Thirty-Day Complications, Unplanned Hospital Encounters, and Mortality after Endosonography and/or Guided Bronchoscopy: A Prospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
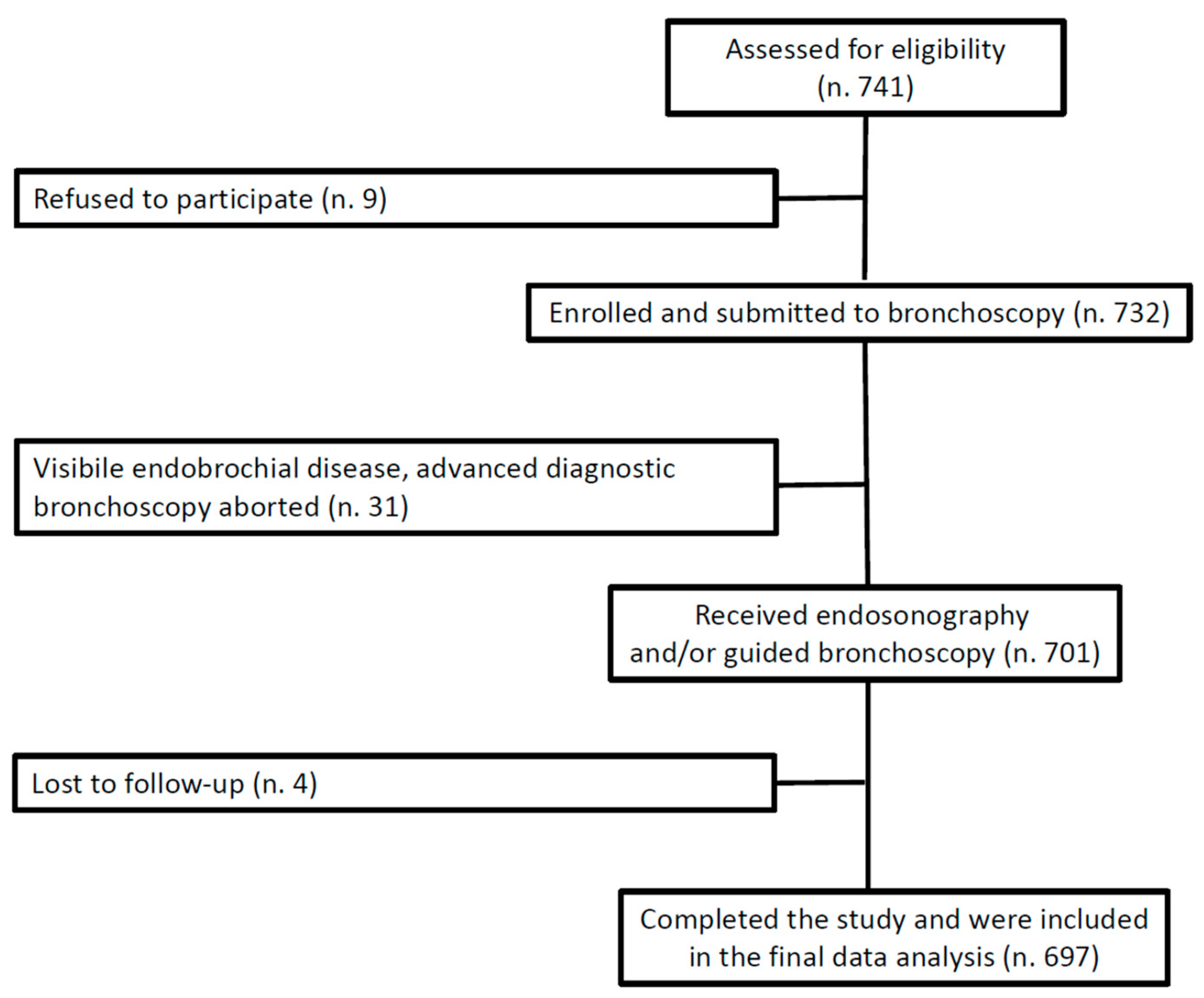
2.1. Study Design, Setting, and Participants
2.2. Anesthesia and Monitoring Protocol
2.3. Advanced Diagnostic Bronchoscopy Protocol
2.4. Data Collection
2.5. Study Outcomes and Their Assessment
2.6. Sample Size Calculation
2.7. Statistical Analysis
3. Results
3.1. Patient and Procedural Characteristics
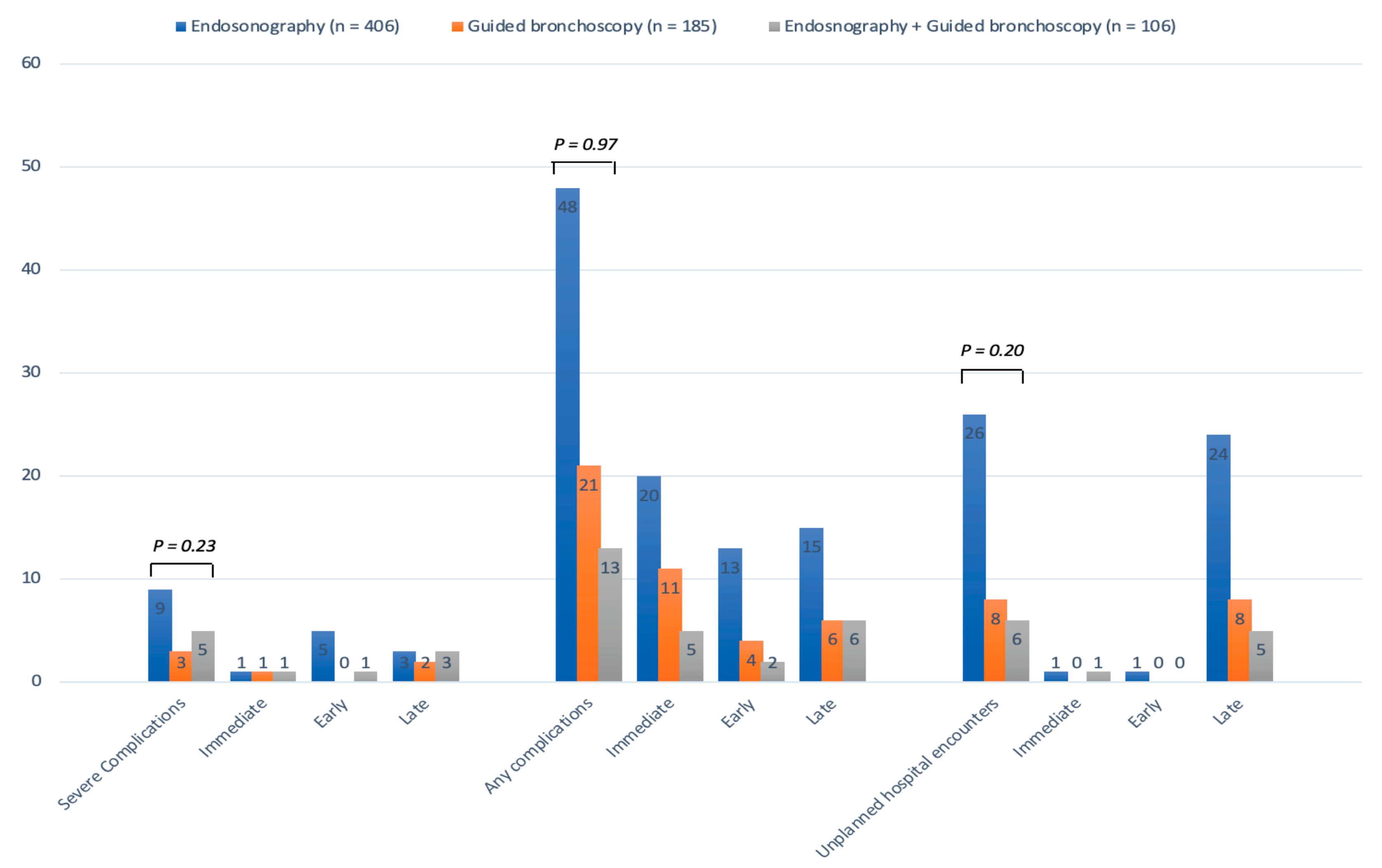
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandrica, S.; Yarmus, L. Recent developments in advanced diagnostic bronchoscopy. Eur. Resp. Rev. 2020, 29, 1901184. [Google Scholar] [CrossRef]
- Kramer, T.; Annema, J.T. Advanced bronchoscopic techniques for the diagnosis and treatment of peripheral lung cancer. Lung Cancer 2020, 161, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Cinquini, M.; Bertolaccini, L.; Del Re, M.; Facchinetti, F.; Ferrara, R.; Franchina, T.; Larici, A.R.; Malapelle, U.; Menis, J.; et al. Benefits and harms of lung cancer screening by chest computed tomography: A systematic review with meta-analysis. J. Clin. Oncol. 2021, 39, 2574–2585. [Google Scholar] [CrossRef]
- Planchard, D.; Papat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic navigation bronchoscopy for the diagnosis of peripheral pulmonary lesions: One-year result of the prospective, multicentric NAVIGATE study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Pastis, N.J., Jr.; Mahajan, A.K.; Khandhar, S.J.; Simoff, M.J.; Machuzak, M.S.; Cicenia, J.; Gildea, T.R.; Silvestri, G.A. Robotic bronchoscopy for peripheral pulmonary lesions: A multicenter pilot and feasibility study (BENEFIT). Chest 2021, 159, 845–852. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.-H.; Husta, B.C.; Adusumili, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-sensing robotic-assisted bronchoscopy in the diagnosis of pulmonary parenchymal lesions. Chest 2022, 161, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, R.L.J.; Futterer, J.J.; Hoefsloot, W.; van der Heijden, E.H.F.M. Cone-beam CT image guidance with and without electromagnetic navigation bronchoscopy for biopsy of peripheral pulmonary lesions. J. Bronchol. Intervent. Pulmonol. 2021, 28, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sundaralingam, A.; Bedawi, E.O.; Harriss, E.K.; Munavvar, M.; Rahman, N.M. Management of complications from pleural procedures. Chest 2022, 161, 1407–1425. [Google Scholar] [CrossRef]
- Asano, F.; Aoe, M.; Ohsaki, Y.; Okada, Y.; Sasada, S.; Sato, S.; Suzuki, E.; Semba, H.; Fukuoka, K.; Fujino, S.; et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: A nationwide survey by the Japan Society of Respiratory Endoscopy. Respir. Res. 2013, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Von Bartheld, M.B.; van Breda, A.; Annema, J.T. Complication rate of endosonography (endobronchial and endoscopic ultrasound): A systematic review. Respiration 2014, 87, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Xu, Y.; Sheu, T.; Volk, R.J.; Tina Shih, Y.-C. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern. Med. 2019, 179, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tina Shih, Y.-C.; Huo, J.; Mehta, H.J.; Wu, Y.; Salloum, R.G.; Alvarado, M.; Zhang, D.; Braithwaite, D.; Guo, Y.; et al. Procedural complications associated with invasive diagnostic procedures after lung cancer screening with low-dose computed tomography. Lung Cancer 2022, 165, 141–144. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, Y.; Huo, J.; Burks, C.; Ost, D.E.; Tina Shih, Y.-C. Updated analysis of complication rates associated with invasive diagnostic procedures after lung cancer screening. JAMA Netw. Open 2020, 3, e2029874. [Google Scholar] [CrossRef] [PubMed]
- Ost, D.; Ernst, A.; Lei, X.; Kovitz, K.L.; Benzaquen, S.; Diaz-Mendoza, J.; Greenhill, S.; Toth, J.; Feller-Kopman, D.; Puchalski, J.; et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016, 193, 68–77. [Google Scholar] [CrossRef]
- Eapen, G.A.; Shah Am Lei, X.; Jimenez, C.A.; Morice, R.C.; Yarmus, L.; Filner, J.; Ray, C.; Michaud, G.; Greenhill, S.R.; Sarkiss, M.; et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration. Results of the AQuIRE Registry. Chest 2013, 143, 1044–1053. [Google Scholar] [CrossRef]
- Colinet, B.; Jacot, W.; Bertrand, D.; Lacombe, S.; Bozonnat, M.C.; Daurès, J.P.; Pujol, J.L.; OncoLR Health Network. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: Description and comparison with the Charlson’s index. Br. J. Cancer 2005, 93, 1098–1105. [Google Scholar] [CrossRef]
- Austin, S.R.; Wong, Y.N.; Uzzo, R.G.; Beck, J.R.; Egleston, B.L. Why Summary Comorbidity Measures Such as the Charlson Comorbidity Index and Elixhauser Score Work. Med. Care 2015, 53, e65–e72. [Google Scholar] [CrossRef]
- Kang, N.; Shin, S.H.; Yoo, H.; Jhun, B.W.; Lee, K.; Um, S.-W.; Kim, H.; Jeong, B.-H. Infectious complications of EBUS-TBNA: A nested case control study using 10-year registry data. Lung Cancer 2021, 161, 1–8. [Google Scholar] [CrossRef]
- Souma, T.; Minezawa, T.; Yatsuya, H.; Okamura, T.; Yamatsuta, K.; Morikawa, S.; Horiguchi, T.; Maeda, S.; Goto, Y.; Hayashi, M.; et al. Risk factors of infectious complications after endobronchial ultrasound-guided transbronchial biopsy. Chest 2020, 158, 797–807. [Google Scholar] [CrossRef]
- Takiguchi, H.; Hayama, N.; Oguma, T.; Harada, K.; Sato, M.; Horio, Y.; Tanaka, J.; Tomomatsu, H.; Tomomatsu, K.; Takihara, T.; et al. Post-bronchoscopy pneumonia in patients suffering from lung cancer: Development and validation of a risk-prediction score. Respir. Investig. 2017, 55, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Taghizadeh, N.; Chee, A.; Hergott, C.A.; Dumoulin, E.; Tremblay, A.; MacEachern, P. Lesion heterogeneity and risk of infectious complications following peripheral endobronchial ultrasound. Respirology 2017, 22, 521–526. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Available online: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed on 27 August 2023).
- Von Bartheld, M.B.; Annema, J.T. Endosonography-related mortality and morbidity for pulmonary indications: A nationwide survey in the Netherlands. Gastrointest. Endosc. 2015, 82, 1009–1015. [Google Scholar] [CrossRef]
- Asano, F.; Aoe, M.; Ohsaki, Y.; Okada, Y.; Sasada, S.; Sato, S.; Suzuki, E.; Senba, H.; Fujino, S.; Ohmori, K. Deaths and complications associated with respiratory endoscopy: A survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2012, 17, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, P.J.; Munavvar, M.; Leuppi, J.D.; Mehta, A.C.; Chhajed, P.N. Endobronchial ultrasound-guided transbronchial needle aspiration: Safe as it sounds. Respirology 2017, 22, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Trick, W.; Mba, B.I.; Mohananey, D.; Sethi, J.; Musani, A. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017, 22, 443–453. [Google Scholar] [CrossRef]
- Folch, E.E.; Mahajan, A.K.; Oberg, C.L.; Maldonado, F.; Toloza, E.; Krimsky, W.S.; Oh, S.; Bowling, M.R.; Benzaquen, S.; Kinsey, C.M.; et al. Standardized definitions of bleeding after transbronchial lung biopsy: A Delphi consensus statement from the Nashville study group. Chest 2020, 158, 393–400. [Google Scholar] [CrossRef]
- Ost, D.E.; Ernst, A.; Grosu, H.; Lei, X.; Diaz-Mendoza, J.; Slade, M.; Gildea, T.R.; Machuzak, M.; Jimenez, C.A.; Toth, J.; et al. Complications following therapeutic bronchoscopy for malignant central airway obstruction. Results of the AQuIRE Registry. Chest 2015, 148, 450–471. [Google Scholar] [CrossRef]
- McCoy, C.E. Understanding the use of composite endpoints in clinical trials. West J. Emerg. Med. 2018, 19, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, R.; Livi, V.; Magnini, D.; Leoncini, F.; Porro, L.M.; Flore, M.C.; Paioli, D.; Sotgiu, G. Pattern of cancer patient referral and organizational model of an interventional pulmonology programme during the COVID-19 pandemic. ERJ Open Res. 2021, 7, 00152–02021. [Google Scholar] [CrossRef]
- Folch, E.E.; Bowling, M.R.; Pritchett, M.A.; Murgu, S.D.; Nead, M.A.; Flandes, J.; Krimsky, W.S.; Mahajan, A.K.; LeMense, G.P.; Murillo, B.A.; et al. NAVIGATE 24-month results: Electromagnetic navigation bronchoscopy for pulmonary lesions at 37 centers in Europe and the United States. J. Thorac. Oncol. 2022, 17, 519–531. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kheraj, R.; Garud, S.; Neeman, N.; Nathanson, L.A.; Kelly, C.P.; Sawhney, M.; Landon, B.; Doyle, R.; Rosenberg, S.; et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch. Inter. Med. 2010, 170, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.R.; Caverly, T.J.; Zhang, S.; Glickman, M.E.; Qian, S.X.; Boudreau, J.H.; Miller, D.; Wiener, R. Invasive procedures and associated complications following initial lung cancer screening in a national cohort of veterans. Chest 2022, 162, 475–484. [Google Scholar] [CrossRef]
- Facciolongo, N.; Patelli, M.; Gasparini, S.; Lazzari Agli, L.; Salio, M.; Simonassi, C.; Del Prato, B.; Zanoni, P. Incidence of complications in bronchoscopy. Multicentre prospective study of 20986 bronchoscopies. Monaldi Arch. Chest Dis. 2009, 71, 8–14. [Google Scholar]
- Ranasinghe, I.; Parzynski, C.S.; Searfoss, R.; Montague, J.; Lin, Z.; Allen, J.; Vender, R.; Bhat, K.; Ross, J.S.; Bernheim, S.; et al. Differences in colonoscopy quality among facilities: Development of a post-colonoscopy risk-standardized rate of unplanned hospital visits. Gastroenterol 2016, 150, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Barakat, M.T.; Girotra, M.; Lee, J.S.; Banerjee, S. Unplanned hospital encounters following endoscopic retrograde cholangiopancreatography in 3 large American states. Gastroenterol 2019, 156, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Rex, D.W. A new “quality indicator” of colonoscopy: Caveat emptor. Gastrointest. Endosc. 2016, 84, 507–511. [Google Scholar] [CrossRef]
- Aburto, M.; Perez-Izquierdo, J.; Agirre, U.; Barredo, I.; Echevarria-Uraga, J.J.; Armendariz, K.; García, S.; Bronte, O.; Gorordo, I.; Egurrola, M.; et al. Complications and hospital admission in the following 90 days after cryobiopsy performed in interstitial lung diasease. Resp. Med. 2020, 165, 105934. [Google Scholar] [CrossRef]
- Sakamoto, K.; Taniguchi, H.; Kondoh, Y.; Wakai, K.; Kimura, T.; Kataoka, K.; Hashimoto, N.; Nishiyama, O.; Hasegawa, Y. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir. Med. 2012, 106, 436–442. [Google Scholar] [CrossRef]
- Abe, M.; Tsushima, K.; Ishii, D.; Shikano, K.; Yoshioka, K.; Sakayori, M.; Suzuki, M.; Hirasawa, Y.; Ishiwata, T.; Kawasaki, T.; et al. Risk factor for acute exacerbation following bronchoalveolar lavage in patients with suspected idiopathic pulmonary fibrosis: A retrospective cohort study. Adv. Respir. Med. 2021, 89, 101–109. [Google Scholar] [CrossRef]
- Casal, R.F.; Lazarus, D.R.; Kuhl, K.; Nogueras-Gonzales, G.; Perusich, S.; Green, L.K.; Ost, D.E.; Sarkiss, M.; Jimenez, C.A.; Eapen, G.A.; et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration under general anesthesia versus moderate sedation. Am. J. Respir. Crit. Care Med. 2015, 191, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Towe, C.W.; Nead, M.A.; Rickman, O.B.; Folch, E.E.; Khandhar, S.J.; Perry, Y.; Linden, P. Safety of electromagnetic navigation bronchoscopy in patients with COPD: Results from the NAVIGATE study. J. Bronchol. Interv. Pulmonol. 2019, 26, 33–40. [Google Scholar] [CrossRef]
- Kim, B.-G.; Jeong, B.-H.; Um, S.-W.; Kim, H.; Yoo, H.; Kim, S.; Lee, K. Using short-term prophylactic antibiotics for prevention of infectious complications after radial endobronchial ultrasound-guided transbronchial biopsy. Respir. Med. 2021, 188, 106609. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nagano, T.; Okuno, K.; Nakata, K.; Takenaka, K.; Kobayashi, K.; Ishikawa, Y.; Sakashita, A.; Kotani, Y.; Funada, Y.; et al. An open-label, prospective clinical study to evaluate the efficacy of prophylactic antibiotics after diagnostic bronchoscopy. Kobe J. Med. Sci. 2012, 58, E110–E118. [Google Scholar] [PubMed]
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax 2013, 68, i1–i44. [Google Scholar] [CrossRef] [PubMed]
- Firat, S.; Bousamra, M.; Gore, E.; Byhardt, R.W. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1047–1057. [Google Scholar] [CrossRef]
- Feinstein, A.R.; Wells, C.K. A clinical-severity staging system for patients with lung cancer. Medicine 1990, 69, 1–33. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients (n = 697) |
|---|---|
| Median (IQR) age, years | 68 (58–75) |
| Males, n (%) | 399 (57.2) |
| Smoking, n (%) | |
| Current | 216 (31.0) |
| Former | 310 (44.5) |
| Never | 171 (24.5) |
| Median (IQR) BMI, kg/m2 | 25 (22–27) |
| Charlson Comorbidity Index, n (%) | |
| 0–1 | 95 (13.6) |
| 2–3 | 239 (34.3) |
| 4–5 | 201 (28.8) |
| >5 | 162 (23.2) |
| ASA score, n (%) | |
| I | 139 (19.9) |
| II | 437 (62.7) |
| III | 115 (16.5) |
| IV | 6 (0.9) |
| Fever, n (%) | 17 (2.4) |
| Dyspnea, mMRC, n (%) | |
| 0 | 91 (13.1) |
| I | 267 (38.3) |
| II | 233 (33.4) |
| III | 97 (13.9) |
| IV | 9 (1.3) |
| Sputum production, n (%) | |
| No | 488 (70.0) |
| Yes, whitish | 150 (21.5) |
| Yes, purulent | 35 (5.0) |
| Yes, hemoptysis | 24 (3.5) |
| Setting, n (%) | |
| Inpatient | 392 (56.2) |
| Outpatient | 305 (43.8) |
| Procedure type, n (%) | |
| Endosonography | 406 (58.3) |
| Guided bronchoscopy | 185 (26.5) |
| Endosonography + guided bronchoscopy | 106 (15.2) |
| Endosonography type, n (%) | |
| None | 185 (26.5) |
| EBUS | 471 (67.6) |
| EUS-B | 34 (4.9) |
| EBUS + EUS-B | 7 (1.0) |
| Median (IQR) procedure duration, minutes | 30 (23–37) |
| Median (IQR) n. of biopsy targets | 1 (1–2) |
| Target lesion, n (%) | |
| Lymph node | 327 (46.9) |
| Lung lesion | 221 (31.7) |
| Lymph node + lung lesion | 149 (21.4) |
| LDA in target lesion at CT, n (%) | |
| No | 531 (76.2) |
| Yes, lymph node(s) | 83 (11.9) |
| Yes, lung lesion(s) | 54 (7.7) |
| Yes, lymph node(s) + lung lesion(s) | 29 (4.2) |
| CNS in target lesion at endosonography, n (%) | 46/512 (9.0) |
| Peri-procedural antibiotic treatment, n (%) | |
| None | 609 (87.4) |
| Prophylactic | 15 (2.1) |
| During/post-procedure | 73 (10.5) |
| Immediate | Early | Late | ||||
|---|---|---|---|---|---|---|
| Severe complications | Type | n | Type | n | Type | n |
| Lidocaine-induced anaphylactic shock | 1 | Respiratory failure | 4 | Pulmonary infection Severe hemoptysis # Pulmonary infection and empyema Mediastinitis Acute exacerbation of pulmonary fibrosis Respiratory failure | 2 2 1 1 1 1 | |
| Air embolism | 1 | Pneumothorax | 1 | |||
| Bleeding grade ≥ 3 # | 1 | Acute coronary syndrome | 1 | |||
| Mild/Moderate complications | Type | n | Type | N | Type | n |
| Laryngospasm or bronchospasm | 17 | Fever ^ Transient (<24 h) RF ° Persistent sore throat Vasovagal syncope Persistent headache/vomiting | 7 2 2 1 1 | Hemoptysis # | 9 | |
| Bleeding grade ≤ 2 # | 16 | Fever ^ | 7 | |||
| Transient but sustained (<80%, >5 min) hypoxemia | 3 | Worsening dyspnea * | 4 |
| Variable |
No Severe Complications (n = 680) |
Severe Complications (n = 17) | p-Value |
|---|---|---|---|
| Median (IQR) age, years | 67 (58–75) | 72 (70–75) | 0.08 |
| Males, n (%) | 387 (56.9) | 12 (70.6) | 0.26 |
| Smoking, n (%) | 0.17 | ||
| Current | 210 (30.9) | 6 (35.3) | |
| Former | 300 (44.1) | 10 (58.8) | |
| Never | 170 (25.0) | 1 (5.9) | |
| Median (IQR) CCI, n (%) | 4 (2–5) | 4 (3–5) | 0.22 |
| Median (IQR) ASA score, n (%) | 2 (2–2) | 2 (2–3) | 0.30 |
| Fever, n (%) | 16 (2.4) | 1 (5.9) | 0.35 |
| Dyspnea, mMRC, n (%) | 0.22 | ||
| 0 | 90 (13.2) | 1 (5.9) | |
| I | 260 (38.2) | 7 (41.2) | |
| II | 229 (33.7) | 4 (23.5) | |
| III | 93 (13.7) | 4 (23.5) | |
| IV | 8 (1.2) | 1 (5.9) | |
| Sputum production, n (%) | 0.15 | ||
| No | 478 (70.3) | 10 (58.8) | |
| Yes, whitish | 145 (21.3) | 5 (29.4) | |
| Yes, purulent | 35 (5.2) | 0 (0.0) | |
| Yes, hemoptysis | 22 (3.2) | 2 (11.8) | |
| Setting, n (%) | 0.23 | ||
| Inpatient | 380 (55.9) | 12 (70.6) | |
| Outpatient | 300 (44.1) | 5 (29.4) | |
| Procedure type, n (%) | 0.23 | ||
| Endosonography | 397 (58.4) | 9 (52.9) | |
| Guided bronchoscopy | 182 (26.8) | 3 (17.7) | |
| Endosonography + guided bronchoscopy | 101 (14.9) | 5 (29.4) | |
| Endosonography type, n (%) | 0.66 | ||
| None | 182 (26.8) | 3 (17.7) | |
| EBUS | 458 (67.4) | 13 (76.5) | |
| EUS-B | 33 (4.9) | 1 (5.9) | |
| EBUS + EUS-B | 7 (1.0) | 0 (0.0) | |
| Median (IQR) procedure duration, minutes | 30 (23–37) | 30 (27–35) | 0.44 |
| Median (IQR) n. of biopsy targets | 1 (1–2) | 2 (1–2) | 0.19 |
| Target lesion, n (%) | 0.13 | ||
| Lymph node | 322 (47.4) | 5 (29.4) | |
| Lung lesion | 216 (31.8) | 5 (29.4) | |
| Lymph node + lung lesion | 142 (20.9) | 7 (41.2) | |
| LDA in target lesion at CT, n (%) | |||
| No | 522 (76.8) | 9 (52.9) | 0.02 |
| Yes, lymph node(s) | 81 (11.9) | 2 (11.8) | 0.99 |
| Yes, lung lesion(s) | 49 (7.2) | 5 (29.4) | <0.0001 |
| Yes, lymph node(s) + lung lesion(s) | 28 (4.1) | 1 (5.9) | 0.15 |
| CNS in target lesion at endosonography, n (%) | 44 (8.8) | 2 (14.3) | 0.36 |
| Peri-procedural antibiotic treatment, n (%) | |||
| None | 599 (88.1) | 10 (58.8) | 0.0003 |
| Prophylactic | 15 (2.2) | 0 (0.0) | 0.54 |
| During/post-procedure | 66 (9.7) | 7 (41.2) | <0.0001 |
| Procedure-Related | n = 11 | Non-Procedure-Related | n = 29 | |
|---|---|---|---|---|
| Unplanned hospital encounters | Respiratory failure Severe hemoptysis Pneumonia Mediastinitis Sepsis from lung abscess Pneumonia with empyema Coma due to air embolism Acute exacerbation of IPF Persistent headache/vomiting ° | 2 2 1 1 1 1 1 1 1 | Neoplastic disease progression | 10 |
| Cardiovascular event | 6 | |||
| Pulmonary embolism | 2 | |||
| Severe dysphagia | 2 | |||
| Chemotherapy-induced leukopenia | 1 | |||
| Urosepsis | 1 | |||
| Severe gastritis | 1 | |||
| Chemotherapy-induced anaphylaxis | 1 | |||
| Endocrine paraneoplastic syndrome | 1 | |||
| Pericardial effusion | 1 | |||
| Chest pain | 1 | |||
| Panic attack | 1 | |||
| Subcutaneous emphysema after lung surgery | 1 | |||
| Procedure-Related | n = 2 | Non-Procedure-Related | n = 22 | |
| Deaths | Acute exacerbation of IPF | 1 | Underlying malignant disease progression | 19 |
| Urosepsis | 1 | |||
| Septic shock from bilateral pneumonia ^ | v | End-stage hepatic disease | 1 | |
| CT-guided TTNA-induced massive hemoptysis | 1 |
| Variable | No Unplanned Hospital Encounters (n = 657) | Unplanned Hospital Encounters (n = 40) | p-Value |
|---|---|---|---|
| Median (IQR) age, years | 67 (58–75) | 71.5 (62–75) | 0.07 |
| Males, n (%) | 375 (57.1) | 24 (60.0) | 0.72 |
| Smoking, n (%) | |||
| Current | 203 (30.9) | 13 (32.5) | |
| Former | 290 (44.1) | 20 (50.0) | 0.56 |
| Never | 164 (25.0) | 7 (17.5) | |
| Median (IQR) CCI, n (%) | 4 (2–5) | 4 (3.0–7.5) | 0.05 |
| Median (IQR) ASA score, n (%) | 2 (2–2) | 2 (2–2) | 0.39 |
| Fever, n (%) | 16 (2.4) | 1 (2.5) | 0.64 |
| Dyspnea, mMRC, n (%) | 0.21 | ||
| 0 | 87 (13.2) | 4 (10.0) | |
| I | 255 (38.8) | 12 (30.0) | |
| II | 217 (33.0) | 16 (40.0) | |
| III | 91 (13.9) | 6 (15.0) | |
| IV | 7 (1.1) | 2 (5.0) | |
| Sputum production, n (%) | 0.07 | ||
| No | 460 (70.0) | 28 (70.0) | |
| Yes, whitish | 142 (21.6) | 8 (20.0) | |
| Yes, purulent | 35 (5.3) | 0 (0.0) | |
| Yes, hemoptysis | 20 (3.0) | 4 (10.0) | |
| Setting, n (%) | 0.03 | ||
| Inpatient | 363 (55.3) | 29 (72.5) | |
| Outpatient | 294 (44.8) | 11 (27.5) | |
| Procedure type, n (%) | 0.61 | ||
| Endosonography | 380 (57.8) | 26 (65.0) | |
| Guided bronchoscopy | 177 (26.9) | 8 (20.0) | |
| Endosonography + guided bronchoscopy | 100 (15.2) | 6 (15.0) | |
| Endosonography type, n (%) | 0.65 | ||
| None | 177 (26.9) | 8 (20.0) | |
| EBUS | 442 (67.3) | 29 (72.5) | |
| EUS-B | 31 (4.7) | 3 (7.5) | |
| EBUS + EUS-B | 7 (1.1) | 0 (0.0) | |
| Median (IQR) procedure duration, minutes | 30 (23–37) | 26.5 (21.5–31.0) | 0.02 |
| Median (IQR) n. of biopsy targets | 1 (1–2) | 1 (1–2) | 0.98 |
| Target lesion, n (%) | |||
| Lymph node | 310 (47.2) | 17 (42.5) | |
| Lung lesion | 209 (31.8) | 12 (30.0) | 0.59 |
| Lymph node + lung lesion | 138 (21.0) | 11 (27.5) | |
| LDA in target lesion at CT, n (%) | |||
| No | 509 (77.5) | 22 (55.0) | 0.001 |
| Yes, lymph node(s) | 76 (11.6) | 7 (17.5) | 0.26 |
| Yes, lung lesion(s) | 44 (6.7) | 10 (25.0) | <0.0001 |
| Yes, lymph node(s) + lung lesion(s) | 28 (4.3) | 1 (2.5) | 0.59 |
| CNS in target lesion at endosonography, n (%) | 43 (9.0) | 3 (9.4) | 0.94 |
| Peri-procedural antibiotic treatment, n (%) | |||
| None | 580 (88.3) | 29 (72.5) | 0.004 |
| Prophylactic | 15 (2.3) | 0 (0.0) | 0.33 |
| During/post-procedure | 62 (9.4) | 11 (27.5) | 0.0003 |
| Variable | No Mortality (n = 673) | Mortality (n = 24) | p-Value |
|---|---|---|---|
| Median (IQR) age, years | 67 (58–75) | 72 (68.0–77.5) | 0.01 |
| Males, n (%) | 384 (57.1) | 15 (62.5) | 0.60 |
| Smoking, n (%) | |||
| Current | 209 (31.1) | 7 (29.2) | |
| Former | 297 (44.1) | 13 (54.2) | 0.56 |
| Never | 167 (24.8) | 4 (16.7) | |
| Median (IQR) CCI, n (%) | 4 (2–5) | 6.5 (5–8) | <0.0001 |
| Median (IQR) ASA score, n (%) | 2 (2–2) | 2 (2–3) | 0.002 |
| Fever, n (%) | 15 (2.2) | 2 (8.3) | 0.11 |
| Dyspnea, mMRC, n (%) | |||
| 0 | 86 (12.8) | 5 (20.8) | 0.25 |
| I | 263 (39.1) | 4 (16.7) | 0.03 |
| II | 227 (33.7) | 6 (25.0) | 0.37 |
| III | 89 (13.2) | 8 (33.3) | 0.01 |
| IV | 8 (1.2) | 1 (4.2) | 0.20 |
| Sputum production, n (%) | |||
| No | 473 (70.3) | 15 (62.5) | |
| Yes, whitish | 143 (21.3) | 7 (29.2) | 0.62 |
| Yes, purulent | 34 (5.1) | 1 (4.2) | |
| Yes, hemoptysis | 23 (3.4) | 1 (4.2) | |
| Setting, n (%) | 0.002 | ||
| Inpatient | 371 (55.1) | 21 (87.5) | |
| Outpatient | 302 (44.9) | 3 (12.5) | |
| Procedure type, n (%) | 0.96 | ||
| Endosonography | 392 (58.3) | 14 (58.3) | |
| Guided bronchoscopy | 179 (26.6) | 6 (25.0) | |
| Endosonography + guided bronchoscopy | 102 (15.2) | 4 (16.7) | |
| Endosonography type, n (%) | 0.69 | ||
| None | 179 (26.6) | 6 (25.0) | |
| EBUS | 455 (67.6) | 16 (66.7) | |
| EUS-B | 32 (4.8) | 2 (8.3) | |
| EBUS + EUS-B | 7 (1.0) | 0 (0.0) | |
| Median (IQR) procedure duration, minutes | 30 (23–37) | 25.5 (20.0–31.5) | 0.06 |
| Median (IQR) n. of biopsy targets | 1 (1–2) | 1 (1–2) | 0.16 |
| Target lesion, n (%) | 0.59 | ||
| Lymph node | 317 (47.1) | 10 (41.7) | |
| Lung lesion | 211 (31.4) | 10 (41.7) | |
| Lymph node + lung lesion | 145 (21.5) | 4 (16.7) | |
| LDA in target lesion at CT, n (%) | |||
| No | 520 (77.3) | 11 (45.8) | 0.0004 |
| Yes, lymph node(s) | 79 (11.7) | 4 (16.7) | 0.46 |
| Yes, lung lesion(s) | 48 (7.1) | 6 (25.0) | 0.001 |
| Yes, lymph node(s) + lung lesion(s) | 26 (3.9) | 3 (12.5) | 0.04 |
| CNS in target lesion at endosonography, n (%) | 44 (8.9) | 2 (11.1) | 0.75 |
| Peri-procedural antibiotic treatment, n (%) | 0.28 | ||
| None | 590 (87.7) | 19 (79.2) | |
| Prophylactic | 14 (2.1) | 1 (4.2) | |
| During/post-procedure | 69 (10.3) | 4 (16.7) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR 95% CI | p-Value | OR 95% CI | p-Value | |
| Age, years | 1.04 (0.99–1.09) | 0.09 | 1.03 (0.99–1.09) | 0.18 |
| Males | 1.82 (0.63–5.21) | 0.27 | 1.56 (0.53–4.64) | 0.42 |
| Smoking habit | ||||
| Never | Ref. | Ref. | Ref. | Ref. |
| Current | 4.86 (0.58–40.74) | 0.15 | - | - |
| Former | 5.67 (0.72–44.65) | 0.10 | - | - |
| CCI | 1.12 (0.93–1.34) | 0.24 | - | - |
| ASA score | 1.62 (0.77–3.39) | 0.20 | - | - |
| Fever | ||||
| Yes | Ref. | Ref. | Ref. | Ref. |
| No | 0.39 (0.05–3.09) | 0.37 | - | - |
| Sputum production | 1.66 (0.62–4.41) | 0.31 | - | - |
| Setting | ||||
| Outpatient | Ref. | Ref. | Ref. | Ref. |
| Inpatient | 1.89 (0.66–5.44) | 0.24 | - | - |
| Procedure type | ||||
| Endosonography + guided bronchoscopy | Ref. | Ref. | Ref. | Ref. |
| Endosonography | 0.46 (0.15–1.40) | 0.17 | - | - |
| Guided bronchoscopy | 0.33 (0.08–1.42) | 0.14 | - | - |
| Endosonography type | ||||
| None | Ref. | Ref. | Ref. | Ref. |
| EBUS/EUS-B/EBUS-EUS-B | 1.71 (0.49–6.00) | 0.41 | - | - |
| Procedure duration, min | 1.02 (0.98–1.06) | 0.35 | - | - |
| N. of biopsy targets | 0.17 (0.73–1.87) | 0.51 | - | - |
| Target lesion | ||||
| Lymph node | Ref. | Ref. | Ref. | Ref. |
| Lung lesion | 1.49 (0.43–5.21) | 0.53 | - | - |
| Lymph node + lung lesion | 3.17 (0.99–10.17) | 0.05 | - | - |
| N. of sampled lymph nodes | 0.99 (0.62–1.57) | 0.95 | - | - |
| N. of sampled lung lesions | 1.82 (0.72–4.59) | 0.21 | - | - |
| N. of needle passes | 1.00 (0.83–1.21) | 0.99 | - | - |
| LDA in target lesion at CT | ||||
| Lymph node/lung/both | 2.94 (1.12–7.74) | 0.003 | 1.95 (0.69–5.49) | 0.21 |
| CNS in target lesion at endosonography | ||||
| Yes | Ref. | Ref. | Ref. | Ref. |
| No | 0.58 (0.13–2.68) | 0.49 | - | - |
| Peri-procedural antibiotic treatment | ||||
| Yes | Ref. | Ref. | Ref. | Ref. |
| No | 0.19 (0.07–0.52) | 0.001 | 0.25 (0.09–0.71) | 0.01 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR 95% CI | p-Value | OR 95% CI | p-Value | |
| Age, years | 1.05 (1.01–1.10) | 0.02 | 0.99 (0.95–1.04) | 0.78 |
| Males | 1.25 (0.54–2.91) | 0.60 | 0.71 (0.28–1.82) | 0.47 |
| Smoking habit | ||||
| Never | Ref. | Ref. | Ref. | Ref. |
| Current | 1.40 (1.40–4.86) | 0.60 | - | - |
| Former | 1.83 (0.59–5.69) | 0.30 | - | - |
| CCI | 1.42 (1.23–1.65) | <0.0001 | 1.37 (1.15–1.63) | <0.0001 |
| ASA score | 2.91 (1.57–5.39) | 0.001 | 2.89 (1.38–6.04) | 0.005 |
| Fever | ||||
| Yes | Ref. | Ref. | Ref. | Ref. |
| No | 0.25 (0.05–1.16) | 0.08 | - | - |
| Dyspnea (mMRC) | ||||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | 0.26 (0.07–1.00) | 0.0 | - | - |
| 2 | 0.46 (0.14–1.53) | 0.2 | - | - |
| 3–4 | 2.0 (0.52–4.95) | 0.42 | - | - |
| Sputum production | 1.42 (0.61–3.30) | 0.42 | - | - |
| Setting, No. (%) | ||||
| Outpatient | Ref. | Ref. | Ref. | Ref. |
| Inpatient | 5.70 (1.68–19.29) | 0.005 | 2.62 (0.71–9.65) | 0.15 |
| Procedure type | ||||
| Endosonography + guided bronchoscopy | Ref. | Ref. | Ref. | Ref. |
| Endosonography | 0.91 (0.29–2.83) | 0.87 | - | - |
| Guided bronchoscopy | 0.86 (0.24–3.10) | 0.81 | - | - |
| Endosonography type | ||||
| None | Ref. | Ref. | Ref. | Ref. |
| EBUS/EUS-B/EBUS-EUS-B | 1.09 (0.42–2.78) | 0.86 | - | - |
| Procedure duration, min | 0.96 (0.92–1.01) | 0.09 | - | - |
| N. of biopsy targets | 1.83 (0.50–1.37) | 0.46 | - | - |
| Target lesion | ||||
| Lymph node | Ref. | Ref. | Ref. | Ref. |
| Lung lesion | 1.50 (0.62–3.67) | 0.37 | - | - |
| Lymph node + lung lesion | 0.85 (0.27–2.84) | 0.82 | - | - |
| N. of sampled lymph nodes | 0.80 (0.52–1.23) | 0.30 | - | - |
| N. of sampled lung lesions | 1.35 (0.62–2.92) | 0.45 | - | - |
| N. of needle passes | 0.95 (0.80–1.12) | 0.51 | - | - |
| LDA in target lesion at CT | ||||
| Lymph node/lung/both | 4.02 (1.76–9.15) | 0.001 | 4.19 (1.74–10.11) | 0.001 |
| CNS in target lesion at endosonography | ||||
| Yes | Ref. | Ref. | Ref. | Ref. |
| No | 0.78 (0.17–3.51) | 0.75 | - | - |
| Peri-procedural antibiotic treatment | ||||
| Yes | Ref. | Ref. | Ref. | Ref. |
| No | 0.54 (0.19–1.47) | 0.23 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnini, D.; Sotgiu, G.; Bello, G.; Puci, M.; Livi, V.; Dell’Anna, A.M.; De Santis, P.; Dell’Ariccia, R.; Viscuso, M.; Flore, M.C.; et al. Thirty-Day Complications, Unplanned Hospital Encounters, and Mortality after Endosonography and/or Guided Bronchoscopy: A Prospective Study. Cancers 2023, 15, 4531. https://doi.org/10.3390/cancers15184531
Magnini D, Sotgiu G, Bello G, Puci M, Livi V, Dell’Anna AM, De Santis P, Dell’Ariccia R, Viscuso M, Flore MC, et al. Thirty-Day Complications, Unplanned Hospital Encounters, and Mortality after Endosonography and/or Guided Bronchoscopy: A Prospective Study. Cancers. 2023; 15(18):4531. https://doi.org/10.3390/cancers15184531
Chicago/Turabian StyleMagnini, Daniele, Giovanni Sotgiu, Giuseppe Bello, Mariangela Puci, Vanina Livi, Antonio Maria Dell’Anna, Paolo De Santis, Ruben Dell’Ariccia, Marta Viscuso, Maria Chiara Flore, and et al. 2023. "Thirty-Day Complications, Unplanned Hospital Encounters, and Mortality after Endosonography and/or Guided Bronchoscopy: A Prospective Study" Cancers 15, no. 18: 4531. https://doi.org/10.3390/cancers15184531
APA StyleMagnini, D., Sotgiu, G., Bello, G., Puci, M., Livi, V., Dell’Anna, A. M., De Santis, P., Dell’Ariccia, R., Viscuso, M., Flore, M. C., Bisanti, A., Paioli, D., Gullì, A., Leoncini, F., Antonelli, M., & Trisolini, R. (2023). Thirty-Day Complications, Unplanned Hospital Encounters, and Mortality after Endosonography and/or Guided Bronchoscopy: A Prospective Study. Cancers, 15(18), 4531. https://doi.org/10.3390/cancers15184531








