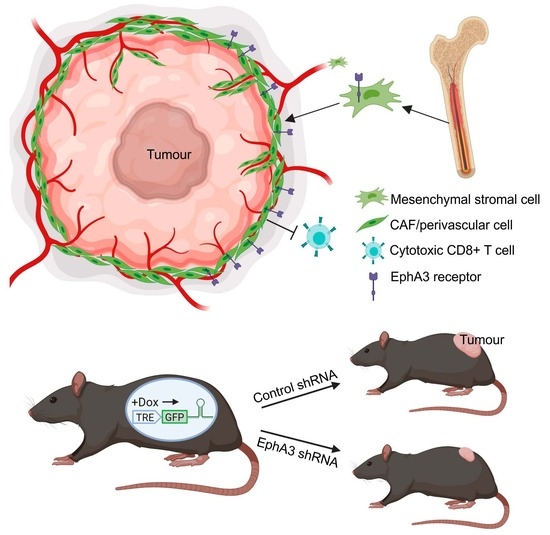
Inhibition of EphA3 Expression in Tumour Stromal Cells Suppresses Tumour Growth and Progression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. shRNA Plasmid Construct and Viral Transduction
2.3. Generation of EphA3 shRNA Mice
2.4. Quantitative RT-PCR (qRT-PCR)
2.5. Syngeneic Tumour Models
2.6. Aortic Ring Sprouting Assay
2.7. Antibodies
2.8. Tissue Immunohistochemistry (IHC) and Immunofluorescence (IF)
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
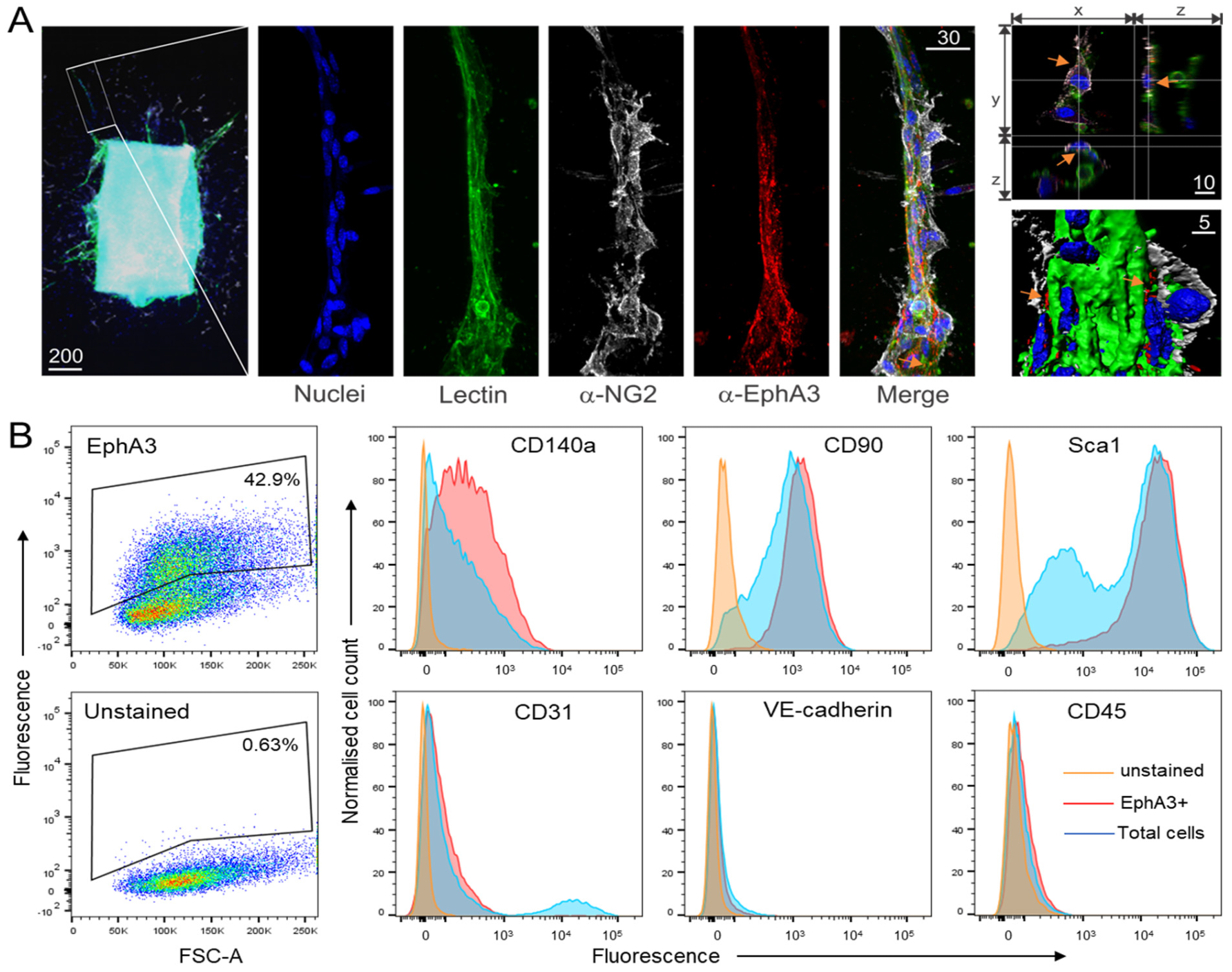
3.1. EphA3 Is Expressed on MSC-like Cells Associated with Angiogenesis
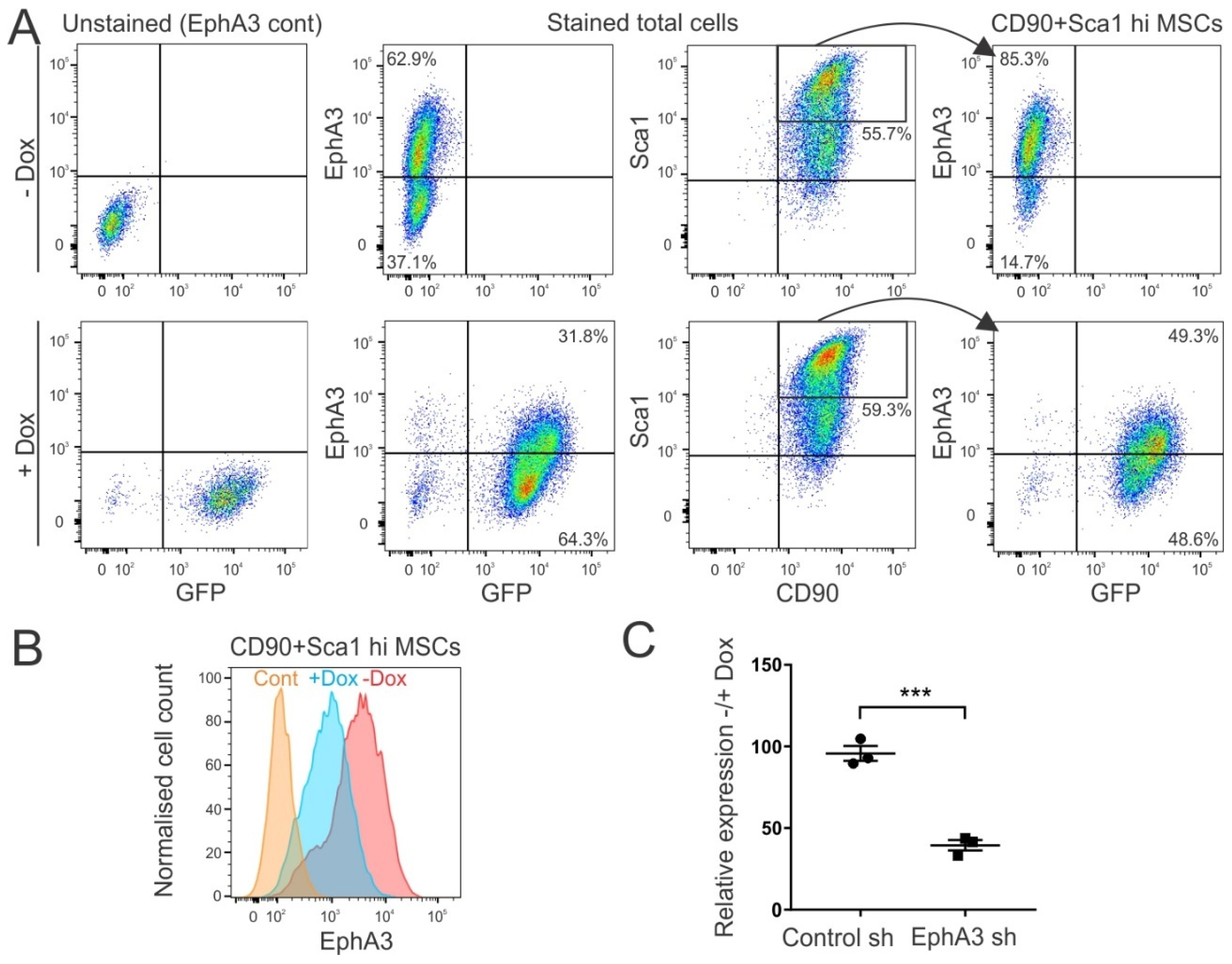
3.2. Generation of Mice with Inducible shRNA-Mediated Knockdown of EphA3
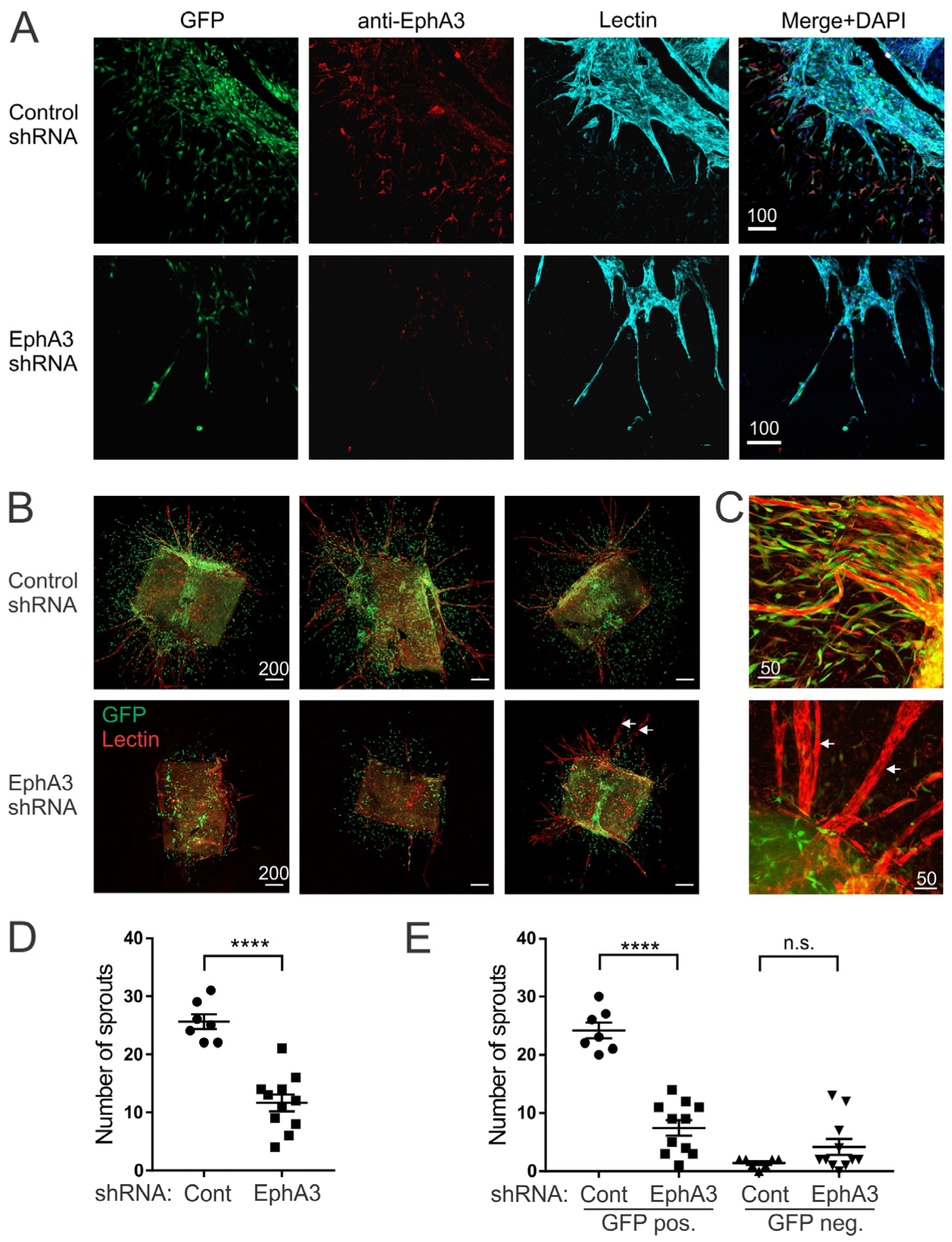
3.3. Reduced Angiogenic Sprouting of Aortic Explants from EphA3 shRNA Mice
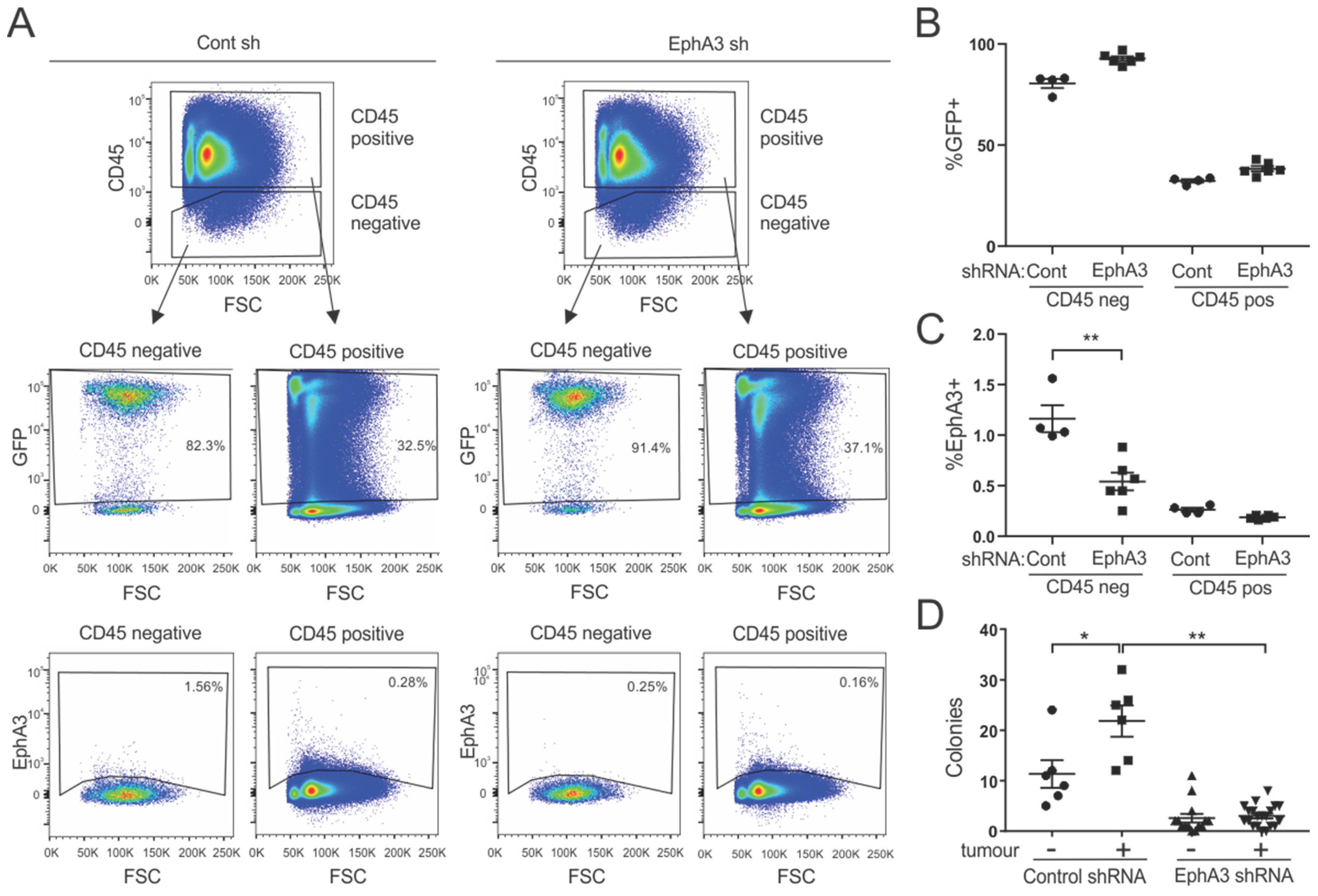
3.4. Bone Marrow-Derived Cells from EphA3 Knockdown Mice Show Reduced Colony-Forming Potential
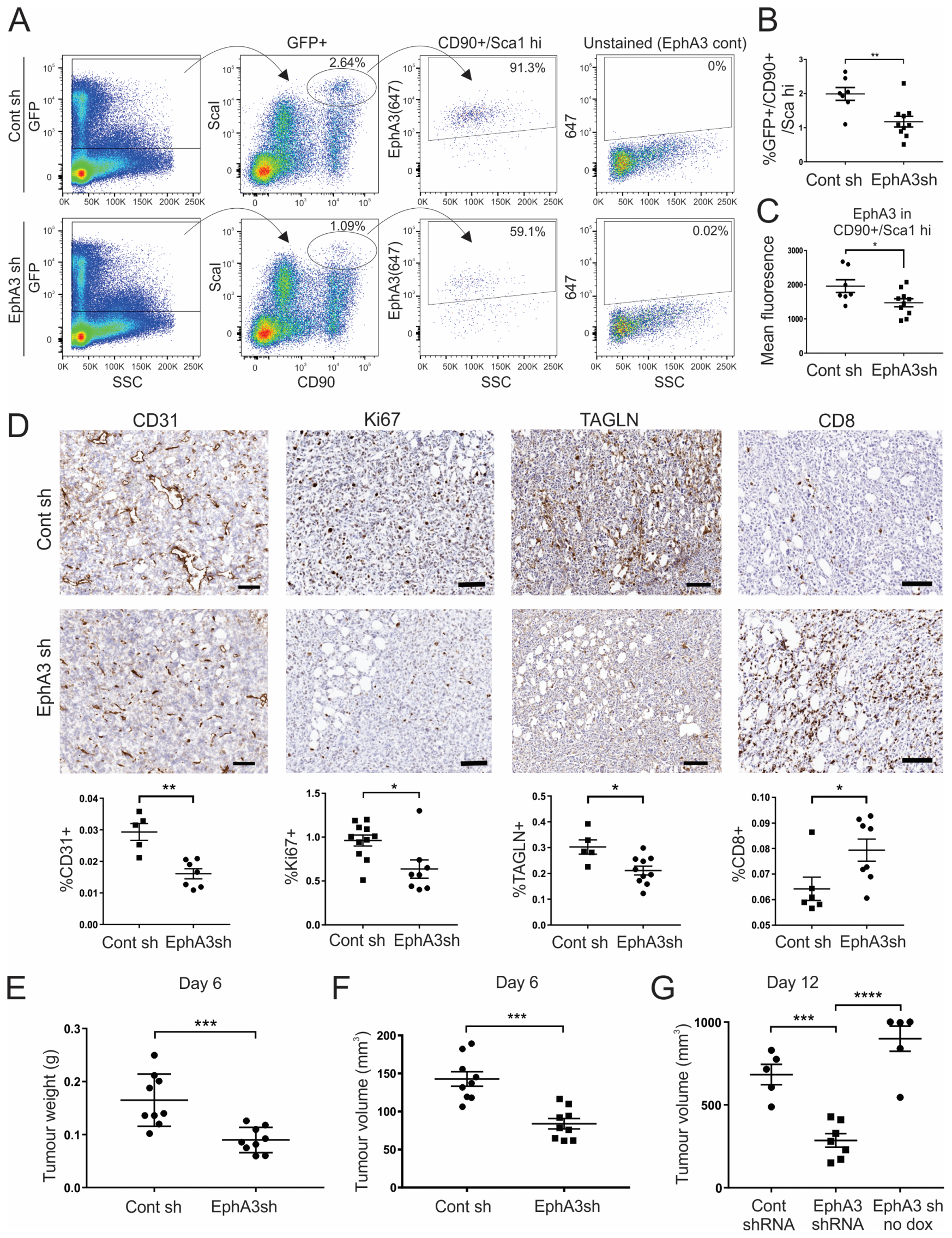
3.5. Tumours from EphA3 Knockdown Mice Exhibit an Altered TME and Reduced Tumour Growth
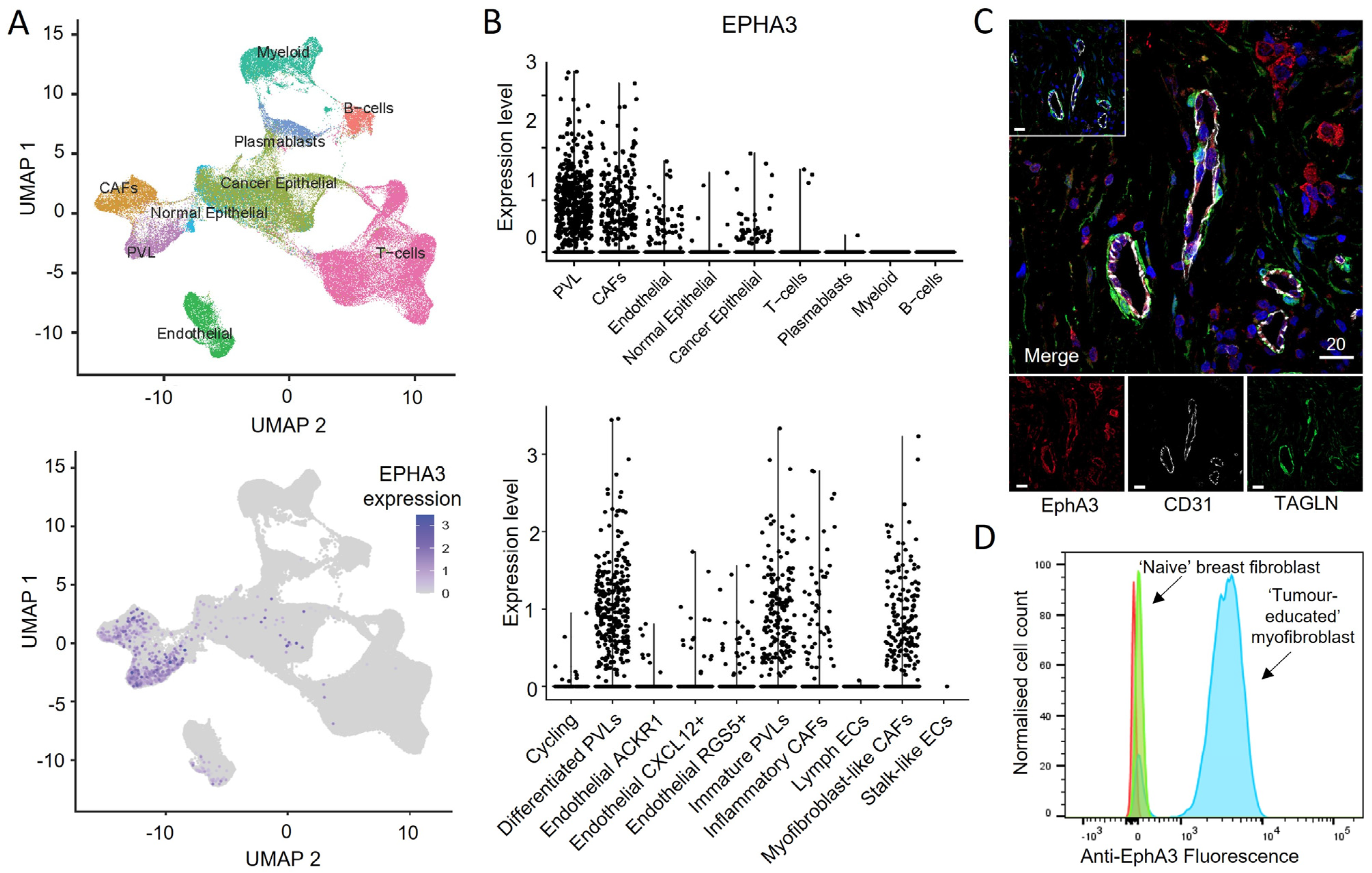
3.6. EphA3 Is Expressed in Distinct CAF Subtypes in Human Tumours
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tape, C.J.; Ling, S.; Dimitriadi, M.; McMahon, K.M.; Worboys, J.D.; Leong, H.S.; Norrie, I.C.; Miller, C.J.; Poulogiannis, G.; Lauffenburger, D.A.; et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell 2016, 165, 1818. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Swarbrick, A. Single-cell advances in stromal-leukocyte interactions in cancer. Immunol. Rev. 2021, 302, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Du, L.; Lin, L.; Wang, Y. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat. Rev. Drug Discov. 2017, 16, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Harney, A.S.; Pollard, J.W. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell 2016, 30, 18–25. [Google Scholar] [CrossRef]
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef]
- Wilkinson, D.G. Regulation of cell differentiation by Eph receptor and ephrin signaling. Cell Adh. Migr. 2014, 8, 339–348. [Google Scholar] [CrossRef]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Nievergall, E.; Lackmann, M.; Janes, P.W. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell Mol. Life Sci. 2012, 69, 1813–1842. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, W.; Amato, K. Eph receptor tyrosine kinases in cancer stem cells. Cytokine Growth Factor Rev. 2015, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mosch, B.; Reissenweber, B.; Neuber, C.; Pietzsch, J. Eph receptors and ephrin ligands: Important players in angiogenesis and tumor angiogenesis. J. Oncol. 2010, 2010, 135285. [Google Scholar] [CrossRef] [PubMed]
- Janes, P.W.; Vail, M.E.; Gan, H.K.; Scott, A.M. Antibody Targeting of Eph Receptors in Cancer. Pharmaceuticals 2020, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Shiuan, E.; Chen, J. Eph Receptor Tyrosine Kinases in Tumor Immunity. Cancer Res. 2016, 76, 6452–6457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tu, P.; Chen, X.; Guo, S.; Wang, J. Eph Receptors: Actors in Tumor Microenvironment. Crit. Rev. Oncog. 2017, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Janes, P.W.; Vail, M.E.; Ernst, M.; Scott, A.M. Eph receptors in the immune-suppressive tumor microenvironment. Cancer Res. 2020, 81, 801–805. [Google Scholar] [CrossRef]
- Ogawa, K.; Pasqualini, R.; Lindberg, R.A.; Kain, R.; Freeman, A.L.; Pasquale, E.B. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene 2000, 19, 6043–6052. [Google Scholar] [CrossRef]
- Brantley, D.M.; Cheng, N.; Thompson, E.J.; Lin, Q.; Brekken, R.A.; Thorpe, P.E.; Muraoka, R.S.; Cerretti, D.P.; Pozzi, A.; Jackson, D.; et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene 2002, 21, 7011–7026. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.M.; Chen, J. Eph receptor tyrosine kinases in angiogenesis: From development to disease. Angiogenesis 2004, 7, 17–28. [Google Scholar] [CrossRef]
- Janes, P.W.; Slape, C.I.; Farnsworth, R.H.; Atapattu, L.; Scott, A.M.; Vail, M.E. EphA3 biology and cancer. Growth Factors 2014, 32, 176–189. [Google Scholar] [CrossRef]
- Vail, M.E.; Murone, C.; Tan, A.; Hii, L.; Abebe, D.; Janes, P.W.; Lee, F.-T.; Baer, M.; Palath, V.; Bebbington, C.; et al. Targeting EphA3 Inhibits Cancer Growth by Disrupting the Tumor Stromal Microenvironment. Cancer Res. 2014, 74, 4470–4481. [Google Scholar] [CrossRef] [PubMed]
- To, C.; Farnsworth, R.; Vail, M.; Chheang, C.; Gargett, C.; Murone, C.; Llerena, C.; Major, A.; Scott, A.; Janes, P.; et al. Hypoxia-controlled EphA3 marks a human endometrium derived multipotent mesenchymal stromal cell that supports vascular growth. PLoS ONE 2014, 9, e112106. [Google Scholar] [CrossRef] [PubMed]
- Dow, L.E.; Premsrirut, P.K.; Zuber, J.; Fellmann, C.; McJunkin, K.; Miething, C.; Park, Y.; Dickins, R.A.; Hannon, G.J.; Lowe, S.W. A pipeline for the generation of shRNA transgenic mice. Nat. Protoc. 2012, 7, 374–393. [Google Scholar] [CrossRef] [PubMed]
- Vert, J.-P.; Foveau, N.; Lajaunie, C.; Vandenbrouck, Y. An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinform. 2006, 7, 520. [Google Scholar] [CrossRef]
- Fellmann, C.; Zuber, J.; McJunkin, K.; Chang, K.; Malone, C.D.; Dickins, R.A.; Xu, Q.; Hengartner, M.O.; Elledge, S.J.; Hannon, G.J.; et al. Functional identification of optimized RNAi triggers using a massively parallel sensor assay. Mol. Cell 2011, 41, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; Luce, M.J.; Bao, L.; Agha-Mohammadi, S.; Reiser, J. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J. Gene Med. 2005, 7, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Premsrirut, P.K.; Dow, L.E.; Kim, S.Y.; Camiolo, M.; Malone, C.D.; Miething, C.; Scuoppo, C.; Zuber, J.; Dickins, R.A.; Kogan, S.C.; et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell 2011, 145, 145–158. [Google Scholar] [CrossRef]
- Beard, C.; Hochedlinger, K.; Plath, K.; Wutz, A.; Jaenisch, R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis 2006, 44, 23–28. [Google Scholar] [CrossRef]
- Zambrowicz, B.P.; Imamoto, A.; Fiering, S.; Herzenberg, L.A.; Kerr, W.G.; Soriano, P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 1997, 94, 3789–3794. [Google Scholar] [CrossRef]
- Takiguchi, M.; Dow, L.E.; Prier, J.E.; Carmichael, C.L.; Kile, B.T.; Turner, S.J.; Lowe, S.W.; Huang, D.C.; Dickins, R.A. Variability of inducible expression across the hematopoietic system of tetracycline transactivator transgenic mice. PLoS ONE 2013, 8, e54009. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Robinson, S.D.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’Amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef]
- Janes, P.W.; Griesshaber, B.; Atapattu, L.; Nievergall, E.; Hii, L.L.; Mensinga, A.; Chheang, C.; Day, B.W.; Boyd, A.W.; Bastiaens, P.I.; et al. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J. Cell Biol. 2011, 195, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.J.; Cowin, A.J.; Kaur, P. Pericytes, mesenchymal stem cells and the wound healing process. Cells 2013, 2, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Dickins, R.A.; McJunkin, K.; Hernando, E.; Premsrirut, P.K.; Krizhanovsky, V.; Burgess, D.J.; Kim, S.Y.; Cordon-Cardo, C.; Zender, L.; Hannon, G.J.; et al. Tissue-specific and reversible RNA interference in transgenic mice. Nat. Genet. 2007, 39, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Stephen, L.J.; Fawkes, A.L.; Verhoeve, A.; Lemke, G.; Brown, A. A critical role for the EphA3 receptor tyrosine kinase in heart development. Dev. Biol. 2007, 302, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Alorro, M.G.; Pierce, T.P.; Eissmann, M.F.; Dijkstra, C.; Dickins, R.A.; Ernst, M.; Buchert, M.; Masson, F. Generation of an inducible mouse model to reversibly silence Stat3. Genesis 2017, 55, e23023. [Google Scholar] [CrossRef]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, S.; Zhou, X.; Cui, Y.; Wang, W.; Wen, L.; Guo, L.; Fu, W.; Tang, F. Single-Cell Multiomics Sequencing Reveals Prevalent Genomic Alterations in Tumor Stromal Cells of Human Colorectal Cancer. Cancer Cell 2020, 38, 818–828.e5. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Manikandan, M.; Almalki, S.; Mahmood, A.; Shinwari, T.; Vishnubalaji, R.; Mobarak, M.; Alfayez, M.; Aldahmash, A.; Kassem, M.; et al. Transgelin is a poor prognostic factor associated with advanced colorectal cancer (CRC) stage promoting tumor growth and migration in a TGFβ-dependent manner. Cell Death Dis. 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Rep. 2020, 31, 107628. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H.; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Acar, A.; Eaton, E.N.; Mellody, K.T.; Scheel, C.; Ben-Porath, I.; Onder, T.T.; Wang, Z.C.; Richardson, A.L.; Weinberg, R.A.; et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 20009–20014. [Google Scholar] [CrossRef] [PubMed]
- Day, B.W.; Stringer, B.W.; Al-Ejeh, F.; Ting, M.J.; Wilson, J.; Ensbey, K.S.; Jamieson, P.R.; Bruce, Z.C.; Lim, Y.C.; Offenhauser, C.; et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell 2013, 23, 238–248. [Google Scholar] [CrossRef]
- Foo, S.S.; Turner, C.J.; Adams, S.; Compagni, A.; Aubyn, D.; Kogata, N.; Lindblom, P.; Shani, M.; Zicha, D.; Adams, R.H. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 2006, 124, 161–173. [Google Scholar] [CrossRef]
- Salvucci, O.; Maric, D.; Economopoulou, M.; Sakakibara, S.; Merlin, S.; Follenzi, A.; Tosato, G. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood 2009, 114, 1707–1716. [Google Scholar] [CrossRef]
- Motegi, S.I.; Ishikawa, O. Mesenchymal stem cells: The roles and functions in cutaneous wound healing and tumor growth. J. Dermatol. Sci. 2017, 86, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rendon, E.; Hale, S.J.; Ryan, D.; Baban, D.; Forde, S.P.; Roubelakis, M.; Sweeney, D.; Moukayed, M.; Harris, A.L.; Davies, K.; et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem. Cells 2007, 25, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Helms, E.; Onate, M.K.; Sherman, M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020, 10, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Swords, R.T.; Greenberg, P.L.; Wei, A.H.; Durrant, S.; Advani, A.S.; Hertzberg, M.S.; Lewis, I.D.; Rivera, G.; Gratzinger, D.; Fan, A.C.; et al. KB004, a first in class monoclonal antibody targeting the receptor tyrosine kinase EphA3, in patients with advanced hematologic malignancies: Results from a phase 1 study. Leuk. Res. 2016, 50, 123–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vail, M.E.; Farnsworth, R.H.; Hii, L.; Allen, S.; Arora, S.; Anderson, R.L.; Dickins, R.A.; Orimo, A.; Wu, S.Z.; Swarbrick, A.; et al. Inhibition of EphA3 Expression in Tumour Stromal Cells Suppresses Tumour Growth and Progression. Cancers 2023, 15, 4646. https://doi.org/10.3390/cancers15184646
Vail ME, Farnsworth RH, Hii L, Allen S, Arora S, Anderson RL, Dickins RA, Orimo A, Wu SZ, Swarbrick A, et al. Inhibition of EphA3 Expression in Tumour Stromal Cells Suppresses Tumour Growth and Progression. Cancers. 2023; 15(18):4646. https://doi.org/10.3390/cancers15184646
Chicago/Turabian StyleVail, Mary E., Rae H. Farnsworth, Linda Hii, Stacey Allen, Sakshi Arora, Robin L. Anderson, Ross A. Dickins, Akira Orimo, Sunny Z. Wu, Alexander Swarbrick, and et al. 2023. "Inhibition of EphA3 Expression in Tumour Stromal Cells Suppresses Tumour Growth and Progression" Cancers 15, no. 18: 4646. https://doi.org/10.3390/cancers15184646
APA StyleVail, M. E., Farnsworth, R. H., Hii, L., Allen, S., Arora, S., Anderson, R. L., Dickins, R. A., Orimo, A., Wu, S. Z., Swarbrick, A., Scott, A. M., & Janes, P. W. (2023). Inhibition of EphA3 Expression in Tumour Stromal Cells Suppresses Tumour Growth and Progression. Cancers, 15(18), 4646. https://doi.org/10.3390/cancers15184646