Treatment of Colorectal Cancer in Certified Centers: Results of a Large German Registry Study Focusing on Long-Term Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim
2.2. Data Source
2.3. Inclusion and Exclusion Criteria
- (a)
- Diagnosis of colorectal cancer according to the ICD-10-GM codes C18 (malignant neoplasm of the colon) or C20 (malignant neoplasm of the rectum).
- (b)
- Age of at least 18 years at the time of diagnosis.
- (c)
- No previous diagnoses of colorectal cancer (a patient was only considered as incident between 2009 and 2017 if there were no earlier diagnoses of colorectal cancer recorded; to avoid issues with missing information concerning earlier tumor diagnoses, patients with a cancer diagnosis between 2006 and 2008 were excluded a priori following the guideline “good practice of secondary data analysis” [23] since it would not have been possible to assess a case’s compliance to this inclusion criterion). Previous diagnoses of other, non-colorectal cancer were no exclusion criterion.
- (d)
- Sufficient information concerning the certification status of the treating hospital (in this context, treatment at a non-certified institution that belongs to an association containing a GCS-certified colorectal cancer center was also considered a certified center treatment).
- (e)
- Consistent histological subtype (only adenocarcinoma, exclusion of, e.g., lymphoma or sarcoma).
2.4. Statistical Analysis
2.5. Data Protection and Ethics
3. Results
3.1. Inclusion Process
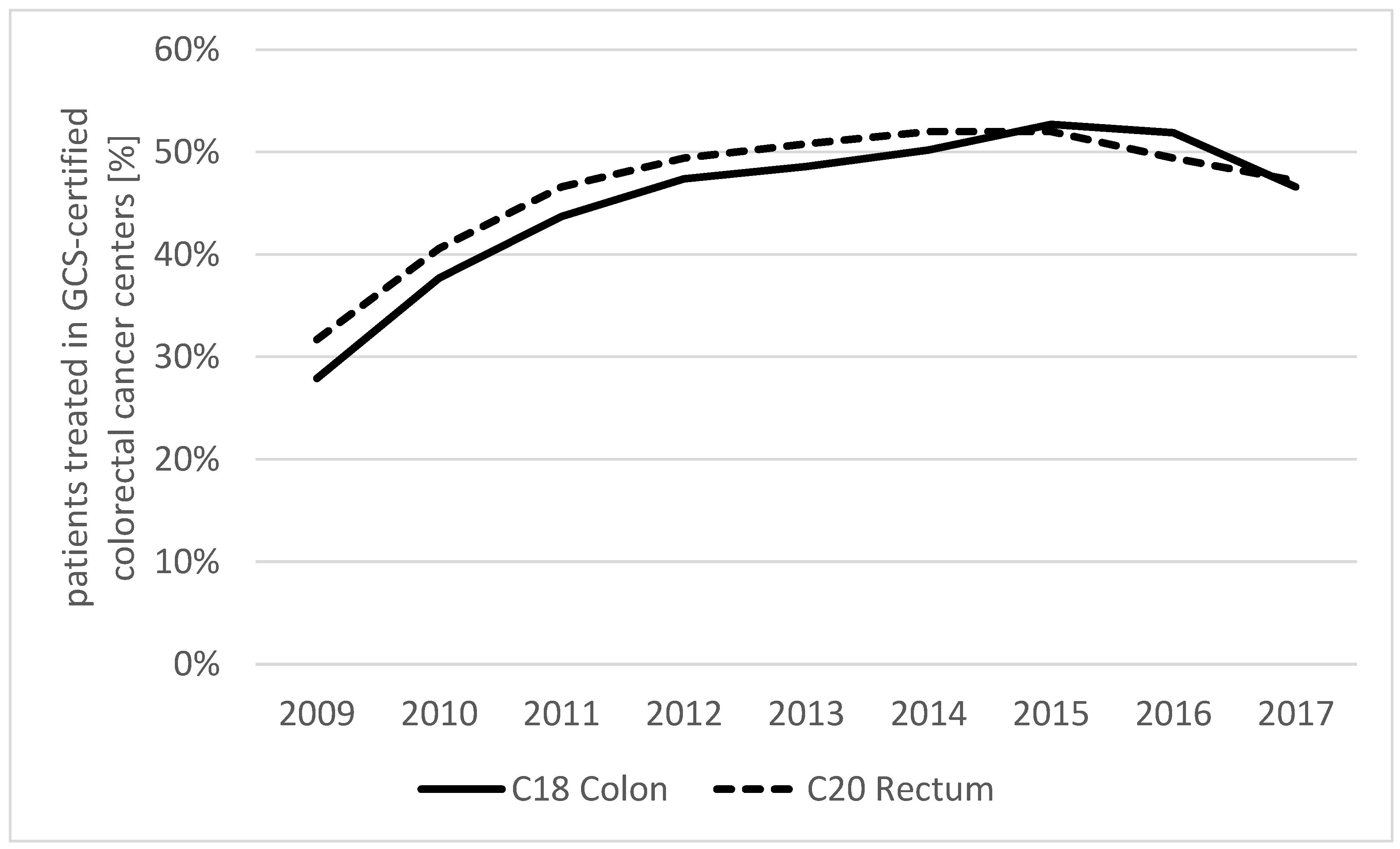
3.2. Share of Patients Treated in GCS-Certified Colorectal Cancer Centers
3.3. Description of Collectives
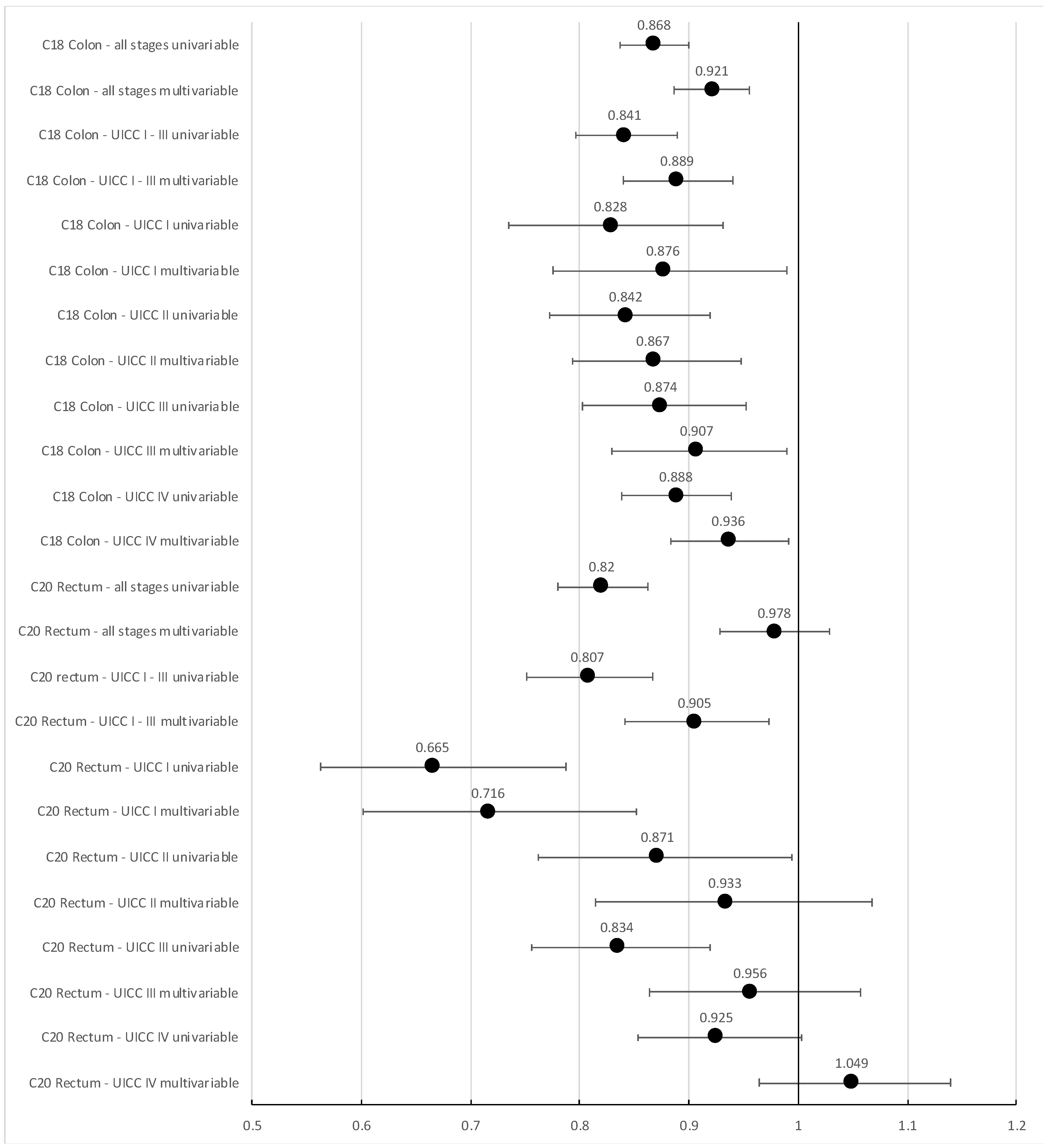
3.4. Survival Analyses
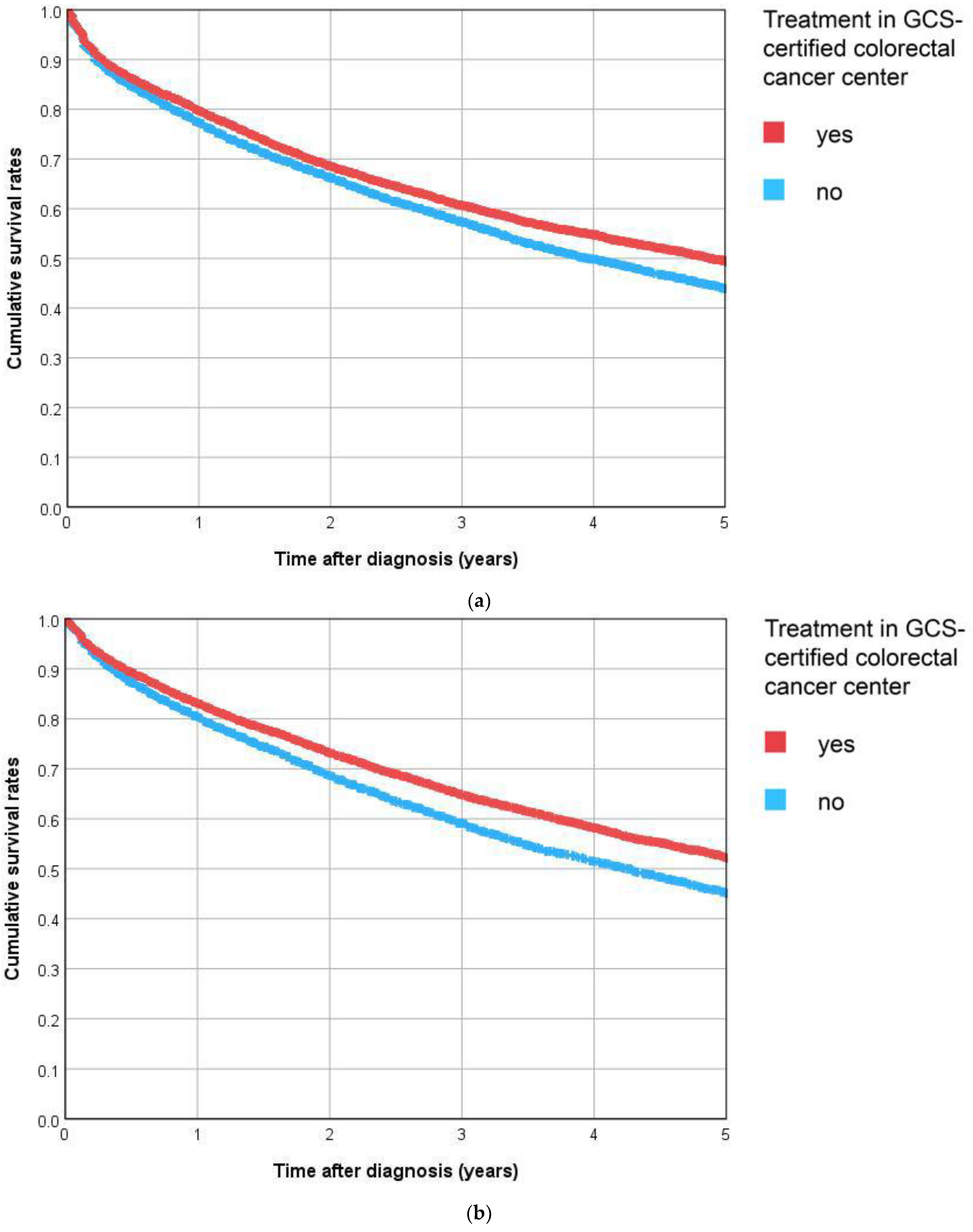
3.4.1. Overall Survival
3.4.2. Recurrence-Free Survival
4. Discussion
4.1. Summary
4.2. Effect of Certification
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Cancer Report: Cancer Research for Cancer Prevention; World Cancer Reports; Wild, C.P., Weiderpass, E., Stewart, B.W., Eds.; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Krebs in Deutschland für 2017/2018, 13th ed.; Robert Koch-Institut; Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. (Eds.) Zentrum für Krebsregisterdaten: Berlin, Germany, 2021; ISBN 978-3-89606-309-0. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Kolorektales Karzinom, Langversion 2.1, 2019, AWMF Registrierungsnummer: 021/007OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/ (accessed on 21 June 2023).
- Griesshammer, E.; Adam, H.; Sibert, N.T.; Wesselmann, S. Implementing quality metrics in European Cancer Centers (ECCs). World J. Urol. 2021, 39, 49–56. [Google Scholar] [CrossRef]
- Wind, A.; Rajan, A.; van Harten, W.H. Quality assessments for cancer centers in the European Union. BMC Health Serv. Res. 2016, 16, 474. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.gov/research/infrastructure/cancer-centers (accessed on 15 January 2023).
- Kato, M. Designated cancer hospitals and cancer control in Japan. J. Natl. Inst. Public Health 2012, 61, 549–555. [Google Scholar]
- Bundesministerium für Gesundheit. Nationaler Krebsplan. Available online: https://www.bundesgesundheitsministerium.de/themen/praevention/nationaler-krebsplan.html (accessed on 15 January 2023).
- Brucker, S.Y.; Schumacher, C.; Sohn, C.; Rezai, M.; Bamberg, M.; Wallwiener, D. Benchmarking the quality of breast cancer care in a nationwide voluntary system: The first five-year results (2003–2007) from Germany as a proof of concept. BMC Cancer 2008, 8, 358. [Google Scholar] [CrossRef]
- Kowalski, C.; Graeven, U.; von Kalle, C.; Lang, H.; Beckmann, M.W.; Blohmer, J.-U.; Burchardt, M.; Ehrenfeld, M.; Fichtner, J.; Grabbe, S.; et al. Shifting cancer care towards Multidisciplinarity: The cancer center certification program of the German cancer society. BMC Cancer 2017, 17, 850. [Google Scholar] [CrossRef]
- GCS-Certification, Center Search. Available online: https://www.krebsgesellschaft.de/deutsche-krebsgesellschaft/zertifizierung/zentrumssuche.html (accessed on 15 January 2023).
- oncoMAP. Available online: https://www.oncomap.de/centers?selectedOrgans=[Darm]&showMap=1 (accessed on 5 July 2023).
- GCS-Certification Documents. Available online: https://www.krebsgesellschaft.de/zertdokumente.html (accessed on 5 July 2023). (In German).
- GCS-Certification, Annual Reports. Available online: https://www.krebsgesellschaft.de/jahresberichte.html (accessed on 8 September 2023).
- Völkel, V.; Draeger, T.; Gerken, M.; Fürst, A.; Klinkhammer-Schalke, M. Long-Term Survival of Patients with Colon and Rectum Carcinomas: Is There a Difference between Cancer Centers and Non-Certified Hospitals? Gesundheitswesen 2019, 81, 801–807. [Google Scholar] [CrossRef]
- Birkmeyer, N.J.O.; Goodney, P.P.; Stukel, T.A.; Hillner, B.E.; Birkmeyer, J.D. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer 2005, 103, 435–441. [Google Scholar] [CrossRef]
- Mehta, R.; Ejaz, A.; Hyer, J.M.; Tsilimigras, D.I.; White, S.; Merath, K.; Sahara, K.; Bagante, F.; Paredes, A.Z.; Cloyd, J.M.; et al. The Impact of Dedicated Cancer Centers on Outcomes among Medicare Beneficiaries Undergoing Liver and Pancreatic Cancer Surgery. Ann. Surg. Oncol. 2019, 26, 4083–4090. [Google Scholar] [CrossRef]
- Butea-Bocu, M.C.; Müller, G.; Pucheril, D.; Kröger, E.; Otto, U. Is there a clinical benefit from prostate cancer center certification? An evaluation of functional and oncologic outcomes from 22,649 radical prostatectomy patients. World J. Urol. 2021, 39, 5–10. [Google Scholar] [CrossRef]
- Richter, M.; Sonnow, L.; Mehdizadeh-Shrifi, A.; Richter, A.; Koch, R.; Zipprich, A. German oncology certification system for colorectal cancer—Relative survival rates of a single certified centre vs. national and international registry data. Innov. Surg. Sci. 2021, 6, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kranz, J.; Grundmann, R.; Steffens, J. Does structural and process quality of certified prostate cancer centers result in better medical care? Urol. A 2021, 60, 59–66. [Google Scholar] [CrossRef]
- Available online: https://innovationsfonds.g-ba.de/downloads/beschluss-dokumente/268/2022-10-17_WiZen_Ergebnisbericht.pdf (accessed on 28 July 2023).
- Arbeitsgruppe Erhebung und Nutzung von Sekundärdaten der Deutschen Gesellschaft fur Sozialmedizin und Prävention. Arbeitsgruppe Epidemiologische Methoden der Deutschen Gesellschaft für Epidemiologie. Deutsche Gesellschaft für Medizinische Informatik Biometrie und Epidemiologie, Deutsche Gesellschaft für Sozialmedizin und Prävention. Good practice of secondary data analysis, first revision. Gesundheitswesen 2008, 70, 54–60. [Google Scholar]
- Fünfte Stellungnahme der Regierungskommission für eine Moderne und bedarfsgerechte Krankenhausversorgung: Verbesserung von Qualität und Sicherheit der Gesundheitsversorgung Potenzialanalyse Anhand exemplarischer Erkrankungen. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/K/Krankenhausreform/5_Stellungnahme_Potenzialanalyse_bf_Version_1.1.pdf (accessed on 11 August 2023).
- Cheng, C.; Datzmann, T.; Hernandez, D.; Schmitt, J.; Schlander, M. Do certified cancer centers provide more cost-effective care? A health economic analysis of colon cancer care in Germany using administrative data. Int. J. Cancer 2021, 149, 1744–1754. [Google Scholar] [CrossRef]
- Trautmann, F.; Reißfelder, C.; Pecqueux, M.; Weitz, J.; Schmitt, J. Evidence-based quality standards improve prognosis in colon cancer care. Eur. J. Surg. Oncol. 2018, 44, 1324–1330. [Google Scholar] [CrossRef]
- Paulson, E.C.; Mitra, N.; Sonnad, S.; Armstrong, K.; Wirtalla, C.; Kelz, R.R.; Mahmoud, N.N. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann. Surg. 2008, 248, 675–686. [Google Scholar] [CrossRef]
- Okawa, S.; Tabuchi, T.; Nakata, K.; Morishima, T.; Koyama, S.; Odani, S.; Miyashiro, I. Three-year survival from diagnosis in surgically treated patients in designated and nondesignated cancer care hospitals in Japan. Cancer Sci. 2021, 112, 2513–2521. [Google Scholar] [CrossRef]
- Ghandourh, W.A. Palliative care in cancer: Managing patients’ expectations. J. Med. Radiat. Sci. 2016, 63, 242–257. [Google Scholar] [CrossRef]
- Bausewein, C.; Simon, S.T.; Pralong, A.; Radbruch, L.; Nauck, F.; Voltz, R. Palliative Care of Adult Patients with Cancer. Dtsch. Arztebl. Int. 2015, 112, 863–870. [Google Scholar] [CrossRef]
- Klinkhammer-Schalke, M.; Kaiser, T.; Apfelbacher, C.; Benz, S.; Dreinhöfer, K.E.; Geraedts, M.; Hauptmann, M.; Hoffmann, F.; Hoffmann, W.; Koller, M.; et al. Manual for Methods and Use of Routine Practice Data for Knowledge Generation. Gesundheitswesen 2020, 82, 716–722. [Google Scholar] [CrossRef]
- Stausberg, J.; Maier, B.; Bestehorn, K.; Gothe, H.; Groene, O.; Jacke, C.; Jänicke, M.; Kostuj, T.; Mathes, T.; Niemeyer, A.; et al. Memorandum Registry for Health Services Research: Update 2019. Gesundheitswesen 2020, 82, e39–e66. [Google Scholar] [CrossRef]
- Hoffmann, F.; Kaiser, T.; Apfelbacher, C.; Benz, S.; Bierbaum, T.; Dreinhöfer, K.; Hauptmann, M.; Heidecke, C.-D.; Koller, M.; Kostuj, T.; et al. Routine Practice Data for Evaluating Intervention Effects: Part 2 of the Manual. Gesundheitswesen 2021, 83, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Lipitz Snyderman, A.N.; Fortier, E.; Li, D.G.; Chimonas, S. What do patients want to know when selecting a hospital for cancer care? JCO 2018, 36, e18810. [Google Scholar] [CrossRef]
- Habibzadeh, F. Disparity in the selection of patients in clinical trials. Lancet 2022, 399, 1048. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Martin, T.; Curtis, S.; Faries, D.; Robinson, S.; Johnston, J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015, 16, 495. [Google Scholar] [CrossRef]
- Diers, J.; Wagner, J.; Baum, P.; Lichthardt, S.; Kastner, C.; Matthes, N.; Löb, S.; Matthes, H.; Germer, C.-T.; Wiegering, A. Nationwide in-hospital mortality following colonic cancer resection according to hospital volume in Germany. BJS Open 2019, 3, 672–677. [Google Scholar] [CrossRef]
- Golder, A.M.; McMillan, D.C.; Horgan, P.G.; Roxburgh, C.S.D. Determinants of emergency presentation in patients with colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 4366. [Google Scholar] [CrossRef]

| C18 Colon | C20 Rectum | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment in GCS-Certified Centers | Yes | No | Yes | No | ||||
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Female | 6043 | 43.9 | 7515 | 44.9 | 2650 | 33.5 | 3353 | 37.1 |
| Male | 7717 | 56.1 | 9222 | 55.1 | 5254 | 66.5 | 5686 | 62.9 |
| Age | ||||||||
| 18–49 | 915 | 6.6 | 765 | 4.6 | 574 | 7.3 | 481 | 5.3 |
| 50–59 | 1801 | 13.1 | 1918 | 11.5 | 1553 | 19.6 | 1611 | 17.8 |
| 60–69 | 3086 | 22.4 | 3569 | 21.3 | 2136 | 27.0 | 2333 | 25.8 |
| 70–79 | 4860 | 35.3 | 6353 | 38.0 | 2480 | 31.4 | 3047 | 33.7 |
| 80+ | 3098 | 22.5 | 4132 | 24.7 | 1161 | 14.7 | 1567 | 17.3 |
| Year of diagnosis | ||||||||
| 2009–2011 | 3725 | 27.1 | 6504 | 38.9 | 2246 | 28.4 | 3409 | 37.7 |
| 2012–2014 | 4883 | 35.5 | 5136 | 30.7 | 2878 | 36.4 | 2793 | 30.9 |
| 2015–2017 | 5152 | 37.4 | 5097 | 30.5 | 2780 | 35.2 | 2837 | 31.4 |
| Total | 13,760 | 100.0 | 16,737 | 100.0 | 7904 | 100.0 | 9039 | 100.0 |
| C18 Colon | C20 Rectum | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment in GCS-Certified Centers | Yes | No | Yes | No | ||||
| n | % | n | % | n | % | n | % | |
| UICC stage | ||||||||
| I | 2622 | 19.1 | 2915 | 17.4 | 1348 | 17.1 | 1511 | 16.7 |
| II | 3694 | 26.8 | 4190 | 25.0 | 1348 | 17.1 | 1577 | 17.4 |
| III | 2892 | 21.0 | 3352 | 20.0 | 2913 | 36.9 | 2770 | 30.6 |
| IV | 3634 | 26.4 | 3344 | 20.0 | 1747 | 22.1 | 1718 | 19.0 |
| X | 918 | 6.7 | 2936 | 17.5 | 548 | 6.9 | 1463 | 16.2 |
| Grade | ||||||||
| G1 | 742 | 5.4 | 1232 | 7.4 | 400 | 5.1 | 683 | 7.6 |
| G2 | 8913 | 64.8 | 10,286 | 61.5 | 5431 | 68.7 | 5704 | 63.1 |
| G3/4 | 3059 | 22.2 | 3932 | 23.5 | 1127 | 14.3 | 1650 | 18.3 |
| GX | 1046 | 7.6 | 1287 | 7.7 | 946 | 12.0 | 1002 | 11.1 |
| Lymphatic invasion | ||||||||
| L0 | 6783 | 49.3 | 7726 | 46.2 | 4297 | 54.4 | 3999 | 44.2 |
| L1 | 4711 | 34.2 | 5905 | 35.3 | 1705 | 21.6 | 2304 | 25.5 |
| LX | 2266 | 16.5 | 3106 | 18.6 | 1902 | 24.1 | 2736 | 30.3 |
| Vein invasion | ||||||||
| V0 | 9405 | 68.4 | 11,075 | 66.2 | 5157 | 65.2 | 5251 | 58.1 |
| V1/2 | 1905 | 13.8 | 2401 | 14.3 | 779 | 9.9 | 1000 | 11.1 |
| VX | 2450 | 17.8 | 3261 | 19.5 | 1968 | 24.9 | 2788 | 30.8 |
| Total | 13,760 | 100.0 | 16,737 | 100.0 | 7904 | 100.0 | 9039 | 100.0 |
| HR | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| C18 Colon all stages univariable | 0.845 | 0.802 | 0.891 |
| C18 Colon all stages multivariable * | 0.878 | 0.832 | 0.927 |
| C20 Rectum all stages univariable | 0.786 | 0.732 | 0.844 |
| C20 Rectum all stages multivariable * | 0.856 | 0.796 | 0.921 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Völkel, V.; Gerken, M.; Kleihues-van Tol, K.; Schoffer, O.; Bierbaum, V.; Bobeth, C.; Roessler, M.; Reissfelder, C.; Fürst, A.; Benz, S.; et al. Treatment of Colorectal Cancer in Certified Centers: Results of a Large German Registry Study Focusing on Long-Term Survival. Cancers 2023, 15, 4568. https://doi.org/10.3390/cancers15184568
Völkel V, Gerken M, Kleihues-van Tol K, Schoffer O, Bierbaum V, Bobeth C, Roessler M, Reissfelder C, Fürst A, Benz S, et al. Treatment of Colorectal Cancer in Certified Centers: Results of a Large German Registry Study Focusing on Long-Term Survival. Cancers. 2023; 15(18):4568. https://doi.org/10.3390/cancers15184568
Chicago/Turabian StyleVölkel, Vinzenz, Michael Gerken, Kees Kleihues-van Tol, Olaf Schoffer, Veronika Bierbaum, Christoph Bobeth, Martin Roessler, Christoph Reissfelder, Alois Fürst, Stefan Benz, and et al. 2023. "Treatment of Colorectal Cancer in Certified Centers: Results of a Large German Registry Study Focusing on Long-Term Survival" Cancers 15, no. 18: 4568. https://doi.org/10.3390/cancers15184568
APA StyleVölkel, V., Gerken, M., Kleihues-van Tol, K., Schoffer, O., Bierbaum, V., Bobeth, C., Roessler, M., Reissfelder, C., Fürst, A., Benz, S., Rau, B. M., Piso, P., Distler, M., Günster, C., Hansinger, J., Schmitt, J., & Klinkhammer-Schalke, M. (2023). Treatment of Colorectal Cancer in Certified Centers: Results of a Large German Registry Study Focusing on Long-Term Survival. Cancers, 15(18), 4568. https://doi.org/10.3390/cancers15184568







