Evaluation of Real-Life Chemoimmunotherapy Combination in Patients with Metastatic Small Cell Lung Carcinoma (SCLC): A Multicentric Case–Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
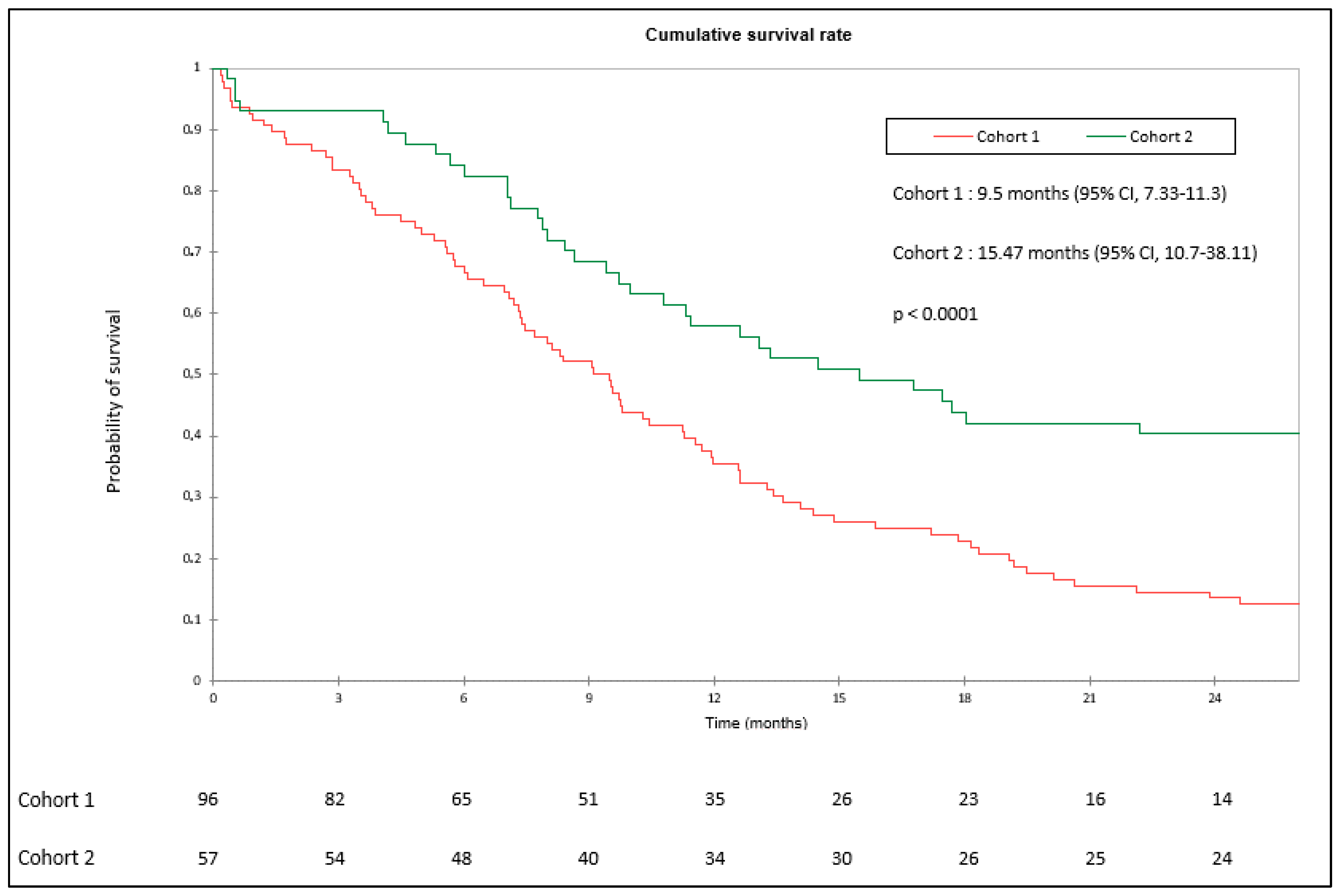
3.1. OS and PFS
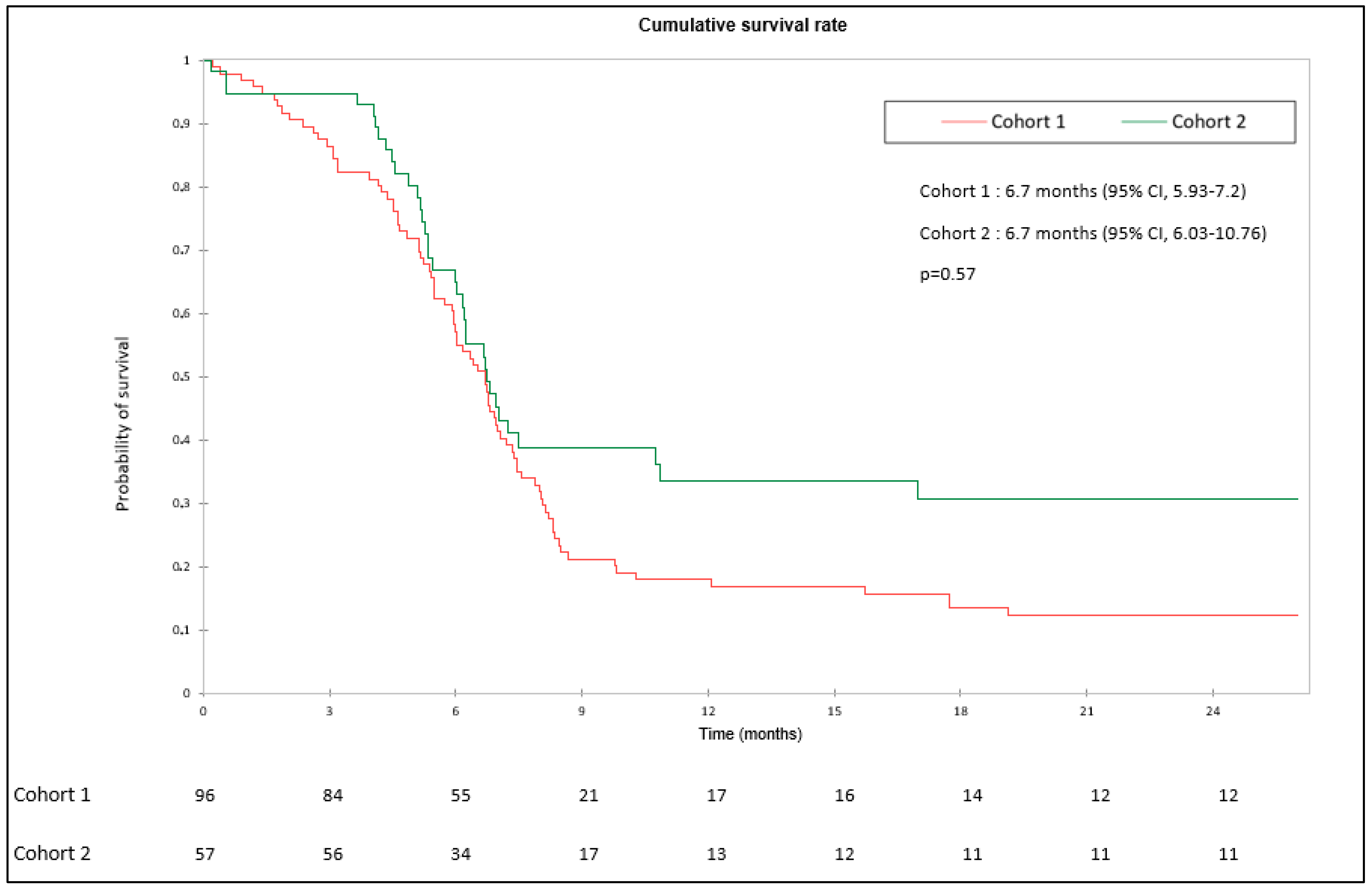
3.2. Objective Response Rate
3.3. Efficacy of 2nd Line Treatment
3.4. Population of Interest
3.4.1. Brain Metastases
3.4.2. Liver Metastases
3.4.3. Poor Performance Status
3.4.4. Elderly Patients
3.5. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Demedts, I.K.; Vermaelen, K.Y.; van Meerbeeck, J.P. Treatment of extensive-stage small cell lung carcinoma: Current status and future prospects. Eur. Respir. J. 2010, 35, 202–215. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Calvert, A.H.; Newell, D.R.; Gumbrell, L.A.; O’Reilly, S.; Burnell, M.; Boxall, F.E.; Siddik, Z.H.; Judson, I.R.; Gore, M.E.; Wiltshaw, E. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 1989, 7, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Stein, M.M.; Kase, M.; Cummings, A.L.; Bharanikumar, R.; Lau, D.; Garon, E.B.; Patel, S.P. Comparison of the tumor immune microenvironment and checkpoint blockade biomarkers between stage III and IV non-small cell lung cancer. Cancer Immunol. Immunother. 2023, 72, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Esposito, G.; Palumbo, G.; Carillio, G.; Manzo, A.; Montanino, A.; Sforza, V.; Costanzo, R.; Sandomenico, C.; La Manna, C.; Martucci, N.; et al. Immunotherapy in Small Cell Lung Cancer. Cancers 2020, 12, 2522. [Google Scholar] [CrossRef]
- Gürbüz, M.; Kutlu, Y.; Akkuş, E.; Köksoy, E.B.; Köse, N.; Öven, B.B.; Uluç, B.O.; Demiray, A.G.; Erdem, D.; Demir, B.; et al. Atezolizumab combined with chemotherapy in the first-line treatment of extensive-stage small cell lung cancer: A real-life data of the Turkish Oncology Group. J. Cancer Res. Clin. Oncol. 2022, 148, 3547–3555. [Google Scholar] [CrossRef]
- Sathiyapalan, A.; Febbraro, M.; Pond, G.R.; Ellis, P.M. Chemo-Immunotherapy in First Line Extensive Stage Small Cell Lung Cancer (ES-SCLC): A Systematic Review and Meta-Analysis. Curr. Oncol. Tor. Ont. 2022, 29, 9046–9065. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, W.C.; Hung, C.M.; Wei, Y.F. Chemotherapy or chemo-immunotherapy as first-line treatment for extensive-stage small-cell lung cancer: A meta-analysis. Immunotherapy 2021, 13, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, A.A.; Gibson, A.J.; Fung, A.S.; Cheung, W.Y.; Dean, M.L.; Bebb, D.G.; Pabani, A. A Real-World Evaluation of Atezolizumab Plus Platinum-Etoposide Chemotherapy in Patients With Extensive-Stage SCLC in Canada. JTO Clin. Res. Rep. 2021, 2, 100249. [Google Scholar] [CrossRef]
- Tagliamento, M.; Frelaut, M.; Baldini, C.; Naigeon, M.; Nencioni, A.; Chaput, N.; Besse, B. The use of immunotherapy in older patients with advanced non-small cell lung cancer. Cancer Treat Rev. 2022, 106, 102394. [Google Scholar] [CrossRef]
- Gomes, F.; Lorigan, P.; Woolley, S.; Foden, P.; Burns, K.; Yorke, J.; Blackhall, F. A prospective cohort study on the safety of checkpoint inhibitors in older cancer patients-the ELDERS study. ESMO Open 2021, 6, 100042. [Google Scholar] [CrossRef]
- Molinier, O.; Besse, B.; Barlesi, F.; Audigier-Valette, C.; Friard, S.; Monnet, I.; Jeannin, G.; Mazières, J.; Cadranel, J.; Hureaux, J.; et al. IFCT-1502 CLINIVO: Real-world evidence of long-term survival with nivolumab in a nationwide cohort of patients with advanced non-small-cell lung cancer. ESMO Open 2022, 7, 100353. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Hartshorn, K.; Rahma, O.; Lin, N.; Snyder-Cappione, J.E. Aging, immune senescence, and immunotherapy: A comprehensive review. Semin. Oncol. 2018, 45, 187–200. [Google Scholar] [CrossRef]
- Sagie, S.; Maixner, N.; Stemmer, A.; Lobachov, A.; Bar, J.; Urban, D. Real-world evidence for immunotherapy in the first line setting in small cell lung cancer. Lung Cancer 2022, 172, 136–141. [Google Scholar] [CrossRef]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Luciani, A. Topotecan or other agents as second-line therapy for relapsed small-cell lung cancer: A meta-analysis of randomized studies. Mol. Clin. Oncol. 2021, 15, 218. [Google Scholar] [CrossRef]
- Toublanc, A.C.; Guecamburu, M.; Veillon, R.; Rosellini, P.; Girodet, P.O.; Zysman, M. Second-line lurbinectedin as a new treatment option for small-cell lung cancer: Preliminary results in real-clinical practice. Thorac. Cancer 2022, 13, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Sekkath Veedu, J. Current Strategies for Extensive Stage Small Cell Lung Cancer Beyond First-line Therapy. Clin. Lung Cancer 2022, 23, 14–20. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cohort 1 (n = 96) | Cohort 2 (n = 57) | p-Value | |
|---|---|---|---|---|
| Age, mean (standard deviation) | 64.9 (8.39) | 67.1 (9.04) | 0.13 | |
| Performance status | 0 | 22 (23) | 14 (25) | 0.21 |
| 1 | 51 (53) | 37 (65) | - | |
| 2 | 20 (21) | 5 (8.8) | - | |
| 3 | 3 (3.1) | 1 (1.8) | - | |
| Sex | Man | 62 (65) | 31 (54) | 0.21 |
| Woman | 34 (35) | 26 (46) | - | |
| Smoking habit | Never | 2 (2.1) | 2 (3.5) | 0.80 |
| Past | 31 (32) | 19 (33) | - | |
| Current | 63 (66) | 36 (63) | - | |
| Brain metastasis | No | 62 (65) | 43 (75) | 0.16 |
| Yes | 34 (35) | 14 (25) | - | |
| Liver metastasis | No | 53 (55) | 32 (56) | 0.91 |
| Yes | 43 (45) | 25 (44) | ||
| Pleural involvement | No | 68 (71) | 28 (49) | <0.01 |
| Yes | 28 (29) | 29 (51) | - | |
| Platinum salt | Cisplatin | 18 (19) | 0 (0) | <0.001 |
| Carboplatin | 67 (70) | 57 (100) | - |
| Cohort 1 (n = 96) | Cohort 2 (n = 57) | p-Value | |
|---|---|---|---|
| Complete response | 6 (6.2) | 6 (11) | 0.04 |
| Partial response | 53 (55) | 40 (70) | - |
| Stability | 12 (12) | 6 (11) | - |
| Progression | 25 (26) | 5 (8.8) | - |
| Grade | Cohort 1 (n = 94) | Cohort 2 (n = 55) | p-Value | |
|---|---|---|---|---|
| Hepatic cytolysis | 1 | 3 (3.2) | 2 (3.5) | 0.28 |
| 2 | 7 (7.4) | 1 (1.8) | - | |
| 3 | 0 (0) | 1 (1.8) | - | |
| Infection | 36 (38) | 29 (51) | 0.13 | |
| Renal failure | 1 | 4 (4.2) | 2 (3.5) | 1.00 |
| 2 | 5 (5.3) | 3 (5.3) | - | |
| 3 | 3 (3.2) | 1 (1.8) | - | |
| 4 | 1 (1.1) | 0 (0) | - | |
| Pneumonitis | 0 (0) | 3 (5.3) | 0.15 | |
| Skin toxicity | 1 | 8 (8.4) | 7 (12) | 0.79 |
| 2 | 5 (5.3) | 2 (3.5) | - | |
| 3 | 1 (1.1) | 1 (1.8) | - | |
| Gastrointestinal toxicity | 1 | 21 (22) | 9 (16) | 0.02 |
| 2 | 4 (4.2) | 9 (16) | - | |
| 3 | 9 (9.5) | 1 (1.8) | - | |
| 4 | 2 (2.1) | 0 (0) | - | |
| Hematological disorders | 1 | 20 (21) | 19 (33) | 0.34 |
| 2 | 16 (17) | 10 (18) | - | |
| 3 | 22 (23) | 8 (14) | - | |
| 4 | 19 (20) | 8 (14) | - | |
| Other toxicity | 3 (3.2) | 10 (18) * | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzedine, R.; Canellas, A.; Naltet, C.; Wislez, M.; Azarian, R.; Seferian, A.; Giroux Leprieur, E. Evaluation of Real-Life Chemoimmunotherapy Combination in Patients with Metastatic Small Cell Lung Carcinoma (SCLC): A Multicentric Case–Control Study. Cancers 2023, 15, 4593. https://doi.org/10.3390/cancers15184593
Ezzedine R, Canellas A, Naltet C, Wislez M, Azarian R, Seferian A, Giroux Leprieur E. Evaluation of Real-Life Chemoimmunotherapy Combination in Patients with Metastatic Small Cell Lung Carcinoma (SCLC): A Multicentric Case–Control Study. Cancers. 2023; 15(18):4593. https://doi.org/10.3390/cancers15184593
Chicago/Turabian StyleEzzedine, Rémy, Anthony Canellas, Charles Naltet, Marie Wislez, Reza Azarian, Andrei Seferian, and Etienne Giroux Leprieur. 2023. "Evaluation of Real-Life Chemoimmunotherapy Combination in Patients with Metastatic Small Cell Lung Carcinoma (SCLC): A Multicentric Case–Control Study" Cancers 15, no. 18: 4593. https://doi.org/10.3390/cancers15184593
APA StyleEzzedine, R., Canellas, A., Naltet, C., Wislez, M., Azarian, R., Seferian, A., & Giroux Leprieur, E. (2023). Evaluation of Real-Life Chemoimmunotherapy Combination in Patients with Metastatic Small Cell Lung Carcinoma (SCLC): A Multicentric Case–Control Study. Cancers, 15(18), 4593. https://doi.org/10.3390/cancers15184593






