Comparing Predicted Toxicities between Hypofractionated Proton and Photon Radiotherapy of Liver Cancer Patients with Different Adaptive Schemes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data and Treatment Plans
2.2. NTCP Models
2.3. NTCP Calculation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Khozouz, R.F.; Huq, S.Z.; Perry, M.C. Radiation-Induced Liver Disease. J. Clin. Oncol. 2008, 26, 4844–4845. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic Toxicity Resulting from Cancer Treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Takatori, K.; Terashima, K.; Yoshida, R.; Horai, A.; Satake, S.; Ose, T.; Kitajima, N.; Kinoshita, Y.; Demizu, Y.; Fuwa, N. Upper Gastrointestinal Complications Associated with Gemcitabine-Concurrent Proton Radiotherapy for Inoperable Pancreatic Cancer. J. Gastroenterol. 2014, 49, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Andronesi, O.C.; Bortfeld, T.R.; Richter, C.; Wolf, R.; Guimaraes, A.R.; Hong, T.S.; Seco, J.; Yuan, Y. Proton Therapy in Liver Cancer Feasibility Study of in Vivo MRI Based Dosimetric Verification of Proton End-of-Range for Liver Cancer Patients. Radiother. Oncol. 2013, 106, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.O.; Knopf, A.; Langendijk, J.A.; Weber, D.C.; Lomax, A.J.; Zhang, Y. Assessment of Dosimetric Errors Induced by Deformable Image Registration Methods in 4D Pencil Beam Scanned Proton Treatment Planning for Liver Tumours. Radiother. Oncol. 2018, 128, 174–181. [Google Scholar] [CrossRef]
- Albertini, F.; Matter, M.; Nenoff, L.; Zhang, Y.; Lomax, A. Online Daily Adaptive Proton Therapy. Br. J. Radiol. 2020, 92, 20190594. [Google Scholar] [CrossRef]
- Paganetti, H.; Botas, P.; Sharp, G.C.; Winey, B. Adaptive Proton Therapy. Phys. Med. Biol. 2021, 66, 22TR01. [Google Scholar] [CrossRef]
- Nenoff, L.; Buti, G.; Bobić, M.; Lalonde, A.; Nesteruk, K.P.; Winey, B.; Sharp, G.C.; Sudhyadhom, A.; Paganetti, H. Integrating Structure Propagation Uncertainties in the Optimization of Online Adaptive Proton Therapy Plans. Cancers 2022, 14, 3926. [Google Scholar] [CrossRef]
- Lalonde, A.; Bobić, M.; Winey, B.; Verburg, J.; Sharp, G.C.; Paganetti, H. Anatomic Changes in Head and Neck Intensity-Modulated Proton Therapy: Comparison between Robust Optimization and Online Adaptation. Radiother. Oncol. 2021, 159, 39–47. [Google Scholar] [CrossRef]
- Nenoff, L.; Matter, M.; Hedlund Lindmar, J.; Weber, D.C.; Lomax, A.J.; Albertini, F. Daily Adaptive Proton Therapy: The Key to Use Innovative Planning Approaches for Paranasal Cancer Treatments. Acta Oncol. 2019, 63, 085018. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Andratschke, N.; Alheit, H.; Holy, R.; Moustakis, C.; Nestle, U.; Sauer, O. Definition of Stereotactic Body Radiotherapy: Principles and Practice for the Treatment of Stage I Non-Small Cell Lung Cancer. Strahlenther. Onkol. 2014, 190, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bijl, E.; Remeijer, P.; Sonke, J.J.; Van Der Heide, U.A.; Janssen, T. Adaptive Margins for Online Adaptive Radiotherapy. Phys. Med. Biol. 2022, 67, 195016. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.E.; Wang, K.; Yan, Y.; Desai, N.; Hannan, R.; Chambers, E.; Cai, B.; Lin, M.H.; Sher, D.J.; Wang, J.; et al. Preliminary Evaluation of PTV Margins for Online Adaptive Radiation Therapy of the Prostatic Fossa. Pract. Radiat. Oncol. 2023, 13, e345–e353. [Google Scholar] [CrossRef]
- Thorwarth, D.; Low, D.A. Technical Challenges of Real-Time Adaptive MR-Guided Radiotherapy. Front. Oncol. 2021, 11, 332. [Google Scholar] [CrossRef]
- Keall, P.J.; Brighi, C.; Glide-Hurst, C.; Liney, G.; Liu, P.Z.Y.; Lydiard, S.; Paganelli, C.; Pham, T.; Shan, S.; Tree, A.C.; et al. Integrated MRI-Guided Radiotherapy—Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2022, 19, 458–470. [Google Scholar] [CrossRef]
- Moazzezi, M.; Rose, B.; Kisling, K.; Moore, K.L.; Ray, X. Prospects for Daily Online Adaptive Radiotherapy via Ethos for Prostate Cancer Patients without Nodal Involvement Using Unedited CBCT Auto-Segmentation. J. Appl. Clin. Med. Phys. 2021, 22, 82–93. [Google Scholar] [CrossRef]
- Lyman, J.T.; Wolbarst, A.B. Optimization of Radiation Therapy, III: A Method of Assessing Complication Probabilities from Dose-Volume Histograms. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 103–109. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of Normal Tissue to Therapeutic Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Kutcher, G.J.; Burman, C. Calculation of Complication Probability Factors for Non-Uniform Normal Tissue Irradiation: The Effective Volume Method Gerald. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1623–1630. [Google Scholar] [CrossRef]
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of Normal Tissue Tolerance Data to an Analytic Function. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Normolle, D.; Balter, J.M.; Mcginn, C.J.; Lawrence, T.S.; Ten Haken, R.K. Analysis of Radiation-Induced Liver Disease Using the Lyman NTCP Model. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Pursley, J.; El Naqa, I.; Sanford, N.N.; Noe, B.; Wo, J.Y.; Eyler, C.E.; Hwang, M.; Brock, K.K.; Yeap, B.Y.; Wolfgang, J.A.; et al. Dosimetric Analysis and Normal-Tissue Complication Probability Modeling of Child-Pugh Score and Albumin-Bilirubin Grade Increase After Hepatic Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Dawson, L.; McGinn, C.J.; Lawrence, T.S.; Ten Haken, R.K. Analysis of Radiation-Induced Gastric and Duodenal Bleeds Using the Lyman-Kutcher-Burman Model. I. J. Radiat. Oncol. Biol. Phys. 2003, 57, S217–S218. [Google Scholar] [CrossRef]
- Holyoake, D.L.P.; Aznar, M.; Mukherjee, S.; Partridge, M.; Hawkins, M.A. Modelling Duodenum Radiotherapy Toxicity Using Cohort Dose-Volume-Histogram Data. Radiother. Oncol. 2017, 123, 431–437. [Google Scholar] [CrossRef]
- Murphy, J.D.; Christman-Skieller, C.; Kim, J.; Dieterich, S.; Chang, D.T.; Koong, A.C. A Dosimetric Model of Duodenal Toxicity after Stereotactic Body Radiotherapy for Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1420–1426. [Google Scholar] [CrossRef]
- Fowler, J.F. 21 Years of Biologically Effective Dose. Br. J. Radiol. 2010, 83, 554–568. [Google Scholar] [CrossRef]
- Yaes, R.J.; Patel, P.; Maruyama, Y. On Using the Linear-Quadratic Model in Daily Clinical Practice. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 1353–1362. [Google Scholar] [CrossRef]
- Elhammali, A.; Patel, M.; Weinberg, B.; Verma, V.; Liu, J.; Olsen, J.R.; Gay, H.A. Late Gastrointestinal Tissue Effects after Hypofractionated Radiation Therapy of the Pancreas. Radiat. Oncol. 2015, 10, 186. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and Analyzing Dose Distributions: A Concept of Equivalent Uniform Dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef]
- Gay, H.A.; Niemierko, A. A Free Program for Calculating EUD-Based NTCP and TCP in External Beam Radiotherapy. Phys. Med. 2007, 23, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Moteabbed, M.; Smeets, J.; Hong, T.S.; Janssens, G.; Labarbe, R.; Wolfgang, J.A.; Bortfeld, T.R. Toward MR-Integrated Proton Therapy: Modeling the Potential Benefits for Liver Tumors. Phys. Med. Biol. 2021, 66, 195004. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.J.; Lee, P.; Low, D.A.; Kim, J.; Mittauer, K.E.; Bassetti, M.F.; Glide-Hurst, C.K.; Raldow, A.C.; Yang, Y.; Portelance, L.; et al. A Multi-Institutional Phase 2 Trial of Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided On-Table Adaptive Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Murr, M.; Brock, K.K.; Fusella, M.; Hardcastle, N.; Hussein, M.; Jameson, M.G.; Wahlstedt, I.; Yuen, J.; McClelland, J.R.; Vasquez Osorio, E. Applicability and Usage of Dose Mapping/Accumulation in Radiotherapy. Radiother. Oncol. 2023, 182, 109527. [Google Scholar] [CrossRef]
- McMahon, S.J. The Linear Quadratic Model: Usage, Interpretation and Challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef]
- Jones, L.; Hoban, P.; Metcalfe, P. The Use of the Linear Quadratic Model in Radiotherapy: A Review. Australas. Phys. Eng. Sci. Med. 2001, 24, 132–146. [Google Scholar] [CrossRef]
- Astrahan, M. Some Implications of Linear-Quadratic-Linear Radiation Dose-Response with Regard to Hypofractionation. Med. Phys. 2008, 35, 4161–4172. [Google Scholar] [CrossRef]
- Chen, Y.; Grassberger, C.; Li, J.; Hong, T.S.; Paganetti, H. Impact of Potentially Variable RBE in Liver Proton Therapy. Phys. Med. Biol. 2018, 63, 195001. [Google Scholar] [CrossRef]
- Ibragimov, B.; Toesca, D.; Chang, D.; Yuan, Y.; Koong, A.; Xing, L. Development of Deep Neural Network for Individualized Hepatobiliary Toxicity Prediction after Liver SBRT. Med. Phys. 2018, 45, 4763–4774. [Google Scholar] [CrossRef]
- Chamseddine, I.; Kim, Y.; De, B.; El Naqa, I.; Duda, D.G.; Wolfgang, J.A.; Pursley, J.; Wo, J.Y.; Hong, T.S.; Paganetti, H.; et al. Predictive Model of Liver Toxicity to Aid the Personalized Selection of Proton versus Photon Therapy in Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1234–1243. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Lambin, P.; De Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of Patients for Radiotherapy with Protons Aiming at Reduction of Side Effects: The Model-Based Approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef]
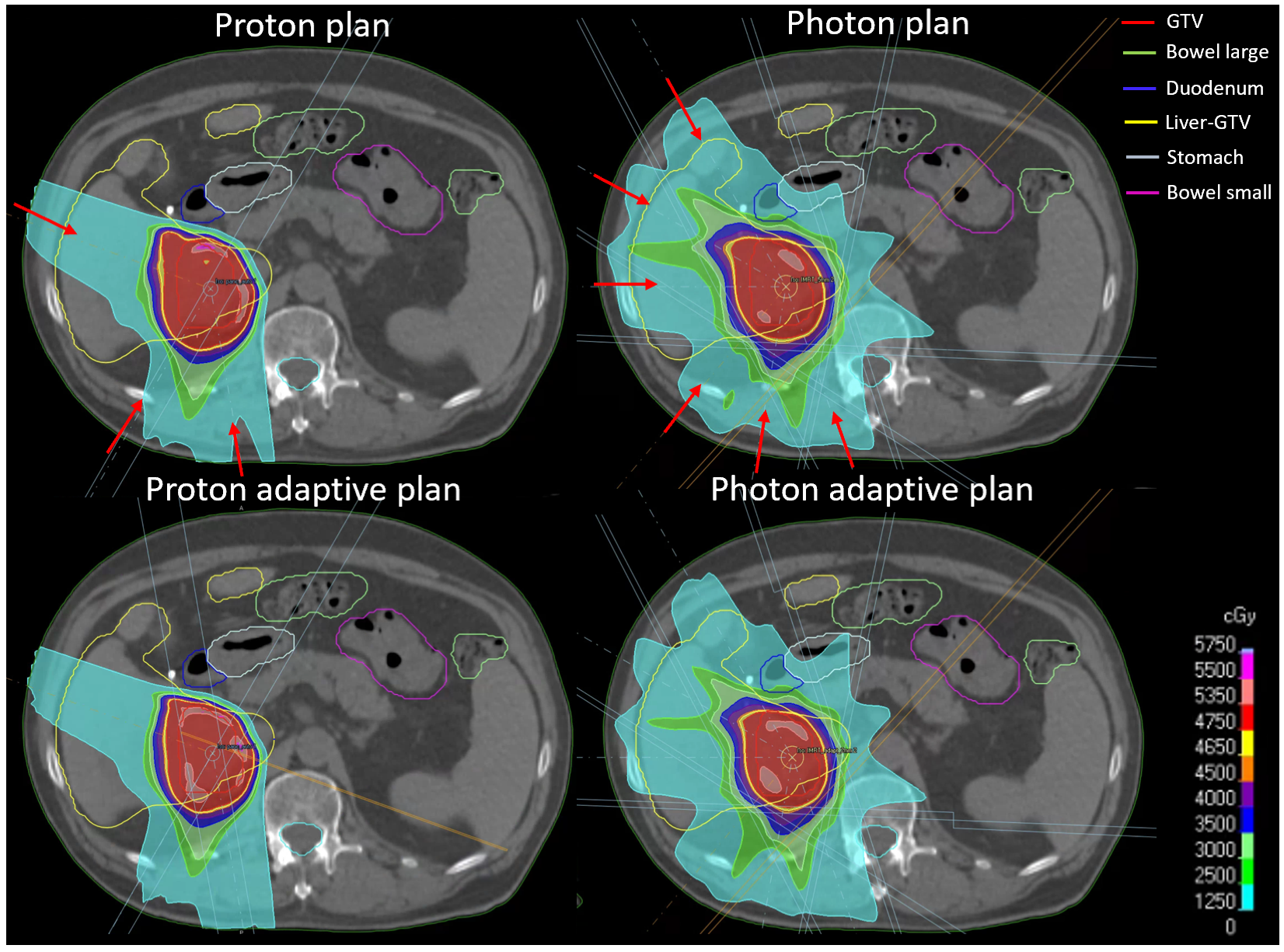

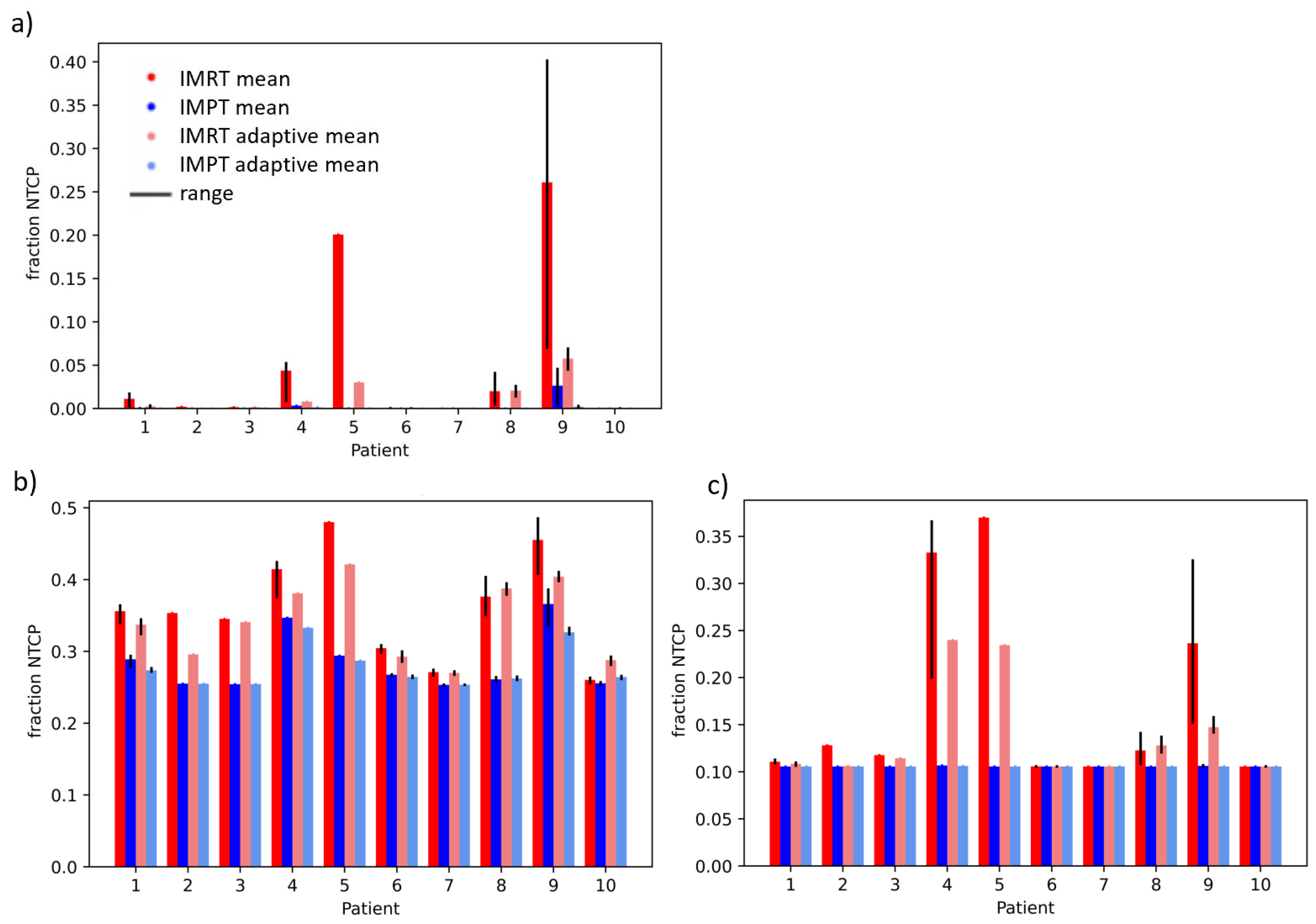
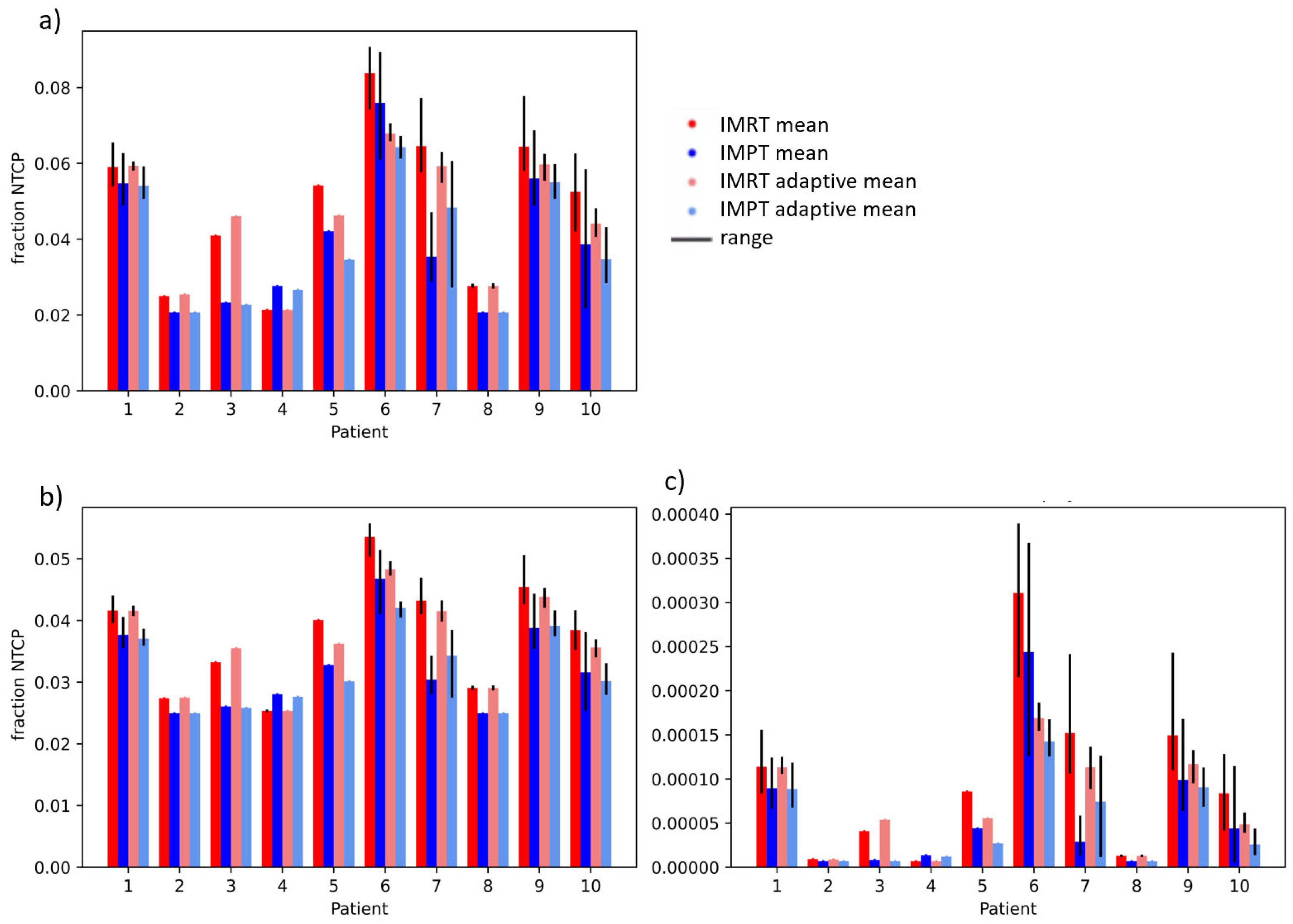
| Patient | GTV Volume in cm3 | Liver-GTV Volume in cm3 |
|---|---|---|
| 1 | 340.05 | 1983.80 |
| 2 | 20.67 | 1434.67 |
| 3 | 92.88 | 1790.45 |
| 4 | 28.94 | 1165.49 |
| 5 | 85.56 | 1590.77 |
| 6 | 100.92 | 2659.70 |
| 7 | 20.54 | 1128.75 |
| 8 | 59.77 | 1061.49 |
| 9 | 89.78 | 964.46 |
| 10 | 26.86 | 1134.98 |
| Structure | NTCP Model | IMRT vs. IMPT, Non-Adaptive | IMRT vs. IMPT, Adaptive | Adaptive vs. Non-Adaptive, IMRT | Adaptive vs. Non-Adaptive, IMPT |
|---|---|---|---|---|---|
| Liver | Dawson RILD | 0.103 | 0.078 | 0.121 | 0.302 |
| Pursley ALBI | 0.001 | 0.001 | 0.065 | 0.145 | |
| Pursely CP A + B | 0.064 | 0.074 | 0.066 | 0.168 | |
| Duodenum | Pan gastric bleed | 0.009 | 0.010 | 0.091 | 0.519 |
| Holyaoke grade 3 tox | 0.002 | 0.001 | 0.098 | 0.433 | |
| Murphy grade 3–4 tox | 0.011 | 0.002 | 0.091 | 0.393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenoff, L.; Sudhyadhom, A.; Lau, J.; Sharp, G.C.; Paganetti, H.; Pursley, J. Comparing Predicted Toxicities between Hypofractionated Proton and Photon Radiotherapy of Liver Cancer Patients with Different Adaptive Schemes. Cancers 2023, 15, 4592. https://doi.org/10.3390/cancers15184592
Nenoff L, Sudhyadhom A, Lau J, Sharp GC, Paganetti H, Pursley J. Comparing Predicted Toxicities between Hypofractionated Proton and Photon Radiotherapy of Liver Cancer Patients with Different Adaptive Schemes. Cancers. 2023; 15(18):4592. https://doi.org/10.3390/cancers15184592
Chicago/Turabian StyleNenoff, Lena, Atchar Sudhyadhom, Jackson Lau, Gregory C. Sharp, Harald Paganetti, and Jennifer Pursley. 2023. "Comparing Predicted Toxicities between Hypofractionated Proton and Photon Radiotherapy of Liver Cancer Patients with Different Adaptive Schemes" Cancers 15, no. 18: 4592. https://doi.org/10.3390/cancers15184592
APA StyleNenoff, L., Sudhyadhom, A., Lau, J., Sharp, G. C., Paganetti, H., & Pursley, J. (2023). Comparing Predicted Toxicities between Hypofractionated Proton and Photon Radiotherapy of Liver Cancer Patients with Different Adaptive Schemes. Cancers, 15(18), 4592. https://doi.org/10.3390/cancers15184592







