Cytology and HPV Co-Testing for Detection of Vaginal Intraepithelial Neoplasia: A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
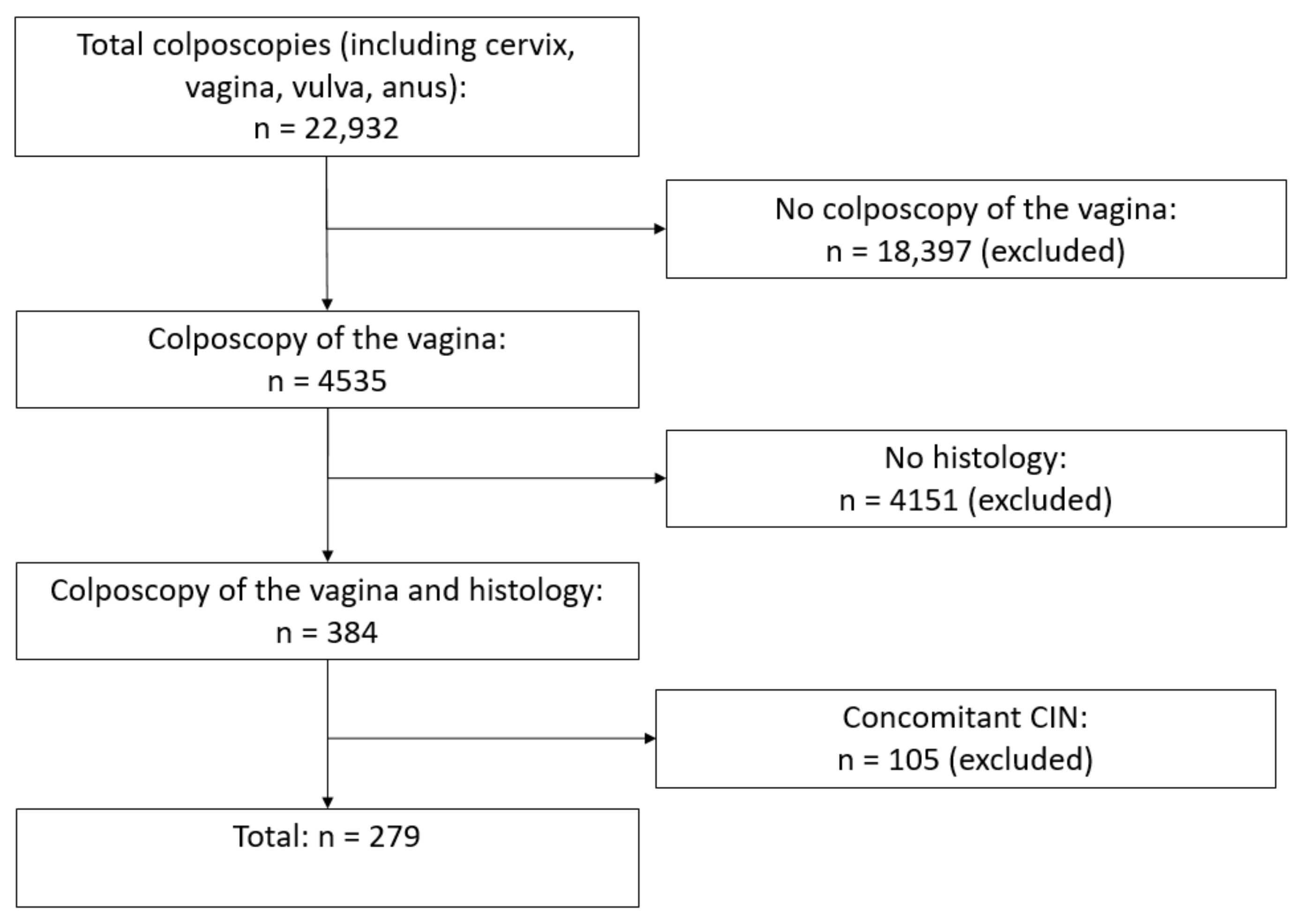
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethical Approval
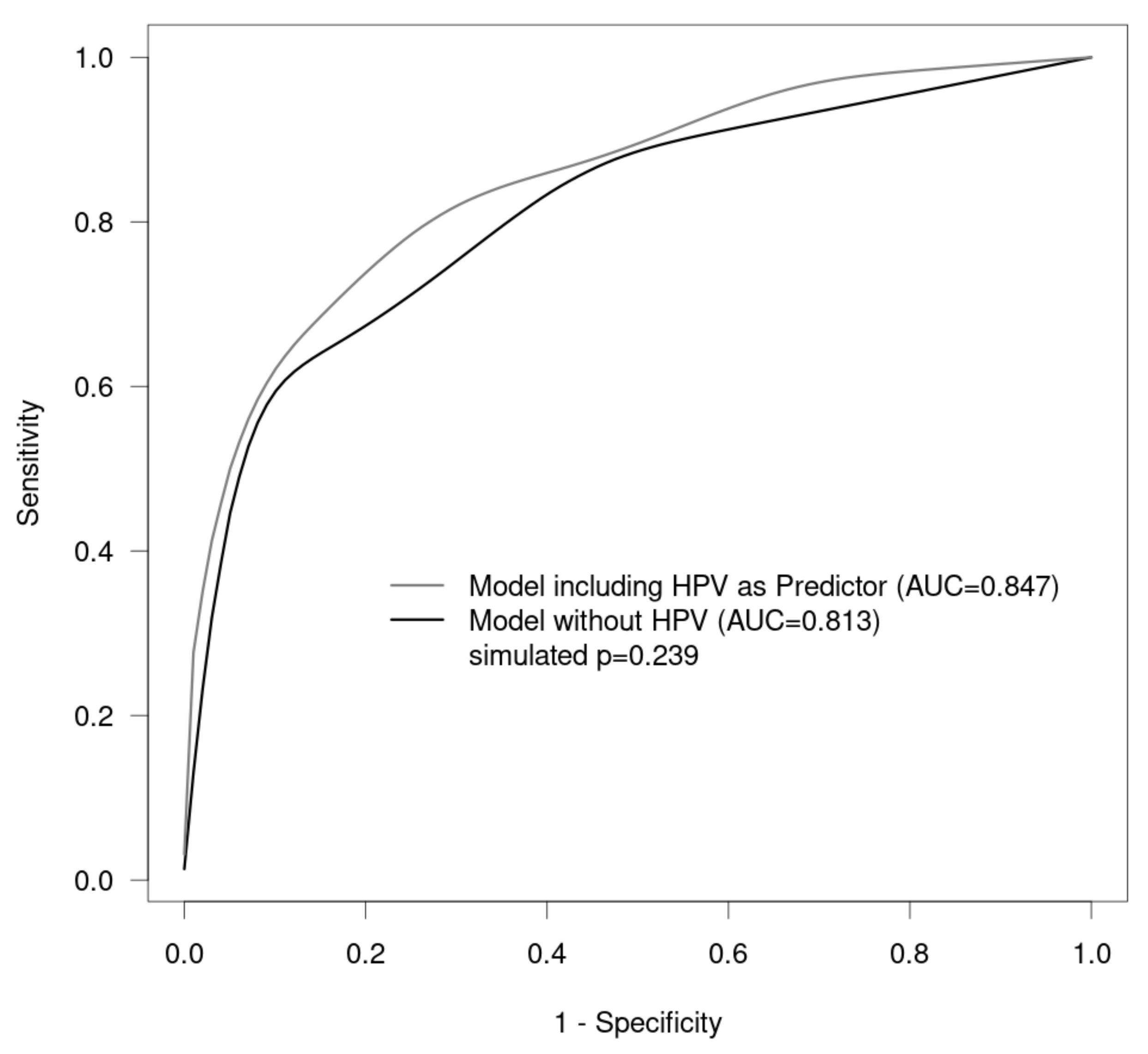
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sopracordevole, F.; Mancioli, F.; Clemente, N.; De Piero, G.; Buttignol, M.; Giorda, G.; Ciavattini, A. Abnormal Pap Smear and Diagnosis of High-Grade Vaginal Intraepithelial Neoplasia: A Retrospective Cohort Study. Medicine 2015, 94, e1827. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.H.; Flowers, L.C. Vulvo-vaginal cancers: Risks, evaluation, prevention and early detection. Obstet. Gynecol. Clin. N. Am. 2007, 34, 783–802. [Google Scholar] [CrossRef]
- Cong, Q.; Song, Y.; Wang, Q.; Zhang, H.; Gao, S.; Sui, L. A Retrospective Study of Cytology, High-Risk HPV, and Colposcopy Results of Vaginal Intraepithelial Neoplasia Patients. Biomed. Res. Int. 2018, 2018, 5894801. [Google Scholar] [CrossRef] [PubMed]
- Stuebs, F.A.; Koch, M.C.; Mehlhorn, G.; Gass, P.; Schulmeyer, C.E.; Hartman, A.; Strehl, J.; Adler, W.; Beckmann, M.W.; Renner, S.K. Accuracy of colposcopic findings in detecting vaginal intraepithelial neoplasia: A retrospective study. Arch. Gynecol. Obstet. 2020, 301, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Madsen, B.S.; Jensen, H.L.; van den Brule, A.J.; Wohlfahrt, J.; Frisch, M. Risk factors for invasive squamous cell carcinoma of the vulva and vagina—Population-based case-control study in Denmark. Int. J. Cancer 2008, 122, 2827–2834. [Google Scholar] [CrossRef]
- Daling, J.R.; Madeleine, M.M.; Schwartz, S.M.; Shera, K.A.; Carter, J.J.; McKnight, B.; Porter, P.L.; Galloway, D.A.; McDougall, J.K.; Tamimi, H. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol. Oncol. 2002, 84, 263–270. [Google Scholar] [CrossRef]
- Sherman, J.F.; Mount, S.L.; Evans, M.F.; Skelly, J.; Simmons-Arnold, L.; Eltabbakh, G.H. Smoking increases the risk of high-grade vaginal intraepithelial neoplasia in women with oncogenic human papillomavirus. Gynecol. Oncol. 2008, 110, 396–401. [Google Scholar] [CrossRef]
- Zeligs, K.P.; Byrd, K.; Tarney, C.M.; Howard, R.S.; Sims, B.D.; Hamilton, C.A.; Stany, M.P. A clinicopathologic study of vaginal intraepithelial neoplasia. Obstet. Gynecol. 2013, 122, 1223–1230. [Google Scholar] [CrossRef]
- Sillman, F.H.; Fruchter, R.G.; Chen, Y.S.; Camilien, L.; Sedlis, A.; McTigue, E. Vaginal intraepithelial neoplasia: Risk factors for persistence, recurrence, and invasion and its management. Am. J. Obstet. Gynecol. 1997, 176, 93–99. [Google Scholar] [CrossRef]
- Sopracordevole, F.; Barbero, M.; Clemente, N.; Fallani, M.G.; Cattani, P.; Agarossi, A.; De Piero, G.; Parin, A.; Frega, A.; Boselli, F.; et al. High-grade vaginal intraepithelial neoplasia and risk of progression to vaginal cancer: A multicentre study of the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV). Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 818–824. [Google Scholar]
- Lamos, C.; Mihaljevic, C.; Aulmann, S.; Bruckner, T.; Domschke, C.; Wallwiener, M.; Paringer, C.; Fluhr, H.; Schott, S.; Dinkic, C.; et al. Detection of Human Papillomavirus Infection in Patients with Vaginal Intraepithelial Neoplasia. PLoS ONE 2016, 11, e0167386. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Tumours of Female Reproductive Organs, 4th ed.; World Health Organization: Geneve, Switzerland, 2014; Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Female-Reproductive-Organs-2014 (accessed on 18 September 2023).
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch. Pathol. Lab. Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef]
- Stuebs, F.A.; Gass, P.; Dietl, A.K.; Schulmeyer, C.E.; Adler, W.; Geppert, C.; Hartmann, A.; Knöll, A.; Beckmann, M.W.; Koch, M.C. Human papilloma virus genotype distribution in women with premalignant or malignant lesions of the uterine cervix. Arch. Gynecol. Obstet. 2021, 304, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, D.; Xu, S.; Wang, X.; Chen, Y.; Lv, W.; Xie, X. Clinical features, treatment and outcomes of vaginal intraepithelial neoplasia in a Chinese tertiary centre. Ir. J. Med. Sci. 2016, 185, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Sugase, M.; Matsukura, T. Distinct manifestations of human papillomaviruses in the vagina. Int. J. Cancer 1997, 72, 412–415. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Nugent, E.K.; Elfrink, S.H.; Gold, M.A.; Moore, K.N. A contemporary analysis of epidemiology and management of vaginal intraepithelial neoplasia. Am. J. Obstet. Gynecol. 2013, 208, 410.e1–410.e6. [Google Scholar] [CrossRef]
- Smith, J.S.; Backes, D.M.; Hoots, B.E.; Kurman, R.J.; Pimenta, J.M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet. Gynecol. 2009, 113, 917–924. [Google Scholar] [CrossRef]
- Bornstein, J.; Bentley, J.; Bosze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Nugent, E.K.; Yunker, A.C.; Rocconi, R.P.; Graybill, W.S.; Erickson, B.K.; Moore, K.N. Vaginal cancer: The experience from 2 large academic centers during a 15-year period. J. Low. Genit. Tract Dis. 2013, 17, 409–413. [Google Scholar] [CrossRef]
- Siegler, E.; Segev, Y.; Mackuli, L.; Auslender, R.; Shiner, M.; Lavie, O. Vulvar and Vaginal Cancer, Vulvar Intraepithelial Neoplasia 3 and Vaginal Intraepithelial Neoplasia 3: Experience of a Referral Institute. The Israel Medical Association journal. Israel Med. Assoc. J. 2016, 18, 286–289. [Google Scholar]
- Rhodes, H.E.; Chenevert, L.; Munsell, M. Vaginal intraepithelial neoplasia (VaIN 2/3): Comparing clinical outcomes of treatment with intravaginal estrogen. J. Low. Genit. Tract Dis. 2014, 18, 115–121. [Google Scholar] [CrossRef]
- Ao, M.; Zheng, D.; Wang, J.; Gu, X.; Xi, M. A retrospective study of cytology and HPV genotypes results of 3229 vaginal intraepithelial neoplasia patients. J. Med. Virol. 2022, 94, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Boonlikit, S.; Noinual, N. Vaginal intraepithelial neoplasia: A retrospective analysis of clinical features and colpohistology. J. Obstet. Gynaecol. Res. 2010, 36, 94–100. [Google Scholar] [CrossRef]
- Schulmeyer, C.E.; Stübs, F.; Gass, P.; Renner, S.K.; Hartmann, A.; Strehl, J.; Mehlhorn, G.; Geppert, C.; Adler, W.; Beckmann, M.W.; et al. Correlation between referral cytology and in-house colposcopy-guided cytology for detecting early cervical neoplasia. Arch. Gynecol. Obstet. 2020, 301, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.W.; Quaas, J.; Bischofberger, A.; Kammerle, A.; Lux, M.P.; Wesselmann, S. Establishment of the Certification System “Gynaecological Dysplasia” in Germany. Geburtshilfe Frauenheilkd 2014, 74, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Stuebs, F.A.; Koch, M.C.; Dietl, A.K.; Adler, W.; Geppert, C.; Hartmann, A.; Knöll, A.; Beckmann, M.W.; Mehlhorn, G.; Schulmeyer, C.E.; et al. Cytology and High-Risk Human Papillomavirus Test for Cervical Cancer Screening Assessment. Diagnostics 2022, 12, 1748. [Google Scholar] [CrossRef]
- Schulmeyer, C.E.; Koch, M.C.; Dietl, A.K.; Stuebs, F.A.; Behrens, A.; Renner, S.K.; Mehlhorn, G.; Geppert, C.C.; Hartmann, A.; Beckmann, M.W.; et al. Standardized Procedures for Patients with Dysplasia and Other Diseases of the Cervix, Vulva, and Vagina at a Certified Dysplasia Unit Prior to the Introduction of the Organized Cervical Cancer Screening Program. Geburtshilfe Frauenheilkd 2023, 83, 1031–1042. [Google Scholar] [CrossRef]
- Griesser, H.K.M.; Jordan, B.; Küppers, V.; Gieseking, F.; Kühn, W. Für die Koordinationskonferenz Zytologie; Das Prozedere bei auffälligen Befunden—Kommentar zur Münchner Nomenklatur III. FRAUENARZT 2015, 56, 10–12. [Google Scholar]
- Sopracordevole, F.; Barbero, M.; Clemente, N.; Fallani, M.G.; Cattani, P.; Agarossi, A.; de Piero, G.; Parin, A.; Frega, A.; Boselli, F.; et al. Colposcopic patterns of vaginal intraepithelial neoplasia: A study from the Italian Society of Colposcopy and Cervico-Vaginal Pathology. Eur. J. Cancer Prev. 2018, 27, 152–157. [Google Scholar] [CrossRef]
- Nayar, R.; Wilbur, D.C. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015, 123, 271–281. [Google Scholar] [CrossRef]
- Stuebs, F.A.; Schulmeyer, C.E.; Mehlhorn, G.; Gass, P.; Kehl, S.; Renner, S.K.; Renner, S.P.; Geppert, C.; Adler, W.; Hartmann, A.; et al. Accuracy of colposcopy-directed biopsy in detecting early cervical neoplasia: A retrospective study. Arch. Gynecol. Obstet. 2018, 299, 525–532. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 15 May 2023).
- Wee, W.W.; Chia, Y.N.; Yam, P.K. Diagnosis and treatment of vaginal intraepithelial neoplasia. Int. J. Gynaecol. Obstet. 2012, 117, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Indraccolo, U.; Baldoni, A. A simplified classification for describing colposcopic vaginal patterns. J. Low. Genit. Tract Dis. 2012, 16, 75–79. [Google Scholar] [CrossRef]
- Cong, Q.; Fu, Z.; Zhang, D.; Sui, L. Importance of Colposcopy Impression in the Early Diagnosis of Posthysterectomy Vaginal Cancer. J. Low. Genit. Tract Dis. 2019, 23, 13–17. [Google Scholar] [CrossRef]
- Wittenborn, J.; Kupec, T.; Iborra, S.; Stickeler, E.; Najjari, L.; Kennes, L.N. HPV High-risk Multiple Infection Is a Key Predictor of Cervical Dysplasia in Diagnostic LEEPs: A Retrospective Cohort Analysis. Geburtshilfe Frauenheilkd 2022, 82, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.W.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Hack, C.C.; et al. Diagnosis, Therapy and Follow-up of Cervical Cancer. Guideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 1 with Recommendations on Epidemiology, Screening, Diagnostics and Therapy. Geburtshilfe Frauenheilkd 2022, 82, 139–180. [Google Scholar] [CrossRef]
- Fehm, T.; Stübs, F.A.; Koch, M.C.; Mallmann, P.; Dannecker, C.; Dietl, A.; Sevnina, A.; Mergel, F.; Lotz, L.; Ehret, A.; et al. Diagnosis, Therapy and Follow-up of Cervical Cancer. Guideline of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL, May 2021)—Part 2 with Recommendations on Psycho-oncology, Rehabilitation, Follow-up, Recurrence, Palliative Therapy and Healthcare Facilities. Geburtshilfe Frauenheilkd 2022, 82, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis, Therapy, and Follow-Up Care of Vulva Cancer and its Precursors. National Guideline of the German Society and Gynecology and Obstetrics (S2k-Level, AWMF No. 015/059, August 2015). Available online: https://www.awmf.org/uploads/tx_szleitlinien/032-042l_S2k_Vaginalkarzinom-Vorstufen-Diagnostik-Therapie-Nachsorge_2018-11.pdf (accessed on 28 July 2023).
- De Vuyst, H.; Clifford, G.M.; Nascimento, M.C.; Madeleine, M.M.; Franceschi, S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int. J. Cancer 2009, 124, 1626–1636. [Google Scholar] [CrossRef]
- So, K.A.; Hong, J.H.; Hwang, J.H.; Song, S.H.; Lee, J.K.; Lee, N.W.; Lee, K.W. The utility of the human papillomavirus DNA load for the diagnosis and prediction of persistent vaginal intraepithelial neoplasia. J. Gynecol. Oncol. 2009, 20, 232–237. [Google Scholar] [CrossRef][Green Version]
- Bansal, M.; Austin, R.M.; Zhao, C. Correlation of histopathologic follow-up findings with vaginal human papillomavirus and low-grade squamous intraepithelial lesion Papanicolaou test results. Arch. Pathol. Lab. Med. 2011, 135, 1545–1549. [Google Scholar] [CrossRef]
- Coughlan, C.; McAuliffe, F.; Bermingham, N.; Gleeson, N. Vaginal cytology following primary hysterectomy for cervical cancer: Is it useful? Ir. J. Med. Sci. 2006, 175, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Tempfer, C.; Beckmann, M.W.; Küppers, V.; Quaas, J. Statement of the AGO and AG-CPC on the Aftercare/Follow-up for Surgical Procedures of the Lower Genital Tract after the Introduction of a New Cancer Screening Guideline. Geburtshilfe Frauenheilkd 2020, 80, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Richtlinie des Gemeinsamen Bundesauschusses für Organisierte Krebsfrüherkennungsprogramme (oKFE-Richtlinie/oKFE-RL) in der Fassung vom 19 Juli 2018 Zuletzt Geändert am 12 Mai 2023. Available online: https://www.g-ba.de/downloads/62-492-3189/oKFE-RL-2023-05-12-iK-2023-07-07.pdf (accessed on 1 September 2023).
| Bethesda | Benign (n = 86) | VaIN I/vLSIL (n = 116) | VaIN II/vHSIL (n = 41) | VaIN III/vHSIL (n = 33) | Carcinoma (n = 3) |
|---|---|---|---|---|---|
| NILM (n = 107) | 50 (46.7%) | 51 (47.7%) | 4 (3.7%) | 2 (1.9%) | 0 |
| ASC-US (n = 17) | 6 (35.3%) | 8 (47.1%) | 2 (11.8%) | 1 (5.9%) | 0 |
| LSIL (n = 70) | 17 (24.3%) | 36 (51.4%) | 11 (15.7%) | 6 (8.6%) | 0 |
| HSIL (n = 57) | 4 (7.0%) | 11 (19.3%) | 22 (38.6%) | 20 (35.1%) | 0 |
| AGC, endocervical favoring neoplasia (n = 1) | 1 (100%) | 0 | 0 | 0 | 0 |
| ASC-H (n = 24) | 8 (33.3%) | 10 (41.6%) | 2 (8.3%) | 3 (12.5%) | 1 (4.2%) |
| HSIL with features suspicious for invasion (n = 1) | 0 | 0 | 0 | 1 (100%) | 0 |
| Squamous cell carcinoma (n = 2) | 0 | 0 | 0 | 0 | 2 (100%) |
| Bethesda | Benign (n = 45) | VaIN I/vLSIL (n = 62) | VaIN II/vHSIL (n = 22) | VaIN III/vHSIL (n = 22) | Carcinoma (n = 2) |
|---|---|---|---|---|---|
| NILM (n = 62) | 28 (45.2%) | 29 (46.8%) | 3 (4.8%) | 2 (3.2%)1 | 0 |
| ASC-US (n = 8) | 3 (37.5%) | 4 (50.0%) | 0 (0%) | 1 (12.5%) | 0 |
| LSIL (n = 33) | 7 (21.2%) | 19 (57.6%) | 5 (15.2%) | 2 (6.1%) | 0 |
| HSIL (n = 37) | 1 (2.7%) | 7 (18.9%) | 13 (35.1%) | 16 (43.2%) | 0 |
| AGC, endocervical favoring neoplasia (n = 0) | 0 | 0 | 0 | 0 | 0 |
| ASC-H (n = 12) | 6 (50.0%) | 3 (25%) | 1 (8.3%) | 1 (8.3%) | 1 (8.3%) |
| HSIL with features suspicious for invasion (n = 0) | 0 | 0 | 0 | 0 | 0 |
| Squamous cell carcinoma (n = 1) | 0 | 0 | 0 | 0 | 1 (100%) |
| Bethesda | Benign (n = 41) | VaIN I/vLSIL (n = 54) | VaIN II/vHSIL (n = 19) | VaIN III/vHSIL (n = 11) | Carcinoma (n = 1) |
|---|---|---|---|---|---|
| NILM (n = 45) | 22 (48.9%) | 22 (48.9%) | 1 (2,2%) | 0 | 0 |
| ASC-US (n = 9) | 3 (33.3%) | 4 (44.4%) | 2 (22.2%) | 0 | 0 |
| LSIL (n = 37) | 10 (27.0%) | 17 (45.9%) | 6 (16.2%) | 4 (10.8%) | 0 |
| HSIL (n = 20) | 3 (15.0%) | 4 (20.0%) | 9 (45.0%) | 4 (20.0%) | 0 |
| AGC, endocervical favoring neoplasia (n = 1) | 1 (100%) | 0 | 0 | 0 | 0 |
| ASC-H (n = 12) | 2 (16.7%) | 7 (58.3%) | 1 (8.3%) | 2 (16.7%) | 0 |
| HSIL with features suspicious for invasion (n = 1) | 0 | 0 (0%) | 0 (0%) | 1 (100%) | 0 |
| Squamous cell carcinoma (n = 1) | 0 | 0 | 0 | 0 | 1 (100%) |
| Histology | hrHPV-Positive | hrHPV-Negative |
|---|---|---|
| Benign (n = 84) | 35 (41.7%) | 49 (58.3%) |
| VaIN I/vLSIL (n = 111) | 60 (54.1%) | 51 (45.9%) |
| VaIN II/vHSIL (n = 40) | 31 (77.5%) | 9 (22.5%) |
| VaIN III/vHSIL (n = 32) | 26 (81.3%) | 6 (18.7%) |
| Carcinoma (n = 3) | 2 (66.7%) | 1 (33.3%) |
| Odds Ratio | 95% Confidence Intervals | p Value | |
|---|---|---|---|
| Cytology LSIL | 3.02 | 1.18 to 7.7 | 0.021 |
| Cytology HSIL+ | 33.80 | 12.74 to 89.68 | <0.001 |
| Cytology unspecific | 3.28 | 1.14 to 9.42 | 0.028 |
| HPV positive | 2.99 | 1.51 to 5.93 | 0.002 |
| Histology of Vagina (n = 153) | Hysterectomy for HPV-Related CIN or Cervical Cancer (n = 80) | Hysterectomy for Non-HPV-Related CIN or Cervical Cancer (n = 32) | Reason for Hysterectomy Unknown (n = 41) |
|---|---|---|---|
| Benign (n = 45) | 19 (42.2%) | 12 (26.7%) | 14 (31.1%) |
| VaIN I/vLSIL (n = 62) | 32 (51.6%) | 14 (22.6%) | 16 (25.8%) |
| VaIN II/vHSIL (n = 22) | 15 (68.2%) | 4 (18.2%) | 3 (13.6%) |
| VaIN III/vHSIL (n = 22) | 14 (63.6%) | 1 (4.5%) | 7 (31.8%) |
| Carcinoma (n = 2) | 0 | 1 (50%) | 1 (50%) |
| Benign (n = 50) | VaIN I/vLSIL (n = 90) | VaIN II/vHSIL (n = 31) | VaIN III/vHSIL (n = 27) | Carcinoma (n = 1) | |
|---|---|---|---|---|---|
| CIN I/cLSIL (n = 10) | 6 (12%) | 2 (2.2%) | 1 (3.2%) | 1 (3.7%) | 0 |
| CIN II/cHSIL (n = 16) | 2 (4%) | 9 (10%) | 4 (12.9%) | 1 (3.7%) | 0 |
| CIN III/cHSIL (n = 56) | 11 (22%) | 26 (28.9%) | 11 (35.5%) | 8 (29.6%) | 0 |
| Cx-Ca. (n = 49) | 17 (34%) | 15 (16.7%) | 7 (22.6%) | 9 (33.3%) | 1 (100%) |
| VaIN I/vLSIL (n = 5) | 2 (4%) | 2 (2.2%) | 1 (3.2%) | 0 | 0 |
| VaIN II/vHSIL (n = 21) | 3 (6%) | 14 (15.6%) | 3 (9.7%) | 1 (3.7%) | 0 |
| VaIN III/vHSIL (n = 23) | 5 (10%) | 12 (13.3%) | 1 (3.2%) | 5 (18.5%) | 0 |
| Vaginal-Ca. (n = 2) | 1 (2%) | 0 | 1 (3.2%) | 0 | 0 |
| VIN I/vuLSIL (n = 0) | 0 | 0 | 0 | 0 | 0 |
| VIN II/vuHSIL (n = 1) | 1 (2%) | 0 | 0 | 0 | 0 |
| VIN III/vuHSIL (n = 12) | 1 (2%) | 8 (8.9%) | 2 (6.5%) | 1 (3.7%) | 0 |
| Vulva-Ca. (n = 4) | 1 (2%) | 2 (2.2%) | 0 (0%) | 1 (3.7%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuebs, F.A.; Dietl, A.K.; Koch, M.C.; Adler, W.; Geppert, C.I.; Hartmann, A.; Knöll, A.; Mehlhorn, G.; Beckmann, M.W.; Schulmeyer, C.E.; et al. Cytology and HPV Co-Testing for Detection of Vaginal Intraepithelial Neoplasia: A Retrospective Study. Cancers 2023, 15, 4633. https://doi.org/10.3390/cancers15184633
Stuebs FA, Dietl AK, Koch MC, Adler W, Geppert CI, Hartmann A, Knöll A, Mehlhorn G, Beckmann MW, Schulmeyer CE, et al. Cytology and HPV Co-Testing for Detection of Vaginal Intraepithelial Neoplasia: A Retrospective Study. Cancers. 2023; 15(18):4633. https://doi.org/10.3390/cancers15184633
Chicago/Turabian StyleStuebs, Frederik A., Anna K. Dietl, Martin C. Koch, Werner Adler, Carol Immanuel Geppert, Arndt Hartmann, Antje Knöll, Grit Mehlhorn, Matthias W. Beckmann, Carla E. Schulmeyer, and et al. 2023. "Cytology and HPV Co-Testing for Detection of Vaginal Intraepithelial Neoplasia: A Retrospective Study" Cancers 15, no. 18: 4633. https://doi.org/10.3390/cancers15184633
APA StyleStuebs, F. A., Dietl, A. K., Koch, M. C., Adler, W., Geppert, C. I., Hartmann, A., Knöll, A., Mehlhorn, G., Beckmann, M. W., Schulmeyer, C. E., Heindl, F., Emons, J., Seibold, A., Behrens, A. S., & Gass, P. (2023). Cytology and HPV Co-Testing for Detection of Vaginal Intraepithelial Neoplasia: A Retrospective Study. Cancers, 15(18), 4633. https://doi.org/10.3390/cancers15184633








