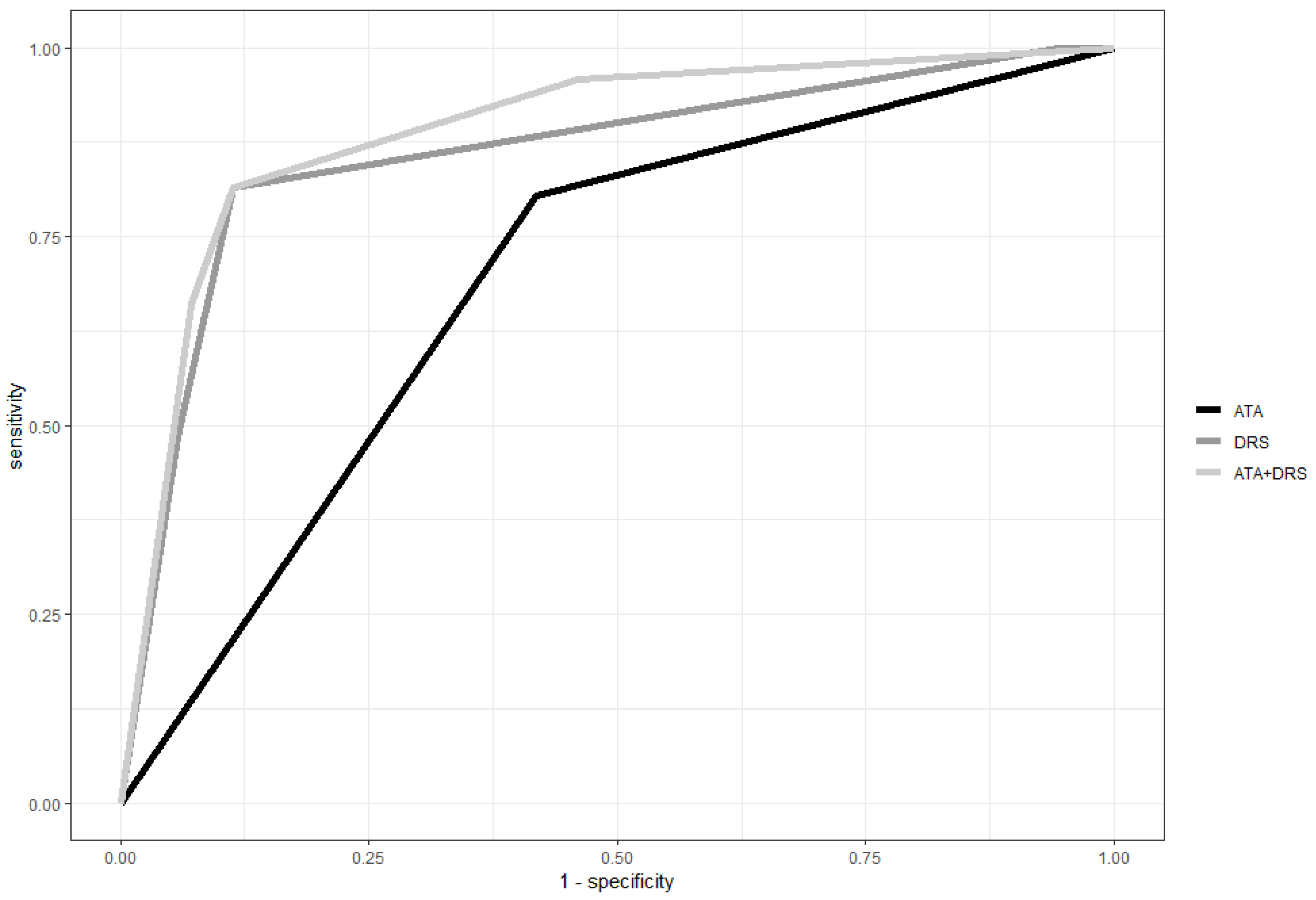
3.5. Prognostic Performance of ATA Risk Class, Response to Initial Therapy and ATA Risk Classes Plus Response to Initial Therapy in PTC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Basolo, F.; Bellantone, R.; Boni, G.; Cannizzaro, M.A.; De Palma, M.; Durante, C.; Elisei, R.; Fadda, G.; Frasoldati, A.; et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: Joint statements of six Italian societies. J. Endocrinol. Investig. 2018, 41, 849–876. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid J. 2022, 11, e210046. [Google Scholar] [CrossRef] [PubMed]
- Blumhardt, R.; Wolin, E.A.; Phillips, W.T.; Salman, U.A.; Walker, R.C.; Stack, B.C., Jr.; Metter, D. Current controversies in the initial post-surgical radioactive iodine therapy for thyroid cancer: A narrative review. Endocr. Relat. Cancer 2014, 21, R473–R484. [Google Scholar] [CrossRef] [PubMed]
- Leboulleux, S.; Bournaud, C.; Chougnet, C.N.; Zerdoud, S.; Al Ghuzlan, A.; Catargi, B.; Do Cao, C.; Kelly, A.; Barge, M.L.; Lacroix, L.; et al. Thyroidectomy without Radioiodine in Patients with Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar]
- Mazzaferri, E.L. Management of low-risk differentiated thyroid cancer. Endocr. Pract. 2007, 13, 498–512. [Google Scholar] [CrossRef]
- Shaha, A.R.; Shah, J.P.; Loree, T.R. Low-risk differentiated thyroid cancer: The need for selective treatment. Ann. Surg. Oncol. 1997, 4, 328–333. [Google Scholar] [CrossRef]
- Tuttle, R.M. Risk-adapted management of thyroid cancer. Endocr. Pract. 2008, 14, 764–774. [Google Scholar] [CrossRef]
- Hay, I.D. Management of patients with low-risk papillary thyroid carcinoma. Endocr. Pract. 2007, 13, 521–533. [Google Scholar] [CrossRef]
- Momesso, D.P.; Vaisman, F.; Yang, S.P.; Bulzico, D.A.; Corbo, R.; Vaisman, M.; Tuttle, R.M. Dynamic Risk Stratification in Patients with Differentiated Thyroid Cancer Treated Without Radioactive Iodine. J. Clin. Endocrinol. Metab. 2016, 101, 2692–2700. [Google Scholar] [CrossRef]
- Lang, B.H.; Lo, C.Y.; Chan, W.F.; Lam, K.Y.; Wan, K.Y. Staging systems for papillary thyroid carcinoma: A review and comparison. Ann. Surg. 2007, 245, 366–378. [Google Scholar] [CrossRef]
- Brierley, J.D.; Panzarella, T.; Tsang, R.W.; Gospodarowicz, M.K.; O’Sullivan, B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer 1997, 79, 2414–2423. [Google Scholar] [CrossRef]
- Verburg, F.A.; Mader, U.; Kruitwagen, C.L.; Luster, M.; Reiners, C. A comparison of prognostic classification systems for differentiated thyroid carcinoma. Clin. Endocrinol. 2010, 72, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Shaha, A.R.; Shah, J.P.; Loree, T.R. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann. Surg. Oncol. 1996, 3, 534–538. [Google Scholar] [CrossRef]
- Tuttle, R.M. Controversial Issues in Thyroid Cancer Management. J. Nucl. Med. 2018, 59, 1187–1194. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Leboeuf, R.; Shaha, A.R. Medical management of thyroid cancer: A risk adapted approach. J. Surg. Oncol. 2008, 97, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Haymart, M.R.; Esfandiari, N.H.; Stang, M.T.; Sosa, J.A. Controversies in the Management of Low-Risk Differentiated Thyroid Cancer. Endocr. Rev. 2017, 38, 351–378. [Google Scholar] [CrossRef]
- Momesso, D.P.; Tuttle, R.M. Update on differentiated thyroid cancer staging. Endocrinol. Metab. Clin. N. Am. 2014, 43, 401–421. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Leboeuf, R. Follow up approaches in thyroid cancer: A risk adapted paradigm. Endocrinol. Metab. Clin. N. Am. 2008, 37, 419–435. [Google Scholar] [CrossRef]
- Byar, D.P.; Green, S.B.; Dor, P.; Williams, E.D.; Colon, J.; van Gilse, H.A.; Mayer, M.; Sylvester, R.J.; van Glabbeke, M. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur. J. Cancer 1979, 15, 1033–1041. [Google Scholar] [CrossRef]
- The American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Tarasova, V.D.; Tuttle, R.M. A Risk-adapted Approach to Follow-up in Differentiated Thyroid Cancer. Rambam Maimonides Med. J. 2016, 7, e0004. [Google Scholar] [CrossRef]
- Castagna, M.G.; Maino, F.; Cipri, C.; Belardini, V.; Theodoropoulou, A.; Cevenini, G.; Pacini, F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur. J. Endocrinol. 2011, 165, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, N.; Aljamei, H.; Aljomaiah, A.; Moria, Y.; Alzahrani, A.S. Natural Course of the American Thyroid Association Response to Therapy Statuses (Dynamic Risk Stratification) in Differentiated Thyroid Cancer. Eur. Thyroid J. 2021, 10, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J. TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Seejore, K.; Mulla, O.; Gerrard, G.E.; Gill, V.M.; Al-Qaissi, A.; Moor, J.W.; Murray, R.D. Outcomes of 756 patients with differentiated thyroid cancer and excellent response to treatment: An evidence-based paradigm for long-term surveillance strategies. Clin. Endocrinol. 2022, 96, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Steinschneider, M.; Pitaro, J.; Koren, S.; Mizrakli, Y.; Benbassat, C.; Muallem Kalmovich, L. Differentiated Thyroid Cancer with Biochemical Incomplete Response: Clinico-Pathological Characteristics and Long Term Disease Outcomes. Cancers 2021, 13, 5422. [Google Scholar] [CrossRef]
- Vaisman, F.; Momesso, D.; Bulzico, D.A.; Pessoa, C.H.; Dias, F.; Corbo, R.; Vaisman, M.; Tuttle, R.M. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin. Endocrinol. 2012, 77, 132–138. [Google Scholar] [CrossRef]
| Total Patients (n = 704) | Low-Risk Patients (n = 372) | Intermediate-Risk Patients (n = 332) | p-Value | |
|---|---|---|---|---|
| Sex, n (%) | 0.3 | |||
| M | 183 (26.0%) | 103 (27.7%) | 80 (24.1%) | |
| F | 521 (74.0%) | 269 (72.3%) | 252 (75.9%) | |
| Age at diagnosis, yrs | 0.1 | |||
| Mean | 46.2 | 47.6 | 44.8 | |
| Median | 45.0 | 48.0 | 43.0 | |
| Surgical treatment, n (%) | <0.05 | |||
| Total thyroidectomy | 694 (98.6%) | 362 (97.3%) | 332 (100%) | |
| Lobectomy | 10 (1.4%) | 10 (2.7%) | 0 (0%) | |
| Histological variant | <0.0001 | |||
| Classical | 18/273 * (6.6%) | 8/142 (5.6%) | 10/131 (7.7%) | |
| Follicular | 171/273 * (62.6%) | 134/142 (94.4%) | 37/131 (28.2%) | |
| Aggressive variant (hurtle cells, tall cells, solid, Warthin-like, columnar cells, trabecular, insular, sclerosant) | 84/273 * (30.8%) | 0/142 (0%) | 84/131 (64.1%) | |
| T stage, n (%) | <0.0001 | |||
| 1 | 375 (53.3%) | 290 (78.0%) | 85 (25.6%) | |
| 2 | 83 (11.8%) | 66 (17.7%) | 17 (5.1%) | |
| 3 | 246 (34.9%) | 16 (4.3%) | 230 (69.3%) | |
| Multifocal, n (%) | 262 (37.2%) | 114 (30.6%) | 148 (44.6%) | 0.0001 |
| Bilateral, n (%) | 185 (26.3%) | 67 (18.0%) | 118 (35.5%) | <0.0001 |
| Extrathyroidal, n (%) | 225 (31.9%) | 0 (0%) | 225 (67.8%) | <0.0001 |
| Lymph node metastases, n (%) | 167 (23.6%) | 0 (0%) | 167 (50.3%) | <0.0001 |
| TNM Stage, n (%) | <0.0001 | |||
| I | 622 (88.4%) | 366 (98.4%) | 256 (77.1%) | |
| II | 82 (11.6%) | 6 (1.6%) | 76 (22.9%) | |
| Post-op I131 treatment, n (%) | 592 (84.1%) | 267 (71.8%) | 325 (97.9%) | <0.0001 |
| Follow-up | 0.06 | |||
| Mean | 8.3 ± 5.0 | 8.6 ± 5.4 | 8.0 ± 4.7 | |
| Median | 8.0 | 8.0 | 7.6 | |
| Range | 1–54 | 1–54 | 1–28.7 |
| Excellent Response | Indeterminate Response | Biochemical Incomplete Response | Structural Incomplete Response | |
|---|---|---|---|---|
| Total patients (n = 704) | 522 (74.2%) | 34 (4.8%) | 82 (11.6%) | 66 (9.4%) |
| Low-risk patients (n = 372) | 320 (86.0%) | 11 (3.0%) | 31 (8.3%) | 10 (2.7%) |
| Intermediate-risk patients (n = 332) | 202 (60.8%) | 23 (6.9%) | 51 (15.4%) | 56 (16.9%) |
| Low- vs. intermediate-risk patients (p-value) | <0.0001 | |||
| Univariate Analysyis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Response to Initial Treatment | Parameters | OR | 95%CI | p Value | OR | 95%CI | p Value |
| Excellent response | Age >55 years | 0.61 | 0.17–1.74 | 0.39 | - | - | - |
| Male gender | 1.61 | 0.55–4.26 | 0.34 | - | - | - | |
| Multifocality | 2.39 | 0.92–6.36 | 0.07 | - | - | - | |
| Bilaterality | 2.15 | 0.77–5.59 | 0.12 | - | - | - | |
| mETE | 3.48 | 1.34–9.31 | 0.01 | - | - | - | |
| Intermediate ATA risk class | 5.88 | 2.07–20.98 | 0.002 | 5.88 | 2.07–20.98 | 0.002 | |
| Biochemical incomplete response | Age >55 years | 1.43 | 0.53–4.05 | 0.48 | - | – | - |
| Male gender | 2.72 | 1.05–7.56 | 0.04 | 2.92 | 1.04–8.48 | 0.03 | |
| Multifocality | 1.01 | 0.41–2.50 | 0.96 | - | – | - | |
| Bilaterality | 1.12 | 0.44–2.91 | 0.80 | - | – | - | |
| mETE | 1.48 | 0.59–3.79 | 0.40 | - | – | - | |
| Intermediate ATA risk class | 2.76 | 1.11–7.09 | 0.03 | 2.95 | 1.15–7.91 | 0.02 | |
| Structural incomplete response | Age >55 years | 2.63 | 081–9.50 | 0.11 | 4.09 | 1.09–20.0 | 0.04 |
| Male gender | 0.96 | 0.34–2.65 | 0.93 | - | – | - | |
| Multifocality | 1.42 | 0.54–3.82 | 0.47 | - | – | - | |
| Bilaterality | 0.84 | 0.30–2.31 | 0.74 | - | – | - | |
| mETE | 2.78 | 1.03–7.86 | 0.04 | - | – | - | |
| Intermediate ATA risk class | 4.61 | 1.04–32.43 | 0.06 | 7.32 | 1.43–62.2 | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, L.; Dalmiglio, C.; Maino, F.; Mattii, E.; Trimarchi, A.; Cartocci, A.; Castagna, M.G. Dynamic Risk Stratification Integrated with ATA Risk System for Predicting Long-Term Outcome in Papillary Thyroid Cancer. Cancers 2023, 15, 4656. https://doi.org/10.3390/cancers15184656
Valerio L, Dalmiglio C, Maino F, Mattii E, Trimarchi A, Cartocci A, Castagna MG. Dynamic Risk Stratification Integrated with ATA Risk System for Predicting Long-Term Outcome in Papillary Thyroid Cancer. Cancers. 2023; 15(18):4656. https://doi.org/10.3390/cancers15184656
Chicago/Turabian StyleValerio, Laura, Cristina Dalmiglio, Fabio Maino, Elisa Mattii, Andrea Trimarchi, Alessandra Cartocci, and Maria Grazia Castagna. 2023. "Dynamic Risk Stratification Integrated with ATA Risk System for Predicting Long-Term Outcome in Papillary Thyroid Cancer" Cancers 15, no. 18: 4656. https://doi.org/10.3390/cancers15184656
APA StyleValerio, L., Dalmiglio, C., Maino, F., Mattii, E., Trimarchi, A., Cartocci, A., & Castagna, M. G. (2023). Dynamic Risk Stratification Integrated with ATA Risk System for Predicting Long-Term Outcome in Papillary Thyroid Cancer. Cancers, 15(18), 4656. https://doi.org/10.3390/cancers15184656






