Venetoclax with Hypomethylating Agents in Newly Diagnosed Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis of Survival Data from Real-World Studies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Real-world studies (i.e., studies conducted out of an experimental setting);
- patients aged at least 18 years;
- previously untreated Acute Myeloid Leukemia (including also secondary AML and therapy-related AML);
- venetoclax used as first line of treatment in association with hypomethylating agents (azacitidine and/or decitabine);
- articles written in the English language.
2.3. Data Extraction and Synthesis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute myeloid leukaemia. Lancet 2023, 401, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Newell, L.F.; Cook, R.J. Advances in Acute Myeloid Leukemia. BMJ 2021, 375, n2026. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G. Older Patients with Acute Myeloid Leukemia Benefit from Intensive Chemotherapy: An Update from the Swedish Acute Leukemia Registry. Clin. Lymphoma Myeloma Leuk. 2011, 11, S54–S59. [Google Scholar] [CrossRef]
- Späth, C.; Neumann, T.; Schmidt, C.A.; Heidel, F.H.; Krüger, W.H. Patients receiving allogeneic haematopoietic stem-cell transplantation and clinical outcomes after early access to palliative care. Lancet Haematol. 2023, 10, e777–e784. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.-P.; Chou, W.-C.; Buckstein, R.; Cermak, J.; et al. Multicenter, Randomized, Open-Label, Phase III Trial of Decitabine Versus Patient Choice, with Physician Advice, of Either Supportive Care or Low-Dose Cytarabine for the Treatment of Older Patients with Newly Diagnosed Acute Myeloid Leukemia. JCO 2012, 30, 2670–2677. [Google Scholar] [CrossRef]
- Schiller, G. A Slow-Go Prognosis for Older Patients with Newly Diagnosed AML. Blood 2021, 138, 501–502. [Google Scholar] [CrossRef]
- Koenig, K.; Mims, A.; Levis, M.J.; Horowitz, M.M. The Changing Landscape of Treatment in Acute Myeloid Leukemia. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 343–354. [Google Scholar] [CrossRef]
- Wei, A.H.; Roberts, A.W. BCL2 Inhibition: A New Paradigm for the Treatment of AML and Beyond. Hemasphere 2023, 7, e912. [Google Scholar] [CrossRef]
- Klanova, M.; Klener, P. BCL-2 Proteins in Pathogenesis and Therapy of B-Cell Non-Hodgkin Lymphomas. Cancers 2020, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Amaya, M.; Strati, P.; Konopleva, M.Y. Venetoclax for AML: Changing the treatment paradigm. Blood Adv. 2019, 3, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.A.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and Preliminary Efficacy of Venetoclax with Decitabine or Azacitidine in Elderly Patients with Previously Untreated Acute Myeloid Leukaemia: A Non-Randomised, Open-Label, Phase 1b Study. Lancet Oncol. 2018, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Qin, Y.; Kuang, P.; Liu, T. Venetoclax combined with hypomethylating agents or low-dose cytarabine as induction chemotherapy for patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 219–227. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Loghavi, S.; Furudate, K.; Montalban-Bravo, G.; Maiti, A.; Kadia, T.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Sasaki, K.; et al. Impact of splicing mutations in acute myeloid leukemia treated with hypomethylating agents combined with venetoclax. Blood Adv. 2021, 5, 2173–2183. [Google Scholar] [CrossRef]
- Konopleva, M.; Thirman, M.J.; Pratz, K.W.; Garcia, J.S.; Recher, C.; Pullarkat, V.; Kantarjian, H.M.; DiNardo, C.D.; Dail, M.; Duan, Y.; et al. Impact of FLT3 Mutation on Outcomes after Venetoclax and Azacitidine for Patients with Treatment-Naïve Acute Myeloid Leukemia. Clin. Cancer Res. 2022, 28, 2744–2752. [Google Scholar] [CrossRef]
- Pollyea, D.A.; DiNardo, C.D.; Arellano, M.L.; Pigneux, A.; Fiedler, W.; Konopleva, M.; Rizzieri, D.A.; Smith, B.D.; Shinagawa, A.; Lemoli, R.M.; et al. Impact of Venetoclax and Azacitidine in Treatment-Naïve Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin. Cancer Res. 2022, 28, 2753–2761. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Recher, C.; Schuh, A.C.; Thirman, M.J.; Garcia, J.S.; DiNardo, C.D.; Vorobyev, V.; Fracchiolla, N.S.; et al. Measurable Residual Disease Response and Prognosis in Treatment-Naïve Acute Myeloid Leukemia with Venetoclax and Azacitidine. J. Clin. Oncol. 2022, 40, 855–865. [Google Scholar] [CrossRef]
- De Bellis, E.; Imbergamo, S.; Candoni, A.; Liço, A.; Tanasi, I.; Mauro, E.; Mosna, F.; Leoncin, M.; Stulle, M.; Griguolo, D.; et al. Venetoclax in Combination with Hypomethylating Agents in Previously Untreated Patients with Acute Myeloid Leukemia Ineligible for Intensive Treatment: A Real-Life Multicenter Experience. Leuk. Res. 2022, 114, 106803. [Google Scholar] [CrossRef] [PubMed]
- Laloi, L.; Billotey, N.C.; Dumas, P.; Paul, F.; Villate, A.; Simand, C.; Fornecker, L.; Puisset, F.; Bertoli, S.; Simonet, M.B.; et al. Retrospective, Real-life Study of Venetoclax plus Azacitidine or Low-dose Cytarabine in French Patients with Acute Myeloid Leukemia Ineligible for Intensive Chemotherapy. Cancer Med. 2023, 12, 7175–7181. [Google Scholar] [CrossRef] [PubMed]
- Roldán Pérez, A.; Vázquez Paganini, J.A.; Penalva Moreno, M.J.; Giménez Mesa, E.; Vilches Moreno, A.S.; Nuñez-Torrón Stock, C.; Herráez García, R. Real-life Experience of Venetoclax and Hypomethylating Agents in Acute Myeloid Leukemia Patients Not Candidates for Intensive Chemotherapy or Who Are Refractory/Relapsed: A Single-centre Experience. Clin. Case Rep. 2022, 10, e6116. [Google Scholar] [CrossRef] [PubMed]
- Todisco, E.; Papayannidis, C.; Fracchiolla, N.; Petracci, E.; Zingaretti, C.; Vetro, C.; Martelli, M.P.; Zappasodi, P.; Di Renzo, N.; Gallo, S.; et al. AVALON: The Italian Cohort Study on Real-life Efficacy of Hypomethylating Agents plus Venetoclax in Newly Diagnosed or Relapsed/Refractory Patients with Acute Myeloid Leukemia. Cancer 2023, 129, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct Individual Patient Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Vachhani, P.; Flahavan, E.M.; Xu, T.; Ma, E.; Montez, M.; Gershon, A.; Onishi, M.; Jin, H.; Ku, G.; Flores, B.; et al. Venetoclax and Hypomethylating Agents as First-Line Treatment in Newly Diagnosed Patients with AML in a Predominately Community Setting in the US. Oncology 2022, 27, 907–918. [Google Scholar] [CrossRef]
- Matthews, A.H.; Perl, A.E.; Luger, S.M.; Loren, A.W.; Gill, S.I.; Porter, D.L.; Babushok, D.V.; Maillard, I.P.; Carroll, M.P.; Frey, N.V.; et al. Real-World Effectiveness of CPX-351 vs Venetoclax and Azacitidine in Acute Myeloid Leukemia. Blood Adv. 2022, 6, 3997–4005. [Google Scholar] [CrossRef]
- Aiba, M.; Shigematsu, A.; Suzuki, T.; Miyagishima, T. Shorter Duration of Venetoclax Administration to 14 Days Has Same Efficacy and Better Safety Profile in Treatment of Acute Myeloid Leukemia. Ann. Hematol. 2023, 102, 541–546. [Google Scholar] [CrossRef]
- Apel, A.; Moshe, Y.; Ofran, Y.; Gural, A.; Wolach, O.; Ganzel, C.; Canaani, J.; Zektser, M.; Duek, A.; Stemer, G.; et al. Venetoclax Combinations Induce High Response Rates in Newly Diagnosed Acute Myeloid Leukemia Patients Ineligible for Intensive Chemotherapy in Routine Practice. Am. J. Hematol. 2021, 96, 790–795. [Google Scholar] [CrossRef]
- Begna, K.H.; Gangat, N.; Al-Kali, A.; Litzow, M.R.; Hogan, W.J.; Patnaik, M.M.; Pardanani, A.; Hook, C.C.; Wolanskyj, A.P.; Elliott, M.A.; et al. Acute Myeloid Leukemia after Age 70 Years: A Retrospective Comparison of Survival Following Treatment with Intensive versus HMA ± Venetoclax Chemotherapy. Am. J. Hematol. 2021, 96, E108–E111. [Google Scholar] [CrossRef]
- Cherry, E.M.; Abbott, D.; Amaya, M.; McMahon, C.; Schwartz, M.; Rosser, J.; Sato, A.; Schowinsky, J.; Inguva, A.; Minhajuddin, M.; et al. Venetoclax and Azacitidine Compared with Induction Chemotherapy for Newly Diagnosed Patients with Acute Myeloid Leukemia. Blood Adv. 2021, 5, 5565–5573. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.; Tremblay, D.; Dougherty, M.; Czaplinska, T.; Sanchez, G.; Brady, C.; Kremyanskaya, M.; Bar-Natan, M.; Keyzner, A.; Marcellino, B.K.; et al. Safety and Efficacy: Clinical Experience of Venetoclax in Combination with Hypomethylating Agents in Both Newly Diagnosed and Relapsed/Refractory Advanced Myeloid Malignancies. HemaSphere 2021, 5, e549. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Scholl, S.; Frietsch, J.J.; Hilgendorf, I.; Schrenk, K.; Hammersen, J.; Prims, F.; Thiede, C.; Hochhaus, A.; Schnetzke, U. Clinical Experience with Venetoclax in Patients with Newly Diagnosed, Relapsed, or Refractory Acute Myeloid Leukemia. J. Cancer Res. Clin. Oncol. 2022, 148, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Garciaz, S.; Hospital, M.-A.; Alary, A.-S.; Saillard, C.; Hicheri, Y.; Mohty, B.; Rey, J.; D’Incan, E.; Charbonnier, A.; Villetard, F.; et al. Azacitidine Plus Venetoclax for the Treatment of Relapsed and Newly Diagnosed Acute Myeloid Leukemia Patients. Cancers 2022, 14, 2025. [Google Scholar] [CrossRef] [PubMed]
- Kwag, D.; Cho, B.-S.; Bang, S.-Y.; Lee, J.H.; Min, G.-J.; Park, S.-S.; Park, S.; Yoon, J.-H.; Lee, S.-E.; Eom, K.-S.; et al. Venetoclax with Decitabine versus Decitabine Monotherapy in Elderly Acute Myeloid Leukemia: A Propensity Score-Matched Analysis. Blood Cancer J. 2022, 12, 169. [Google Scholar] [CrossRef]
- Mirgh, S.; Sharma, A.; Shaikh, M.R.M.A.; Kadian, K.; Agrawal, N.; Khushoo, V.; Mehta, P.; Ahmed, R.; Bhurani, D. Hypomethylating Agents+venetoclax Induction Therapy in Acute Myeloid Leukemia Unfit for Intensive Chemotherapy—Novel Avenues for Lesser Venetoclax Duration and Patients with Baseline Infections from a Developing Country. Am. J. Blood Res. 2021, 11, 290–302. [Google Scholar]
- Morsia, E.; McCullough, K.; Joshi, M.; Cook, J.; Alkhateeb, H.B.; Al-Kali, A.; Begna, K.; Elliott, M.; Hogan, W.; Litzow, M.; et al. Venetoclax and Hypomethylating Agents in Acute Myeloid Leukemia: Mayo Clinic Series on 86 Patients. Am. J. Hematol. 2020, 95, 1511–1521. [Google Scholar] [CrossRef]
- Mustafa Ali, M.K.; Corley, E.M.; Alharthy, H.; Kline, K.A.F.; Law, J.Y.; Lee, S.T.; Niyongere, S.; Duong, V.H.; Emadi, A.; Baer, M.R. Outcomes of Newly Diagnosed Acute Myeloid Leukemia Patients Treated with Hypomethylating Agents with or without Venetoclax: A Propensity Score-Adjusted Cohort Study. Front. Oncol. 2022, 12, 858202. [Google Scholar] [CrossRef]
- Salhotra, A.; Aribi, A.; Ngo, D.; Zhang, J.; Sandhu, K.; Al-Malki, M.; Ali, H.; Koller, P.; Arslan, S.; Budde, E.; et al. Outcome of Secondary Acute Myeloid Leukemia Treated with Hypomethylating Agent plus Venetoclax (HMA-Ven) or Liposomal Daunorubicin-cytarabine ( CPX -351). Am. J. Hematol. 2021, 96, E196–E200. [Google Scholar] [CrossRef]
- Shah, M.V.; Chhetri, R.; Dholakia, R.; Kok, C.H.; Gangat, N.; Alkhateeb, H.B.; Al-Kali, A.; Patnaik, M.M.; Baranwal, A.; Greipp, P.T.; et al. Outcomes Following Venetoclax-based Treatment in Therapy-related Myeloid Neoplasms. Am. J. Hematol. 2022, 97, 1013–1022. [Google Scholar] [CrossRef]
- Venugopal, S.; Shoukier, M.; Konopleva, M.; Dinardo, C.D.; Ravandi, F.; Short, N.J.; Andreeff, M.; Borthakur, G.; Daver, N.; Pemmaraju, N.; et al. Outcomes in Patients with Newly Diagnosed TP53-mutated Acute Myeloid Leukemia with or without Venetoclax-based Therapy. Cancer 2021, 127, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.C.; Gutman, J.A.; Purev, E.; Nakic, M.; Tobin, J.; Chase, S.; Kaiser, J.; Lyle, L.; Boggs, C.; Halsema, K.; et al. Real-World Experience of Venetoclax with Azacitidine for Untreated Patients with Acute Myeloid Leukemia. Blood Adv. 2019, 3, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Pelcovits, A.; Moore, J.; Bakow, B.; Niroula, R.; Egan, P.; Reagan, J.L. Tumor Lysis Syndrome Risk in Outpatient versus Inpatient Administration of Venetoclax and Hypomethlators for Acute Myeloid Leukemia. Support. Care Cancer 2021, 29, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, C.; Yan, J. The Efficacy and Safety of Venetoclax and Azacytidine Combination Treatment in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome: Systematic Review and Meta-Analysis. Hematology 2023, 28, 2198098. [Google Scholar] [CrossRef]
- Rausch, C.R.; DiNardo, C.D.; Maiti, A.; Jammal, N.J.; Kadia, T.M.; Marx, K.R.; Borthakur, G.; Savoy, J.M.; Pemmaraju, N.; DiPippo, A.J.; et al. Duration of Cytopenias with Concomitant Venetoclax and Azole Antifungals in Acute Myeloid Leukemia. Cancer 2021, 127, 2489–2499. [Google Scholar] [CrossRef]
- Pervitsky, V.; Guglielmo, J.; Moskoff, B.; Kneen, R.; Leija, C.; Sawicky, D.; Krackeler, M.L.; Jonas, B.A.; Beechinor, R. Characterization of a Multidisciplinary Team’s Role in Hospital Discharge for Patients Receiving Hypomethylating Agents with Venetoclax as Induction Therapy for Acute Myeloid Leukemia. Support. Care Cancer 2023, 31, 224. [Google Scholar] [CrossRef]
- Agarwal, S.K.; DiNardo, C.D.; Potluri, J.; Dunbar, M.; Kantarjian, H.M.; Humerickhouse, R.A.; Wong, S.L.; Menon, R.M.; Konopleva, M.Y.; Salem, A.H. Management of Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients: Evaluation of Dose Adjustments. Clin. Ther. 2017, 39, 359–367. [Google Scholar] [CrossRef]
- Stemler, J.; Mellinghoff, S.C.; Khodamoradi, Y.; Sprute, R.; Classen, A.Y.; Zapke, S.E.; Hoenigl, M.; Krause, R.; Schmidt-Hieber, M.; Heinz, W.J.; et al. Primary Prophylaxis of Invasive Fungal Diseases in Patients with Haematological Malignancies: 2022 Update of the Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). J. Antimicrob. Chemother. 2023, 78, 1813–1826. [Google Scholar] [CrossRef]
- Italian Medicines Agency. Italian Medicines Agency—AIFA Approves Venetoclax in Combination for AML in Adults. Available online: https://www.aifa.gov.it/-/attivazione-web-e-pubblicazione-schede-di-monitoraggio-registro-venclxto-lma- (accessed on 3 July 2023).
- Haute Autorité de Santé. Haute Autorité de Santé—HAS Approves Venetoclax in Combination for AML in Adults. Available online: https://www.has-sante.fr/jcms/p_3284614/fr/venclyxto-venetoclax-leucemie-aigue-myeloide (accessed on 3 July 2023).
- National Institute for Health and Care Excellence. National Institute for Health and Care Excellence—NICE Approves Venetoclax in Combination for AML in Adults. Available online: https://www.nice.org.uk/guidance/ta765/resources/venetoclax-with-azacitidine-for-untreated-acute-myeloid-leukaemia-when-intensive-chemotherapy-is-unsuitable-pdf-82611437583301 (accessed on 3 July 2023).
- Food and Drug Administration. FDA Approves Venetoclax in Combination for AML in Adults. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-venetoclax-combination-untreated-acute-myeloid-leukemia (accessed on 3 July 2023).
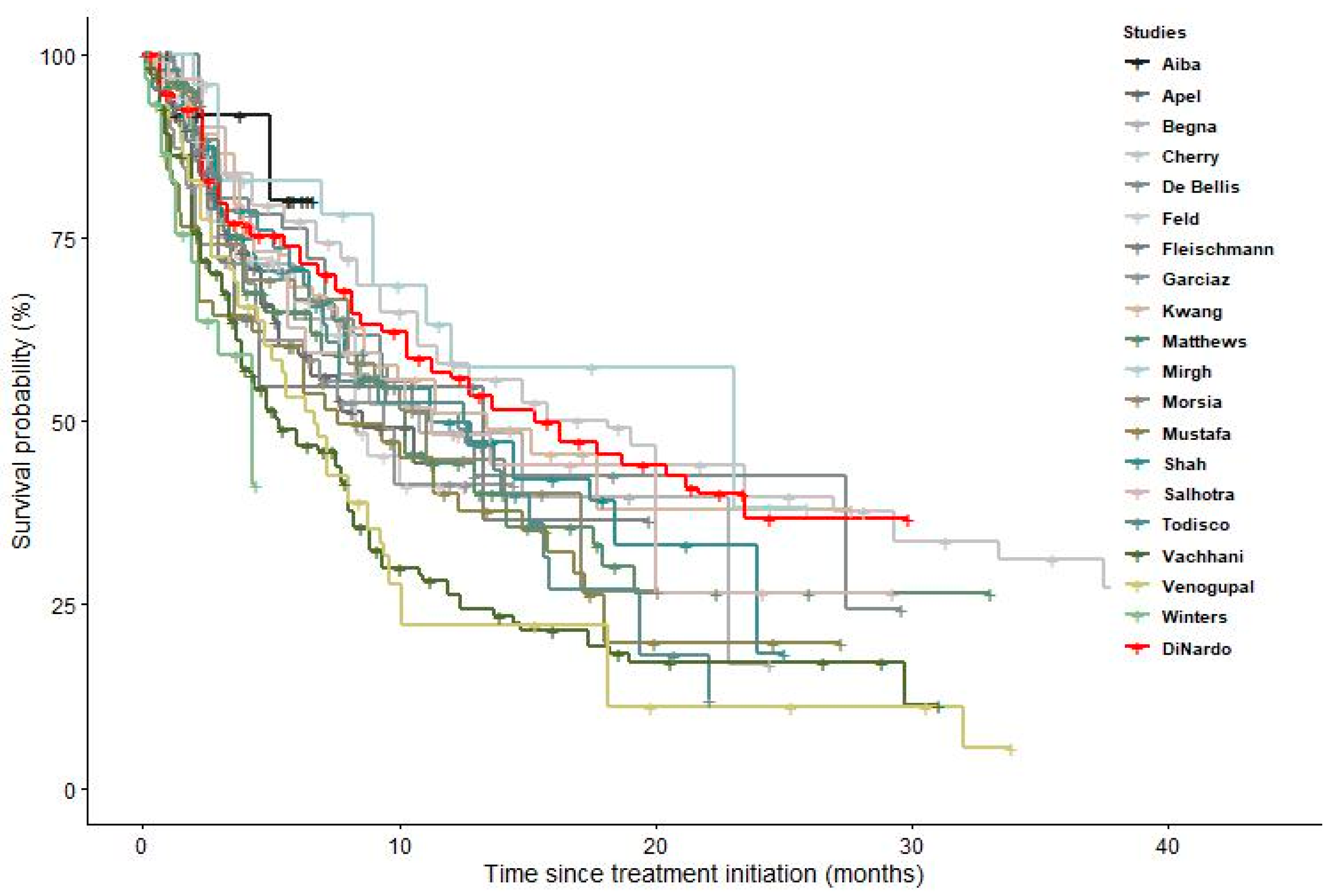
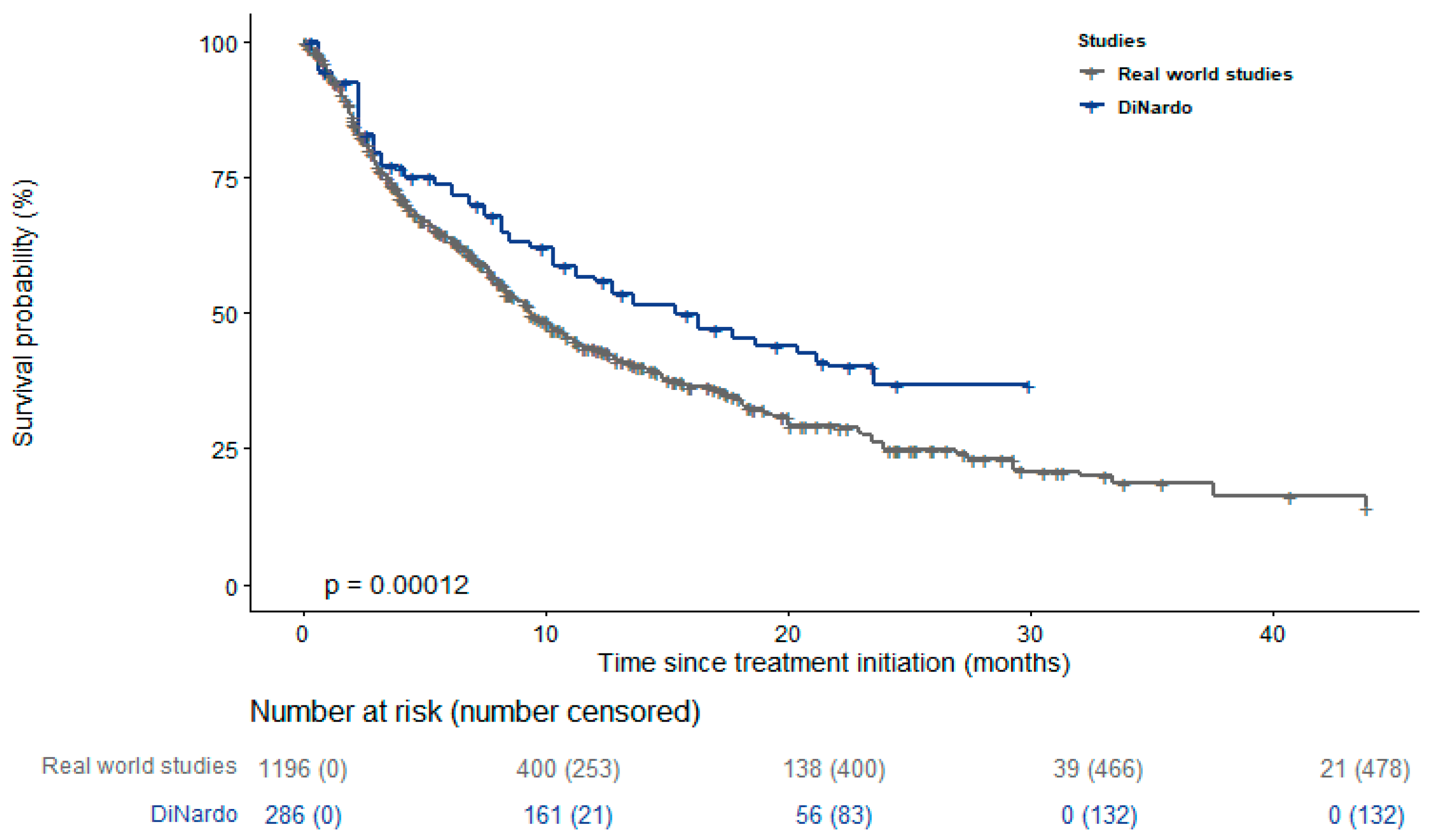
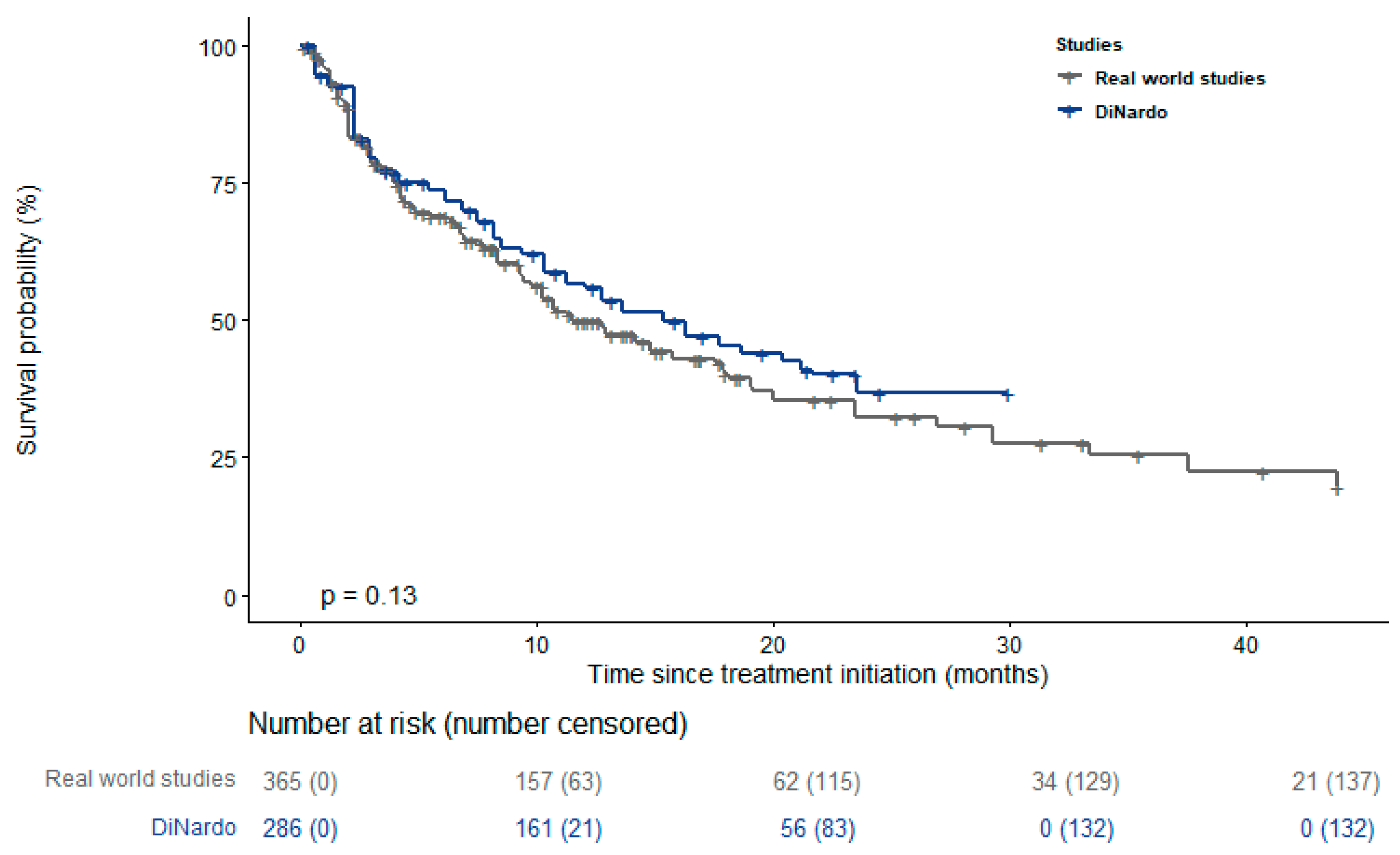
| Study | Number of Centers | N. Patients | Age— Mean (Range) | Males % | AML Type | Treatment | Follow Up—Months |
|---|---|---|---|---|---|---|---|
| Aiba, 2023 [28] | 1 | 13 | 79 (72–86) | 77 | Any AML | VEN+AZA | 6 |
| Apel, 2021 [29] | 11 | 133 | 77 (52–95) | 53 | Any AML | VEN+HMA | 17 |
| Begna, 2021 [30] | 1 | 28 | 75 (71–92) | nr | Any AML | VEN+HMA | 24 |
| Cherry, 2021 [31] | 1 | 143 | 70 (22–91) | 50 | Any AML | VEN+AZA | 83 |
| De Bellis, 2022 [21] | 8 | 51 | 75 (55–82) | 52 | Any AML | VEN+HMA | 33 |
| Feld, 2021 [32] | 1 | 26 | 72 | 64 | Any AML | VEN+HMA | 31 |
| Fleishmann, 2022 [33] | 1 | 17 | 67 (34–83) | 57 | Any AML | VEN+HMA | 15 |
| Garciaz, 2022 [34] | 1 | 39 | 73 (61–81) | 55 | Any AML | VEN+AZA | 15 |
| Kwang, 2022 [35] | 1 | 74 | 71 | 43 | Any AML | VEN+DEC | 48 |
| Matthews, 2022 [27] | 285 | 129 | nr | nr | Any AML | VEN+AZA | 33 |
| Mirgh, 2021 [36] | 1 | 24 | 60 (30–79) | 46 | Any AML | VEN+HMA | 26 |
| Morsia, 2020 [37] | 1 | 44 | 74 (37–91) | 61 | Any AML | VEN+HMA | 23 |
| Mustafa Ali, 2022 [38] | 1 | 51 | nr | 59 | Any AML | VEN+HMA | 28 |
| Salhotra, 2021 [39] | 1 | 30 | 63 (35–72) | 46 | Secondary AML | VEN+HMA | 29 |
| Shah, 2022 [40] | 4 | 32 | 72 (61–75) | 54 | Therapy-related AML | VEN+HMA | 26 |
| Todisco, 2023 [24] | 32 | 43 | 74 | 47 | Any AML | VEN+HMA | 24 |
| Vachhani, 2022 [26] | 280 | 169 | 77 (39–85) | 56 | Any AML | VEN+HMA | 31 |
| Venugopal, 2021 [41] | 1 | 58 | 73 (26–85) | 48 | TP53-mutated AML | VEN+HMA | 43 |
| Winters, 2019 [42] | 1 | 30 | 72 | nr | Any AML | VEN+AZA | 13 |
| DiNardo, 2020 [14] | 134 | 286 | 76 (49–91) | 60 | Any AML | VEN+AZA | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ucciero, A.; Pagnoni, F.; Scotti, L.; Pisterna, A.; Barone-Adesi, F.; Gaidano, G.; Patriarca, A.; Lunghi, M. Venetoclax with Hypomethylating Agents in Newly Diagnosed Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis of Survival Data from Real-World Studies. Cancers 2023, 15, 4618. https://doi.org/10.3390/cancers15184618
Ucciero A, Pagnoni F, Scotti L, Pisterna A, Barone-Adesi F, Gaidano G, Patriarca A, Lunghi M. Venetoclax with Hypomethylating Agents in Newly Diagnosed Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis of Survival Data from Real-World Studies. Cancers. 2023; 15(18):4618. https://doi.org/10.3390/cancers15184618
Chicago/Turabian StyleUcciero, Andrealuna, Federico Pagnoni, Lorenza Scotti, Alessia Pisterna, Francesco Barone-Adesi, Gianluca Gaidano, Andrea Patriarca, and Monia Lunghi. 2023. "Venetoclax with Hypomethylating Agents in Newly Diagnosed Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis of Survival Data from Real-World Studies" Cancers 15, no. 18: 4618. https://doi.org/10.3390/cancers15184618
APA StyleUcciero, A., Pagnoni, F., Scotti, L., Pisterna, A., Barone-Adesi, F., Gaidano, G., Patriarca, A., & Lunghi, M. (2023). Venetoclax with Hypomethylating Agents in Newly Diagnosed Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis of Survival Data from Real-World Studies. Cancers, 15(18), 4618. https://doi.org/10.3390/cancers15184618







