Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors
Abstract
Simple Summary
Abstract
1. Introduction
2. Angiogenesis Affects the Lung Cancer Pattern through Several Mechanisms
2.1. Tumor Growth Enhancement
2.2. Metastasis Promotion
2.3. Changes in Immunological Response in the Microenvironment
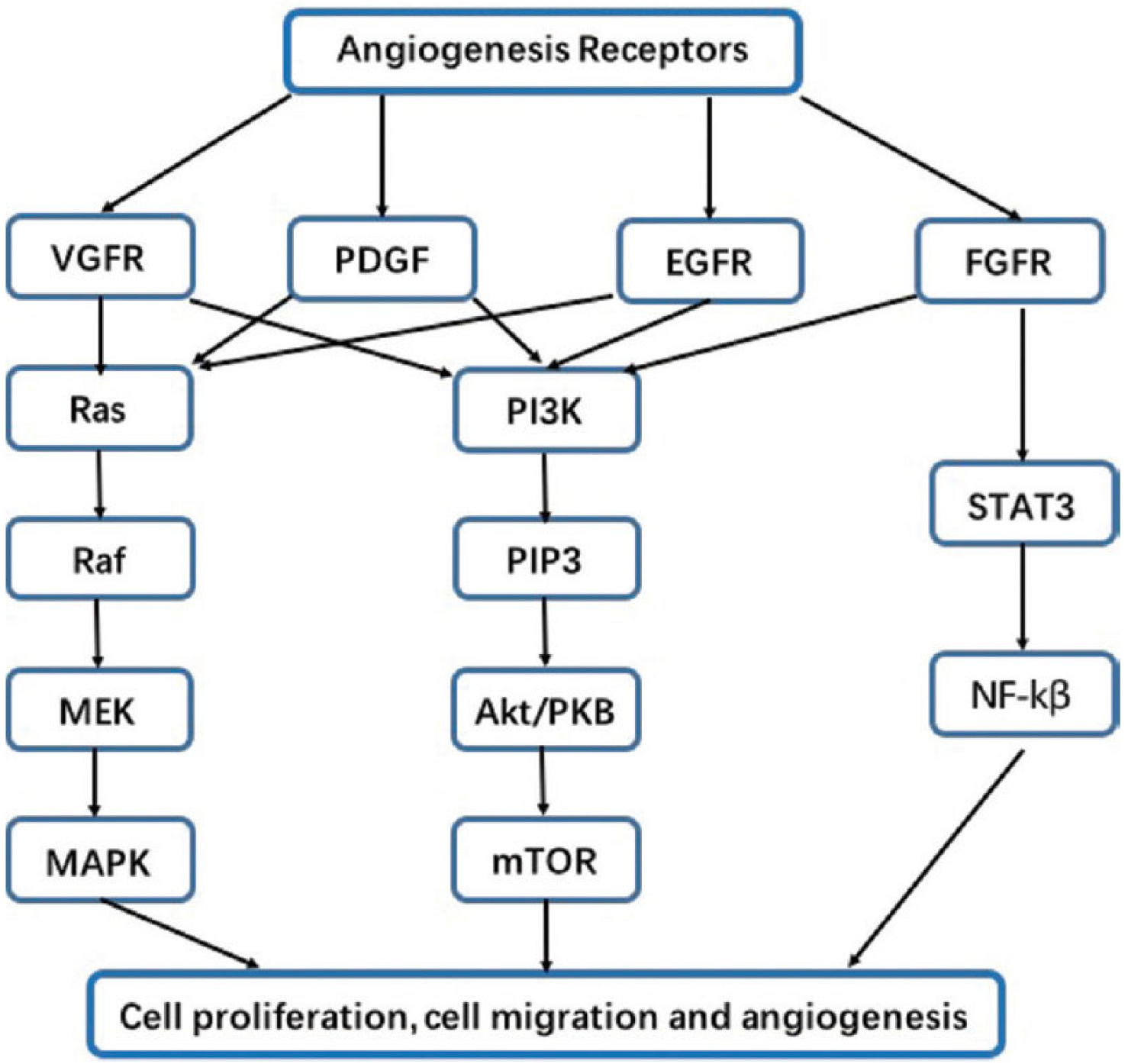
3. The Epidermal Growth Factor (EGF) Family, Their Receptors, and the Downstream
4. Vascular Endothelial Growth Factor (VEGF)
5. Colony Stimulating Factors (CSF)
- -
- Granulocyte colony-stimulating factor (G-CSF): A cytokine that promotes the creation and development of neutrophils, a kind of white blood cell, from bone marrow progenitor cells;
- -
- Granulocyte-macrophage colony-stimulating factor (GM-CSF): A cytokine that stimulates the development and differentiation of bone marrow progenitor cells into neutrophils, monocytes, and macrophages;
- -
- Macrophage colony-stimulating factor (M-CSF): A cytokine that induces the production and maturation of macrophages from bone marrow progenitor cells;
- -
- Interleukin 3 (IL-3 or multi-CSF): A hematopoietic cytokine and colony-stimulating factor that aids in the growth and maturation of erythroid, myeloid, megakaryocyte, and lymphoid progenitors.
6. Bone Morphogenetic Protein (BMP)
7. Fibroblast Growth Factors 1 and 2 (FGF1 and FGF2)
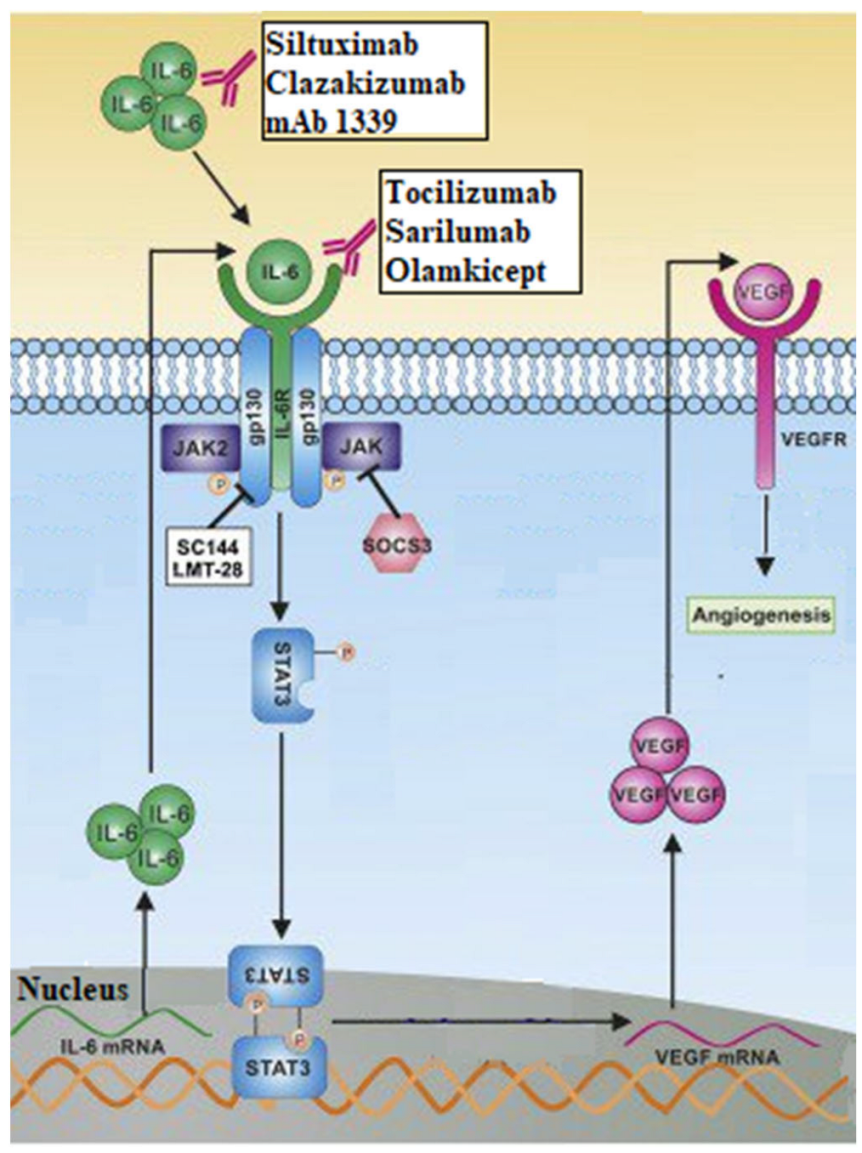
8. Interleukins (IL)
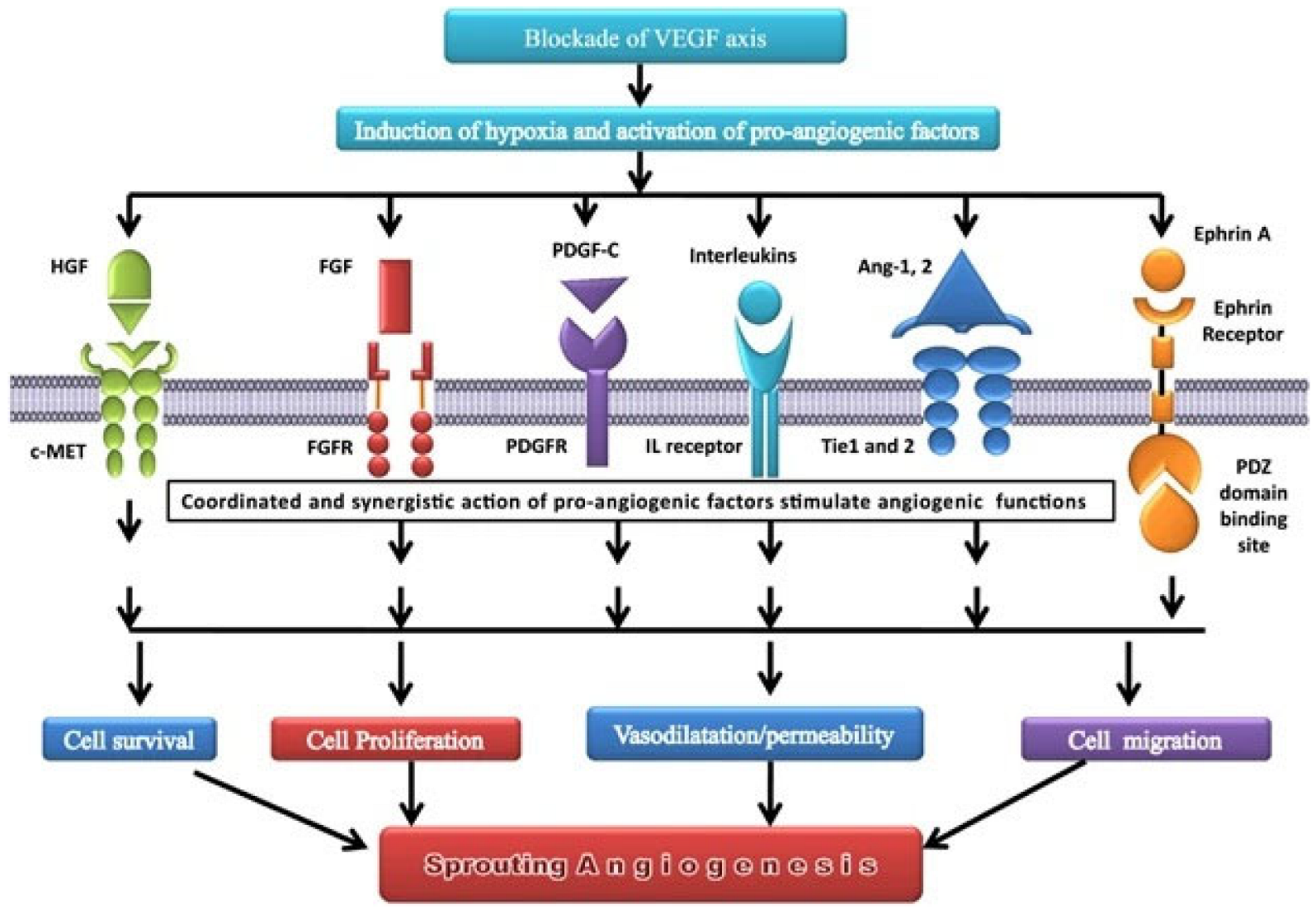
9. Others Growth Factors
- -
- Hepatocyte Growth Factor (HGF): It is a cytokine with two different domains, one N-terminal and one C-terminal, each with its own set of biological activity. The C-terminal domain of HGF mediates its ability to induce angiogenesis by activating subsequent signaling pathways, such as the PI3K/Akt and MAPK/ERK pathways [115,116]. Its rise in lung cancer has been linked to a poor prognosis and resistance to anti-angiogenic therapy. Recent research found that an anti-HGF monoclonal antibody can inhibit HGF-induced angiogenesis and tumor growth in preclinical models of lung cancer, providing a potential therapeutic strategy for lung cancer patients [116,117].
- -
- Human Epidermal Growth Factor Receptors 2 and 3 (HER2 and HER3): These two belong to the family of tyrosine kinases receptors and are overexpressed or mutated in many cancers and increase angiogenesis by activating both the PI3K/Akt and MAPK/ERK signaling pathways [118]. Several HER2-targeting therapy treatments, including monoclonal antibodies and tyrosine kinase inhibitors (TKIs), such as afatinib and neratinib, have demonstrated success in preclinical and clinical trials. Moreover, many researchers are working to bring out new therapies targeting HER-2 in the field of lung cancer [119,120].
- -
- Platelet Derived Growth Factor (PDGF) α/β: They belong to the PDGF receptor tyrosine kinase family and have been linked to lung cancer angiogenesis. PDGFR-alpha and PDGFR-beta are both overexpressed in lung cancer, and their presence has been linked to a bad prognosis. In preclinical lung cancer models, blocking PDGF signaling has been shown to diminish tumor formation and angiogenesis [22,121]. As for the others, combination treatments targeting both the PDGF and VEGF signaling pathways in lung cancer have been examined. In one trial, the anti-PDGF agent nintedanib was coupled with the anti-VEGF agent bevacizumab in lung cancer patients, resulting in an improvement in progression-free survival when compared to bevacizumab alone [122].
- -
- Soluble Tie 2 (sTie2) is a shortened version of the Tie2 receptor, which is an angiopoietin receptor expressed on endothelial cells and is involved in angiogenesis and vascular stabilization [123]. Its expression has been linked to unfavorable outcomes in several malignancies, including lung cancer, and research is being conducted to see how it can be targeted for therapy [124].
- -
- Soluble Neuropilin 1 (sNRP1) is a shortened version of the neuropilin 1 receptor that is produced on endothelial cells and impacts angiogenesis by acting as a VEGF coreceptor [125]. As with soluble Tie 2, large levels of sNRP1 expression have been linked to a worse prognosis, and it is also a molecule of interest in the realm of targeted therapeutics for lung cancer [126,127].
10. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| Ang 1 and 2 | Angiopoietin 1 and 2 |
| AREG | amphiregulin |
| bFGF | basic Fibroblast Growth Factor |
| BMP | Bone Morphogenetic Protein |
| BTC | beta-cellulin |
| CEA | Carcinoembryonic antigen |
| c-MET | cellular Mesenchymal-Epithelial Transition factor |
| CSF | Colony Stimulating Factor |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| EPCs | bone marrow-derived endothelial progenitor cells |
| EPGN | Epigene protein |
| EREG | Epiregulin |
| ERK | Extracellular Signal-Regulated Kinase |
| FGF (1 and 2) | Fibroblast Growth Factor (1 and 2) |
| FGFR (1 and 2) | Fibroblast Growth Factor Receptor (1 and 2) |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GLOBOCAN | Global Cancer Observatory |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GP | glycoprotein |
| HB-EGF | Heparin-Binding EGF-like Growth Factor |
| HER (2 and 3) | Human Epidermal Growth Factor Receptors (2 and 3) |
| HGF | Hepatocyte Growth Factor |
| HIF | Hypoxia-Inducible Factors |
| IL | Interleukin |
| JAK | Janus Kinase |
| MAPK | Mitogen Activated Protein Kinase |
| M-CSF | Macrophage Colony-Stimulating Factor |
| MRI | Magnetic Resonance Imaging |
| NSCLC | Non-Small Cell Lung Cancer |
| PDGF | Platelet-Derived Growth Factor |
| PDGF-c | Platelet-Derived Growth Factor C |
| PDGFR | Platelet-Derived Growth Factor Receptor |
| PDZ | Postsynaptic density protein of 95 kDa (PSD95), Drosophila disc large tumor suppressor (DlgA), and Zonula occludens-1 protein (Zo-1) |
| PI3k | Phosphatidylinositol 3-kinase |
| PIGF | Phosphatidylinositol-glycan F or Placental Growth Factor |
| PLC | Phospholipase C |
| SCLC | Small Cell Lung Cancer |
| sNRP1 | Soluble Neuropilin 1 |
| SOCS3 | Suppressor Of Cytokine Signaling 3 |
| STAT | Signal Transducer and Activator of Transcription |
| sTie 2 | Soluble Angiopoietin receptor |
| TGF-alpha | Transforming growth factor alpha |
| TGF-beta | Transforming Growth Factor-beta |
| Tie 2 | Angiopoietin receptor |
| TKI | Tyrosine Kinase Inhibitors |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Navada, S.; Lai, P.; Schwartz, A.G.; Kalemkerian, G.P. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J. Clin. Oncol. 2006, 24, 7082. [Google Scholar] [CrossRef]
- Sher, T.; Dy, G.K.; Adjei, A.A. Small Cell Lung Cancer. Mayo Clin. Proc. 2008, 83, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Adair, T.H.; Montani, J.-P. Overview of Angiogenesis. In Angiogenesis; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Hyodo, F.; Chandramouli, G.V.; Matsumoto, S.; Matsumoto, K.; Mitchell, J.B.; Krishna, M.C.; Munasinghe, J.P. Krishna Estimation of tumor microvessel density by MRI using a blood pool contrast agent. Int. J. Oncol. 2009, 35, 797–804. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 1985; Volume 43, pp. 175–203. ISBN 978-0-12-006643-8. [Google Scholar]
- Gupta, M.K. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144. [Google Scholar] [CrossRef]
- Shoari, A.; Khodabakhsh, F.; Ahangari Cohan, R.; Salimian, M.; Karami, E. Anti-angiogenic peptides application in cancer therapy; a review. Res. Pharm. Sci. 2021, 16, 559. [Google Scholar] [CrossRef]
- Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int. J. Mol. Sci. 2020, 21, 455. [Google Scholar] [CrossRef]
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and assays for the study of angiogenesis. Exp. Biol. Med. 2014, 239, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, L.C.; West, J.L. Studying the influence of angiogenesis in in vitro cancer model systems. Adv. Drug Deliv. Rev. 2016, 97, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular Mediators of Angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Horn, L.; Sandler, A.B. Angiogenesis in the Treatment of Non-Small Cell Lung Cancer. Proc. Am. Thorac. Soc. 2009, 6, 206–217. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular Endothelial Growth Factor as an Anti-Angiogenic Target for Cancer Therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Raica, M.; Cimpean, A.M. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017, 4, 19–24. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez De La Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 2009, 13, 2822–2833. [Google Scholar] [CrossRef]
- Pekarek, L.; Torres-Carranza, D.; Fraile-Martinez, O.; García-Montero, C.; Pekarek, T.; Saez, M.A.; Rueda-Correa, F.; Pimentel-Martinez, C.; Guijarro, L.G.; Diaz-Pedrero, R.; et al. An Overview of the Role of MicroRNAs on Carcinogenesis: A Focus on Cell Cycle, Angiogenesis and Metastasis. Int. J. Mol. Sci. 2023, 24, 7268. [Google Scholar] [CrossRef]
- Aspriţoiu, V.M.; Stoica, I.; Bleotu, C.; Diaconu, C.C. Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment. Front. Cell Dev. Biol. 2021, 9, 689962. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-angiogenic Agents in Combination with Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhao, W.; Ye, B.; Chen, D. Combination of Immune Checkpoint Inhibitors and Anti-Angiogenic Agents in Brain Metastases from Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 670313. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Schneider, M.R.; Wolf, E. The epidermal growth factor receptor ligands at a glance. J. Cell. Physiol. 2009, 218, 460–466. [Google Scholar] [CrossRef]
- Stoll, S.W.; Rittié, L.; Johnson, J.L.; Elder, J.T. Heparin-Binding EGF-Like Growth Factor Promotes Epithelial–Mesenchymal Transition in Human Keratinocytes. J. Investig. Dermatol. 2012, 132, 2148–2157. [Google Scholar] [CrossRef]
- Liu, T.-C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187–202. [Google Scholar]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The Role of Epidermal Growth Factor Receptor in Cancer Metastasis and Microenvironment. BioMed Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef]
- Spano, J.P.; Fagard, R.; Soria, J.-C.; Rixe, O.; Khayat, D.; Milano, G. Epidermal growth factor receptor signaling in colorectal cancer: Preclinical data and therapeutic perspectives. Ann. Oncol. 2005, 16, 189–194. [Google Scholar] [CrossRef]
- Ardizzone, A.; Bova, V.; Casili, G.; Repici, A.; Lanza, M.; Giuffrida, R.; Colarossi, C.; Mare, M.; Cuzzocrea, S.; Esposito, E.; et al. Role of Basic Fibroblast Growth Factor in Cancer: Biological Activity, Targeted Therapies, and Prognostic Value. Cells 2023, 12, 1002. [Google Scholar] [CrossRef]
- Khodabakhsh, F.; Merikhian, P.; Eisavand, M.R.; Farahmand, L. Crosstalk between MUC1 and VEGF in angiogenesis and metastasis: A review highlighting roles of the MUC1 with an emphasis on metastatic and angiogenic signaling. Cancer Cell Int. 2021, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, F.-E.; Chen, Q.-X.; Liu, B.-H. Molecular mechanisms involved in angiogenesis and potential target of antiangiogenesis in human glioblastomas. Glioma 2018, 1, 35. [Google Scholar] [CrossRef]
- Nan, X.; Xie, C.; Yu, X.; Liu, J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget 2017, 8, 75712–75726. [Google Scholar] [CrossRef]
- Nakagawa, K.; Garon, E.B.; Gao, L.; Callies, S.; Zimmermann, A.; Walgren, R.; Visseren-Grul, C.; Reck, M. RELAY, ramucirumab plus erlotinib versus placebo plus erlotinib in untreated EGFR-mutated metastatic non-small cell lung cancer: Exposure–response relationship. Cancer Chemother. Pharmacol. 2022, 90, 137–148. [Google Scholar] [CrossRef]
- Nakagawa, K.; Garon, E.B.; Seto, T.; Nishio, M.; Ponce Aix, S.; Paz-Ares, L.; Chiu, C.-H.; Park, K.; Novello, S.; Nadal, E.; et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1655–1669. [Google Scholar] [CrossRef]
- Subbiah, V.; Dumbrava, E.I.; Jiang, Y.; Thein, K.Z.; Naing, A.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Tsimberidou, A.M.; Janku, F.; et al. Dual EGFR blockade with cetuximab and erlotinib combined with anti-VEGF antibody bevacizumab in advanced solid tumors: A phase 1 dose escalation triplet combination trial. Exp. Hematol. Oncol. 2020, 9, 7. [Google Scholar] [CrossRef]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; AL-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal. 2022, 20, 49. [Google Scholar] [CrossRef]
- Mehta, V.B.; Besner, G.E.; Mehta, V.B.; Besner, G.E. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 2007, 25, 253–263. [Google Scholar] [CrossRef]
- Rosell, R.; Dafni, U.; Felip, E.; Curioni-Fontecedro, A.; Gautschi, O.; Peters, S.; Massutí, B.; Palmero, R.; Aix, S.P.; Carcereny, E.; et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): An international, multicentre, single-arm, phase 2 trial. Lancet Respir. Med. 2017, 5, 435–444. [Google Scholar] [CrossRef]
- Wang, X.; Goldstein, D.; Crowe, P.J.; Yang, J.-L. Next-generation EGFR/HER tyrosine kinase inhibitors for the treatment of patients with non-small-cell lung cancer harboring EGFR mutations: A review of the evidence. OncoTargets Ther. 2016, 9, 5461–5473. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Bai, R.; Cui, J. Precision targeted therapy for EGFR mutation-positive NSCLC: Dilemmas and coping strategies. Thorac. Cancer 2023, 14, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine Home|Beta ClinicalTrials.gov. Available online: https://beta.clinicaltrials.gov/ (accessed on 7 June 2023).
- European Medicines Agency Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search (accessed on 7 June 2023).
- Holmes, D.I.R.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, X.; Gaucher, J.-F.; Vidal, M.; Broussy, S. A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics. Molecules 2021, 26, 6759. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Cébe-Suarez, S.; Zehnder-Fjällman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006, 63, 601. [Google Scholar] [CrossRef]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Evensen, L.; Micklem, D.R.; Blois, A.; Berge, S.V.; Aarsæther, N.; Littlewood-Evans, A.; Wood, J.; Lorens, J.B. Mural Cell Associated VEGF Is Required for Organotypic Vessel Formation. PLoS ONE 2009, 4, e5798. [Google Scholar] [CrossRef]
- Stratman, A.N.; Malotte, K.M.; Mahan, R.D.; Davis, M.J.; Davis, G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114, 5091–5101. [Google Scholar] [CrossRef]
- Lind, J.S.W.; Smit, E.F. Angiogenesis inhibitors in the treatment of non-small cell lung cancer. Ther. Adv. Med. Oncol. 2009, 1, 95–107. [Google Scholar] [CrossRef]
- Horn, L.; Dahlberg, S.E.; Sandler, A.B.; Dowlati, A.; Moore, D.F.; Murren, J.R.; Schiller, J.H. Phase II Study of Cisplatin Plus Etoposide and Bevacizumab for Previously Untreated, Extensive-Stage Small-Cell Lung Cancer: Eastern Cooperative Oncology Group Study E3501. J. Clin. Oncol. 2009, 27, 6006–6011. [Google Scholar] [CrossRef] [PubMed]
- Cetean, S.; Căinap, C.; Constantin, A.-M.; Căinap, S.; Gherman, A.; Oprean, L.; Hangan, A.; Oprean, R. The importance of the granulocyte-colony stimulating factor in oncology. Med. Pharm. Rep. 2015, 88, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Ray, A. Cytokines and their Role in Health and Disease: A Brief Overview. MOJ Immunol. 2016, 4, 00121. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Achuthan, A.; Hamilton, J.A. Colony Stimulating Factors (CSFs). In Encyclopedia of Immunobiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 586–596. ISBN 978-0-08-092152-5. [Google Scholar]
- George, A.L.; Bangalore-Prakash, P.; Rajoria, S.; Suriano, R.; Shanmugam, A.; Mittelman, A.; Tiwari, R.K. Endothelial progenitor cell biology in disease and tissue regeneration. J. Hematol. Oncol. 2011, 4, 24. [Google Scholar] [CrossRef]
- Gao, D.; Nolan, D.; McDonnell, K.; Vahdat, L.; Benezra, R.; Altorki, N.; Mittal, V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim. Biophys. Acta BBA Rev. Cancer 2009, 1796, 33–40. [Google Scholar] [CrossRef]
- Aapro, M.; Crawford, J.; Kamioner, D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: Where are we now? Support. Care Cancer 2010, 18, 529–541. [Google Scholar] [CrossRef][Green Version]
- Karagiannidis, I.; Salataj, E.; Said Abu Egal, E.; Beswick, E.J. G-CSF in tumors: Aggressiveness, tumor microenvironment and immune cell regulation. Cytokine 2021, 142, 155479. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.; Chen, R.; Yuan, S.; Chen, L.; Qiu, X.; Qian, X.; Zhang, X.; Xiao, Z.; Wang, Q.; et al. rhG-CSF is associated with an increased risk of metastasis in NSCLC patients following postoperative chemotherapy. BMC Cancer 2022, 22, 741. [Google Scholar] [CrossRef]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef]
- Terceiro, L.E.L.; Edechi, C.A.; Ikeogu, N.M.; Nickel, B.E.; Hombach-Klonisch, S.; Sharif, T.; Leygue, E.; Myal, Y. The Breast Tumor Microenvironment: A Key Player in Metastatic Spread. Cancers 2021, 13, 4798. [Google Scholar] [CrossRef]
- Dentelli, P.; Rosso, A.; Olgasi, C.; Camussi, G.; Brizzi, M.F. IL-3 is a novel target to interfere with tumor vasculature. Oncogene 2011, 30, 4930–4940. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Dyer, L.A.; Pi, X.; Patterson, C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol. Metab. 2014, 25, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Montagne, K.; Nishihara, A.; Watabe, T.; Miyazono, K. BMPs Promote Proliferation and Migration of Endothelial Cells via Stimulation of VEGF-A/VEGFR2 and Angiopoietin-1/Tie2 Signalling. J. Biochem. 2008, 143, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chung, C.-H.; Lu, Y.-C.; Wu, M.-H.; Chou, P.-H.; Yen, J.-Y.; Lai, Y.-W.; Wang, G.-S.; Liu, S.-C.; Cheng, J.-K.; et al. BMP-2 induces angiogenesis by provoking integrin α6 expression in human endothelial progenitor cells. Biochem. Pharmacol. 2018, 150, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Facts, Challenges, and Future Perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; Van Dinther, M.; Liu, Z.; Van Bezooijen, R.L.; Zhao, Q.; Pukac, L.; Löwik, C.W.G.M.; Ten Dijke, P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 2007, 120, 964–972. [Google Scholar] [CrossRef]
- Meng, W.; Xiao, H.; Zhao, R.; Li, D.; Li, K.; Meng, Y.; Chen, J.; Wang, Y.; Liao, Y. The Prognostic Value of Bone Morphogenetic Proteins and Their Receptors in Lung Adenocarcinoma. Front. Oncol. 2021, 11, 608239. [Google Scholar] [CrossRef]
- Shen, W.; Pang, H.; Xin, B.; Duan, L.; Liu, L.; Zhang, H. Biological effects of BMP7 on small-cell lung cancer cells and its bone metastasis. Int. J. Oncol. 2018, 53, 1354–1362. [Google Scholar] [CrossRef]
- Ehata, S.; Miyazono, K. Bone Morphogenetic Protein Signaling in Cancer; Some Topics in the Recent 10 Years. Front. Cell Dev. Biol. 2022, 10, 883523. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. WIREs Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Korc, M.; Friesel, R. The Role of Fibroblast Growth Factors in Tumor Growth. Curr. Cancer Drug Targets 2009, 9, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Pardo, O.E.; Wellbrock, C.; Khanzada, U.K.; Aubert, M.; Arozarena, I.; Davidson, S.; Bowen, F.; Parker, P.J.; Filonenko, V.V.; Gout, I.T.; et al. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCε, B-Raf and S6K2. EMBO J. 2006, 25, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.; Lin, H.Y.; Lee, J.J.; Raso, M.G.; Hong, W.K.; Wistuba, I.I.; Lotan, R. Immunohistochemical Expression of Basic Fibroblast Growth Factor and Fibroblast Growth Factor Receptors 1 and 2 in the Pathogenesis of Lung Cancer. Clin. Cancer Res. 2008, 14, 6014–6022. [Google Scholar] [CrossRef] [PubMed]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef]
- Zahra, F.T.; Sajib, M.S.; Mikelis, C.M. Role of bFGF in Acquired Resistance upon Anti-VEGF Therapy in Cancer. Cancers 2021, 13, 1422. [Google Scholar] [CrossRef]
- Terai, H.; Soejima, K.; Yasuda, H.; Nakayama, S.; Hamamoto, J.; Arai, D.; Ishioka, K.; Ohgino, K.; Ikemura, S.; Sato, T.; et al. Activation of the FGF2-FGFR1 Autocrine Pathway: A Novel Mechanism of Acquired Resistance to Gefitinib in NSCLC. Mol. Cancer Res. 2013, 11, 759–767. [Google Scholar] [CrossRef]
- Gacche, R.N. Compensatory angiogenesis and tumor refractoriness. Oncogenesis 2015, 4, e153. [Google Scholar] [CrossRef]
- Chae, Y.K.; Pai, S.G.; Sun, P.; Costa, R.; Matsangou, M.; Agulnik, M.; Giles, F. Fibroblast growth factor receptor (FGFR) as a therapeutic target in lung and head and neck cancer. Am. J. Hematol. Oncol. 2016, 12, 13–19. [Google Scholar]
- Zheng, J.; Zhang, W.; Li, L.; He, Y.; Wei, Y.; Dang, Y.; Nie, S.; Guo, Z. Signaling Pathway and Small-Molecule Drug Discovery of FGFR: A Comprehensive Review. Front. Chem. 2022, 10, 860985. [Google Scholar] [CrossRef] [PubMed]
- Kubick, N.; Klimovich, P.; Flournoy, P.H.; Bieńkowska, I.; Łazarczyk, M.; Sacharczuk, M.; Bhaumik, S.; Mickael, M.-E.; Basu, R. Interleukins and Interleukin Receptors Evolutionary History and Origin in Relation to CD4+ T Cell Evolution. Genes 2021, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.L.; Borba, H.H.; Bonetti, A.D.F.; Leonart, L.; Pontarolo, R. Cytokines and Interferons: Types and Functions. In Autoantibodies and Cytokines; Ali Khan, W., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78984-852-6. [Google Scholar]
- Chen, J.J.W.; Yao, P.-L.; Yuan, A.; Hong, T.-M.; Shun, C.-T.; Kuo, M.-L.; Lee, Y.-C.; Yang, P.-C. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: Its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 729–737. [Google Scholar]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Author Correction: Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2020, 10, 8808. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Duan, L.; Qian, X.; Fan, J.; Lv, Z.; Zhang, X.; Han, J.; Wu, F.; Guo, M.; Hu, G.; et al. IL-17 Promotes Angiogenic Factors IL-6, IL-8, and Vegf Production via Stat1 in Lung Adenocarcinoma. Sci. Rep. 2016, 6, 36551. [Google Scholar] [CrossRef]
- Leung, J.H.; Ng, B.; Lim, W.-W. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells 2022, 11, 2257. [Google Scholar] [CrossRef]
- Zhang, J.; Veeramachaneni, N. Targeting interleukin-1β and inflammation in lung cancer. Biomark. Res. 2022, 10, 5. [Google Scholar] [CrossRef]
- Song, L.; Smith, M.A.; Doshi, P.; Sasser, K.; Fulp, W.; Altiok, S.; Haura, E.B. Antitumor Efficacy of the Anti-Interleukin-6 (IL-6) Antibody Siltuximab in Mouse Xenograft Models of Lung Cancer. J. Thorac. Oncol. 2014, 9, 974–982. [Google Scholar] [CrossRef]
- Gao, S.P.; Mark, K.G.; Leslie, K.; Pao, W.; Motoi, N.; Gerald, W.L.; Travis, W.D.; Bornmann, W.; Veach, D.; Clarkson, B.; et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J. Clin. Investig. 2007, 117, 3846–3856. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Heo, T.-H.; Wahler, J.; Suh, N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget 2016, 7, 15460–15473. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Kim, S.-K.; Kim, D.-S.; Zhang, D.; Park, J.-A.; Yi, H.; Kim, J.-S.; Shin, H.-C. Anti-proliferative action of IL-6R-targeted antibody tocilizumab for non-small cell lung cancer cells. Oncol. Lett. 2015, 9, 2283–2288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 1–16. [Google Scholar] [CrossRef]
- Mohammed, A.; Dashwood, R.H.; Dickinson, S.; Disis, M.L.; Jaffee, E.M.; Johnson, B.D.; Khleif, S.N.; Pollak, M.N.; Schlom, J.; Shoemaker, R.H.; et al. Translational Advances in Cancer Prevention Agent Development (TACPAD) Virtual Workshop on Immunomodulatory Agents: Report. J. Cancer Prev. 2021, 26, 309–317. [Google Scholar] [CrossRef]
- Ray, K.; Ujvari, B.; Ramana, V.; Donald, J. Cross-talk between EGFR and IL-6 drives oncogenic signaling and offers therapeutic opportunities in cancer. Cytokine Growth Factor Rev. 2018, 41, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Han, L.; Zhao, R.; Fatima, S.; Zhao, L.; Gao, F. Prognosis value of IL-6, IL-8, and IL-1β in serum of patients with lung cancer: A fresh look at interleukins as a biomarker. Heliyon 2022, 8, e09953. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Stabile, L.P.; Rothstein, M.E.; Keohavong, P.; Jin, J.; Yin, J.; Land, S.R.; Dacic, S.; Luong, T.M.; Kim, K.J.; Dulak, A.M.; et al. Therapeutic targeting of human hepatocyte growth factor with a single neutralizing monoclonal antibody reduces lung tumorigenesis. Mol. Cancer Ther. 2008, 7, 1913–1922. [Google Scholar] [CrossRef]
- Marmor, M.D.; Skaria, K.B.; Yarden, Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int. J. Radiat. Oncol. 2004, 58, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Riudavets, M.; Sullivan, I.; Abdayem, P.; Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021, 6, 100260. [Google Scholar] [CrossRef] [PubMed]
- Uy, N.F.; Merkhofer, C.M.; Baik, C.S. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers 2022, 14, 4155. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Siddik, Z.H. Platelet-derived growth factor (PDGF) signalling in cancer: Rapidly emerging signalling landscape: PDGF-induced Signalling Cascades. Cell Biochem. Funct. 2015, 33, 257–265. [Google Scholar] [CrossRef]
- Paluri, R.; Madan, A.; Li, P.; Jones, B.; Saleh, M.; Jerome, M.; Miley, D.; Keef, J.; Robert, F. Phase 1b trial of nintedanib in combination with bevacizumab in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2019, 83, 551–559. [Google Scholar] [CrossRef]
- Makinde, T.; Agrawal, D.K. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J. Cell. Mol. Med. 2008, 12, 810–828. [Google Scholar] [CrossRef]
- Khan, K.A.; Wu, F.T.; Cruz-Munoz, W.; Kerbel, R.S. Ang2 inhibitors and Tie2 activators: Potential therapeutics in perioperative treatment of early stage cancer. EMBO Mol. Med. 2021, 13, e08253. [Google Scholar] [CrossRef]
- Mehta, V.; Fields, L.; Evans, I.M.; Yamaji, M.; Pellet-Many, C.; Jones, T.; Mahmoud, M.; Zachary, I. VEGF (Vascular Endothelial Growth Factor) Induces NRP1 (Neuropilin-1) Cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to Generate Novel Carboxy-Terminal NRP1 Fragments That Regulate Angiogenic Signaling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1845–1858. [Google Scholar] [CrossRef]
- Hong, T.-M.; Chen, Y.-L.; Wu, Y.-Y.; Yuan, A.; Chao, Y.-C.; Chung, Y.-C.; Wu, M.-H.; Yang, S.-C.; Pan, S.-H.; Shih, J.-Y.; et al. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 4759–4768. [Google Scholar] [CrossRef]
- Liu, S.-D.; Zhong, L.-P.; He, J.; Zhao, Y.-X. Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chin. Med. J. 2021, 134, 508–517. [Google Scholar] [CrossRef]
| Last Update | Location and Study Identifier | Study Type | Study Title | Condition | Intervention | Status | Findings |
|---|---|---|---|---|---|---|---|
| May 2023 | United Kingdom NCT04179890 | Observational and retrospective | The study observes how long patients with non-small cell lung cancer (NSCLC) benefit from treatment with epidermal growth factor tyrosine kinase inhibitor (EGFR-TKI) when given either for uncommon mutations or for common mutations in the sequence afatinib followed by osimertinib (UpSwinG) | Non-squamous, Non-Small Cell Lung Cancer, | Observation of EGFR-TKI: -Afatinib -Erlotinib -Gefitinib -Osimertinib | Complete | treatment with EGFR-TKI should be considered as standard for most patients with uncommon mutations |
| February 2023 | USA NCT05062980 | Clinical Trial | Quaratusugene Ozeplasmid (Reqorsa) in combination with Pembrolizumab in previously treated Non-Small Cell Lung Cancer (Acclaim-2) Phase I/II | Non-Small Cell Lung Cancer | A: Quaratusugene ozeplasmid (pan-TKI: EGFR and Akt inhibitor) + Pembrolizumab (VEGFR downstream inhibitor: PD1 inhibitor) B: Docetaxel (microtubule inhibitor) + ramucirumab (VEGFR inhibitor) + 3rd molecule proposed by physician | On going | / |
| May 2019 | United Kingdom NCT02109016 | Clinical Trial | A single arm, open-label, phase II study to assess the efficacy of the dual VEGFR-FGFR tyrosine kinase inhibitor, Lucitanib, given orally as a single agent to patients with FGFR1-driven lung cancer. | Advance stage of Small and Non-small cell lung cancer with adenomatous, squamous, and large cell histologies, as well as FGF, VEGF, or PDGF genetic alterations. | Lucitanib, a VEGFR-FGFR tyrosine kinase inhibitor | Terminated | Interim analysis was either impossible (due to short time data collection) or showed low probability of clinically significant result |
| January 2013 | USA NCT00862134 | Clinical Trial | Randomized, Multi-center, Open-label, Study of PR104 Versus PR104/Docetaxel in Non-Small Cell Lung Cancer (NSCLC) Phase II | Non-Small Cell Lung Cancer | A: Docetaxel (microtubule inhibitor) B: Docetaxel + PR104 (hypoxia-activated prodrug) + G-CSF for prophylaxis | Terminated | Interim analysis indicated low probability of clinically significant result |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngaha, T.Y.S.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Abah, M.O.; Fossa, L.T.; Sangadzhieva, Z.D.; D. Sanikovich, V.; S. Rusanov, A.; N. Pirogova, Y.; et al. Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers 2023, 15, 4648. https://doi.org/10.3390/cancers15184648
Ngaha TYS, Zhilenkova AV, Essogmo FE, Uchendu IK, Abah MO, Fossa LT, Sangadzhieva ZD, D. Sanikovich V, S. Rusanov A, N. Pirogova Y, et al. Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers. 2023; 15(18):4648. https://doi.org/10.3390/cancers15184648
Chicago/Turabian StyleNgaha, Tchawe Yvan Sinclair, Angelina V. Zhilenkova, Freddy Elad Essogmo, Ikenna K. Uchendu, Moses Owoicho Abah, Lionel Tabola Fossa, Zaiana D. Sangadzhieva, Varvara D. Sanikovich, Alexander S. Rusanov, Yuliya N. Pirogova, and et al. 2023. "Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors" Cancers 15, no. 18: 4648. https://doi.org/10.3390/cancers15184648
APA StyleNgaha, T. Y. S., Zhilenkova, A. V., Essogmo, F. E., Uchendu, I. K., Abah, M. O., Fossa, L. T., Sangadzhieva, Z. D., D. Sanikovich, V., S. Rusanov, A., N. Pirogova, Y., Boroda, A., Rozhkov, A., Kemfang Ngowa, J. D., N. Bagmet, L., & I. Sekacheva, M. (2023). Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers, 15(18), 4648. https://doi.org/10.3390/cancers15184648








