The Role of Injectables in the Treatment and Prevention of Cancer-Associated Thrombosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology of Cancer-Associated Thrombosis
3. Pathogenesis
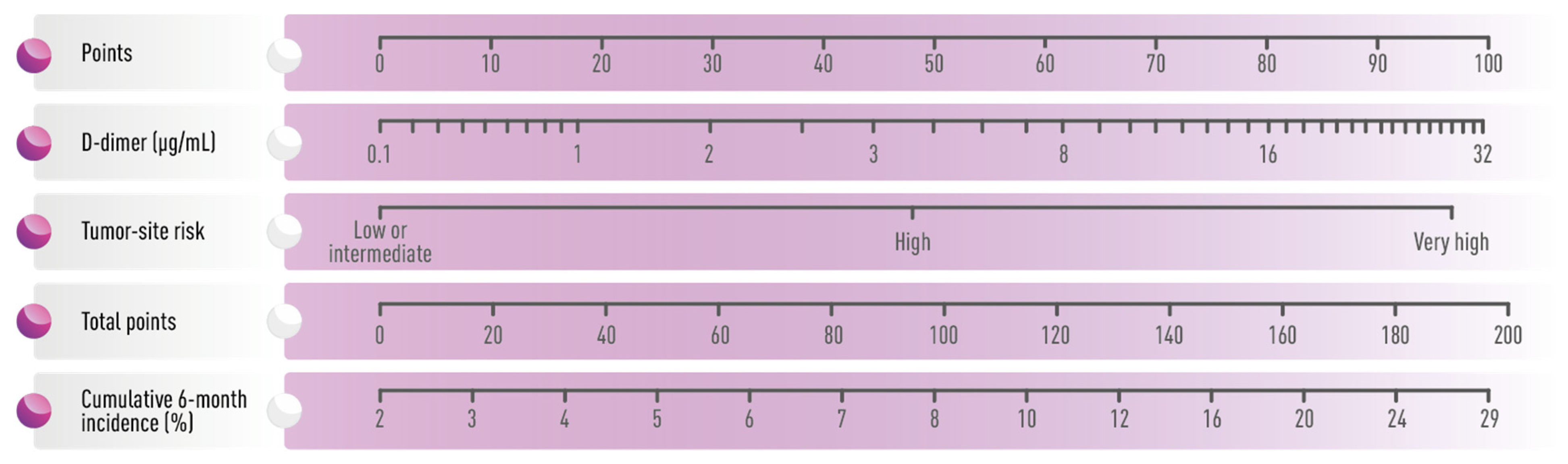
4. Risk Assessment Models in the Prevention of Cancer-Associated Thrombosis
5. The Impact of Cancer Type, Stage, and Treatment
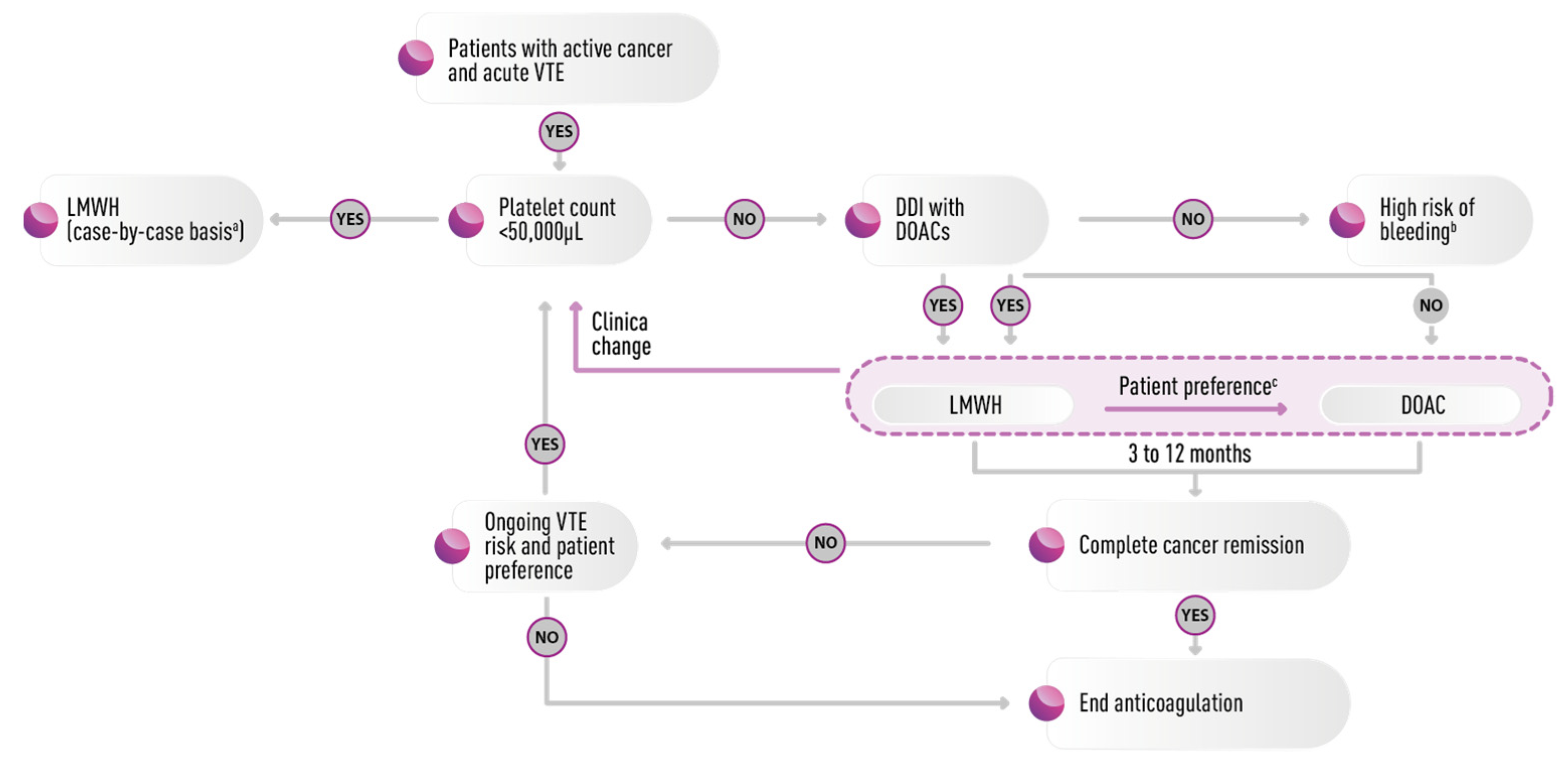
6. Treatment and Prophylaxis
7. Special Clinical Settings
7.1. Drug–Drug Interaction
7.2. Cerebral Involvement
7.3. Low and Unstable Platelet Count
7.4. Frail Patients
7.5. Renal Insufficiency
7.6. Liver Impairment
8. Injectables
8.1. Unfractionated Heparin
8.2. Low molecular Weight Heparins
8.2.1. Dalteparin
8.2.2. Tinzaparin
8.2.3. Enoxaparin
8.3. Fondaparinux
8.4. Anticancer Effect
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falanga, A.; Gal, G.L.; Carrier, M.; Abdel-Razeq, H.; Ay, C.; Martin, A.J.M.; Rocha, A.T.C.; Agnelli, G.; Elalamy, I.; Brenner, B. Management of Cancer-Associated Thrombosis: Unmet Needs and Future Perspectives. TH Open 2021, 5, e376–e386. [Google Scholar] [CrossRef]
- Lee, A.Y.; Levine, M.N. Venous thromboembolism and cancer: Risks and outcomes. Circulation 2003, 107, I17–I21. [Google Scholar] [CrossRef]
- Abu Zaanona, M.I.; Mantha, S. Cancer-associated Thrombosis. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gervaso, L.; Dave, H.; Khorana, A.A. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021, 3, 173–190. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mackman, N.; Falanga, A.; Pabinger, I.; Noble, S.; Ageno, W.; Moik, F.; Lee, A.Y.Y. Cancer-associated venous thromboembolism. Nat. Rev. Dis. Primers 2022, 8, 11. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Munoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Gates, L.E.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; Bounameaux, H.; Doerschug, K.; Geersing, G.J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest 2021, 160, 2247–2259. [Google Scholar] [CrossRef]
- Streiff, M.B.; Abutalib, S.A.; Farge, D.; Murphy, M.; Connors, J.M.; Piazza, G. Update on Guidelines for the Management of Cancer-Associated Thrombosis. Oncologist 2021, 26, e24–e40. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef]
- Mulder, F.I.; Horvath-Puho, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Buller, H.R.; Sorensen, H.T. Venous thromboembolism in cancer patients: A population-based cohort study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef]
- Grilz, E.; Posch, F.; Nopp, S.; Konigsbrugge, O.; Lang, I.M.; Klimek, P.; Thurner, S.; Pabinger, I.; Ay, C. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer-a nationwide analysis. Eur. Heart J. 2021, 42, 2299–2307. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Kushner, A.; West, W.P.; Khan Suheb, M.Z.; Pillarisetty, L.S. Virchow Triad. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Canonico, M.E.; Santoro, C.; Avvedimento, M.; Giugliano, G.; Mandoli, G.E.; Prastaro, M.; Franzone, A.; Piccolo, R.; Ilardi, F.; Cameli, M.; et al. Venous Thromboembolism and Cancer: A Comprehensive Review from Pathophysiology to Novel Treatment. Biomolecules 2022, 12, 259. [Google Scholar] [CrossRef]
- Connolly, G.C.; Phipps, R.P.; Francis, C.W. Platelets and cancer-associated thrombosis. Semin. Oncol. 2014, 41, 302–310. [Google Scholar] [CrossRef]
- Lucchesi, A.; Napolitano, R.; Bochicchio, M.T.; Giordano, G.; Napolitano, M. Platelets Contribution to Thrombin Generation in Philadelphia-Negative Myeloproliferative Neoplasms: The "Circulating Wound" Model. Int. J. Mol. Sci. 2021, 22, 11343. [Google Scholar] [CrossRef]
- Setiawan, B.; Budianto, W.; Sukarnowati, T.W.; Rizky, D.; Pangarsa, E.A.; Santosa, D.; Setiabudy, R.D.; Suharti, C. Correlation of Inflammation and Coagulation Markers with the Incidence of Deep Vein Thrombosis in Cancer Patients with High Risk of Thrombosis. Int. J. Gen. Med. 2022, 15, 6215–6226. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, V.; Bochicchio, M.T.; Giordano, G.; Napolitano, M.; Lucchesi, A. Genetics and Pathogenetic Role of Inflammasomes in Philadelphia Negative Chronic Myeloproliferative Neoplasms: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 561. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.E.; Radivoyevitch, T.; McCrae, K.R.; Khorana, A.A. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am. J. Hematol. 2019, 94, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.H.; Feng, L.; Su, X.; Brassard, A.; Dhoparee-Doomah, I.; Ferri, L.E.; Spicer, J.D.; Cools-Lartigue, J.J. Neutrophil Extracellular Traps in Cancer Therapy Resistance. Cancers 2022, 14, 1359. [Google Scholar] [CrossRef] [PubMed]
- Tafur, A.J.; Wysokinski, W.E.; McBane, R.D.; Wolny, E.; Sutkowska, E.; Litin, S.C.; Daniels, P.R.; Slusser, J.P.; Hodge, D.O.; Heit, J.A. Cancer effect on periprocedural thromboembolism and bleeding in anticoagulated patients. Ann. Oncol. 2012, 23, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Kacimi, S.E.O.; Moeinafshar, A.; Haghighi, S.S.; Saghazadeh, A.; Rezaei, N. Venous thromboembolism in cancer and cancer immunotherapy. Crit. Rev. Oncol. Hematol. 2022, 178, 103782. [Google Scholar] [CrossRef]
- Mukai, M.; Oka, T. Mechanism and management of cancer-associated thrombosis. J. Cardiol. 2018, 72, 89–93. [Google Scholar] [CrossRef]
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Khorana, A.A.; DeSancho, M.T.; Liebman, H.; Rosovsky, R.; Connors, J.M.; Zwicker, J. Prediction and Prevention of Cancer-Associated Thromboembolism. Oncologist 2021, 26, e2–e7. [Google Scholar] [CrossRef]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Doggen, C.J.; Osanto, S.; Rosendaal, F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Konigsbrugge, O.; Pabinger, I.; Ay, C. Risk factors for venous thromboembolism in cancer: Novel findings from the Vienna Cancer and Thrombosis Study (CATS). Thromb. Res. 2014, 133 (Suppl. S2), S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Osanto, S.; Rosendaal, F.R. The risk of a venous thrombotic event in lung cancer patients: Higher risk for adenocarcinoma than squamous cell carcinoma. J. Thromb. Haemost. 2004, 2, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-induced thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef]
- Ahlbrecht, J.; Dickmann, B.; Ay, C.; Dunkler, D.; Thaler, J.; Schmidinger, M.; Quehenberger, P.; Haitel, A.; Zielinski, C.; Pabinger, I. Tumor grade is associated with venous thromboembolism in patients with cancer: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2012, 30, 3870–3875. [Google Scholar] [CrossRef]
- Dickmann, B.; Ahlbrecht, J.; Ay, C.; Dunkler, D.; Thaler, J.; Scheithauer, W.; Quehenberger, P.; Zielinski, C.; Pabinger, I. Regional lymph node metastases are a strong risk factor for venous thromboembolism: Results from the Vienna Cancer and Thrombosis Study. Haematologica 2013, 98, 1309–1314. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Hinyard, L.; Schoen, M.W.; Geneus, C.J.; Armbrecht, E.S.; Buckhold, F.R.; Burroughs, T.E. Description of Venous Thromboembolism in Hospitalized Patients With Metastatic Cancer: A National Sample. J. Natl. Compr. Cancer Netw. 2018, 16, 136–143. [Google Scholar] [CrossRef]
- Asdahl, P.H.; Sundboll, J.; Adelborg, K.; Rasmussen, T.B.; Seesaghur, A.M.; Hernandez, R.K.; Sorensen, H.T.; Pedersen, A.B. Cardiovascular events in cancer patients with bone metastases-A Danish population-based cohort study of 23,113 patients. Cancer Med. 2021, 10, 4885–4895. [Google Scholar] [CrossRef]
- Grover, S.P.; Hisada, Y.M.; Kasthuri, R.S.; Reeves, B.N.; Mackman, N. Cancer Therapy-Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1291–1305. [Google Scholar] [CrossRef]
- Agnelli, G.; Bolis, G.; Capussotti, L.; Scarpa, R.M.; Tonelli, F.; Bonizzoni, E.; Moia, M.; Parazzini, F.; Rossi, R.; Sonaglia, F.; et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: The @RISTOS project. Ann. Surg. 2006, 243, 89–95. [Google Scholar] [CrossRef]
- Groot, O.Q.; Ogink, P.T.; Janssen, S.J.; Paulino Pereira, N.R.; Lozano-Calderon, S.; Raskin, K.; Hornicek, F.; Schwab, J.H. High Risk of Venous Thromboembolism After Surgery for Long Bone Metastases: A Retrospective Study of 682 Patients. Clin. Orthop. Relat. Res. 2018, 476, 2052–2061. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Hu, W. Incidence, risk factors, and outcomes of venous thromboembolism after oncologic surgery: A systematic review and meta-analysis. Thromb. Res. 2019, 173, 48–56. [Google Scholar] [CrossRef]
- Munoz Martin, A.J.; Ramirez, S.P.; Moran, L.O.; Zamorano, M.R.; Beneitez, M.C.V.; Salcedo, I.A.; Escobar, I.G.; Fernandez, J.M.S. Pharmacological cancer treatment and venous thromboembolism risk. Eur. Heart J. Suppl. 2020, 22, C2–C14. [Google Scholar] [CrossRef]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Sorensen, H.T.; Pedersen, L.; Jacobsen, J.; Lash, T.L. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: A Danish population-based cohort study. Cancer 2009, 115, 4442–4449. [Google Scholar] [CrossRef]
- Pritchard, K.I.; Paterson, A.H.; Paul, N.A.; Zee, B.; Fine, S.; Pater, J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J. Clin. Oncol. 1996, 14, 2731–2737. [Google Scholar] [CrossRef]
- Miroddi, M.; Sterrantino, C.; Simmonds, M.; Caridi, L.; Calapai, G.; Phillips, R.S.; Stewart, L.A. Systematic review and meta-analysis of the risk of severe and life-threatening thromboembolism in cancer patients receiving anti-EGFR monoclonal antibodies (cetuximab or panitumumab). Int. J. Cancer 2016, 139, 2370–2380. [Google Scholar] [CrossRef]
- Petrelli, F.; Cabiddu, M.; Borgonovo, K.; Barni, S. Risk of venous and arterial thromboembolic events associated with anti-EGFR agents: A meta-analysis of randomized clinical trials. Ann. Oncol. 2012, 23, 1672–1679. [Google Scholar] [CrossRef]
- Deschenes-Simard, X.; Richard, C.; Galland, L.; Blais, F.; Desilets, A.; Malo, J.; Cvetkovic, L.; Belkaid, W.; Elkrief, A.; Gagne, A.; et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: A retrospective multicentric cohort study. Thromb. Res. 2021, 205, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; Khorana, A.A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymoss, S.; et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019, 20, e566–e581. [Google Scholar] [CrossRef] [PubMed]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201. [Google Scholar] [CrossRef]
- Hutchinson, A.; Rees, S.; Young, A.; Maraveyas, A.; Date, K.; Johnson, M.J. Oral anticoagulation is preferable to injected, but only if it is safe and effective: An interview study of patient and carer experience of oral and injected anticoagulant therapy for cancer-associated thrombosis in the select-d trial. Palliat. Med. 2019, 33, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.; Matzdorff, A.; Maraveyas, A.; Holm, M.V.; Pisa, G. Assessing patients’ anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica 2015, 100, 1486–1492. [Google Scholar] [CrossRef]
- Picker, N.; Lee, A.Y.; Cohen, A.T.; Maraveyas, A.; Beyer-Westendorf, J.; Mantovani, L.G.; Abdelgawwad, K.; Fatoba, S.; Thate-Waschke, I.M.; Bach, M.; et al. Anticoagulation Treatment in Cancer-Associated Venous Thromboembolism: Assessment of Patient Preferences Using a Discrete Choice Experiment (COSIMO Study). Thromb. Haemost. 2021, 121, 206–215. [Google Scholar] [CrossRef]
- Ay, C.; Beyer-Westendorf, J.; Pabinger, I. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann. Oncol. 2019, 30, 897–907. [Google Scholar] [CrossRef]
- Grzesk, G.; Rogowicz, D.; Wolowiec, L.; Ratajczak, A.; Gilewski, W.; Chudzinska, M.; Sinkiewicz, A.; Banach, J. The Clinical Significance of Drug-Food Interactions of Direct Oral Anticoagulants. Int. J. Mol. Sci. 2021, 22, 8531. [Google Scholar] [CrossRef]
- Napolitano, M.; Saccullo, G.; Marietta, M.; Carpenedo, M.; Castaman, G.; Cerchiara, E.; Chistolini, A.; Contino, L.; De Stefano, V.; Falanga, A.; et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: An expert consensus. Blood Transfus. 2019, 17, 171–180. [Google Scholar] [CrossRef]
- Short, N.J.; Connors, J.M. New oral anticoagulants and the cancer patient. Oncologist 2014, 19, 82–93. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Connors, J.M. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019, 3, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, N.; Brito-Dellan, N.; Font, C.; Butler, T.; Rojas-Hernandez, C.M.; Butler, T.; Escalante, C.; on behalf of the, M.H.S.G. Complexity and clinical significance of drug–drug interactions (DDIs) in oncology: Challenging issues in the care of patients regarding cancer-associated thrombosis (CAT). Support. Care Cancer 2022, 30, 8559–8573. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.T.; Schiff, D.; Perry, J.R. Thrombosis in brain tumors. Semin. Thromb. Hemost. 2014, 40, 325–331. [Google Scholar] [CrossRef]
- Kabashneh, S.; Alkassis, S.; Shanah, L.; Alkofahi, A.A. Venous Thromboembolism in Patients With Brain Cancer: Focus on Prophylaxis and Management. Cureus 2020, 12, e8771. [Google Scholar] [CrossRef]
- Kimmell, K.T.; Walter, K.A. Risk factors for venous thromboembolism in patients undergoing craniotomy for neoplastic disease. J. Neuro-Oncol. 2014, 120, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Atiemo, A.; Diran, L.K.; Licholai, G.P.; McLaren Black, P.; Creager, M.A.; Goldhaber, S.Z. Venous Thromboembolism Occurs Frequently in Patients Undergoing Brain Tumor Surgery Despite Prophylaxis. J. Thromb. Thrombolysis 1999, 8, 139–142. [Google Scholar] [CrossRef]
- Iyengar, V.; Patell, R.; Zwicker, J. Challenges in anticoagulation for patients with brain tumors. Best. Pract. Res. Clin. Haematol. 2022, 35, 101350. [Google Scholar] [CrossRef]
- Patell, R.; Zwicker, J.I. Evidence-Based Minireview: Full dose, modified dose, or no anticoagulation for patients with cancer and acute VTE and thrombocytopenia. Hematol. Am. Soc. Hematol. Educ. Program. 2022, 2022, 312–315. [Google Scholar] [CrossRef]
- Hsu, C.; Patell, R.; Zwicker, J.I. The Prevalence of Thrombocytopenia in Patients with Acute Cancer-Associated Thrombosis. Blood Adv. 2022, 7, 4721–4727. [Google Scholar] [CrossRef]
- Leader, A.; Hofstetter, L.; Spectre, G. Challenges and Advances in Managing Thrombocytopenic Cancer Patients. J. Clin. Med. 2021, 10, 1169. [Google Scholar] [CrossRef]
- Proietti, M.; Cesari, M. Frailty: What Is It? Adv. Exp. Med. Biol. 2020, 1216, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scotte, F.; Leroy, P.; Chastenet, M.; Aumont, L.; Benatar, V.; Elalamy, I. Treatment and Prevention of Cancer-Associated Thrombosis in Frail Patients: Tailored Management. Cancers 2019, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Pabinger, I.; Ay, C. How I treat cancer-associated thrombosis. ESMO Open 2020, 5, e000610. [Google Scholar] [CrossRef]
- Moustafa, F.; Giorgi Pierfranceschi, M.; Di Micco, P.; Bucherini, E.; Lorenzo, A.; Villalobos, A.; Nieto, J.A.; Valero, B.; Samperiz, A.L.; Monreal, M.; et al. Clinical outcomes during anticoagulant therapy in fragile patients with venous thromboembolism. Res. Pract. Thromb. Haemost. 2017, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Launay-Vacher, V.; Janus, N.; Deray, G. Renal insufficiency and cancer treatments. ESMO Open 2016, 1, e000091. [Google Scholar] [CrossRef]
- Wang, T.F.; Billett, H.H.; Connors, J.M.; Soff, G.A. Approach to Cancer-Associated Thrombosis: Challenging Situations and Knowledge Gaps. Oncologist 2021, 26, e17–e23. [Google Scholar] [CrossRef]
- Kooiman, J.; den Exter, P.L.; Cannegieter, S.C.; le Cessie, S.; del Toro, J.; Sahuquillo, J.C.; Pedrajas, J.M.; Huisman, M.V. Impact of chronic kidney disease on the risk of clinical outcomes in patients with cancer-associated venous thromboembolism during anticoagulant treatment. J. Thromb. Haemost. 2013, 11, 1968–1976. [Google Scholar] [CrossRef]
- Lee, A.Y.; Peterson, E.A. Treatment of cancer-associated thrombosis. Blood 2013, 122, 2310–2317. [Google Scholar] [CrossRef]
- Hughes, S.; Szeki, I.; Nash, M.J.; Thachil, J. Anticoagulation in chronic kidney disease patients-the practical aspects. Clin. Kidney J. 2014, 7, 442–449. [Google Scholar] [CrossRef]
- Smrke, A.; Gross, P.L. Cancer-Associated Venous Thromboembolism: A Practical Review Beyond Low-Molecular-Weight Heparins. Front. Med. 2017, 4, 142. [Google Scholar] [CrossRef]
- Carrier, M.; Blais, N.; Crowther, M.; Kavan, P.; Le Gal, G.; Moodley, O.; Shivakumar, S.; Suryanarayan, D.; Tagalakis, V.; Wu, C.; et al. Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus. Curr. Oncol. 2021, 28, 5434–5451. [Google Scholar] [CrossRef] [PubMed]
- Berlioz, B.E.; Sanghavi, D. Bivalirudin. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mahat, K.C.; Sedhai, Y.R.; Krishnan, P. Argatroban. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kaliel, H.; Mior, M.; Quan, S.; Ghosh, S.; Wu, C.; Bungard, T.J. Retrospective Review of Prescribing Patterns in Cancer-Associated Thrombosis: A Single Center Experience in Edmonton, Alberta, Canada. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620975489. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.A.; Berry, L.R.; Monagle, P.T.; Chan, A.K. Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br. J. Haematol. 2002, 116, 178–186. [Google Scholar] [CrossRef]
- Hirsh, J.; Bauer, K.A.; Donati, M.B.; Gould, M.; Samama, M.M.; Weitz, J.I. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 141S–159S. [Google Scholar] [CrossRef]
- Fareed, J.; Jeske, W.; Fareed, D.; Clark, M.; Wahi, R.; Adiguzel, C.; Hoppensteadt, D. Are all low molecular weight heparins equivalent in the management of venous thromboembolism? Clin. Appl. Thromb. Hemost. 2008, 14, 385–392. [Google Scholar] [CrossRef]
- Matar, C.F.; Kahale, L.A.; Hakoum, M.B.; Tsolakian, I.G.; Etxeandia-Ikobaltzeta, I.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Barba, M.; Schunemann, H.; et al. Anticoagulation for perioperative thromboprophylaxis in people with cancer. Cochrane Database Syst. Rev. 2018, 7, CD009447. [Google Scholar] [CrossRef] [PubMed]
- Van Matre, E.T.; Reynolds, P.M.; MacLaren, R.; Mueller, S.W.; Wright, G.C.; Moss, M.; Burnham, E.L.; Ho, P.M.; Vandivier, R.W.; Kiser, T.H. Evaluation of unfractionated heparin versus low-molecular-weight heparin and fondaparinux for pharmacologic venous thromboembolic prophylaxis in critically ill patients with cancer. J. Thromb. Haemost. 2018, 16, 2492–2500. [Google Scholar] [CrossRef]
- Oyakawa, T.; Fukumitsu, M.; Ebihara, A.; Shiga, T. Relevance of Non-Bridging Therapy with Heparin during Temporary Interruption of Direct Oral Anticoagulants in Patients with Cancer-Associated Venous Thromboembolism. Ann. Vasc. Dis. 2022, 15, 121–125. [Google Scholar] [CrossRef]
- Liu, D.; Song, D.; Ning, W.; Zhang, X.; Chen, S.; Zhang, H. Efficacy and safety of prophylaxis for venous thromboembolism in brain neoplasm patients undergoing neurosurgery: A systematic review and Bayesian network meta-analysis. J. Thromb. Thrombolysis 2023, 55, 710–720. [Google Scholar] [CrossRef]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef]
- Kahale, L.A.; Hakoum, M.B.; Tsolakian, I.G.; Matar, C.F.; Terrenato, I.; Sperati, F.; Barba, M.; Yosuico, V.E.; Schunemann, H.; Akl, E.A. Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst. Rev. 2018, 6, CD006650. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Mansueto, M.F.; Raso, S.; Siragusa, S. Quality of Life in Patients With Cancer Under Prolonged Anticoagulation for High-Risk Deep Vein Thrombosis: A Long-Term Follow-Up. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620918290. [Google Scholar] [CrossRef] [PubMed]
- Bratt, G.; Tornebohm, E.; Widlund, L.; Lockner, D. Low molecular weight heparin (KABI 2165, Fragmin): Pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb. Res. 1986, 42, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Linkins, L.A. Management of venous thromboembolism in patients with cancer: Role of dalteparin. Vasc. Health Risk Manag. 2008, 4, 279–287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gould, M.K.; Dembitzer, A.D.; Doyle, R.L.; Hastie, T.J.; Garber, A.M. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 1999, 130, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Leizorovicz, A. Comparison of the efficacy and safety of low molecular weight heparins and unfractionated heparin in the initial treatment of deep venous thrombosis. An updated meta-analysis. Drugs 1996, 52 (Suppl. S7), 30–37. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, D.J.; McQuillan, A.; Eikelboom, J.W. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2004, 140, 175–183. [Google Scholar] [CrossRef]
- Monreal, M.; Zacharski, L.; Jimenez, J.A.; Roncales, J.; Vilaseca, B. Fixed-dose low-molecular-weight heparin for secondary prevention of venous thromboembolism in patients with disseminated cancer: A prospective cohort study. J. Thromb. Haemost. 2004, 2, 1311–1315. [Google Scholar] [CrossRef]
- Noble, S.I.; Hood, K.; Finlay, I.G. The use of long-term low-molecular weight heparin for the treatment of venous thromboembolism in palliative care patients with advanced cancer: A case series of sixty two patients. Palliat. Med. 2007, 21, 473–476. [Google Scholar] [CrossRef]
- Francis, C.W.; Kessler, C.M.; Goldhaber, S.Z.; Kovacs, M.J.; Monreal, M.; Huisman, M.V.; Bergqvist, D.; Turpie, A.G.; Ortel, T.L.; Spyropoulos, A.C.; et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: The DALTECAN Study. J. Thromb. Haemost. 2015, 13, 1028–1035. [Google Scholar] [CrossRef]
- Dimakakos, E.P.; Vathiotis, I.; Syrigos, K. The Role of Tinzaparin in Oncology. Clin. Appl. Thromb. Hemost. 2018, 24, 697–707. [Google Scholar] [CrossRef]
- Johansen, K.B.; Balchen, T. Tinzaparin and other low-molecular-weight heparins: What is the evidence for differential dependence on renal clearance? Exp. Hematol. Oncol. 2013, 2, 21. [Google Scholar] [CrossRef]
- Hull, R.D.; Pineo, G.F.; Brant, R.F.; Mah, A.F.; Burke, N.; Dear, R.; Wong, T.; Cook, R.; Solymoss, S.; Poon, M.C.; et al. Self-managed long-term low-molecular-weight heparin therapy: The balance of benefits and harms. Am. J. Med. 2007, 120, 72–82. [Google Scholar] [CrossRef]
- Hull, R.D.; Pineo, G.F.; Brant, R.F.; Mah, A.F.; Burke, N.; Dear, R.; Wong, T.; Cook, R.; Solymoss, S.; Poon, M.C.; et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am. J. Med. 2006, 119, 1062–1072. [Google Scholar] [CrossRef]
- Lee, A.Y.Y.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A.; Investigators, C. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA 2015, 314, 677–686. [Google Scholar] [CrossRef]
- Laporte, S.; Bertoletti, L.; Romera, A.; Mismetti, P.; Perez de Llano, L.A.; Meyer, G. Long-term treatment of venous thromboembolism with tinzaparin compared to vitamin K antagonists: A meta-analysis of 5 randomized trials in non-cancer and cancer patients. Thromb. Res. 2012, 130, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Romera, A.; Cairols, M.A.; Vila-Coll, R.; Marti, X.; Colome, E.; Bonell, A.; Lapiedra, O. A randomised open-label trial comparing long-term sub-cutaneous low-molecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 349–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Zapata, M.J.; Mathioudakis, A.G.; Mousa, S.A.; Bauersachs, R. Tinzaparin for Long-Term Treatment of Venous Thromboembolism in Patients With Cancer: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2018, 24, 226–234. [Google Scholar] [CrossRef]
- Jara-Palomares, L.; Solier-Lopez, A.; Elias-Hernandez, T.; Asensio-Cruz, M.; Blasco-Esquivias, I.; Marin-Barrera, L.; de la Borbolla-Artacho, M.R.; Praena-Fernandez, J.M.; Montero-Romero, E.; Navarro-Herrero, S.; et al. Tinzaparin in cancer associated thrombosis beyond 6months: TiCAT study. Thromb. Res. 2017, 157, 90–96. [Google Scholar] [CrossRef]
- Frere, C.; Crichi, B.; Rueda-Camino, J.A.; Cajfinger, F.; Spiess, N.; Janus, N.; Le Maignan, C.; Marjanovic, Z.; Farge, D. Long-term use of tinzaparin for the treatment of cancer-associated thrombosis in clinical practice: Insights from the prospective TROPIQUE study. J. Med. Vasc. 2022, 47, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.D.; Pineo, G.F.; Brant, R.; Liang, J.; Cook, R.; Solymoss, S.; Poon, M.C.; Raskob, G.; Investigators, L.T. Home therapy of venous thrombosis with long-term LMWH versus usual care: Patient satisfaction and post-thrombotic syndrome. Am. J. Med. 2009, 122, 762–769 e763. [Google Scholar] [CrossRef] [PubMed]
- Amerali, M.; Politou, M. Tinzaparin-a review of its molecular profile, pharmacology, special properties, and clinical uses. Eur. J. Clin. Pharmacol. 2022, 78, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, A.; Ardavanis, A.; Papandreou, C.; Koumakis, G.; Papatsimpas, G.; Papakotoulas, P.; Tsoukalas, N.; Andreadis, C.; Samelis, G.; Papakostas, P.; et al. Prophylaxis of cancer-associated venous thromboembolism with low-molecular-weight heparin-tinzaparin: Real world evidence. Oncol. Lett. 2022, 23, 115. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Laporte, S.; Chapelle, C.; Falvo, N.; Falchero, L.; Cloarec, N.; Monnet, I.; Burnod, A.; Tomasini, P.; Boulon, C.; et al. Failure of the Ottawa Score to Predict the Risk of Recurrent Venous Thromboembolism in Cancer Patients: The Prospective PREDICARE Cohort Study. Thromb. Haemost. 2022, 122, 151–157. [Google Scholar] [CrossRef]
- Jupalli, A.; Iqbal, A.M. Enoxaparin. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Deitcher, S.R.; Kessler, C.M.; Merli, G.; Rigas, J.R.; Lyons, R.M.; Fareed, J.; Investigators, O. Secondary prevention of venous thromboembolic events in patients with active cancer: Enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin. Appl. Thromb. Hemost. 2006, 12, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Marjanovic, Z.; Valcke, J.; Lorcerie, B.; Gruel, Y.; Solal-Celigny, P.; Le Maignan, C.; Extra, J.M.; Cottu, P.; Farge, D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch. Intern. Med. 2002, 162, 1729–1735. [Google Scholar] [CrossRef]
- Trujillo-Santos, J.; Farge-Bancel, D.; Pedrajas, J.M.; Gomez-Cuervo, C.; Ballaz, A.; Braester, A.; Mahe, I.; Villalobos, A.; Porras, J.A.; Monreal, M.; et al. Enoxaparin versus dalteparin or tinzaparin in patients with cancer and venous thromboembolism: The RIETECAT study. Res. Pract. Thromb. Haemost. 2022, 6, e12736. [Google Scholar] [CrossRef]
- Vardanyan, R.; Hruby, V. Chapter 24—Antithrombotic Drugs (Anticoagulants, Antiplatelets, and Thrombolytics). In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 383–412. [Google Scholar]
- Zhang, Y.; Zhang, M.; Tan, L.; Pan, N.; Zhang, L. The clinical use of Fondaparinux: A synthetic heparin pentasaccharide. Prog. Mol. Biol. Transl. Sci. 2019, 163, 41–53. [Google Scholar] [CrossRef]
- Crowther, M.A.; Warkentin, T.E. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: Focus on new anticoagulant agents. Blood 2008, 111, 4871–4879. [Google Scholar] [CrossRef]
- van Doormaal, F.F.; Raskob, G.E.; Davidson, B.L.; Decousus, H.; Gallus, A.; Lensing, A.W.; Piovella, F.; Prins, M.H.; Buller, H.R. Treatment of venous thromboembolism in patients with cancer: Subgroup analysis of the Matisse clinical trials. Thromb. Haemost. 2009, 101, 762–769. [Google Scholar]
- Turpie, A.G.; Bauer, K.A.; Eriksson, B.I.; Lassen, M.R. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: A meta-analysis of 4 randomized double-blind studies. Arch. Intern. Med. 2002, 162, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.; Ali, Z.; Amjad, W.; Alirhayim, Z.; Farooq, H.; Qadir, S.; Khalid, F.; Al-Mallah, M.H. Venous Thromboembolism in Cancer: An Update of Treatment and Prevention in the Era of Newer Anticoagulants. Front. Cardiovasc. Med. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Barginear, M.F.; Gralla, R.J.; Bradley, T.P.; Ali, S.S.; Shapira, I.; Greben, C.; Nier-Shoulson, N.; Akerman, M.; Lesser, M.; Budman, D.R. Investigating the benefit of adding a vena cava filter to anticoagulation with fondaparinux sodium in patients with cancer and venous thromboembolism in a prospective randomized clinical trial. Support. Care Cancer 2012, 20, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.N.; Mao, Z.X.; Wu, Y.; Liang, M.X.; Wang, D.D.; Chen, X.; Chang, P.A.; Zhang, W.; Tang, J.H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Choi, S.H.; Varki, A. Differential metastasis inhibition by clinically relevant levels of heparins--correlation with selectin inhibition, not antithrombotic activity. Clin. Cancer Res. 2005, 11, 7003–7011. [Google Scholar] [CrossRef]
- Stolting, D.P.; Jaehde, U.; Wiese, M.; Bendas, G. Are low molecular weight heparins able to sensitize chemoresistant tumor cells? Int. J. Clin. Pharmacol. Ther. 2013, 51, 70–73. [Google Scholar] [CrossRef]
- Pfankuchen, D.B.; Baltes, F.; Batool, T.; Li, J.P.; Schlesinger, M.; Bendas, G. Heparin antagonizes cisplatin resistance of A2780 ovarian cancer cells by affecting the Wnt signaling pathway. Oncotarget 2017, 8, 67553–67566. [Google Scholar] [CrossRef]
- Karamouzis, M.V.; Athanasiadis, I.; Samelis, G.; Vallilas, C.; Bokas, A.; Nikolaidi, A.; Dimitriadou, A.; Sarantis, P.; Pistamaltzian, N.; Schizas, D.; et al. The Impact of Thromboprophylaxis on the Survival of Patients with Advanced Pancreatic Cancer. The Pancreatic Cancer and Tinzaparin (PaCT) Study. Cancers 2021, 13, 2884. [Google Scholar] [CrossRef]
- Ma, L.; Qiao, H.; He, C.; Yang, Q.; Cheung, C.H.; Kanwar, J.R.; Sun, X. Modulating the interaction of CXCR4 and CXCL12 by low-molecular-weight heparin inhibits hepatic metastasis of colon cancer. Investig. New Drugs 2012, 30, 508–517. [Google Scholar] [CrossRef]
- Delate, T.; Charlu, M.; Zhu, S.; Pai, A.; Clark, N.P.; Witt, D.M.; King, J.M.; King, J.B. Temporal trends in first-line outpatient anticoagulation treatment for cancer-associated venous thromboembolism. Thromb. Res. 2020, 196, 367–370. [Google Scholar] [CrossRef]
- Gornicki, T.; Buldys, K.; Zielinska, D.; Chabowski, M. Direct-Acting Oral Anticoagulant Therapy in Cancer Patients-A Review. Cancers 2023, 15, 2697. [Google Scholar] [CrossRef] [PubMed]
| Vitamin K Antagonists | Low Molecular Weight Heparins | Direct Oral Anticoagulants | |
|---|---|---|---|
| Route of intake | Oral | Subcutaneous injection | Oral |
| Problems with oral intake in cancer | Yes | No | Yes |
| Problems with absorption in cancer | Yes | No | Yes |
| Renal clearance | No | Yes (except tinzaparin) | Yes |
| Food interactions | Yes | No | Yes |
| Influence of fasted/fed status | No | No | Yes * |
| Pharmacokinetic drug–drug interactions | Yes, with chemotherapeutics | No | Yes |
| Need to monitor | Yes | Not routine | No |
| Unfractionated Heparin | Low Molecular Weight Heparins | Fondaparinux | |
|---|---|---|---|
| Origin | Natural compound isolated from animal liver | Depolymerization of heparin | Synthetic compound |
| Molecular weight | 15–19 kDa | 3–6.5 kDa | 1.7 kDa |
| Target of inhibition | Factors Xa and IIa, a weak anti-platelet effect | 2–4 times more efficient at inhibiting factor Xa than IIa | Factor Xa |
| Half-life | ~1 h | 3–6 h * | 17–21 h * |
| Metabolism/excretion | Reticuloendothelial and renal | 10–40% renal | Renal |
| Antidote | Protamine | Protamine (partial reversal **) | None |
| Interaction with platelets | Strong | Weak | None |
| Risk of HIT | Strong | Weak | None |
| Anticoagulant | Clinical Setting | ||
|---|---|---|---|
| Inpatients | Patients Undergoing Surgery | Outpatients | |
| Primary Prophylaxis | |||
| Unfractionated heparin | 5000 IU every 8 h | 5000 IU 2–4 h before surgery and every 8 h thereafter | NA |
| Dalteparin | 5000 anti-Xa IU | 5000 anti-XaIU 12 h before surgery and 5000 anti-Xa IU once daily thereafter | 5000 anti-Xa IU once daily |
| Enoxaparin | 4000 anti-Xa IU | 4000 anti-Xa IU 12 h before surgery and 4000 anti-Xa IU once daily thereafter | 4000 anti-Xa IU once daily |
| Tinzaparin | 4500 anti-Xa IU | 4500 anti-Xa IU once daily starting 12 h post-surgery | 4500 anti-Xa IU once daily |
| Fondaparinux | 2.5 mg once daily | 2.5 mg once daily starting 6–8 h post-surgery | No data |
| Initial treatment | |||
| Unfractionated heparin | 80 IU/kg intravenous bolus, next 18 IU/kg/h intravenously; dose adjusted based on aPTT | ||
| Dalteparin | 100 anti-Xa IU/kg every 12 h, or 200 anti-Xa IU/kg once daily up to day 30 | ||
| Enoxaparin | 100 anti-Xa IU/kg every 12 h, or 150 anti-Xa IU/kg once daily | ||
| Tinzaparin | 175 anti-Xa IU/kg once daily | ||
| Fondaparinux | Not listed in this setting | ||
| Extended treatment | |||
| Unfractionated heparin | Not completed | ||
| Dalteparin | 150 anti-Xa IU/kg once daily beyond day 30 | ||
| Enoxaparin | 100 anti-Xa IU/kg every 12 h, or 150 anti-Xa IU/kg once daily | ||
| Tinzaparin | 175 anti-Xa IU/kg once daily | ||
| Fondaparinux | Not listed in this setting | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, M.; Siragusa, S. The Role of Injectables in the Treatment and Prevention of Cancer-Associated Thrombosis. Cancers 2023, 15, 4640. https://doi.org/10.3390/cancers15184640
Napolitano M, Siragusa S. The Role of Injectables in the Treatment and Prevention of Cancer-Associated Thrombosis. Cancers. 2023; 15(18):4640. https://doi.org/10.3390/cancers15184640
Chicago/Turabian StyleNapolitano, Mariasanta, and Sergio Siragusa. 2023. "The Role of Injectables in the Treatment and Prevention of Cancer-Associated Thrombosis" Cancers 15, no. 18: 4640. https://doi.org/10.3390/cancers15184640
APA StyleNapolitano, M., & Siragusa, S. (2023). The Role of Injectables in the Treatment and Prevention of Cancer-Associated Thrombosis. Cancers, 15(18), 4640. https://doi.org/10.3390/cancers15184640







