Post-Chemoradiation Metastatic, Persistent and Resistant Nodes in Locally Advanced Rectal Cancer: Metrics and Their Impact on Long-Term Outcome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
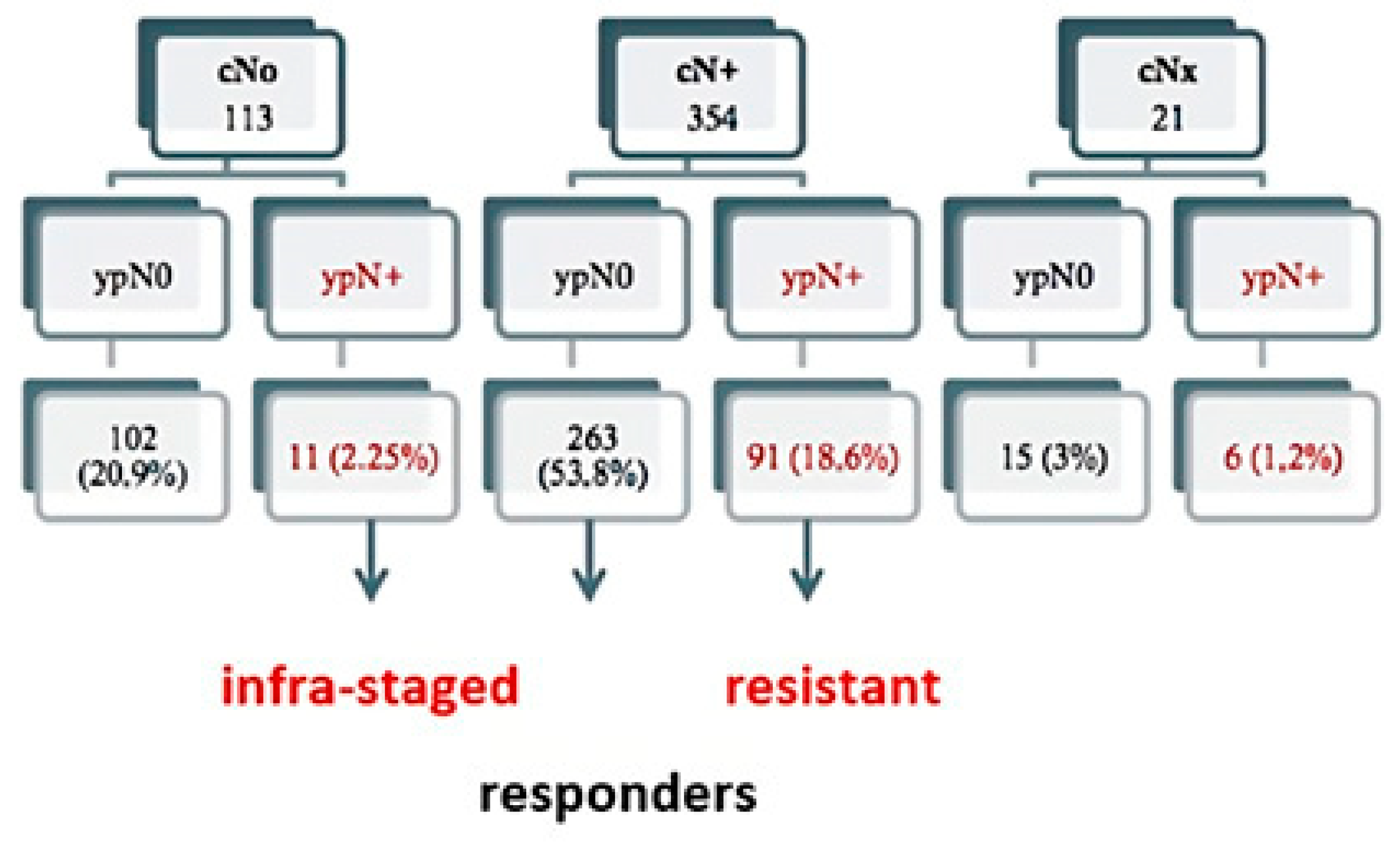
3.1. Demographic Characteristics
3.2. Complete Responders in T Category (ypT0)
3.3. T-Downstaging
3.4. N-Downstaging
3.5. Radial Circumferential Margin
3.6. Perineural and Lymphovascular Invasion
3.7. Patterns of Progression
- Systemic recurrence (metastases only): seen in 90 patients; 58 (15.5%) in the ypN0 group and in 32 patients (30.2%) in the ypN+ group;
- Local recurrence (exclusively local): this was found in 16 patients, 9 (2.4%) in the ypN0 group and in 7 patients (6.6%) in the ypN+ group;
- Mixed relapse (local + systemic): 25 patients were targeted, 15 (4%) in the ypN0 group and in 10 patients (9.4%) in the ypN+ group. Statistically significant differences were observed between the two groups (p = 0.001), with a higher percentage of recurrences of any type in the ypN+ group;
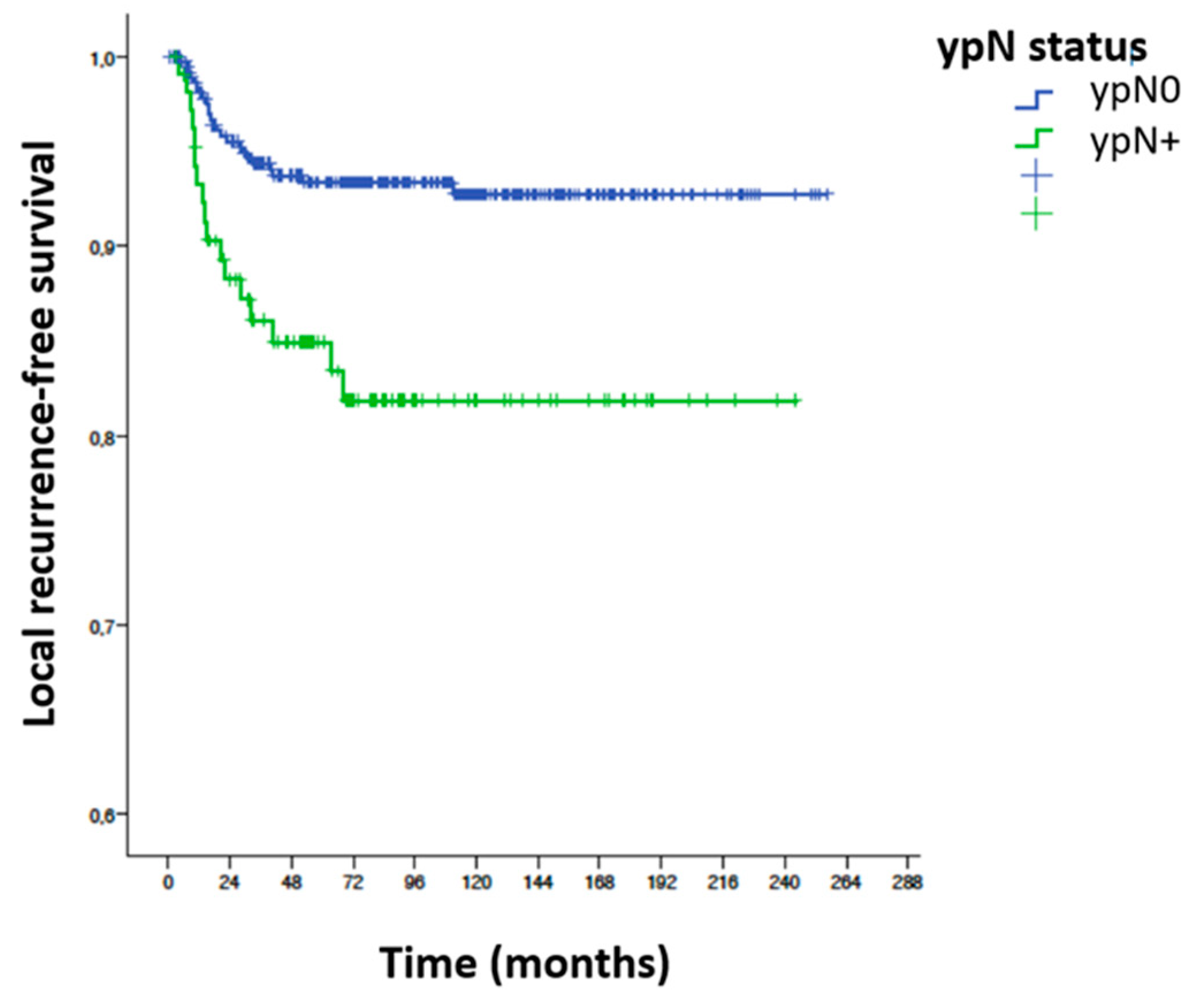
- Local recurrence-free survival (LRFS): a total of 41 local recurrences (either only LR or local associated with metastases) were reported, giving an LRFS of 91.4%. In the ypN0 group there were 24 local recurrences (6.4%) and in the ypN+ group 17 patients (16%) had some form of LR (p = 0.002).
- Anterior recurrence: There were 3 (0.8%) among ypN0 patients and 0 (0%) in ypN+ patients;
- Central recurrence: 11 (2.9%) were diagnosed among ypN0 patients and 3 (2.9%) among ypN+ patients;
- Lateral recurrence: There was 1 (0.3%) among ypN0 patients and 5 (4.8%) in ypN+ patients;
- Presacral recurrence: 9 (2.4%) were detected among ypN0 patients and 8 (7.6%) among ypN0 patients.
3.8. Univariable Analysis
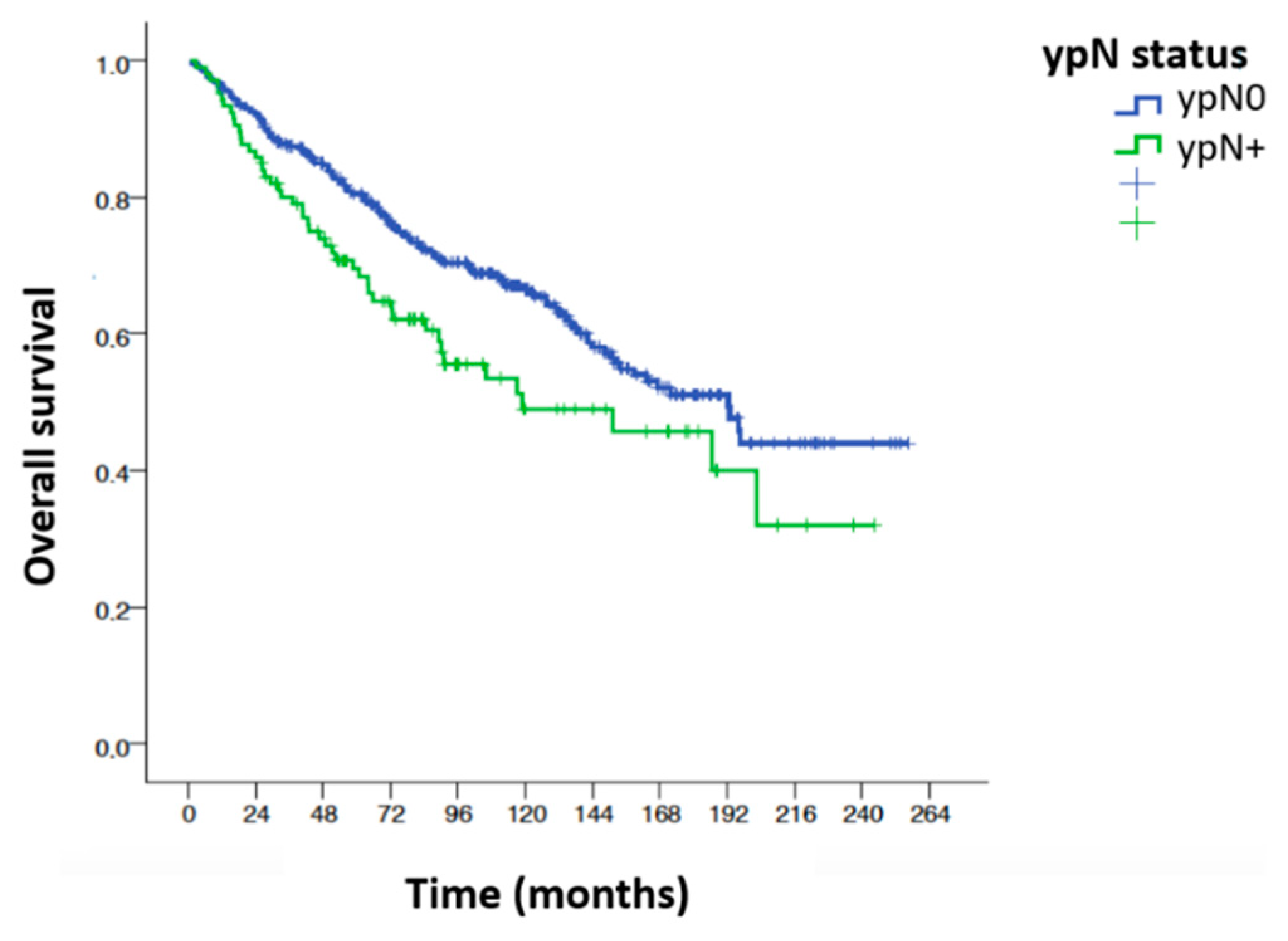
Overall Survival
3.9. Disease-Free Survival (DFS)
3.9.1. Local Recurrence-Free Survival (LRFS)
3.9.2. Univariable Analysis ypN0/ypN+
4. Discussion
4.1. Impact of the Nodal Quotient
4.2. Total Number of Nodes Assessed
4.3. Total Number of Metastatic Nodes
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heald, R.J.; Karanjia, N.D. Results of radical surgery for rectal cancer. World J. Surg. 1992, 16, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Haak, H.E.; Beets, G.L.; Peeters, K.; Nelemans, P.J.; Valentini, V.; Rödel, C.; Kuo, L.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; et al. Prevalence of nodal involvement in rectal cancer after chemoradiotherapy. Br. J. Surg. 2021, 108, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.A.; Serrano, F.J.; Diaz-González, J.A.; Gomez-Espi, M.; Lozano, E.; Garcia, R.; de la Mata, D.; Arranz, J.A.; García-Alfonso, P.; Pérez-Manga, G.; et al. Improved incidence of pT0 downstaged surgical specimens in locally advanced rectal cancer (LARC) treated with induction oxaliplatin plus 5-fluorouracil and preoperative chemoradiation. Ann. Oncol. 2006, 17, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, J.A.; Calvo, F.A.; Cortés, J.; García-Sabrido, J.L.; Gómez-Espí, M.; Del Valle, E.; Muñoz-Jiménez, F.; Alvarez, E. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.A.; Sole, C.V.; Rutten, H.J.; Poortmans, P.; Asencio, J.M.; Serrano, J.; Aristu, J.; Roeder, F.; Dries, W.J. ESTRO/ACROP IORT recommendations for intraoperative radiation therapy in primary locally advanced rectal cancer. Clin. Transl. Radiat. Oncol. 2020, 25, 29–36. [Google Scholar] [CrossRef]
- Sole, C.V.; Calvo, F.A.; Serrano, J.; Del Valle, E.; Rodriguez, M.; Muñoz-Calero, A.; Turégano, F.; García-Sabrido, J.L.; Garcia-Alfonso, P.; Peligros, I.; et al. Post-chemoradiation intraoperative electron-beam radiation therapy boost in resected locally advanced rectal cancer: Long-term results focused on topographic pattern of locoregional relapse. Radiother. Oncol. 2014, 112, 52–58. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Capirci, C.; Valentini, V.; Cionini, L.; De Paoli, A.; Rodel, C.; Glynne-Jones, R.; Coco, C.; Romano, M.; Mantello, G.; Palazzi, S.; et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 99–107. [Google Scholar] [CrossRef]
- Beppu, N.; Matsubara, N.; Noda, M.; Yamano, T.; Kakuno, A.; Doi, H.; Kamikonya, N.; Yamanaka, N.; Yanagi, H.; Tomita, N. Pathologic evaluation of the response of mesorectal positive nodes to preoperative chemoradiotherapy in patients with rectal cancer. Surgery 2015, 157, 743–751. [Google Scholar] [CrossRef]
- Wexner, S.D.; Rotholtz, N.A. Surgeon influenced variables in resectional rectal cancer surgery. Dis. Colon Rectum 2000, 43, 1606–1627. [Google Scholar] [CrossRef]
- Onaitis, M.W.; Noone, R.B.; Hartwig, M.; Hurwitz, H.; Morse, M.; Jowell, P.; McGrath, K.; Lee, C.; Anscher, M.S.; Clary, B.; et al. Neoadjuvant chemoradiation for rectal cancer: Analysis of clinical outcomes from a 13-year institutional experience. Ann. Surg. 2001, 233, 778–785. [Google Scholar] [CrossRef] [PubMed]
- LAFranca, A.; Muttillo, E.M.; Madaffari, I.; Massimi, F.; Longo, G.; Ceccacci, A.; Angelicone, I.; De Giacomo, F.; Sperduti, I.; Balducci, G.; et al. Lymph Node Metastasis in Extraperitoneal Rectal Cancer After Neoadjuvant Therapy: An Unsolved Problem? Anticancer Res. 2023, 43, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Rodel, C.; Martus, P.; Papadoupolos, T.; Fuzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Baik, S.H.; Seong, J.S.; Kim, H.; Roh, J.K.; Lee, K.Y.; Sohn, S.K.; Cho, C.H. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: Impact of postirradiated pathologic downstaging on local recurrence and survival. Ann. Surg. 2006, 244, 1024–1030. [Google Scholar] [CrossRef]
- Kim, T.H.; Chang, H.J.; Kim, D.Y.; Jung, K.H.; Hong, Y.S.; Kim, S.Y.; Park, J.W.; Oh, J.H.; Lim, S.-B.; Choi, H.S.; et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1158–1165. [Google Scholar] [CrossRef]
- Park, I.J.; You, Y.N.; Agarwal, A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Eng, C.; Feig, B.W.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J. Clin. Oncol. 2012, 30, 1770–1776. [Google Scholar] [CrossRef]
- Chan, A.K.; Wong, A.; Jenken, D.; Heine, J.; Buie, D.; Johnson, D. Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 665–677. [Google Scholar] [CrossRef]
- Valentini, V.; Coco, C.; Picciocchi, A.; Morganti, A.G.; Trodella, L.; Ciabattoni, A.; Cellini, F.; Barbaro, B.; Cogliandolo, S.; Nuzzo, G.; et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 664–674. [Google Scholar] [CrossRef]
- Kuo, L.J.; Liu, M.C.; Jian, J.J.; Horng, C.F.; Cheng, T.I.; Chen, C.M.; Fang, W.-T.; Chung, Y.-L. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann. Surg. Oncol. 2007, 14, 2766–2772. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.H.; Yoon, S.M.; Choi, E.K.; Ahn, S.D.; Lee, S.W.; Kim, J.C.; Yu, C.S.; Kim, H.C.; Kim, T.W.; et al. Lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total mesorectal excision followed by chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 796–802. [Google Scholar] [CrossRef]
- Dekker, J.W.; Peeters, K.C.; Putter, H.; Vahrmeijer, A.L.; van de Velde, C.J. Metastatic lymph node ratio in stage III rectal cancer; prognostic significance in addition to the 7th edition of the TNM classification. Eur. J. Surg. Oncol. 2010, 36, 1180–1186. [Google Scholar] [CrossRef]
- Zuo, Z.-G.; Zhang, X.-F.; Wang, H.; Liu, Q.-Z.; Ye, X.-Z.; Xu, C.; Wu, X.-B.; Cai, J.-H.; Zhou, Z.-H.; Li, J.-L.; et al. Prognostic Value of Lymph Node Ratio in Locally Advanced Rectal Cancer Patients After Preoperative Chemoradiotherapy Followed by Total Mesorectal Excision. Medicine 2016, 95, e2988. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, S.H.; Baek, J.H.; Lee, W.K.; Lee, J.N.; Kim, N.R.; Park, Y.H. What does absence of lymph node in resected specimen mean after neoadjuvant chemoradiation for rectal cancer. Radiat. Oncol. 2013, 8, 202. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Proscurshim, I.; Rawet, V.; Pereira, D.D.; Sousa, A.H.; Kiss, D.; Cecconello, I. Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: What does it mean? Dis. Colon Rectum 2008, 51, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kiran, R.P.; Kirat, H.T.; Burgess, A.N.; Nisar, P.J.; Kalady, M.F.; Lavery, I.C. Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann. Surg. Oncol. 2012, 19, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Moretto, R.; Bucci, L.; Pepe, S.; Romano, F.J.; Cella, A.C.; Attademo, L.; Rosanova, M.; De Falco, S.; Fiore, G.; et al. Adjuvant treatment for locally advanced rectal cancer patients after preoperative chemoradiotherapy: When, and for whom? Clin. Color. Cancer 2014, 13, 185–191. [Google Scholar] [CrossRef]
- Chen, P.; Yao, Y.; Gu, J. Rectal cancer patients after neoadjuvant radiotherapy (30Gy/10f) with negative lymph node may not benefit from postoperative adjuvant chemotherapy: A retrospective study. Int. J. Color. Dis. 2015, 30, 1695–1704. [Google Scholar] [CrossRef]
- Baird, D.L.H.; Denost, Q.; Simillis, C.; Pellino, G.; Rasheed, S.; Kontovounisios, C.; Tekkis, P.P.; Rullier, E. The effect of adjuvant chemotherapy on survival and recurrence after curative rectal cancer surgery in patients who are histologically node negative after neoadjuvant chemoradiotherapy. Color. Dis. 2017, 19, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.G.; Barsky, A.R.; Hwang, W.T.; Deville, C.; Wang, X.; Both, S.; Bekelman, J.E.; Christodouleas, J.P.; Vapiwala, N. Comparative toxicity outcomes of proton-beam therapy versus intensity-modulated radiotherapy for prostate cancer in the postoperative setting. Cancer 2019, 125, 4278–4293. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Sato, H.; Ieko, Y.; Miyasaka, Y.; Kanai, T.; Yano, N.; Ono, T.; Akamatsu, H.; Harada, M.; Ichikawa, M.; et al. In silico comparison of the dosimetric impacts of a greater omentum spacer for abdominal and pelvic tumors in carbon-ion, proton and photon radiotherapy. Radiat. Oncol. 2019, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T. Surgical organ displacement: What is the best “materials and methods” for proton radiotherapy? Chin. J. Cancer Res. 2013, 25, 267–268. [Google Scholar] [PubMed]
- Jesseph, J.M.; Fitzek, M.M.; Shahnazi, K.; Ko, S.C.; Howe, J.R.; Thornton, A.F.; Chang, A.L. Surgical organ displacement for proton radiothera-py. Transl. Cancer Res. 2012, 1, 247–254. [Google Scholar]
- Martin-Aragon, T.; Serrano, J.; Benedi, J.; Meirino, R.M.; Garcia-Alonso, P.; Calvo, F.A. The value of oxaliplatin in the systemic treatment of locally advanced rectal cancer. J. Gastrointest Oncol. 2018, 9, 631–640. [Google Scholar] [CrossRef]
- Sole, C.V.; Calvo, F.A.; Ferrer, C.; Alvarez, E.; Carreras, J.L.; Ochoa, E. Human cytomegalovirus and Epstein-Barr virus infection impact on (18)F-FDG PET/CT SUVmax, CT volumetric and KRAS-based parameters of patients with locally advanced rectal cancer treated with neoadjuvant therapy. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.A.; Ayestaran, A.; Serrano, J.; Cambeiro, M.; Palma, J.; Meiriño, R.; Morcillo, M.A.; Lapuente, F.; Chiva, L.; Aguilar, B.; et al. Practice-oriented solutions integrating intraoperative electron irradiation and personalized proton therapy for recurrent or unresectable cancers: Proof of concept and potential for dual FLASH effect. Front. Oncol. 2023, 12, 1116433. [Google Scholar] [CrossRef]

| Variable | Categories | YpN0 n (%) | ypN+ n (%) | Total n (%) | p Value |
|---|---|---|---|---|---|
| Sex | Male Female | 233 (61.3) 147 (0.7) | 64 (59.3) 44 (40.7) | 297 (60.9) 191 (39.1) | 0.699 |
| Age | <65 ≥65 | 161 (42.4) 219 (57.6) | 61 (56.5) 47 (43.5) | 222 (45, 5) 266 (54.5) | 0.009 |
| ASA | 1–2 3–4 | 133 (76.9) 40 (23.1) | 53 (88.3) 7 (11.7) | 186 (79.8) 47 (20.2) | 0.057 |
| Previous Surgery | No Yes | 276 (73.4) 100 (26.6) | 80 (74.8) 27 (25.2) | 356 (73.7) 127 (26.3) | 0.778 |
| ypN0 | ypN+ | Total | |||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | p | ||
| Segment | Lower Medium Upper | 131 (34.5) 204 (53.7) 45 (11.8) | 44 (40.7) 53 (49.1) 11 (10.2) | 175 (35.9) 257 (52.7) 56 (11.5) | 0.482 |
| Anal margin distance | 6.93 (3.16) | 6.62 (3.01) | 6.86 (3.14) | 0.365 | |
| Degree of differentiation | G1 G2 G3 | 108 (30.3) 234 (65.5) 15 (4.2) | 26 (25.2) 68 (66) 9 (8.7) | 134 (29.1) 302 (65.7) 24 (5.2) | 0.146 |
| cN | cN0 cN+ cNx | 102 (26.8) 263 (69.2) 15 (3.9) | 11 (10.2) 91 (84.3) 6 (5.6) | 113 (23.2) 354 (72.5) 21 (4.3) | 0.001 |
| cT | cT2 cT3 cT4 cTx | 26 (6.8) 288 (75.8) 59 (15.5) 7 (1.8) | 3 (2.8) 76 (70.4) 25 (23.1) 4 (3.7) | 29 (5.9) 364 (74.6) 84 (17.2) 11 (2.3) | 0.075 |
| Clinical Stage | II III | 102 (27.9) 263 (72.1) | 11 (10.8) 91 (89.2) | 113 (24.2) 354 (75.8) | <0.001 |
| Pre-operative RT dose | <5040 ≥5040 | 47 (12.4) 331 (87.6) | 19 (17.6) 89 (82.4) | 66 (13.6) 420 (86.4) | 0.168 |
| Full chemotherapy | No Yes | 70 (20.3) 275 (79.7) | 21 (22.3) 73 (77.7) | 91 (20.7) 348 (79.3) | 0.664 |
| Toxicity RT | No Yes | 67 (18.2) 302 (81.8) | 21 (19.8) 85 (80.2) | 88 (18.5) 387 (81.5) | 0.699 |
| Time to surgery | <8 ≥8 | 305 (80.5) 74 (19.5) | 86 (79.6) 22 (20.4) | 391 (80.3) 96 (19.7) | 0.846 |
| ypN0 | ypN+ | Total | |||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | p | ||
| ypT | ypT1/T2 ypT3/T4 | 206 (62.2) 115 (35.8) | 26 (24.1) 82 (75.9) | 232 (54.1) 197 (45.9) | <0.001 |
| No. of identified nodes | <12 ≥12 | 270 (78.0) 76 (22.0) | 64 (61.5) 40 (38.5) | 334 (74.2) 116 (25.8) | <0.001 |
| N-downstaging | No Yes Other | 102 (28.0) 262 (72) 0 (0) | 91 (89.2) 0 (0) 11 (10.8) | 193(41.2) 262 (56.2) 11(2.4) | <0.001 |
| T-downstaging | No Descent 1 T Decrease 2 T Downturn 3 T Worsening | 95 (25.5) 150 (40.3) 54 (14.5) 63 (16.9) 10 (2.7) | 62 (59.6) 33 (31.7) 5 (4.8) 0 (0) 4 (3.8) | 157 (33.0) 183 (38.4) 59 (12.4) 63 (13.2) 14 (2.9) | <0.001 |
| Mesorectum Quality | Incomplete + almost full Full | 50 (30.1) 116 (69.9) | 31 (47.7) 34 (52.3) | 81 (35.1) 150 (64.9) | 0.012 |
| Type of resection | R1 R0 | 3 (0.8) 377 (99.2) | 5 (4.6) 103 (95.4) | 8 (1.6) 480 (98.4) | 0.015 |
| Tumor regression grade (TRG) | 3–4 (few tumor cells and fibrosis/no tumor cells, only fibrotic tissue) 1–2 (tumor dominant tissue and evidence of fibrosis) | 218 (57.8) 159 (42.2) | 17 (16) 89 (84) | 235 (48.7) 248 (51.3) | <0.001 |
| Circumferential resection margin (CRM) | Free Affect | 376 (99.2) 3 (0.8) | 102 (95.3) 5 (4.7) | 478 (98.4) 8 (1.6) | 0.015 |
| Perineural invasion | No Yes | 238 (91.9) 21 (8.1) | 40 (51.3) 38 (48.7) | 278 (82.5) 59 (17.5) | <0.001 |
| Lymphovascular invasion | No Yes | 244 (92.4) 20 (7.6) | 35 (45.5) 42 (54.5) | 279 (81.8) 62 (18.2) | <0.001 |
| Adjuvant chemotherapy | No Yes | 102 (26.8) 278 (73.2) | 16 (14.8) 92 (85.2) | 118 (24.2) 370 (75.8) | 0.010 |
| Type adjuvant chemotherapy | Mayo Folfox Xeloda Folfiri Xelox Other | 99 (26.3) 141 (37.4) 17 (4.5) 1 (0.3) 15 (4.0) 8 (2.1) | 25 (23.4) 49 (45.8) 5 (4.7) 0 (0.0) 9 (8.4) 4 (3.7) | 124 (25.6) 190 (39.3) 22 (4.5) 1 (0.2) 24 (2.9) 12 (2.5) | <0.001 |
| Exitus | IC | |||||
|---|---|---|---|---|---|---|
| Variable | n | HR | Inf | Sup | p | |
| Pre-surgical | ||||||
| Age | <65 | 220 | 0.48 | 0.36 | 0.66 | <0.001 |
| ≥65 | 260 | 1 | ||||
| ASA | 1–2 | 186 | 1 | |||
| 3–4 | 45 | 2.11 | 1.15 | 3.88 | 0.016 | |
| Height Tumor | Lower | 174 | 1.49 | 1.10 | 2.04 | 0.010 |
| Medium | 251 | 1 | ||||
| Upper | 55 | 1.12 | 0.68 | 1.82 | 0.646 | |
| Toxicity RT neoadjuvant | No | 87 | 1 | |||
| Yes | 380 | 0.92 | 0.63 | 1.33 | 0.648 | |
| Surgical | ||||||
| Surgical Technique | Sphincter-preserving | 329 | 1 | |||
| AAP | 145 | 1.75 | 1.30 | 2.35 | <0.001 | |
| Laparoscopy | No | 352 | 1 | |||
| Yes | 135 | 0.38 | 0.23 | 0.644 | <0.001 | |
| Total mesorectal excision (TME) | No | 55 | 1.05 | 0.66 | 1.68 | 0.80 |
| Yes | 425 | 1 | ||||
| Resection R0 vs. R1 | No | 9 | 5.13 | 2.39 | 11.00 | <0.001 |
| Yes | 471 | 1 | ||||
| Complication | No | 286 | 1 | |||
| Yes | 169 | 1.79 | 1.311 | 2.442 | <0.001 | |
| Post-surgical | ||||||
| Quality Mesorectum | Incomplete + almost complete | 79 | 1.81 | 1.08 | 3.01 | 0.023 |
| Complete | 148 | 1 | ||||
| Mesorectal circumferential margin | Free | 470 | 1 | |||
| Involved | 8 | 7.12 | 3.30 | 15.38 | <0.001 | |
| Tumor regression grade | 3–4 | 232 | 1 | |||
| 1–2 | 243 | 1.55 | 1.15 | 2.09 | 0.004 | |
| ypT | ypT1/T2 | 227 | 1 | |||
| ypT3/T4 | 194 | 1.52 | 1.12 | 2.07 | 0.007 | |
| ypN | ypN0 | 373 | 1 | |||
| ypN+ | 106 | 1.51 | 1.08 | 2.10 | 0.016 | |
| No. of isolated lymph nodes | <12 | 328 | 0.79 | 0.519 | 1.22 | 0.295 |
| ≥12 | 113 | 1 | ||||
| No. nodes + | 0–2 | 438 | 1 | |||
| ≥3 | 40 | 2.48 | 1.61 | 3.82 | <0.001 | |
| Node ratio + 28% | <0.28 | 382 | 1 | |||
| ≥0.28 | 53 | 2.11 | 1.42 | 3.14 | <0.001 | |
| Node quotient + 50% | <50% | 406 | 1 | |||
| ≥50% | 29 | 1.91 | 1.16 | 3.17 | 0.012 | |
| Perineural Invasion | No | 275 | 1 | |||
| Yes | 58 | 2.57 | 1.69 | 3.89 | <0.001 | |
| Lymphovascular Invasion | No | 276 | 1 | |||
| Yes | 61 | 1.88 | 1.22 | 2.91 | 0.004 | |
| Adjuvant chemotherapy | No | 110 | 2.08 | 1.54 | 2.82 | <0.001 |
| Yes | 370 | 1 | ||||
| R. Global | IC | |||||
|---|---|---|---|---|---|---|
| Variable | n | HR | Inf | Sup | p | |
| Pre-surgical | ||||||
| Age | <65 | 220 | 1.15 | 0.82 | 1.62 | 0.412 |
| ≥65 | 260 | 1 | ||||
| ASA | 1–2 | 186 | 1 | |||
| 3–4 | 45 | 1.19 | 0.63 | 2.24 | 0.600 | |
| Height Tumor | Lower | 174 | 1.19 | 0.82 | 1.71 | 0.353 |
| Medium | 251 | 1 | ||||
| Upper | 55 | 1.16 | 0.67 | 2.01 | 0.582 | |
| Toxicity RT neoadjuvant | No | 87 | 1 | |||
| Yes | 380 | 0.77 | 0.51 | 1.17 | 0.221 | |
| Surgical | ||||||
| Surgical Technique | Sphincter-preserving | 329 | 1 | |||
| AAP | 149 | 1.48 | 1.04 | 2.10 | 0.031 | |
| Laparoscopy | No | 345 | 1 | |||
| Yes | 135 | 0.77 | 0.51 | 1.16 | 0.216 | |
| Total mesorectal excision (TME) | No | 55 | 0.99 | 0.547 | 1.55 | 0.762 |
| Yes | 425 | 1 | ||||
| Resection R0 vs. R1 | No | 9 | 4.35 | 1.91 | 9.89 | <0.001 |
| Yes | 471 | 1 | ||||
| Complication | No | 286 | 1 | |||
| Yes | 169 | 1.61 | 1.13 | 2.29 | 0.009 | |
| Post-surgical | ||||||
| Quality Mesorectum | Incomplete + almost complete | 79 | 1.60 | 0.93 | 2.73 | 0.088 |
| Complete | 148 | 1 | ||||
| Mesorectal circumferential margin | Free | 470 | 1 | |||
| Involved | 8 | 5.72 | 2.51 | 13.05 | <0.001 | |
| Tumor regression grade | 3–4 | 232 | 1 | |||
| 1–2 | 243 | 2.66 | 1.83 | 3.89 | <0.001 | |
| ypT | ypT1/T2 | 227 | 1 | |||
| ypT3/T4 | 194 | 2.29 | 1.59 | 3.31 | <0.001 | |
| ypN | ypN0 | 373 | 1 | |||
| ypN+ | 106 | 2.46 | 1.73 | 3.51 | <0.001 | |
| No. of isolated lymph nodes | <12 | 328 | 1.09 | 0.72 | 1.64 | 0.679 |
| ≥12 | 113 | 1 | ||||
| No. nodes + | 0–2 | 438 | 1 | |||
| ≥3 | 40 | 3.27 | 2.08 | 5.14 | <0.001 | |
| Node ratio + 16% | <0.16 | 362 | 1 | |||
| ≥16 | 73 | 3.061 | 2.07 | 4.51 | <0.001 | |
| Node quotient + 50% | <50% | 406 | 1 | |||
| ≥50% | 29 | 2.35 | 1.37 | 4.05 | 0.002 | |
| Perineural Invasion | No | 275 | 1 | |||
| Yes | 58 | 2.95 | 1.91 | 4.54 | <0.001 | |
| Lymphovascular Invasion | No | 276 | 1 | |||
| Yes | 61 | 2.73 | 1.78 | 4.19 | <0.001 | |
| Adjuvant chemotherapy | No | 110 | 1.26 | 0.85 | 1.87 | 0.252 |
| Yes | 370 | 1 | ||||
| R. Local | IC | |||||
|---|---|---|---|---|---|---|
| Variable | n | HR | Inf | Sup | p | |
| Pre-surgical | ||||||
| Age | <65 | 220 | 1.03 | 0.56 | 1.88 | 0.931 |
| ≥65 | 260 | 1 | ||||
| ASA | 1–2 | 186 | 1 | |||
| 3–4 | 45 | 2.76 | 1.00 | 7.61 | 0.049 | |
| Height Tumor | Lower | 174 | 1.23 | 0.65 | 2.35 | 0.511 |
| Medium | 251 | 1 | ||||
| Upper | 55 | 0.89 | 0.30 | 2.61 | 0.841 | |
| Toxicity RT neoadjuvant | No | 87 | 1 | |||
| Yes | 380 | 0.41 | 0.21 | 0.78 | 0.007 | |
| Surgical | ||||||
| Surgical Technique | Sphincter preserving | 329 | 1 | |||
| AAP | 149 | 2.39 | 1.28 | 4.44 | 0.006 | |
| Laparoscopy | No | 345 | 1 | |||
| Yes | 135 | 0.51 | 0.22 | 1.16 | 0.112 | |
| Total mesorectal excision | No | 55 | 1.22 | 0.43 | 3.42 | 0.704 |
| Yes | 425 | 1 | ||||
| Resection R0 vs. R1 | No | 9 | 9.72 | 3.45 | 27.41 | <0.001 |
| Yes | 471 | 1 | ||||
| Complication | No | 286 | 1 | |||
| Yes | 169 | 2.15 | 1.16 | 3.40 | 0.014 | |
| Post-surgical | ||||||
| Quality Mesorectum | Incomplete + almost complete | 79 | 0.87 | 0.30 | 2.51 | 0.801 |
| Complete | 148 | 1 | ||||
| Mesorectal circumferential margin | Free | 470 | 1 | |||
| Affect | 8 | 7.69 | 2.35 | 25.12 | 0.001 | |
| TRG | 3–4 | 232 | 1 | |||
| 1–2 | 243 | 3.16 | 1.55 | 6.45 | 0.002 | |
| ypT | ypT1/T2 | 227 | 1 | |||
| ypT3/T4 | 194 | 1.58 | 0.83 | 2.99 | 0.162 | |
| ypN | ypN0 | 373 | 1 | |||
| ypN+ | 106 | 2.71 | 1.46 | 5.06 | 0.002 | |
| No. of isolated lymph nodes | <12 | 328 | 1.03 | 0.48 | 2.21 | 0.931 |
| ≥12 | 113 | 1 | ||||
| No. nodes + | 0–2 | 438 | 1 | |||
| ≥3 | 40 | 2.66 | 1.18 | 6.02 | 0.018 | |
| Node ratio + 25% | <0.25 | 376 | 1 | |||
| ≥0.25 | 59 | 4.13 | 2.09 | 8.17 | <0.001 | |
| Node quotient + 50% | <50% | 406 | 1 | |||
| ≥50% | 29 | 3.00 | 1.25 | 7.21 | 0.014 | |
| Perineural Invasion | No | 275 | 1 | |||
| Yes | 58 | 2.47 | 1.21 | 5.02 | 0.013 | |
| Lymphovascular Invasion | No | 276 | 1 | |||
| Yes | 61 | 2.36 | 1.16 | 4.83 | 0.018 | |
| Adjuvant chemotherapy | No | 110 | 1.88 | 0.99 | 3.57 | 0.054 |
| Yes | 370 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, F.A.; Tudela, M.; Serrano, J.; Muñoz-Fernández, M.; Peligros, M.I.; Garcia-Alfonso, P.; del Valle, E. Post-Chemoradiation Metastatic, Persistent and Resistant Nodes in Locally Advanced Rectal Cancer: Metrics and Their Impact on Long-Term Outcome. Cancers 2023, 15, 4591. https://doi.org/10.3390/cancers15184591
Calvo FA, Tudela M, Serrano J, Muñoz-Fernández M, Peligros MI, Garcia-Alfonso P, del Valle E. Post-Chemoradiation Metastatic, Persistent and Resistant Nodes in Locally Advanced Rectal Cancer: Metrics and Their Impact on Long-Term Outcome. Cancers. 2023; 15(18):4591. https://doi.org/10.3390/cancers15184591
Chicago/Turabian StyleCalvo, Felipe A., María Tudela, Javier Serrano, Mercedes Muñoz-Fernández, María Isabel Peligros, Pilar Garcia-Alfonso, and Emilio del Valle. 2023. "Post-Chemoradiation Metastatic, Persistent and Resistant Nodes in Locally Advanced Rectal Cancer: Metrics and Their Impact on Long-Term Outcome" Cancers 15, no. 18: 4591. https://doi.org/10.3390/cancers15184591
APA StyleCalvo, F. A., Tudela, M., Serrano, J., Muñoz-Fernández, M., Peligros, M. I., Garcia-Alfonso, P., & del Valle, E. (2023). Post-Chemoradiation Metastatic, Persistent and Resistant Nodes in Locally Advanced Rectal Cancer: Metrics and Their Impact on Long-Term Outcome. Cancers, 15(18), 4591. https://doi.org/10.3390/cancers15184591






