Implementation of an Enhanced Recovery after Surgery Protocol in Advanced and Recurrent Rectal Cancer Patients after beyond Total Mesorectal Excision Surgery: A Feasibility Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
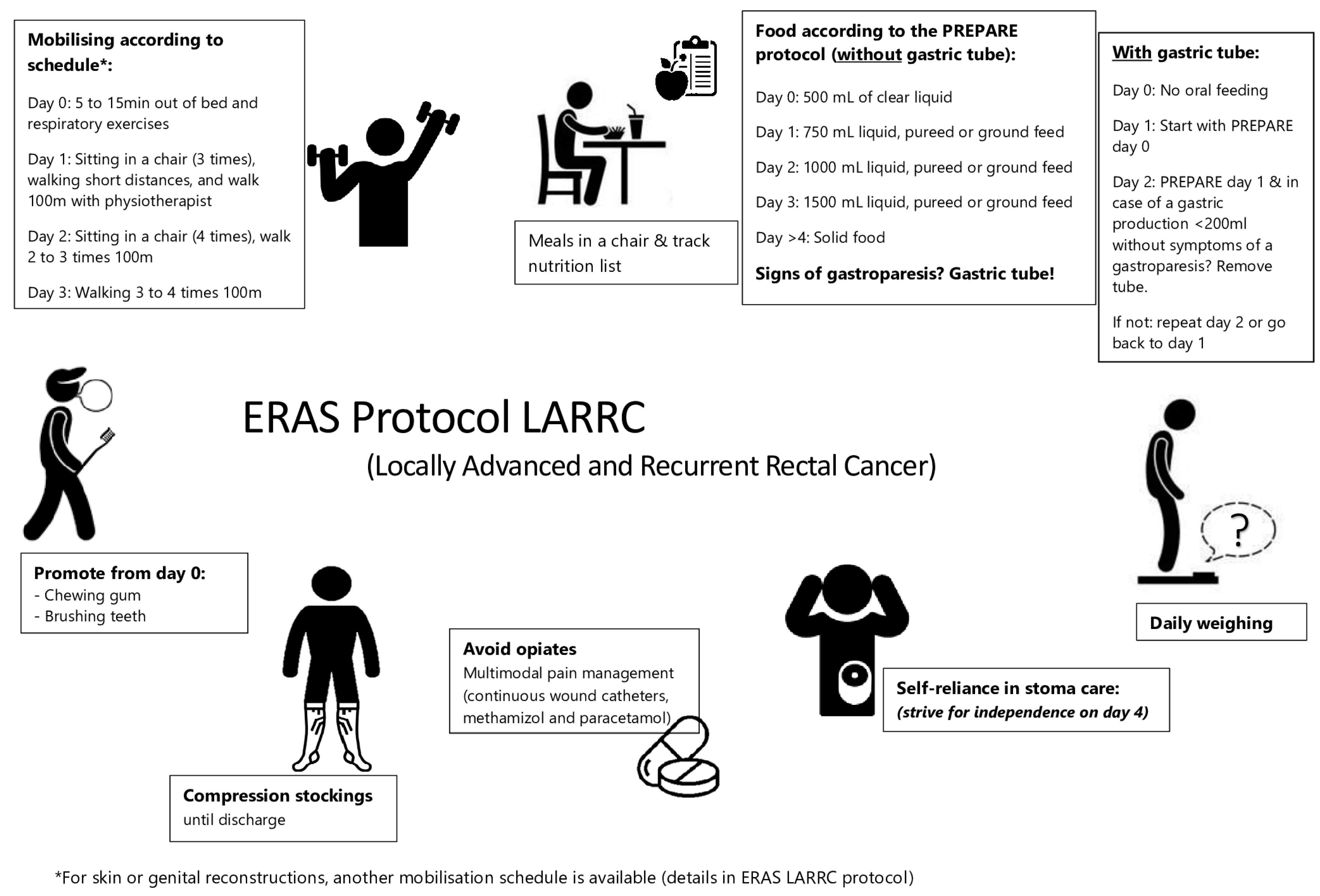
2.2. Enhanced Recovery after Surgery Protocol
2.3. Data Collection and Follow-Up
2.4. Statistical Analyses
3. Results
3.1. Compliance to the ERAS LARRC Protocol
3.2. Comparison of ERAS-Related Outcomes: Anaesthesia and Pain Management
3.3. Comparison of ERAS-Related Outcomes: Nasogastric Tube Management
3.4. Comparison of ERAS-Related Outcomes: Urological Management
3.5. Outcomes, Functional Recovery, and Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Hausel, J.; Thorell, A.; Ljungqvist, O.; Soop, M.; Nygren, J.; Group, E.R.A.S.S. Adherence to the Enhanced Recovery After Surgery Protocol and Outcomes After Colorectal Cancer Surgery. Arch. Surg. 2011, 146, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Collaborative, P. Perioperative management and anaesthetic considerations in pelvic exenterations using Delphi methodology: Results from the PelvEx Collaborative. BJS Open 2021, 5, zraa055. [Google Scholar] [CrossRef] [PubMed]
- Denost, Q.; Rullier, E.; Maillou-Martinaud, H.; Tuech, J.-J.; Ghouti, L.; Cotte, E.; Panis, Y.; Lelong, B.; Rouanet, P.; Broc, G.; et al. International variation in managing locally advanced or recurrent rectal cancer: Prospective benchmark analysis. Br. J. Surg. 2020, 107, 1846–1854. [Google Scholar] [CrossRef]
- Tekkis, P.; Road, F. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br. J. Surg. 2013, 100, 1009–1014. [Google Scholar] [CrossRef]
- Mariathasan, A.B.; Boye, K.; Giercksky, K.E.; Brennhovd, B.; Gullestad, H.P.; Emblemsvåg, H.L.; Grøholt, K.K.; Dueland, S.; Flatmark, K.; Larsen, S.G. Beyond total mesorectal excision in locally advanced rectal cancer with organ or pelvic side-wall involvement. Eur. J. Surg. Oncol. 2018, 44, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Harji, D.; Mauriac, P.; Bouyer, B.; Berard, X.; Gille, O.; Salut, C.; Rullier, E.; Celerier, B.; Robert, G.; Denost, Q. The feasibility of implementing an enhanced recovery programme in patients undergoing pelvic exenteration. Eur. J. Surg. Oncol. 2021, 47, 3194–3201. [Google Scholar] [CrossRef]
- Ketelaers, S.H.J.; Voogt, E.L.K.; Simkens, G.A.; Bloemen, J.G.; Nieuwenhuijzen, G.A.P.; de Hingh, I.H.J.; Rutten, H.J.T.; Burger, J.W.A.; Orsini, R.G. Age-related differences in morbidity and mortality after surgery for primary clinical T4 and locally recurrent rectal cancer. Color. Dis. 2021, 23, 1141–1152. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Cecire, J.; Hitos, K.; Shedden, K.; Gavegan, F.; Pathmanathan, N.; El Khoury, T.; Di Re, A.; Cocco, A.; Limmer, A.; et al. The impact of variations in care and complications within a colorectal enhanced recovery after surgery (ERAS) program on length of stay. Ann. Coloproctol. 2021, 38, 36–46. [Google Scholar] [CrossRef]
- Nordkamp, S.; Ketelaers, S.H.J.; Piqeur, F.; Scholten, H.J.; van de Calseijde, S.; Tolenaar, J.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T.; Burger, J.W.A.; Bloemen, J.G. Current perioperative care in patients undergoing bTME for rectal cancer: What are the differences with the colorectal Enhanced Recovery After Surgery protocol? Color. Dis. under review.
- Robella, M.; Tonello, M.; Berchialla, P.; Sciannameo, V.; Ilari Civit, A.M.; Sommariva, A.; Sassaroli, C.; Di Giorgio, A.; Gelmini, R.; Ghirardi, V.; et al. Enhanced Recovery after Surgery (ERAS) Program for Patients with Peritoneal Surface Malignancies Undergoing Cytoreductive Surgery with or without HIPEC: A Systematic Review and a Meta-Analysis. Cancers 2023, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.H.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J. Surg. 2013, 37, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef]
- Federation of Medical Specialists. Dutch National Guidelines Colorectal Cancer. 2020. Available online: https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/startpagina_-_crc.html (accessed on 6 September 2023).
- Voogt, E.L.K.; Nordkamp, S.; Rutten, H.J.T.; Burger, J.W.A.; Collaborative, P. Induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as neoadjuvant treatment for locally recurrent rectal cancer: The PelvEx II study. Eur. J. Surg. Oncol. 2021, 47, e22–e23. [Google Scholar] [CrossRef]
- Van den Berg, K.; Schaap, D.P.; Voogt, E.L.K.; Buffart, T.E.; Verheul, H.M.W.; de Groot, J.W.B.; Verhoef, C.; Melenhorst, J.; Roodhart, J.M.L.; de Wilt, J.H.W.; et al. Neoadjuvant FOLFOXIRI prior to chemoradiotherapy for high-risk (“ugly”) locally advanced rectal cancer: Study protocol of a single-arm, multicentre, open-label, phase II trial (MEND-IT). BMC Cancer 2022, 22, 957. [Google Scholar] [CrossRef]
- Kendall, M.C.; Alves, L.J.; Pence, K.; Mukhdomi, T.; Croxford, D.; De Oliveira, G.S. The Effect of Intraoperative Methadone Compared to Morphine on Postsurgical Pain: A Meta-Analysis of Randomized Controlled Trials. Anesthesiol. Res. Pract. 2020, 2020, 6974321. [Google Scholar] [CrossRef]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Marymont, J.H.; Shear, T.; Parikh, K.N.; Patel, S.S.; Gupta, D.K. Intraoperative Methadone for the Prevention of Postoperative Pain: A Randomized, Double-blinded Clinical Trial in Cardiac Surgical Patients. Anesthesiology 2015, 122, 1112–1122. [Google Scholar] [CrossRef]
- Richlin, D.M.; Reuben, S.S. Postoperative pain control with methadone following lower abdominal surgery. J. Clin. Anesth. 1991, 3, 112–116. [Google Scholar] [CrossRef]
- Ketelaers, S.H.J.; Dhondt, L.; van Ham, N.; Harms, A.S.; Scholten, H.J.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T.; Burger, J.W.A.; Bloemen, J.G.; Vogelaar, F.J. A prospective cohort study to evaluate continuous wound infusion with local analgesics within an enhanced recovery protocol after colorectal cancer surgery. Color. Dis. 2022, 24, 1172–1183. [Google Scholar] [CrossRef]
- Ramirez, J.A.; McIntosh, A.G.; Strehlow, R.; Lawrence, V.A.; Parekh, D.J.; Svatek, R.S. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: A systematic review. Eur. Urol. 2013, 64, 588–597. [Google Scholar] [CrossRef]
- Marcq, G.; Kassouf, W. Postoperative ileus: A systematic pathway for radical cystectomy candidates? Can. Urol. Assoc. J. 2021, 15, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Raynor, M.C.; Pruthi, R.S. Postoperative Ileus After Radical Cystectomy: Looking for Answers to an Age-old Problem. Eur. Urol. 2014, 66, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar]
- Bellato, V.; An, Y.; Cerbo, D.; Campanelli, M.; Franceschilli, M.; Khanna, K.; Sensi, B.; Siragusa, L.; Rossi, P.; Sica, G.S. Feasibility and outcomes of ERAS protocol in elective cT4 colorectal cancer patients: Results from a single-center retrospective cohort study. World J. Surg. Oncol. 2021, 19, 196. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, J.L.; Gómez-Hidalgo, N.R.; Pérez-Benavente, A.; Carbonell-Socias, M.; Manrique-Muñoz, S.; Serrano, M.P.; Gutiérrez-Barceló, P.; Bradbury, M.; Nelson, G.; Gil-Moreno, A. Importance of Enhanced Recovery After Surgery (ERAS) Protocol Compliance for Length of Stay in Ovarian Cancer Surgery. Ann. Surg. Oncol. 2021, 28, 8979–8986. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, P.; González Ayora, S.; Ortega López, M.; León Arellano, M.; Guadalajara, H.; García-Olmo, D.; Pastor, C. Implementation barriers for Enhanced Recovery After Surgery (ERAS) in rectal cancer surgery: A comparative analysis of compliance with colon cancer surgeries. Updates Surg. 2021, 73, 2161–2168. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Ulrich, A.B.; Bruckner, T.; Münter, M.; Nickles, A.; Contin, P.; Löffler, T.; Reissfelder, C.; Koch, M.; Büchler, M.W.; et al. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: Is there still a chance for cure? Ann. Surg. 2011, 253, 522–533. [Google Scholar] [CrossRef]
- Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. Br. J. Surg. 2018, 105, 650–657. [CrossRef]
- Liccardo, F.; Baird, D.L.H.; Pellino, G.; Rasheed, S.; Kontovounisios, C.; Tekkis, P.P. Predictors of short-term readmission after beyond total mesorectal excision for primary locally advanced and recurrent rectal cancer. Updates Surg. 2019, 71, 477–484. [Google Scholar] [CrossRef]
- Steffens, D.; Solomon, M.J.; Lee, P.; Austin, K.; Koh, C.; Byrne, C.; Karunaratne, S.; Hatcher, S.; Taylor, K.; McBride, K. Surgical, survival and quality of life outcomes in over 1000 pelvic exenterations: Lessons learned from a large Australian case series. ANZ J. Surg. 2023, 93, 1232–1241. [Google Scholar] [CrossRef]
- Assi, H.; Persson, A.; Palmquist, I.; Öberg, M.; Buchwald, P.; Lydrup, M.-L. Short-term outcomes following beyond total mesorectal excision and reconstruction using myocutaneous flaps: A retrospective cohort study. Eur. J. Surg. Oncol. 2022, 48, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Sharma, Y.; Yaxley, A.; Baldwin, C.; Miller, M. Use of the Patient-Generated Subjective Global Assessment to Identify Pre-Frailty and Frailty in Hospitalized Older Adults. J. Nutr. Health Aging 2021, 25, 1229–1234. [Google Scholar] [PubMed]
- Van Rooijen, S.; Carli, F.; Dalton, S.; Thomas, G.; Bojesen, R.; Le Guen, M.; Barizien, N.; Awasthi, R.; Minnella, E.; Beijer, S.; et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: The first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019, 19, 98. [Google Scholar]
- Ghignone, F.; Hernandez, P.; Mahmoud, M.N.; Ugolini, G. Functional recovery in senior adults undergoing surgery for colorectal cancer: Assessment tools and strategies to preserve functional status. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 387–393. [Google Scholar]
- Brady, M.; Kinn, S.; Stuart, P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst. Rev. 2003, CD004423. [Google Scholar] [CrossRef]
- Smith, M.D.; McCall, J.; Plank, L.; Herbison, G.P.; Soop, M.; Nygren, J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst. Rev. 2014, CD009161. [Google Scholar] [CrossRef]
- Carli, F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: Implications of the stress response. Can. J. Anaesth. 2015, 62, 110–119. [Google Scholar] [CrossRef]
- Abis, G.S.A.; Stockmann, H.B.A.C.; van Egmond, M.; Bonjer, H.J.; Vandenbroucke-Grauls, C.M.J.E.; Oosterling, S.J. Selective decontamination of the digestive tract in gastrointestinal surgery: Useful in infection prevention? A systematic review. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2013, 17, 2172–2178. [Google Scholar]
- Abis, G.S.A.; Stockmann, H.B.A.C.; Bonjer, H.J.; van Veenendaal, N.; van Doorn-Schepens, M.L.M.; Budding, A.E.; Wilschut, J.A.; van Egmond, M.; Oosterling, S.J.; de Lange, E.S.M.; et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br. J. Surg. 2019, 106, 355–363. [Google Scholar]
- Kwon, S.; Thompson, R.; Dellinger, P.; Yanez, D.; Farrohki, E.; Flum, D. Importance of perioperative glycemic control in general surgery: A report from the Surgical Care and Outcomes Assessment Program. Ann. Surg. 2013, 257, 8–14. [Google Scholar] [CrossRef]
- Weibel, S.; Rücker, G.; Eberhart, L.H.; Pace, N.L.; Hartl, H.M.; Jordan, O.L.; Mayer, D.; Riemer, M.; Schaefer, M.S.; Raj, D.; et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 10, CD012859. [Google Scholar] [PubMed]
- Watanabe, J.; Miki, A.; Koizumi, M.; Kotani, K.; Sata, N. Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4394. [Google Scholar] [CrossRef] [PubMed]
- Short, V.; Herbert, G.; Perry, R.; Atkinson, C.; Ness, A.R.; Penfold, C.; Thomas, S.; Andersen, H.K.; Lewis, S.J. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst. Rev. 2015, CD006506. [Google Scholar] [CrossRef]
- Felder, S.; Rasmussen, M.S.; King, R.; Sklow, B.; Kwaan, M.; Madoff, R.; Jensen, C. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst. Rev. 2019, 3, CD004318. [Google Scholar]
- Sachdeva, A.; Dalton, M.; Amaragiri, S.V.; Lees, T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst. Rev. 2010, CD001484. [Google Scholar] [CrossRef]
- Changchien, C.R.; Yeh, C.Y.; Huang, S.T.; Hsieh, M.-L.; Chen, J.-S.; Tang, R. Postoperative urinary retention after primary colorectal cancer resection via laparotomy: A prospective study of 2355 consecutive patients. Dis. Colon Rectum 2007, 50, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.S.; Lapitan, M.C.M. Drugs for treatment of urinary retention after surgery in adults. Cochrane Database Syst. Rev. 2010, CD008023. [Google Scholar] [CrossRef] [PubMed]
- McPhail, M.J.W.; Abu-Hilal, M.; Johnson, C.D. A meta-analysis comparing suprapubic and transurethral catheterization for bladder drainage after abdominal surgery. Br. J. Surg. 2006, 93, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | n = 72 (%) |
|---|---|
| Age, mean in years (SD) | 63.9 (10.7) |
| Gender | |
| Male | 48 (66.7) |
| Female | 24 (33.3) |
| ASA class | |
| I–II | 59 (82.0) |
| III–IV | 13 (18.1) |
| Induction chemotherapy | 36 (50.0) |
| Neoadjuvant radiotherapy | 72 (100) |
| Pathological Tumour stage | |
| T0–T2 | 6 (8.3) |
| T3–T4 | 37 (51.4) |
| N.A. * | 29 (40.3) |
| Pathological Nodal stage | |
| N0 | 26 (36.1) |
| N1/2 | 17 (13.4) |
| N.A. * | 29 (40.3) |
| Main procedure | |
| LAR/Re-resection with anastomosis | 18 (25.0) |
| APR | 27 (37.5) |
| Total exenteration | 23 (31.9) |
| Tumour resection n.o.s. ** | 23 (31.9) |
| Intraoperative radiotherapy | 58 (80.6) |
| Bladder resection | 24 (33.3) |
| Urologic reconstruction | 34 (47.2) |
| Partial sacral resection | 14 (19.4) |
| Prostate and/or vesicles resection (male only) | 35 (72.9) |
| Uterus and/or ovaria resection (female only) | 13 (54.2) |
| Vagina resection (female only) | 7 (29.2) |
| Pelvic sidewall resection | 36 (50.0) |
| Lateral lymph node resection | 9 (12.5) |
| Length of operation, median in mL (IQR) | 306.0 (219.3–368.8) |
| Intraoperative blood loss, median in mL (IQR) | 1550.0 (762.5–2875.0) |
| Omentoplasty | 51 (70.8) |
| Preadmission Compliance Care Elements | Compliance % | |
|---|---|---|
| 1 | Preoperative nutritional status assessed | 98.6 |
| 2 | Preoperative nutritional treatment in case of (risk for) malnutrition | 14.3 |
| 3 | Alcohol (quitted before surgery) | 93.1 |
| 4 | Preadmission patient education | 94.4 |
| 5 | Ostomy introduction | 100 |
| 6 | Patient screened for anaemia preoperatively | 94.4 |
| 7 | Anaemia treatment given when applicable | 60 |
| 8 | Smoker (quitted before surgery) | 81.9 |
| 9 | Prehabilitation (in case of vulnerability) | 100 |
| Preoperative compliance care elements | ||
| 10 | No oral bowel preparation used unless patients received an LAR | 94.4 |
| 11 | Preoperative oral carbohydrate treatment | 91.7 |
| 12 | No preoperative sedative medication < 65 years | 95.8 |
| 13 | Thrombosis prophylaxis administered until outpatient at 28 days + compression socks | 77.8 |
| 14 | Antibiotic prophylaxis before incision | 100 |
| 15 | PONV prophylaxis administered | 100 |
| 16 | Date of admission = date of surgery (unless they received ureter stents) | 81.9 |
| 17 | SDD administered | 73.6 |
| Intraoperative compliance care elements | ||
| 18 | No epidural or spinal anaesthesia but use of multimodal anaesthesia | 75 |
| 19 | Nerve blocks or local anaesthesia and continuous wound infusion | 75 |
| 20 | Forced-air heating cover used | 100 |
| 21 | No nasogastric tube placed intraoperatively and used postoperatively unless ileus appeared; it was then removed according to protocol | 44.2 |
| 22 | No resection-site drainage placed according to protocol (pelvic exenteration) | 56.9 |
| Postoperative compliance care elements | ||
| 23 | Termination of urinary drainage at end of operation; SPC was used in case of potential retention bladder, Otherwise it was handled according to protocol | 84.7 |
| 24 | Stimulation of gut motility: laxatives and non-medicamental treatment used according to protocol | 100 |
| 25 | Weighted on POD 0 | 43.1 |
| 26 | Weighted on POD 1 | 29.2 |
| 27 | Weighted on POD 2 | 65.3 |
| 28 | Weighted on POD 3 | 72.2 |
| 29 | Pain management with CWI, metamizole and paracetamol | 72.2 |
| 30 | PONV prevention | 100 |
| 31 | Energy intake on POD 0 > 500 ml | 20.8 |
| 32 | Energy intake on POD 1 > 750 ml | 43.1 |
| 33 | Energy intake on POD 2 > 1000 mL | 37.5 |
| 34 | Energy intake on POD 3 > 1500 mL | 41.7 |
| 35 | Mobilisation on day of surgery | 58.3 |
| 36 | Mobilisation on POD1 according to protocol | 52.8 |
| 37 | Mobilisation on POD2 according to protocol | 69.4 |
| 38 | Mobilisation on POD3 according to protocol | 77.8 |
| 39 | Follow-up control performed around 30 days postoperatively | 100 |
| Anaesthesia | Multimodal Management | Epidural | |
|---|---|---|---|
| n = 54 (75.0%) | n = 18 (25.0%) | p-Value | |
| Postoperative analgesics | |||
| Continuous wound infusion | 53 (98.1) | 1 (5.6) | <0.001 |
| Patient-controlled analgesia (PCA) | 16 (29.6) | 8 (44.4) | 0.456 |
| Median duration of PCA in days (IQR) | 4.0 (3.0–5.0) | 3.0 (1.0–4.0) | 0.269 |
| Epidural | NA | 3.0 (2.0–3.0) | |
| Postoperative NSAID (Metamizole iv) | 49 (90.7) | 13 (72.2) | 0.063 |
| Median duration of metamizole in days (IQR) | 5.0 (3.0–6.0) | 5.0 (3.0–6.5) | 0.641 |
| Postoperative opioids * POD 0 | 53 (98.1) | 18 (100) | 0.750 |
| Postoperative opioids * POD 1 | 23 (42.6) | 18 (100) | <0.001 |
| Postoperative opioids * POD 2 | 15 (27.8) | 18 (100) | <0.001 |
| Postoperative opioids * POD 3 | 14 (25.9) | 17 (94.4) | <0.001 |
| Postoperative opioids * POD 4 | 13 (24.1) | 11 (64.7) | 0.002 |
| Postoperative opioids * POD 5 | 12 (22.2) | 7 (41.2) | 0.112 |
| Nausea POD 0 | 10 (18.6) | 1 (5.6) | 0.380 |
| Nausea POD 1 | 15 (27.8) | 3 (16.7) | 0.314 |
| Nausea POD 2 | 16 (45.6) | 5 (27.8) | 0.987 |
| Nausea POD 3 | 12 (22.2) | 6 (33.3) | 0.049 |
| Mobilisation POD 0 | 33 (61.1) | 9 (50.0) | 0.554 |
| Mobilisation POD 1 | 31 (57.4) | 7 (38.9) | 0.173 |
| Mobilisation POD 2 | 40 (74.1) | 10 (55.6) | 0.153 |
| Mobilisation POD 3 | 45 (83.3) | 11 (61.1) | 0.050 |
| Complication < 30 days | 38 (70.4) | 12 (66.7) | 0.768 |
| Complication > 30 and <90 days | 24 (45.3) | 5 (27.8) | 0.192 |
| Median time to passage of stool in days (IQR) | 3.0 (2.0–4.0) | 2.0 (1.0–4.0) | 0.411 |
| Median time to tolerating solid food in days (IQR) | 4.5 (2.0–6.3) | 5.0 (2.8–7.3) | 0.987 |
| Median time to recover ADL in days (IQR) | 6.0 (4.0–8.0) | 6.5 (4.8–9.0) | 0.976 |
| Median time to termination of urinary drainage in days (IQR) | 5.0 (2.0–10.5) | 8.5 (3.8–13.0) | 0.798 |
| Median time to functional recovery in days (IQR) | 7.0 (5.0–14.0) | 10 (7.0–14.3) | 0.328 |
| Median length of postoperative ICU stay in days (IQR) | 1.0 (0.0–1.0) | 2.0 (1.0–2.3) | 0.004 |
| Median length of hospital stay in days (IQR) | 7.0 (5.5–14.0) | 10.0 (6.8–15.5) | 0.440 |
| Functional Outcomes and Complications | n = 72 (%) |
|---|---|
| Median time to oral pain control in days (IQR) | 4.0 (3.0–4.0) |
| Median time to passage of stool in days (IQR) | 3.0 (2.0–4.0) |
| Median time to tolerating solid food in days (IQR) | 5.0 (2.0–7.0) |
| Median time to recover ADL in days (IQR) | 6.0 (4.0–8.0) |
| Median time to termination of urinary drainage in days (IQR) | 6.0 (3.0–12.0) |
| Median time to termination of resection-site drain in days (IQR) | 3.0 (2.0–4.0) |
| Median time to termination of nasogastric tube in days (IQR) | 3.0 (2.0–6.5) |
| Mobilisation according to protocol | 47 (65.3) |
| Median length of hospital stay in days (IQR) | 9.0 (6.0–14.0) |
| Complications | 51 (70.8) |
| Complications < 30 days | 44 (61.1) |
| Most severe complication < 30 days (Clavien–Dindo) | |
| None | 21 (29.2) |
| I–IIIa | 39 (54.2) |
| IIIb–IV | 12 (16.7) |
| Complications > 30 days <90 days | 29 (40.3) |
| Gastro-intestinal complications | |
| Gastroparesis/paralytic ileus | 18 (25.0) |
| Intra-abdominal abscess | 8 (11.1) |
| Leakage anastomosis | 6 (8.3) |
| Wound infection/dehiscence | 12 (16.7) |
| Mechanical ileus | 4 (5.6) |
| Urological complications | |
| Bladder retention | 5 (6.9) |
| Urinary tract infection | 9 (12.5) |
| Other | 6 (8.3) |
| Neurological complications | 9 (12.5) |
| Cardio-pulmonary complications | 10 (13.9) |
| Vascular complications | 3 (4.2) |
| Reoperations | 14 (19.4) |
| Readmissions | 19 (26.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordkamp, S.; Creemers, D.M.J.; Glazemakers, S.; Ketelaers, S.H.J.; Scholten, H.J.; van de Calseijde, S.; Nieuwenhuijzen, G.A.P.; Tolenaar, J.L.; Crezee, H.W.; Rutten, H.J.T.; et al. Implementation of an Enhanced Recovery after Surgery Protocol in Advanced and Recurrent Rectal Cancer Patients after beyond Total Mesorectal Excision Surgery: A Feasibility Study. Cancers 2023, 15, 4523. https://doi.org/10.3390/cancers15184523
Nordkamp S, Creemers DMJ, Glazemakers S, Ketelaers SHJ, Scholten HJ, van de Calseijde S, Nieuwenhuijzen GAP, Tolenaar JL, Crezee HW, Rutten HJT, et al. Implementation of an Enhanced Recovery after Surgery Protocol in Advanced and Recurrent Rectal Cancer Patients after beyond Total Mesorectal Excision Surgery: A Feasibility Study. Cancers. 2023; 15(18):4523. https://doi.org/10.3390/cancers15184523
Chicago/Turabian StyleNordkamp, Stefi, Davy M. J. Creemers, Sofie Glazemakers, Stijn H. J. Ketelaers, Harm J. Scholten, Silvie van de Calseijde, Grard A. P. Nieuwenhuijzen, Jip L. Tolenaar, Hendi W. Crezee, Harm J. T. Rutten, and et al. 2023. "Implementation of an Enhanced Recovery after Surgery Protocol in Advanced and Recurrent Rectal Cancer Patients after beyond Total Mesorectal Excision Surgery: A Feasibility Study" Cancers 15, no. 18: 4523. https://doi.org/10.3390/cancers15184523
APA StyleNordkamp, S., Creemers, D. M. J., Glazemakers, S., Ketelaers, S. H. J., Scholten, H. J., van de Calseijde, S., Nieuwenhuijzen, G. A. P., Tolenaar, J. L., Crezee, H. W., Rutten, H. J. T., Burger, J. W. A., & Bloemen, J. G. (2023). Implementation of an Enhanced Recovery after Surgery Protocol in Advanced and Recurrent Rectal Cancer Patients after beyond Total Mesorectal Excision Surgery: A Feasibility Study. Cancers, 15(18), 4523. https://doi.org/10.3390/cancers15184523






