Simple Summary
This research study focuses on the P-cadherin protein, which is found in many types of tumors, and could be a potential target for therapy. FF-21101, a human–mouse chimeric monoclonal antibody that targets P-cadherin, was labeled with indium-111(111In) and investigated for biodistribution and radiation doses in six cynomolgus macaques. To assess the radiation profile distribution and clearance of FF-21101(111In), we performed whole-body imaging and generated time–activity curves. The lungs, spleen, liver, kidneys, and heart wall received the highest radiation dose estimates for FF-21101(111In). These findings led us to estimate the radiation doses that would be delivered to different organs if this antibody was labeled with yttrium-90 (90Y) and used in targeted radioimmunotherapy in patients. FF-21101 shows potential for clinical application in humans, according to biodistribution data obtained from macaques. Based on these results, an investigational new drug application was filed with the FDA for a Phase I clinical trial.
Abstract
P-cadherin is associated with a wide range of tumor types, making it an attractive therapeutic target. FF-21101 is a human–mouse chimeric monoclonal antibody (mAb) directed against human P-cadherin, which has been radioconjugated with indium-111 (111In) utilizing a DOTA chelator. We investigated the biodistribution of FF-21101(111In) in cynomolgus macaques and extrapolated the results to estimate internal radiation doses of 111In- and yttrium-90 (90Y)-FF-21101 for targeted radioimmunotherapy in humans. Whole-body planar and SPECT imaging were performed at 0, 2, 24, 48, 72, 96, and 120 h post-injection, using a dual-head gamma camera. Volumes of interest of identifiable source organs of radioactivity were defined on aligned reference CT and serial SPECT images. Organs with the highest estimated dose values (mSv/MBq) for FF-21101(111In) were the lungs (0.840), spleen (0.816), liver (0.751), kidneys (0.629), and heart wall (0.451); and for FF-21101(90Y) dose values were: lungs (10.49), spleen (8.21), kidneys (5.92), liver (5.46), and heart wall (2.61). FF-21101(111In) exhibits favorable biodistribution in cynomolgus macaques and estimated human dosimetric characteristics. Data obtained in this study were used to support the filing of an investigational new drug application with the FDA for a Phase I clinical trial.
1. Introduction
Cadherins are cell–cell adhesion glycoproteins that form calcium-dependent intercellular junctions and play an essential role in morphogenesis, development, and maintenance of the integrity of tissues and organs [1]. The cadherin family is subdivided into various subfamilies, including the classical E-, P-, and N-cadherins, each demonstrating a specific tissue distribution [2]. E-cadherin is expressed in all epithelial tissues; however, the expression of P-cadherin is restricted to the basal or inner layers of stratified epithelia, including prostate and skin, and to breast myoepithelial cells [3]. Several studies have demonstrated that aberrant P-cadherin expression is associated with cell proliferation and with tumors of the colon, breast, lung, thyroid, pancreas, and cervix, making P-cadherin (encoded by the gene CDH3) an excellent tumor-associated target [4,5].
The development of P-cadherin anticancer therapy commenced with PF-03732010, a humanized anti-CDH3 monoclonal antibody (mAb) with demonstrated antitumor activity confirmed by carcinoma mouse models [6]. More recently, a clinical trial (ClinicalTrials.gov accession no. NCT00557505) revealed that this antibody was well tolerated in humans but demonstrated no significant anti-tumor activity in solid tumors to warrant further development as a naked antibody [7]. Nonetheless, due to the favorable toxicity profile of the anti-CDH3 antibody and the interesting features of the target molecule, a radioimmunotherapy approach was proposed to achieve enhanced anti-tumor activity. The method of conjugating targeting antibodies or peptides with radionuclides has been successful in treating well-differentiated neuroendocrine tumors, prostate cancer, and other cancers [8,9,10]. Since high accumulation and homogeneous distribution at the target site and rapid elimination from non-target tissues are desirable for radiopharmaceutical therapy (RPT), the half-life of the radionuclide should be long enough to allow the radiolabeled monoclonal antibody (or peptide) to reach the tumor, and short enough to limit radiation exposure to normal tissues [11]. In addition, the specificity of the selected antibody to the cellular target enables increased therapeutic efficacy while minimizing toxicity to normal tissues [12,13,14,15].
FF-21101, a human–mouse chimeric immunoglobulin G1 (IgG1) monoclonal antibody (mAb) targeting human P-cadherin is being developed as a radio-immuno-therapeutic (RIT) for the treatment of patients with advanced solid tumors. In this paper, we describe FF-21101 as the mAb conjugate to the polydentate macrocyclic ligand, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) for use in dosimetry, imaging (e.g., with 111In), or as a radioimmunotherapeutic (e.g, with 90Y). The DOTA chelator forms stable metal complexes with a number of divalent and trivalent metals, including the lanthanide, indium-111 (111In) [16].
Tumor geometry is considered to be a significant variable for the success of RIT, and it has been proposed that the emission characteristics of the radionuclide should be matched appropriately to the lesion size/volume to be treated in order to focus energy within the tumor rather than in the surrounding tissues [17]. Therefore, for our study, FF-21101 was labeled with the theranostic pair, 111In and 90Y. The nuclear properties of 111In (physical half-life of 67.3 h and decay via electron capture with gamma emissions of 172 and 245 keV to stable cadmium-111) are well suited for multi-day imaging studies using planar gamma camera imaging and single photon emission computed tomography (SPECT). These nuclear properties are well matched with the 64.1 h physical half-life of yttrium-90 (90Y) and the biological half-life of the IgG form of the FF-21101 product [18]. 90Y emits beta particles, which penetrate soft tissue to a relatively long spatial depth of 5.3 mm. Therefore, 90Y-labeled mAbs cause DNA damage to the targeted cells as well as to adjacent or more distant cells due to their long range [19]. As a result of this cross-fire effect, there is an expectation that FF-21101(90Y) will have a more positive therapeutic impact on treating solid tumors than anti-CDH3 therapies alone, making 90Y ideal for this purpose [20]. 90Y is also used clinically for radioembolization of hepatocellular carcinoma and metastatic disease to the liver from colon cancer using microspheres [21]. Unfortunately, while 90Y has a favorable characteristic as a therapeutic radionuclide, the lack of gamma emission makes it suboptimal for imaging and dosimetry and dictates the need to delineate its biodistribution with a γ-emitting surrogate [22,23]. 111In has, therefore, been the surrogate radionuclide of choice for 90Y for gamma camera imaging due to the similarities in metabolic handling [24] and coordination chemistry with 90Y [25,26].
In this study, we evaluated the biodistribution and dosimetry of FF-21101(111In) in cynomolgus macaques. We estimated human adult male and female organ radiation absorbed doses by computing non-human primate (NHP) residence times based on serial gamma camera imaging and mass-scaled them to estimate the human equivalents. This was followed by the calculation of human absorbed dose estimates according to the methodology developed by the Medical Internal Radiation Dose (MIRD) Committee [18]. In order to compute internal radiation dose estimates for FF-21101(90Y) for radioimmunotherapy, 111In residence times obtained from FF-21101(111In) were converted to 90Y equivalents.
2. Material and Methods
2.1. Radiolabeling Preparation of FF-21101(111In)
The monoclonal antibody (FF-21101) conjugated to DOTA was provided as a 5 mg/mL solution in 250 mM sodium acetate, pH 5.5, by FUJIFILM Diosynth Biotechnologies, USA, Inc., Morrisville, NC, USA. Indium-111 chloride (111InCl3) was purchased from Mallinckrodt, Inc., St. Louis, MO, USA, and stored securely according to manufacturer specifications until ready for use. A preset volume of FF-21101 was transferred to a sterile 10 mL reaction vial (Greer Laboratories, Lenoir, NC, USA) using aseptic techniques and mixed with 111InCl3 in a shielded laminar air flow hood; the specific radioactivity of the mixture was 37 MBq/mg at the time of injection. The radioactive dose volume at the time of preparation was calculated based on the radioactive concentration of 111InCl3 at the time of preparation and added to the reaction vial. The reaction mixture was incubated at 40 °C in a thermomixer for 20 min. Following incubation, clinical formulation buffer (25 mmol/mL sodium acetate, 2 mg/mL ascorbic acid, 0.3 mg/mL diethylene triamine pentaacetic acid, and 0.72% saline; AMRI, Burlington, MA, USA) was added to bring the total volume to 10 mL. Approximately 111 MBq of FF-21101(111In) in 5 mL was drawn from the reaction vial into a pre-labeled 10 mL syringe, assayed in a dose calibrator, and delivered for injection. Labeled antibody was purified using a Biospin Column 6 (Bio-Rad, Tokyo, Japan) according to the manufacturer’s instructions. The labeling processes did not result in degradation of these antibodies.
2.2. Determination of Specific Activity
The specific activity of FF-21101(111In) was calculated based on measurements of radioactivity concentration and molar concentration in the dosing injectate. The radioactivity concentration of FF-21101(111In) was determined using a Packard 5500 gamma spectrometer (Perkin-Elmer, Waltham, MA, USA).
2.3. Experimental Animals
After a five-week quarantine/acclimation period, six adult (3 male and 3 female) cynomolgus macaques were housed separately in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments were performed following published guidelines under a protocol approved by the Institutional Animal Care and Use Committee. Prior to imaging, the animals were fasted overnight but had free access to water. The animals were anesthetized with an intramuscular injection of ketamine (10–15 mg/kg) plus atropine sulfate (0.04 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained via inhalation of isoflurane (1% to 3%) and oxygen delivered by a Fabius Tiro gas anesthesia system (Drager Medical, Inc., Tokyo, Japan). Cannulas were placed in both saphenous veins of each animal: one cannula was used for administration of the FF-21101(111In) and the other was used for repetitive blood sampling. Body temperature was maintained at 37 ± 0.8 °C using an air-circulating heating device (model 505; Arizant Healthcare Inc., Eden Prairie, MN, USA). The electrocardiogram, pulse oximetry, and respiration rates were continuously monitored using an Infinity Gamma XL Monitor (Drager Medical, Inc.). The stage of menstrual cycle in females was not monitored. The use of a vacuum immobilization system bag was instrumental in helping to maintain NHP position throughout each planar/SPECT and subsequent CT scan imaging sessions over several days.
2.4. Gamma Camera and SPECT System
All animal whole-body (WB) planar and SPECT gamma camera imaging studies were performed using a Siemens Symbia S scanner (Siemens Medical Solutions USA, Hoffman Estates, IL, USA) equipped with two 3/8″ NaI detectors and medium-energy low-penetration collimators. The reported NEMA 111In system planar sensitivity for that system is 430 cpm/µCi.
2.5. Gamma Camera and SPECT Imaging Protocol
Each primate was first placed in a vacuum immobilization bag to maintain consistent positioning during each planar/SPECT and CT imaging session. All primates were positioned supine/feet first into the gantry. Approximately 111 MBq of 111In-FF-21101 was administered to each primate as a continuous infusion over 10 min. A (0 h) planar/SPECT scanning session commenced immediately after the end of the infusion and consisted of a head-to-foot whole-body planar sweep followed by either a single-bed (female) or two-bed (male), head–neck–trunk SPECT scan. This planar-plus-SPECT-imaging paradigm was repeated multiple times according to the following schedule: 2, 24, 48, 72, 96, and 120 h from the end of infusion. A 10 mL liquid vial 111In calibration source (approximately 1.85 MBq) was acquired along with the NHP at each time point, to convert reconstructed SPECT counts to activity.
For whole-body planar imaging, the acquisition parameters were set as follows: a 256 × 1024 acquisition matrix, and a scan speed of 10 cm/min (0, 2, 24 h), 7 cm/min (48, 72 h), or 5 cm/min (96, 120 h), with 15% (±7.5%) energy window widths centered over the two 111In photopeaks (172 and 245 keV). For SPECT imaging, the acquisition parameters were: 128 views over 360° (64 views/head and 180° total gantry rotation), 15 sec/view (0, 2, 24 h), 21 sec/view (48, 72 h), or 30 sec/view (96, 120 h). Images were acquired and reconstructed in a 128×128 matrix with a pixel size of 4.8 mm. In addition to the two photopeak images, images from a scatter window below the 245 keV photopeak (211 keV/11%) and scatter windows below and above the 172 keV photopeak (144 keV/18% and 190 keV/7%) were also acquired, for dual- and triple-energy-window scatter compensation, respectively. An ordered-subset expectation-maximum iterative algorithm (8 iterations, 16 subsets, and 9.6 mm full-width at half-maximum Gaussian post-filter), incorporating CT-based attenuation correction (AC) and energy-window-based scatter and system spatial resolution compensations, was employed for reconstruction of the SPECT images.
2.6. CT Imaging
The CT imaging was performed on a GE 750 HD scanner (General Electric Healthcare, Waukesha, WI, USA) and was used for SPECT attenuation correction and to help identify the contours of the source organ’s volumes of interest (VOIs). Primates were positioned centrally on the CT scanner table. A scout scan (AP, 120 kVp, 80 mA, 100 cm, 10 cm/s) was first acquired to determine animal positioning and anatomical coverage. This was followed by a CT scan with the following parameters: 120 kVp, Smart mA tube current modulation, 0.5 s rotation, 64 × 0.625 mm nominal beam width at isocenter, and 1.375 pitch. The AC CT-filtered back-projection reconstruction parameters were 5 mm slice thickness and spacing, 50 cm diameter, and STANDARD filter; while those for the organ-contouring CT were 3.75 mm slice thickness, 3.27 mm slice spacing, 50 cm diameter, and SOFT filter.
2.7. Image Analysis for Dosimetry Calculations
Three-dimensional VOIs of identifiable source organs of radioactivity were manually defined on the first-day CT scan and all serial SPECT scans were manually registered to that CT scan using MIM version 6.5 (MIM Software Inc., Beachwood, OH, USA). These VOIs were used for both CT-based organ mass estimation and SPECT organ activity versus time (time–activity curve, [TAC]) generation. The identifiable source organs were heart contents, lungs, liver, spleen, kidneys, and testes. Two-dimensional total-body regions of interest were defined in an analogous fashion using the planar whole-body scan images, for total-body TAC generation.
2.8. Blood Sampling
Measurement of radioactivity concentrations in the blood was based on 0.5 mL venous blood samples obtained via the catheterized vein at 1, 2, 5, 10, 15, 20, and 30 min, and 2, 24, 48, 72, 96, and 120 h after FF-21101(111In) injection. Approximately 50 mg of each blood sample was assayed along with an 111In counting standard, to derive radioactivity concentration versus time using a gamma counter (Cobra Quantum, Perkin-Elmer, Waltham, MA, USA), expressed as a Fraction of Injected Activity per mL (FIA/mL).
2.9. Residence Time and Absorbed Dose Calculations
Human organ radiation absorbed dose estimates for FF-21101(111In) (mGy/MBq) were obtained using the MIRD methodology. The MIRD equation for estimating mGy/MBq to a target from one or more sources is
where Dt is the dose to a target (mGy/MBq); Ts is the residence time (or normalized cumulative activity, equal to the total number of disintegrations per unit of administered activity, in h) in source s; and Si(t ← s) is the average absorbed dose in target t per unit of cumulative activity, , in source s (dose factor, in mGy/MBq-h). Cumulative activity and residence time Ts are related as follows:
where A0 is administered activity. Residence time was computed based on the imaging data acquired from all macaques as the fraction of administered activity in the source, fs(t), integrated from time t = 0 to ∞. The dose factors employed in the dose calculations were those defined in the FDA-cleared and de facto industry-standard program OLINDA/EXM 1.1 (Vanderbilt University, Nashville, TN, USA). Human radiation doses were calculated from all cynomolgus macaques and the normalized number of disintegrations using OLINDA/EXM 1.1 for the adult male and female phantoms. Supplementary Materials describe the dosimetry assumptions in detail.
The calculated absorbed doses for FF-21101(111In) and FF-21101(90Y) were then compared with the published absorbed doses for ibritumomab tiuxetan (Zevalin; Acrotech Biopharma, LLC, East Windsor, NJ, USA) labeled with the same radionuclides (111In and 90Y) [18].
3. Results
3.1. FF-21101(111In) Synthesis
Radiolabeling of FF-21101 with 111InCl3 was carried out by the clinical site radiopharmacy. The prescribed radiolabeled activities were assayed using a pre-calibrated dose calibrator, prepared in the empty reaction vial, drawn into an appropriately sized sterile syringe, and sterile-filtered prior to infusion into the subject. Radiochemical purity of the product was assayed using radio-thin layer radiochromatography and typically exceeded 98% up to 24 h post-compounding. Each primate was administered 103.6–189.8 MBq of FF-21101(111In) in a volume of 5 mL. FF-21101 antibody concentration administered to each animal ranged from 0.48 to 0.54 mg/mL. Antibody dose ranged from 0.29 to 0.63 mg/kg (mean of 0.34 mg/kg for males and mean of 0.59 mg/kg for females). Simultaneously, with the FF-21101(111In) injection, up to 15 mL of saline was infused using a second syringe through the same catheter port; this infusion of saline was continued for ~30 s after the end of the FF-21101(111In) administration to steadily flush the infusion line.
3.2. Planar/SPECT Imaging of FF-21101(111In)
FF-21101(111In) accumulated in the spleen, lungs, liver, kidneys, and heart contents in all six primates (Figure 1A). It is noteworthy that the testes of the male NHPs were considered a source organ due to FF-21101(111In)-specific accumulation being visible in the whole-body planar images. This necessitated a second bed position SPECT scan over the pelvic region to properly account for the radioactivity in the testes (Figure 1B). Due to the size of male NHPs, the axial extent of the first bed position SPECT scan only covered the head through to the mid-lower abdomen. In contrast, the ovaries of female NHPs did not show radiotracer accumulation above that of the surrounding body tissue, and, hence, were not considered as a source organ. As a result, only a single-bed position SPECT scan was required (Figure 1C). In addition, we have included a representative image of a female from the Phase-I clinical trial with an adenocarcinoma of the vagina, in which tumors surround the urinary bladder (Figure 1D). As a reference, subsequent FDG(18F) PET imaging obtained as standard of care demonstrates increased radiotracer uptake localizing to the vaginal tumor that corresponded to the area of FF-21101(111In) uptake (Figure 1E).
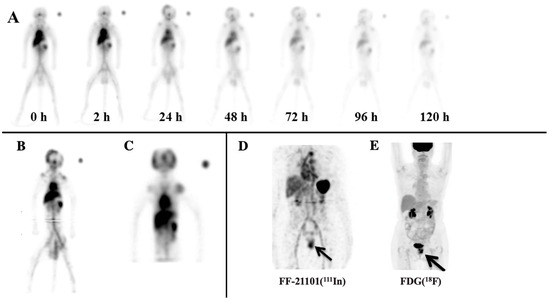
Figure 1.
Representative NHP FF-21101(111In) scans: (A) Male NHP anterior whole-body planar images (female biodistribution was the same, aside from the testes); (B) summed coronal view of the 24 h, two-bed SPECT of the same NHP male; and (C) summed coronal view of a female 24 h, single-bed SPECT. The scans demonstrate uptake in blood pool, lungs, liver, spleen, kidneys, and testes. (D) FF-21101(111In) MIP SPECT image of a 69-year-old female with an adenocarcinoma of the vagina. A black arrow indicates that FF-21101(111In) activity is increased in the recurrent tumor in the mid-to-lower left vaginal wall. (E) Subsequent MIP FDG(18F) PET demonstrated high uptake within both urinary bladder and the uterine tumor, thus confirming the location of the adenocarcinoma of the vagina.
3.3. Pharmacokinetics of FF-21101(111In)
The 0 to 120 h planar/SPECT imaging demonstrated that the hepatobiliary system was the primary route for clearance of FF-21101(111In). Hence, despite accumulation in the kidneys, radiotracer activity in the urinary bladder activity was not prominent.
The time-dependent biologic FF-21101(111In)-derived NHP FIA/mL in whole blood (Figure 2A) reached a mean peak value of 0.0037 ± 0.00093 standard error of the mean (SEM) within 2 h. Radioactivity counts decreased 40% mono-exponentially to a mean of 0.0022 ± 0.00042 SEM within 24 h after injection. At the end of the measurements, blood FIA/mL decreased by an average of 78% to a mean of 0.00079 ± 0.00016 SEM at 120 h. The corresponding time-dependent biologic FF-21101(111In)-derived humanized FIA/mL in whole blood are presented in Figure 2B, following the same pattern of decrease in radioactivity.
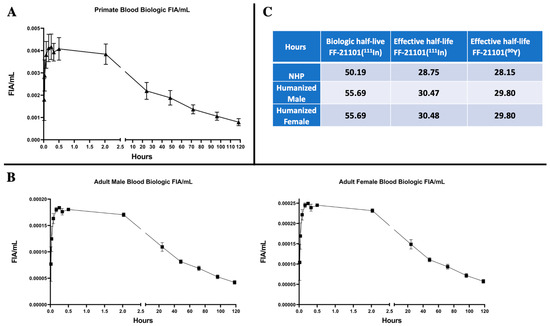
Figure 2.
Primate (A) and Humanized (B) Biologic (decay-corrected) fraction of injected activity per mL (FIA/mL) of FF-21101(111In) in the blood. Data points: Mean ± SEM. Biologic and effective half-life (C) calculated from FIAs.
As expected, relatively low accumulation of FF-21101(111In) was observed in most organs not involved in the biological clearance of FF-21101(111In)-derived radioactivity (Figure 3). We did not observe retention or accumulation of FF-21101(111In) in the brain.
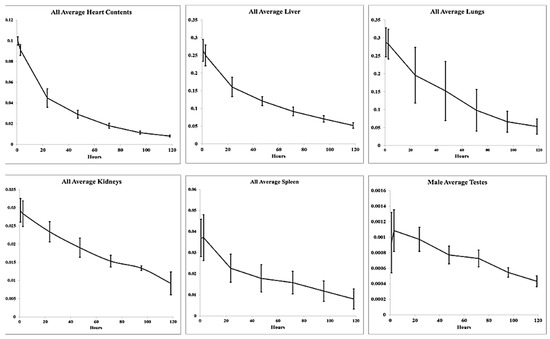
Figure 3.
Humanized fraction of FF-21101(111In) versus time in identified source organs. Data points: mean ± SD.
3.4. Radiation Dosimetry
Source organ residence times for FF-21101(111In) and FF-21101(90Y) are shown in Table 1 and Table 2, respectively; the corresponding organ doses are shown in Table 3 and Table 4.

Table 1.
Humanized FF-21101(111In) source organ residence times (h) for the derived from NHP dosimetric data.

Table 2.
Humanized FF-21101(90Y) source organ residence times (h) derived from those for FF-21101(111In).

Table 3.
OLINDA/EXM 1.1 organ radiation absorbed dose estimates for FF-21101(111In).

Table 4.
OLINDA/EXM 1.1 organ radiation absorbed dose estimates for FF-21101(90Y).
The median biologic half-life for the circulating antibody in whole blood of NHPs was 50.19 h with a median effective half-life of 28.75 h for FF-21101(111In). The corresponding median effective half-life for FF-21101(90Y) in NHPs was 28.15 h. In comparison, the derived humanized median biologic half-life of FF-21101(111In) was 55.69 h with an effective half-life of 29.8 h for FF-21101(90Y) (Figure 2C).
Although OLINDA/EXM 1.1 computes the equivalent dose (mSv/MBq), the radiation weighting factors for all the emissions (photon and electron) from 111In and 90Y are in unity, thus, the equivalent dose is the same as the radiation absorbed dose for both, i.e., 1 mSv = 1 mGy. The results are tabulated for a reference adult human (male and female). In each case, a comparison with the published absorbed doses for ibritumomab tiuxetan labeled with the same radionuclide is also provided [18]. The organs with the five-highest estimated absorbed doses for FF-21101(111In) were the lungs (0.840 mSv/MBq), spleen (0.816 mSv/MBq), liver (0.751 mSv/MBq), kidneys (0.629 mSv/MBq), and heart wall (0.451 mSv/MBq). The organs with the five-highest estimated absorbed doses for FF-21101(90Y) were similar to those for FF-21101(111In): lungs (10.49 mSv/MBq), spleen (8.21 mSv/MBq), kidneys (5.92 mSv/MBq), liver (5.46 mSv/MBq), and heart wall (2.61 mSv/MBq). Comparison between the male and female dose estimates shows similar organ doses, as well as the 111In effective dose, suggesting similar biodistribution profiles for the two genders. Similar trends were observed in human dosimetry for 111In/90Y-ibritumomab tiuxetan (Table 3 and Table 4). For all NHPs, the humanized estimates for FF-21101(90Y)-administered activity were below the applicable limits for external beam radiotherapy (<3 Gy to red marrow, <30 Gy to the liver, and <20 Gy to the kidneys and lungs, <1 Gy to the testes, <2 Gy to the ovaries) [27,28].
4. Discussion
Radioimmunotherapy and peptide receptor radionuclide therapy are both unique approaches for the targeting and treatment of tumors with a high degree of specificity. Both approaches offer great promise to deliver a lethal dose of radiation to cancer cells while significantly reducing the radiation dose to normal organs. Compounds that have been shown to have such favorable therapeutic abilities and safety profiles are 90Y-ibritumomab tiuxetan (as an example for radioimmunotherapy) and 177Lu-DOTATATE (Lutathera, Advanced Accelerator Application, USA, Inc., Millburn, NJ, USA, as an example of peptide receptor radionuclide therapy) [29]. FF-21101 is a human–mouse chimeric immunoglobulin of the IgG1 subclass that targets human P-cadherin and may prove to be another successful therapeutic agent for radioimmunotherapy applications.
This study provides information on non-target uptake levels of FF-21101(111In/90Y) in normal organs and was conducted to facilitate a Phase I clinical trial with FF-21101 radiolabeled with 111In for dosimetry and 90Y for therapy. All animals tolerated the imaging procedures and intravenous infusion of FF-21101(111In) well, with minimal discomfort. The use of the immobilizer was instrumental and helped in the fusion alignment of the planar (WB) and SPECT images, and, thus, decreased the possibility of significant errors due to organ volume contouring or dose calculation errors because of image misregistration. No significant weight loss was observed in the animals. Biodistribution and radiation dosimetry were evaluated after intravenous infusion of FF-21101(111In) in six cynomolgus macaques, with an activity range of 103.6–189.8 MBq. The radiation absorbed doses were increased in organs affected by blood flow. The source organs identified by FF-21101(111In) in decreasing order by dose were the lungs, spleen, liver, kidneys, and heart wall; and for FF-21101(90Y), the lungs, spleen, kidneys, liver, and heart wall. The amount of FF-21101(111In) that was associated with the ovaries and testes was similar, given that most of its dose came from surrounding source organs; however, since the testes are in fact source organs (whereas ovaries are not), the dose absorbed by testes from FF-21101(90Y) is much higher (1.86 in testes vs. 0.306 mSv/MBq in ovaries), as 90Y is a pure beta emitter. Thus, it is important to evaluate the dosimetry profile of animal models in both genders.
The FDA package insert specifies an effective half-life of 30 h for 90Y-ibritumomab tiuxetan, which is in agreement with our calculations [30]. The pattern of biodistribution and clearance of FF-21101(111In) in NHPs was similar to that reported in humans for 111In-ibritumomab tiuxetan [18]. All source organs exhibited uptake and mono-exponential clearance typical of an intact mAb, with the five-highest absorbed dose estimates (D) (mSv/MBq) of FF-21101(111In) in the lungs, spleen, liver, kidneys, and heart wall. The order of organs with the five-highest absorbed D values in the 111In-ibritumomab tiuxetan studies was slightly different, with the highest exposure to the liver, spleen, kidneys, osteogenic cells, and red marrow. The radiation absorbed dose from 90Y-ibritumomab tiuxetan in the lungs was low in comparison to FF-21101(90Y) (0.761 and 10.495 mSv/MBq, respectively). The spleen was shown to have the highest absorbed radiation dose from 90Y-ibritumomab tiuxetan, yet the dose from FF-21101(90Y) was double that of the spleen. The doses to the kidneys and liver followed a similar pattern to that of the spleen. The dose from FF-21101(90Y) in red marrow was significantly lower than that of 90Y-ibritumomab tiuxetan (0.857 vs. 2.73 mSv/MBq, respectively). The difference in absorbed D values is not unexpected given the mechanism of action of ibritumomab (anti-CD20) versus FF-21101 (anti-P-cadherin). 111In-ibritumomab tiuxetan is an immunoconjugate of a murine anti-CD20 mAb approved in 2002 by the FDA for assessing its biodistribution in low-grade or follicular B-cell non-Hodgkin’s lymphoma patients prior to 90Y-ibritumomab tiuxetan (Zevalin®) therapy [31].
In RPT, tumor cell killing is achieved by delivering ionizing radiation [32]. In our case, we used 90Y for the therapeutic component. This radionuclide emits high-energy beta particles (2300 keV maximum beta energy (Eβmax)), with 90% of its energy being absorbed within a sphere corresponding to 100–200 cell diameters. This gives 90Y a broad crossfire effect locally, which is ideal for treating solid tumors [20]. The favorable imaging and dosimetry results of this study further support the possibility of exploring the integration of FF-21101 in future clinical trials. One such Phase I study has already been conducted at the MD Anderson Cancer Center (ClinicalTrials.gov Identifier: NCT02454010) to investigate a number of parameters, including the determination of radiation dose estimates prior to radioimmunotherapy, its human biodistribution properties, and dosimetry profiles in primary solid tumors and metastases [33].
Table 5 compares humanized NHP organ radiation absorbed dose estimates for FF-21101(90Y) to reported human-normalized FF-21101(90Y) organ radiation absorbed dose estimates based on FF-21101(111I) dosimetric imaging reported by Subbiah et al. from a Phase I clinical trial [33]. The NHP values in this table are reported in mGy/mCi to match the units of the published human dose estimates. The table shows that most organ dose estimates correlate relatively well within acceptable uncertainties. The difference between NHP and human values for kidneys, liver, red marrow, and osteogenic cells were all within less than 30% of each other. It was previously reported that the degree of uncertainty associated with the dosimetry data in human and preclinical studies was about 20–47% [34,35,36]. The biggest discrepancies in dose estimates are noted for the spleen, testes, and lungs. Several reasons could be attributed to these discrepancies. For example, the difference in the estimated spleen dose could be attributed to the differences in the subject population between the two studies. For the patient studies, human data were obtained from 16 patients (7 male and 9 female) with advanced solid tumors, while the data from the NHP were acquired from 6 healthy subjects. Subbiah et al. [33] reported P-cadherin expression in tumors and normal tissues and demonstrated a moderate amount of P-cadherin expression in normal splenic tissue; however, as previously reported by others, nonspecific binding of mAbs as well as macrophage uptake [37,38] may cause increased tracer accumulation in the spleen, in addition to possible aggregation/complexation, which, when combined with patient tumor burden, may explain the 70% higher spleen human dose estimates. The difference in testicular values between the NHP and the human study, on the other hand, could be due to the size of cynomolgus macaques’ testes in comparison to human testes. To delineate the ROIs in NHP, testes present several challenges due to their small size and resultant coarse images that lead to increased partial volume effects, thereby potentially causing the observed difference in dose estimates. Finally, the difference in observed lung dose estimates between the NHP and human study (129%) could be attributed to the effects of placing the primate in a vacuum immobilization bag to maintain consistent positioning during each planar/SPECT- and CT-imaging session. The tracheal intubation and administration of anesthesia could cause the increase in radiotracer accumulation in the lungs due to potential atelectasis of the lung.

Table 5.
Humanized OLINDA/EXM 1.1 organ radiation absorbed dose estimates for FF-21101(90Y) compared to reported human-normalized FF-21101(90Y) organ radiation absorbed dose estimates based on FF-21101(111I) dosimetric imaging.
5. Conclusions
Overexpression of P-cadherin in several types of tumors provides an attractive target for RIT [4,5]. The biodistribution and radiation dosimetry characteristics of FF-21101(111In), as determined in NHPs, indicate that the radiation dose of this radiotracer to potential human patients is similar to that of other mAb-based agents used for planar and SPECT imaging (e.g., ibritumomab tiuxetan). The highest radiation dose is delivered to the lungs, followed by the spleen, liver, kidneys, and heart wall but all are within acceptable ranges. However, in this study, we assess that NHP internal dosimetry underestimated the radiation dose estimated in humans by 70% in the spleen and testes and overestimated the dose estimates in the lungs. Yet, the comparison of results in other organs between NHP and human data may be justified, despite differences in the acquisition parameters in tumor-bearing vs. healthy NHP subjects. Taken together, the results of this study confirm that FF-21101 conjugated to 111In for imaging and to 90Y for radioimmunotherapy can be safely translated to clinical trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15184532/s1. References [39,40,41] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, G.R., W.E., L.D.P., J.M. and O.M.; Methodology, G.R., W.E., V.S., J.M., A.P.M., J.G. and O.M.; Formal analysis, W.E., L.M. and O.M.; Investigation, V.S.; Data curation, V.P., A.P.M. and J.G.; Writing—original draft, G.R., W.E. and L.M.; Writing—review & editing, G.R., L.D.P., L.M. and O.M.; Visualization, L.M.; Supervision, G.R., V.S. and O.M.; Project administration, L.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
Research support for this study was provided by FUJIFILM Pharmaceuticals USA., Inc., Cambridge, MA, USA.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer (protocol number 2015-0220), Houston, TX, USA.
Informed Consent Statement
The image of a patient depicted on this manuscript was sourced from a clinical trial conducted at the University of Texas MD Anderson Cancer Center under the approval of the local Institutional Review Board. The specific written informed consent utilized on this clinical trial addresses the utilization of deanonymized images for publication purposes.
Data Availability Statement
The data presented in this study is available upon request from the corresponding author.
Acknowledgments
The authors would like to thank Masahiko Tokura for providing assistance during the execution of the experiments. We express our gratitude to Aaron Cherry, Adina Feldman, Jennifer Miller, Lisa Moore, and Dana Toomey for their technical assistance.
Conflicts of Interest
G. Ravizzini reports grants from FUJIFILM Pharmaceuticals U.S.A., Inc., during the conduct of the study as well as grants from Blue Earth Diagnostics, Inc. (U.S.A.), ABX GmbH, Alfasigma S.p.A., Novartis, Bayer, Amgen, Regeneron Pharmaceuticals Inc, Curium, and Clarity outside the submitted work. W.D. Erwin reports grants from FUJIFILM Pharmaceuticals U.S.A. during the conduct of the study, as well as grants from Oncosil Medical, Ltd., Ipsen Pharma SAS, and Alfasigma S.p.A. outside the submitted work. V. Subbiah reports grants from FUJIFILM Pharmaceuticals U.S.A., Inc., (clinical trials research support) during the conduct of the study; grants from FUJIFILM Pharmaceuticals U.S.A. outside the submitted work; research funding/grant support for clinical trials from Roche/Genentech, Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Northwest Biotherapeutics, Berghealth, Incyte, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfasigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Loxo Oncology, Medimmune, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, UT MD Anderson Cancer Center, Turning Point Therapeutics, and Boston Pharmaceuticals; travel support from Novartis, Pharmamar, ASCO, ESMO, Helsinn, and Incyte; consultancy/advisory board relationships with Helsinn, Loxo Oncology/Eli Lilly, R-Pharma US, Incyte, QED Pharma, Medimmune, Novartis, and Signant Health; and other from Medscape. A. McCoy and J. Gonzalez report other from FUJIFILM Pharmaceuticals U.S.A. during the conduct of the study. O. Mawlawi reports grants from FUJIFILM Pharmaceuticals U.S.A during the conduct of the study. No potential conflicts of interest were disclosed by the other authors.
References
- Conacci-Sorrell, M.E.; Ben-Yedidia, T. Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002, 16, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. The cadherins: Cell-cell adhesion molecules controlling animal morphogenesis. Development 1988, 102, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y.; Hirohashi, S. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989, 49, 2128–2133. [Google Scholar] [PubMed]
- Gamallo, C.; Moreno-Bueno, G. The prognostic significance of P-cadherin in infiltrating ductal breast carcinoma. Mod. Pathol. 2001, 14, 650–654. [Google Scholar] [CrossRef]
- Stefansson, I.M.; Salvesen, H.B. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J. Clin. Oncol. 2004, 22, 1242–1252. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yan, Z. PF-03732010: A fully human monoclonal antibody against P-cadherin with antitumor and antimetastatic activity. Clin. Cancer Res. 2010, 16, 5177–5188. [Google Scholar] [CrossRef]
- A Study of PF-03732010 in Patients with Advanced Solid Tumors. ClinicalTrials.gov ID NCT00557505. Available online: https://clinicaltrials.gov/study/NCT00557505?tab=results (accessed on 21 June 2023).
- Strosberg, J.; Krenning, E. 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 1391–1392. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Emmanouilides, C.; Witzig, T.E. Safety and efficacy of yttrium-90 ibritumomab tiuxetan in older patients with non-Hodgkin’s lymphoma. Cancer Biother. Radiopharm. 2007, 22, 684–691. [Google Scholar] [CrossRef]
- Oriuchi, N.; Higuchi, T. Current status of cancer therapy with radiolabeled monoclonal antibody. Ann. Nucl. Med. 2005, 19, 355–365. [Google Scholar] [CrossRef]
- Basch, E. Toward patient-centered drug development in oncology. N. Engl. J. Med. 2013, 369, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Stokke, C.; Gabina, P.M. Dosimetry-based treatment planning for molecular radiotherapy: A summary of the 2017 report from the Internal Dosimetry Task Force. EJNMMI Phys. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Bastiaannet, R.; van Roekel, C. First Evidence for a Dose-Response Relationship in Patients Treated with (166)Ho Radioembolization: A Prospective Study. J. Nucl. Med. 2020, 61, 608–612. [Google Scholar] [CrossRef]
- Heppeler, A.; Froidevaux, S. Radiometal-labelled macrocyclic chelator-derivatized somatostatin analogue with superb tumour-targeting properties and potential for receptor mediated internal radiotherapymediated. Chem. Eur. J. 1999, 5, 1974–1981. [Google Scholar] [CrossRef]
- O’Donoghue, J.A.; Bardies, M. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J. Nucl. Med. 1995, 36, 1902–1909. [Google Scholar]
- Fisher, D.R.; Shen, S. MIRD dose estimate report No. 20: Radiation absorbed-dose estimates for 111In- and 90Y-ibritumomab tiuxetan. J. Nucl. Med. 2009, 50, 644–652. [Google Scholar] [CrossRef]
- Lehnert, M.; Ludwig, H. Update on the rational use of Y-ibritumomab tiuxetan in the treatment of follicular lymphoma. Onco. Targets Ther. 2009, 2, 199–208. [Google Scholar]
- Howell, R.W.; Rao, D.V. Macroscopic dosimetry for radioimmunotherapy: Nonuniform activity distributions in solid tumors. Med. Phys. 1989, 16, 66–74. [Google Scholar] [CrossRef]
- Murthy, R.; Kamat, P. Radioembolization of yttrium-90 microspheres for hepatic malignancy. Semin. Interv. Radiol. 2008, 25, 48–57. [Google Scholar] [CrossRef]
- Camera, L.; Kinuya, S. Comparative biodistribution of indium- and yttrium-labeled B3 monoclonal antibody conjugated to either 2-(p-SCN-Bz)-6-methyl-DTPA (1B4M-DTPA) or 2-(p-SCN-Bz)-1,4,7,10-tetraazacyclododecane tetraacetic acid (2B-DOTA). Eur. J. Nucl. Med. 1994, 21, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Zhang, J. Theranostic Imaging of Yttrium-90. Biomed. Res. Int. 2015, 2015, 481279. [Google Scholar] [CrossRef] [PubMed]
- Naruki, Y.; Carrasquillo, J.A. Differential cellular catabolism of 111In, 90Y and 125I radiolabeled T101 anti-CD5 monoclonal antibody. Int. J. Rad. Appl. Instrum. B. 1990, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Maecke, H.R.; Riesen, A. The molecular structure of indium-DTPA. J. Nucl. Med. 1989, 30, 1235–1239. [Google Scholar]
- Adams, G.P.; Shaller, C.C. A single treatment of yttrium-90-labeled CHX-A″-C6.5 diabody inhibits the growth of established human tumor xenografts in immunodeficient mice. Cancer Res. 2004, 64, 6200–6206. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Rubin, P.; Casarett, G.W. Clinical radiation pathology as applied to curative radiotherapy. Cancer 1968, 22, 767–778. [Google Scholar] [CrossRef]
- Hope, T.A.; Abbott, A. NANETS/SNMMI Procedure Standard for Somatostatin Receptor-Based Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE. J. Nucl. Med. 2019, 60, 937–943. [Google Scholar] [CrossRef]
- Tiuxetan, I. ZEVALINTM. 2001, USA/Package Insert/ZEVALIN. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/ibriide021902lb.pdf (accessed on 27 April 2023).
- FDA. First radiopharmaceutical for non-Hodgkin’s lymphoma. FDA Consum. 2002, 36, 3. Available online: https://permanent.access.gpo.gov/lps1609/www.fda.gov/fdac/departs/2002/302_upd.html#lymphoma (accessed on 26 April 2023).
- Sgouros, G.; Dewaraja, Y.K. Tumor Response to Radiopharmaceutical Therapies: The Knowns and the Unknowns. J. Nucl. Med. 2021, 62 (Suppl. S3), 12S–22S. [Google Scholar]
- Subbiah, V.; Erwin, W. Phase I Study of P-cadherin-targeted Radioimmunotherapy with (90)Y-FF-21101 Monoclonal Antibody in Solid Tumors. Clin. Cancer Res. 2020, 26, 5830–5842. [Google Scholar] [CrossRef] [PubMed]
- Kranz, M.; Sattler, B. Radiation dosimetry of the alpha(4)beta(2) nicotinic receptor ligand (+)-[(18)F]flubatine, comparing preclinical PET/MRI and PET/CT to first-in-human PET/CT results. EJNMMI Phys. 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Garrow, A.A.; Andrews, J.P.M. Preclinical dosimetry models and the prediction of clinical doses of novel positron emission tomography radiotracers. Sci. Rep. 2020, 10, 15985. [Google Scholar] [CrossRef]
- Doss, M.; Kolb, H.C. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J. Nucl. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lobo, E.D.; Hansen, R.J. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef]
- Malik, P.; Phipps, C. Pharmacokinetic Considerations for Antibody-Drug Conjugates against Cancer. Pharm. Res. 2017, 34, 2579–2595. [Google Scholar] [CrossRef]
- Sgouros, G. Bone marrow dosimetry for radioimmunotherapy: Theoretical considerations. J. Nucl Med. 1993, 34, 689–694. [Google Scholar]
- Macey, D.J.; Williams, L.E. AAPM Report No 71, A Primer for Radioimmunotherapy and Radionuclide Therapy; Medical Physics Publishing: Madison, WI, USA, 2001. [Google Scholar]
- ICoR Protection. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values; ICRP Report No. 89; ICRP Publication: Pergamon, Turkey; Oxford, UK; New York, NY, USA, 2001. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).