Simple Summary
Lung cancer (LC) is considered one of the most common cancers globally. Numerous studies have determined the relations between E-cigarette, or vaping, products (EVPs) and many proven environmental toxicants in LC development. Even though tobacco smoke remains the chief cause of LC, there is increasing concern that EVPs use could also increase LC risk. Consumption of EVPs has been dramatically increasing world-wide, particularly among younger people and non-smokers. This review seeks to consolidate the known environmental toxicants and EVPs contributing to LC to ensure that future research endeavors may identify key focus areas. Thus, EVPs are a highly potential risk factor for LC and an area of significant concern for the future. Since these factors have been linked to the development of LC, more research is needed to determine the mechanisms by which they affect lung pathology. Discovering the pathophysiology of EVPs use and environmental toxicant exposure in LC development can facilitate the adoption of exposure reduction strategies.
Abstract
Lung cancer (LC) is the second-most prevalent tumor worldwide. According to the most recent GLOBOCAN data, over 2.2 million LC cases were reported in 2020, with an estimated new death incident of 1,796,144 lung cancer cases. Genetic, lifestyle, and environmental exposure play an important role as risk factors for LC. E-cigarette, or vaping, products (EVPs) use has been dramatically increasing world-wide. There is growing concern that EVPs consumption may increase the risk of LC because EVPs contain several proven carcinogenic compounds. However, the relationship between EVPs and LC is not well established. E-cigarette contains nicotine derivatives (e.g., nitrosnornicotine, nitrosamine ketone), heavy metals (including organometal compounds), polycyclic aromatic hydrocarbons, and flavorings (aldehydes and complex organics). Several environmental toxicants have been proven to contribute to LC. Proven and plausible environmental carcinogens could be physical (ionizing and non-ionizing radiation), chemicals (such as asbestos, formaldehyde, and dioxins), and heavy metals (such as cobalt, arsenic, cadmium, chromium, and nickel). Air pollution, especially particulate matter (PM) emitted from vehicles and industrial exhausts, is linked with LC. Although extensive environmental exposure prevention policies and smoking reduction strategies have been adopted globally, the dangers remain. Combined, both EVPs and toxic environmental exposures may demonstrate significant synergistic oncogenicity. This review aims to analyze the current publications on the importance of the relationship between EVPs consumption and environmental toxicants in the pathogenesis of LC.
1. Introduction
Lung cancer (LC) is the second-most prevalent tumor worldwide and is considered the underlying cause of cancer-related death [1,2]. According to the most recent GLOBOCAN data, in 2020, there were 2,206,771 new cases of LC and 1,796,144 recorded deaths globally [3]. Lung cancer is the most prevalent cancer in males, followed by prostate and colorectal cancer [4]. While breast cancer is the most frequently diagnosed cancer in females, followed by LC and colorectal cancer [4]. Lung tumor is a global problem and is more frequent in 37 countries, including China, Russia, the Middle East, Eastern Europe, and Southeast Asia [5]. Despite significant advances in diagnostic strategies and effective new treatment lines, the 5-year survival rate of LC is only 10–20% [6]. Lung cancer has a poor prognosis, and more than 75% of lung cancers are diagnosed in a late advanced stage with multiple systemic metastatic, particularly in developing countries [7,8].
Several extrinsic and intrinsic factors play a significant role in LC pathogenesis. Extrinsic factors included lifestyle, environmental toxicants, occupational exposure, and some specific infections, while intrinsic influences involved sex, immune, and genetic factors [2,9,10,11]. The potential role of genetic sustainability and gene mutations and alteration in developing and progression of LC was described in numerous published studies [12,13,14]. It is proven that causally associate LC with active and passive smoking and various occupational and environmental toxic agents [10]. The respiratory tract is considered a sensitive organ that is characterized by a sizeable absorbent area exposed to several toxic agents. Exposure to these agents over time may eventually lead to oncogenesis in that tissue [15].
Traditional and electronic smoking, as well as further risk factors such as environmental toxicants, exposure to arsenic, asbestos, and air pollution, remain significant contributors to LC development as demonstrated in Figure 1 [5]. A more profound comprehension of the epidemiology and risk factors for LC can guide preventative strategies and reduce the rising disease burden globally [5,16]. E-cigarette, or vaping, products (EVPs) use has been dramatically increasing worldwide, remarkably among younger non-smokers and more females [17,18]. Unfortunately, adults aged 18 to 24 had the highest rate of EVP use, with over 2 million middle and high school students reporting use [19,20]. In the United States (US), a 900% surge in the use of EVPs among high school students was documented between 2011 and 2015 [20]. According to the Centers for Disease Control and Prevention (CDC) recent surveillance, electronic cigarettes (ECs) were the most widely used tobacco product among US teenagers in 2020 [21]. In 2019, CDC reported that over 2,500 patients were diagnosed with ECs, or vaping, product use-associated lung injury (EVALI), and they were males under 35 using vaping tetrahydrocannabinol (THC)-containing counterfeit street ECs products [22]. There is growing concern that EVPs consumption may increase the risk of LC because EVPs contain several proven carcinogenic compounds [15]. However, the relationship between EVPs and LC is not well established, and the long-term effects will take years to develop [15,23].
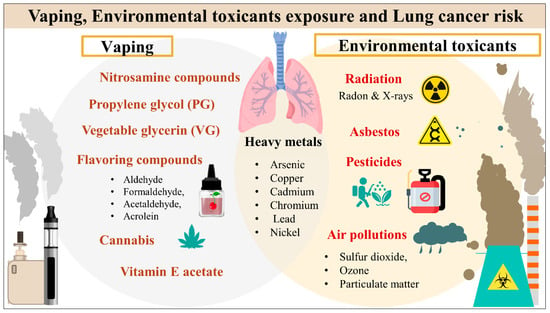
Figure 1.
Vaping products and environmental toxicants exposure and lung cancer risk.
A recent study detected more than 500 chemicals in tested vaping cartridges, and most were categorized as carcinogens [24]. Basically, E-Liquid is composed of four main ingredients: nicotine, water, flavorings and humectants, propylene glycol (PG) and vegetable glycerin (VG) [25]. An ECs contains nicotine derivatives (e.g., nitrosnornicotine, nitrosamine ketone), heavy metals, and flavorings (aldehydes and complex organics) [24]. Other proved toxins such as formaldehyde, acrolein, acetaldehyde, metallic nanoparticles, benzene, toluene, ethylbenzene, and xylene [24,26,27]. The oncogenicity of EVPs has been attributed to several distinct molecular pathways. The direct chemical reactions or carcinogenic products generated by combustion and pyrolysis could induce oxidative stress, epithelial-mesenchymal transition, and mitochondrial DNA genotoxicity [23].
There is an increase in the incidence of LC among nonsmokers, which could be attributed to environmental exposure to several known toxic or carcinogenic compounds [10]. Because of global industrialization and increased pollution, the etiological factors of LC have become increasingly complex [10]. Chronic low-dose environmental toxicants exposures have been associated with various cancers, including thyroid carcinoma [28,29], liver [28], breast [30], bladder [31], skin [32], and kidney [33]. Several experimental studies revealed that various compounds found in the environment could induce cancer by triggering cellular, gene mutation and molecular alterations [34,35]. Numerous toxic agents were classified as carcinogenic to humans based on the Environmental Protection Agency of the United States (EPA) and the International Agency for Research on Cancer (IARC) [35,36,37]. Environmental health risks are related to chemical, physical, and biological factors. Among these compounds include pesticides, asbestos, particulate matter (PM), polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons, heavy metals and physical (ionizing and non-ionizing radiations such as exposure to radon or ultraviolet (UV) radiation, respectively) [10,38]. Also, prolonged environmental exposure to air pollution increases LC risk [39]. A study in the USA observed a 40% increased risk of LC among six US cities with the highest PM levels in the air [5]. According to the IACR, arsenic has been implicated in LC, and a 3.6-fold increased risk of LC mortality has been reported among individuals living in Chile who were exposed to increased arsenic levels in their drinking water during the 1950s–1970s [40]. Hence, arsenic and PM is classified as group I carcinogen by the IARC [37].
Despite various studies on the risk factors, classification, and pathogenesis of LC, little data is devoted to the interplay between EVPs consumption and environmental toxicants that determine the development of malignancy. Combined, both toxic exposures may demonstrate significant synergistic oncogenicity [41]. The present review aims to explore the current publications on the importance of the relationship between EVPs consumption and environmental toxicants in the pathogenesis of LC.
2. Materials and Methods
The association between environmental toxicants exposure, vaping, and lung cancer was investigated by looking for research in international databases such as Scopus, PubMed, and Web of Science. Vaping device/e-liquid contents, potential carcinogens, effects of vaping smoke/e-liquid products, environmental risk factors/exposure, lung, cancer, pesticides, persistent organic pollutants (POPs), including heavy metals, polychlorinated biphenyls (PCBs), bisphenol A (BPA), phthalates, and radiation were some of the keywords applied during the search.
3. Epidemiology of Lung Cancer
Lung cancer is the second-most frequent malignance diagnosed worldwide and is considered one of the underlying causes of death [1,2]. According to the most recent GLOBOCAN data, over 2.2 million LC cases were reported in 2020, with an estimated new death incident of 1,796,144 lung cancer cases [3]. Sex variation, geographical differences, environmental contamination, occupational exposure, and histologic subtypes of LC also exhibited noticeable differences in incidence patterns [42]. Although LC is the most common male cancer, there are increasing trends in women’s LC incidence and mortality [43]. From its geographical distribution, Polynesia had the highest LC incidence, followed by Micronesia and Eastern Asia. While regarding the mortality rates, Micronesia had the highest mortality followed by Polynesia and Eastern Asia [43]. Early detection and recent advancements in LC targeting therapy decrease mortality in some high-income nations, including the United Kingdom, the United States, and Australia [43,44]. Several epidemiological studies and reports have stated that LC etiology is related to proven and potential risk factors [42]. Lung cancer risk has been connected to several jobs and industries, including metallurgy, driving, mining, and construction [45]. Smoking is the most significant and well-documented risk factor for LC. Smoking has been linked to more than 90% of LC in men, while occupational exposures are responsible for 10% to 20% [46,47]. A synergistic effect has been detected between several occupational exposures and smoking [45]. Occupational LC represents nearly 75% of all occupational cancers [47]. Smoking has been identified as the primary risk factor for LC in several studies [48,49,50]. Tobacco smoking is a known risk factor for lung cancer, with more than 70 human carcinogens identified based on the IARC report. Moreover, IARC monographs summarized the epidemiologic research findings supporting a causal link between tobacco use and lung cancer [51]. It has been observed that there is a stronger association between smoking and the LC types SCC and SCLC than adenocarcinoma and LCC [39]. It has been proven that exposure to secondhand tobacco smoke from parents or in the workplace is linked with an elevated risk of LC. However, the evidence linking childhood exposure to tobacco smoke and an increased risk of LC is scarce [52,53,54]. The mechanisms through which smoking and environmental exposures lead to raised risk of LC are not yet well established. It is suggested that exposure to carcinogenic agents could lead to oxidative stress, DNA damage, chronic inflammatory activity, growth factors, elevated cytokines, and DNA repair dysfunction [55].
Lung cancer is a heterogeneous illness with various clinicopathological characteristics [56]. Histologically, LC is categorized into two groups: small-cell lung (SCLC) and non-small-cell lung (NSCLC) [57]. The total diagnosis percentage for NSCLC is 85%, while only 15% for SCLC. The tumor origin of SCLC is poorly differentiated neuroendocrine, while NSCLC sub-types of cancer derived from lung epithelia. In addition, NSCLC is subdivided into three sub-types based mainly on the morphology of the transformed cells: adenocarcinoma (LUAD), squamous-cell carcinoma (LUSC), and large-cell carcinoma (LCC) [58]. The NSCLC subtypes developed from alveolar type II epithelial cells in LUAD and airway basal epithelial cells in LUSC [59] (Figure 2). According to the 2015 WHO classification, the most frequent subtype of LC is LUAD, followed by LUSC [60]. Unfortunately, the prognosis for all LC types is poor, specifically, LUSC and SCLC, which are predictably detected in tobacco-using male smokers [61]. The SCLC is characterized by rapid metastasis, poor prognosis, and poorly responsive to therapy [5,62].
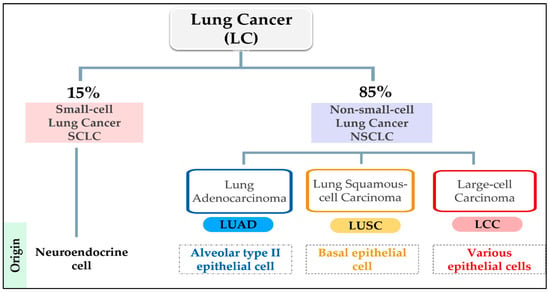
Figure 2.
Histological classification of lung cancer (LC); Small-cell Lung Cancer (SCLC); Non-small cell Lung Cancer (NSCLC); Adenocarcinoma (LUAD); Squamous-cell Carcinoma (LUSC) and Large-cell Carcinoma (LCC).
Regarding genetic susceptibility to LC, it has been recognized that nearly 85% of LC risk was linked to cigarette smoking. Hence, LC develops in 15% of smokers, proposing a differential susceptibility to the effects of tobacco carcinogens. Additionally, 10% to 15% of LC arise in non-smokers. Differences in genetic profiles probably have a role in this differential sustainability [12]. In literature, synergized smoking and environmental toxicant exposures produced carcinogenic effects and were accompanied by several somatic mutations in LC. Known mutations and loss of heterozygosity in oncogenes and tumor suppressor genes involved in lung carcinogenesis accumulate in individual somatic cells during lung tumor initiation and progression [12,13,63]. The genetic complexity of LC was stated in numerous published articles. According to the Cancer Genome Atlas, sequencing 178 squamous cell carcinomas confirmed the complexity of LC, with a mean of 165 genomic rearrangements, 360 exonic mutations, and 323 copy number alterations per tumor [64]. In smokers, numerous mutations were identified, including Kirsten rat sarcoma (KRAS), tumor protein p53 (TP53), epidermal growth factor receptor (EGFR), v-raf murine sarcoma viral oncogene homolog B (BRAF), and serine/ threonine kinase 11 (STK11) [12]. Adenocarcinoma is more likely to arise in women and individuals with a smoking history. It occurs peripherally and tests positive for targetable driver mutations such as anaplastic lymphoma kinase (ALK), EGFR, ROS1, and BRAF [5,62]. LC continues to be the most prevalent underlying cause of cancer-related death. Although there has been rapid advancement in LC screening and target treatment, further research focusing on the genetic contribution to LC susceptibility is required.
4. Vaping and Environmental Toxicants Interact as Lung Cancer Risk Factors
Co-exposure to various lung carcinogens could play a more synergistic or additive role in lung carcinogenesis than single carcinogen exposure [65,66]. Nowadays, the prevalence of vaping is on the rise accompanied by polluted air and contaminated environment. Additionally, the smoke from vaping contains several carcinogenic compounds that contribute to environmental contamination [67]. With the rapid increase in ECs users worldwide, secondhand exposure to ECs aerosols has become a serious public health concern [67]. It is proven that both smokers and second-hand exposure who live in contaminated environment are more prone to develop LC than others [67]. The incidence of LC is rising among nonsmokers or second-hand exposure which could be attributed to exposure to environmental carcinogenic compounds [10]. Importantly, more than 70 percent of inhaled E-cigarette aerosols are eventually exhaled, which may jeopardize active and negative smokers’ health [68]. The smoke from vaping contains several carcinogenic compounds that increase the risk of LC in both smoker and second-hand exposure. Vaping smoke and waste products share in environmental contamination and consequently increase the risk of LC. Exposures to specific environmental toxicants, either from vaping or other resources such as air pollution, heavy metals, and asbestos, have been reported to have a negative impact on pulmonary function and enhance lung carcinogenesis [10]. Exposure to PM2.5 is a well-established LC risk factor, and many studies have confirmed high concentrations of PM2.5 resulting from Ecs. In most cases, the reported indoor PM2.5 levels during Ecs use are above the WHO recommended threshold (25 μg/m3) [67,69]. Notably, a study detected 600 to 800 μg/m3 of PM2.5 concentrations in vape shops and vaping conventions [67,70]. The presence of carcinogenic aldehyde compounds such as acrolein, formaldehyde, and acetaldehyde in emitted smoke is detected in the indoor environment [69]. Acetaldehyde and formaldehyde are categorized by the IARC as possibly carcinogenic to humans (Group 2B) and carcinogenic to humans (Group 1), respectively [54]. Recently, electronic waste (e-waste) has received considerable attention due to its known risks to the environment and public health. Basic recycling processes release several toxicants, in particular, metals and microplastics, into the environment, impacting human health [71]. Electronic cigarette waste products such as (disposable vapes, pods or cartridges, e-liquid containers, and vape batteries) can leach contaminants into water, soil, and air [72]. Vape batteries and metal-coated wires can leach heavy metals (including lithium, lead, arsenic, aluminum, mercury, and bromines), battery acid, and nicotine into the local environment, affecting human health [67,73]. The impact of vaping smoke and waste products is exhibited in (Figure 3) Although the long-term effects of vaping on human health are not yet well established, the high levels of indoor air pollutants produced by E-cigarettes are raising alarms for public health.
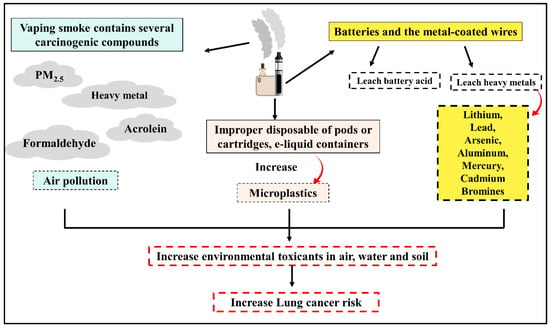
Figure 3.
Relationship between using vape and rise of environmental toxicants exposure.
5. Vaping and Lung Cancer
The term “vaping” refers to the usage of ECs or other devices to inhale a range of heated and aerosolized substances [74]. Vaping or ECs products can be used to deliver nicotine, flavorings, cannabis (CBD) and other chemicals [75]. Vaping devices were manufactured as nicotine replacement therapy to facilitate smoking cessation and decrease the negative health impact of traditional nicotine smoking [76]. The fundamental design of all vaping devices consists of three central components: a refillable or disposable liquid reservoir, a heating element, and a power source [15]. Classically, ECs convert a liquid solution comprising nicotine, VG, PG, and flavors into aerosols [77,78]. There are many various designs and styles available in the market ranging from disposable devices that resemble cigarettes to pod mods that are refillable and rechargeable using a USB cable [79,80]. Recently, modern devices such as JUUL have become more prestigious, rechargeable, stylized, controlled flavors, colorful, socially acceptable alternatives to conventional cigarettes, and equipped with attractive accessories. The rising trend of vaping among the public could be based on perceptions of the safety of flavorants and inhaling aerosol substances [81,82]. The absence of the production of carbon monoxide (CO) or other combustion-related toxic substances during vaping might increase its use. A vast improvement in the palatability of E-cigarette liquids (ELS) was observed; as nicotine-alone-based ELs have a bitter taste, there was a subsequent shift towards flavored ELs [15]. Furthermore, despite prior gains in lowering smoking rates and nicotine usage, the current data point to an alarming rise in vaping among younger people, especially adolescents [83]. From 2017 to 2018, high school students’ current usage of ECs increased from 11.7% to 20.8% and from 3.3% to 4.9% for middle school students. Current use of ECs is defined as having vaped within the previous 30 days [84]. Although the association between vaping and the development of LC is not well established, the carcinogenicity of EVPs such as nitrosamine compounds, humectants (PG and VG), flavoring compounds, CBD and vitamin E acetate has been attributed to several possible mechanisms.
5.1. Nitrosamine Compounds
Although vaping devices are marketed as safer than nicotine products, the correlation between ECs vaping and lung oncogenicity is still unknown [23]. It is documented that nitrosamine compounds attain adequate local concentrations within the distal bronchioles and alveoli, thus potentiating adduct formation and DNA damage [15]. The induced DNA methylation changes were supported by Lee et al.’s findings that ECs result from DNA adduct formation in murine bronchogenic tissues [85]. Tang et al. 2019, reported that mice exposed to ECs fumes for three months developed lung adenocarcinomas (9 of 40 mice, 22.5%); however, this tumor was particularly rare in mice exposed to filtered air or vehicle control. They suggested that ECs induces DNA damage in the lungs and inhibits DNA repair in lung tissues, implicate ECs as a lung carcinogen in mice [42]. However, another study found that stream air-vaporized nicotine is not lung carcinogenic in rats [86]. This discrepancy in findings could be explained by the fact that the aerosol size of ECs is smaller than the aerosols generated in traditional tobacco, and the small size of ECs aerosol allows nicotine to penetrate deeply into bronchioloalveolar cells [42]. Moreover, ECs induce mutagenic DNA adducts (cyclic 1,N2-γ-hydroxy-propano-deoxyguanosine [γ-OH-PdG] and O6-methyl-dG) in the mice lungs resulting in DNA damage [85]. It is suggested that tobacco smoke-generated ROS may result in lung epithelial cells’ DNA damage, prompting apoptosis and leading to the development of LC [87,88]. It has been that ECs induce similar respiratory epithelial toxicity and oxidative stress, which play a chief role in malignant transformation. This pathway appears to have multifactorial oncogenicity, with inflammation being directly associated with LC and with the adverse inflammasome/macrophage activation inducing an overall immunosuppressive “cold” environment, hostile to T-Cells—proven to be important in LC oncogenesis and malignant potential [89]. In addition, thermal breakdown of flavoring ELs creates carcinogenic organic aldehydes such as formaldehyde, acetaldehyde and acrolein [27,90]. It is recognized that formaldehyde, acrolein, and acetaldehyde are carcinogens [54,91,92].
Compared to conventional smoking, ECs create lower levels of carcinogens and toxic elements such as nicotine, PM, polycyclic aromatic hydrocarbons (PCA), formaldehyde nitrosamines, and heavy metals [15,25]. However, long-term exposure to low levels of the aforementioned elements poses serious health effects. Moreover, vaping devices can deliver substantial nicotine levels that could become addictive [25,93]. Several potential carcinogenic mechanisms could play a role in LC development, including increased DNA methylation, mutations, and binding to the nicotinic acetylcholine receptor that could induce tumorigenesis, survival, and invasion [94]. Furthermore, 80% of inhaled nicotine is metabolized into a nontoxic compound, cotinine which is excreted in urine [95]. At the same time, nearly 10% of inhaled nicotine undergoes endogenous conversion to nitrosamine compounds such as nitrosonornicotine and nitrosamine ketone [96,97]. These nitrosamine compounds are potent human carcinogens [54]. Tang et al. determined substantial nitrosamine ketone derivative levels 4-(methylnitrosoamino)-4-(3-pyidyl)-1-butanol in pulmonary tissues [98].
5.2. Propylene Glycol and Vegetable Glycerin
Propylene glycol (PG) and vegetable glycerin (VG) are humectants commonly used in ELs with different ratios to produce aerosols that simulate traditional tobacco cigarette smoke [99]. The PG/VG ratio in the ELs is modified according to preferred plume (higher concentration of VG) or flavor (higher concentration of PG) [75]. The Food and Drug Administration (FDA) has approved the dermal or oral use of PG and VG in food, cosmetics and medications [100]. Although the FDA has categorized these humectants as generally recognized as safe (GRAS), the safety data of inhalation exposure to these elements and their thermal degradation products in ECs is limited [101]. The heated PG and VG in ECs are known to undergo thermal degradation, producing pulmonary irritants, free radicals and suspected carcinogenic carbonyl compounds (acetaldehyde, formaldehyde, and acrolein) [102]. It has been proposed that chronic PG/VG exposure damages epithelial barrier function by reducing lung cell volume and membrane fluidity, which could contribute to airway damage [103]. Many studies have observed that exposure to aerosols from PG and VG leads to an increase in pro-inflammatory and oxidative stress reactions [104,105,106]. A recent study found that PG is metabolized to methylglyoxal (MGO) in airway epithelia leading to altering mucociliary function via reduction of Ca2+ activated and voltage-dependent K+ (BK) channels [107]. It is suggested that MGO is a potent glycation agent in the human body resulting glycation of proteins, DNA and lipids and the gradual accumulation of advanced glycation end products (AGEs) in cells and tissues [108]. Recently, emerging evidence indicated that MGO plays a role in cancer development and progression [109]. Furthermore, Huynh et al. 2020 demonstrated the lung colonization-promoting effects of ECs on human breast cancer cells, indicating the risks of ECs on the lung metastasis of various cancers [110].
5.3. Flavoring Compounds
Vaping offers a diverse range of flavors, which is one of the main ECS attractions among youth and non-smokers [27]. The traditional mint flavor was recently replaced by a wide range of artificial contaminants and flavoring liquids with a high risk of pulmonary toxicities [25]. Several flavoring additives are aldehydes, and recent studies have examined the impact of toxic aldehyde emissions on human health during vaping [27]. There are growing concerns regarding the safety profile of ELs flavors compounds [111]. Importantly, ECs generate vapors using a heating element, which can lead to the decomposition of ELs ingredients. As coil temperatures rise, the liability of oxidation, pyrolysis, and thermal decomposition of organic aldehydes increases [100,101]. Thermal breakdown of flavoring ELs creates toxic organic aldehydes during vaping at high concentration levels that exceed occupational safety guidelines [90]. Numerous studies have exhibited the formation of toxic aldehydes, especially formaldehyde, in ECs vapors during vaping [27]. Gillman et al. discovered that the aldehyde emission levels in flavored ELs were 150–200% greater than those in unflavored e-liquids [112]. Many organic aldehyde derivatives are included (formaldehyde, acetaldehyde, acrolein, glyoxal, hexanaldehyde, and methanol) [27]. The data regarding the impact of ELs flavors mixture on inhalation toxicity and lung oncogenesis are insufficiently well established. Also, it is unclear whether the concentration limits are relevant to human exposure levels [111]. It is recognized that formaldehyde, acrolein, and acetaldehyde are carcinogens [54,91,92]. Several mechanisms have been suggested by which flavoring compounds in ECs could contribute to LC [15,27]. It is found that flavoring compounds in ELs induce intense toxic activity, an inflammatory response with macrophage activation and chemotaxis, vascular injury, dyslipidemia, and increased platelet reactivity) [113,114]. These inflammatory and toxic processes lead to the formation of reactive oxygen species (ROS) and encourage oxidative stress-induced lung tissue damage [115]. The ROS could be generated intracellularly (via mitochondrial oxidative phosphorylation) or from exogenous sources (E-cigarette aerosols or cigarette smoke) [116]. ROS plays a significant role in modulating the immune-inflammatory system and causes damage to DNA, [95] cellular membranes, lipids, and proteins [117,118]. Raised ROS levels could lead to the activation of polymorphonuclear neutrophils (PMNs), which generate further ROS in lung tissue [119]. A study by Zahedi et al. demonstrated increased epithelial-mesenchymal transition (EMT) in A549 CCL-185 lung cancer cells, with resultant increased invasive/metastatic potential, on exposure to various flavored ELs including menthol based [120]. Additionally, Nair et al. found that the pro-inflammatory effects of menthol were mediated directly via TRPM8, resulting in calcium influx in a BEAS-2B cell-line model [121]. Remarkably, altered intracellular calcium through TRPM8 has been previously shown to induce a neoplastic phenotype in LC [122]. Another suggested ECS adverse effect is direct suppression on pulmonary epithelial cilia movement leading to impairment of clearance of toxic particles and increase the risk of respiratory infections [123].
5.4. Cannabidiol (CBD) Vaping Products
Not only nicotine is used in ECs, but other vaporized substances, especially cannabis derivatives, are widely used worldwide [124]. Cannabidiol (CBD) vaping products have become extensively accessible in the United state since their legalization in 2018. However, data are scarce on the relationship between LC risk and vaping cannabis. Other known risk factors for LC, such as chronic tobacco use and flavoring compounds, could confound with cannabis and play a chief role in LC carcinogenesis [125]. Even though most ELs contain nicotine, CBD and cannabinoid based ELs consumption has increased significantly, especially among the younger population [126]. The consumption rates jump by 16.6% in Canada, 13.8% in the US, and 9.0% in the UK [124,126]. It is unclear how vaping CBD negatively affects respiratory cell function. Cannabis ELs are prone to thermal decomposition and pyrolysis, yielding diverse potentially toxic organic compounds [127]. Cannabidiol vaping products oxidized into a reactive CBD quinone (CBDQ), which generates adducts with protein cysteine residues, altering protein function [112]. CBDQ was found to induce cytotoxicity, apoptosis in specific cells, liver toxicity, and inhibit topoisomerase II and angiogenesis. Thus, CBD can potentially have harmful adverse effects on lung cells [128]. It has been revealed that aerosolized CBD induces apoptosis, pro-inflammatory reactions, ROS generation, and enhanced cytotoxicity in bronchial epithelial cell lines [129,130]. The potential for cannabis oncogenicity could be attributed to toxic and pro-inflammatory effects on respiratory functions and can cause pulmonary irritation [15]. Notably, the meta-analysis of CBD smoking and LC risk by Zhang et al. failed to determine an increased risk of LC with cannabis use [131]. Another study by Thomas et al. explored that synthetic cannabinoid-based ECs result in significant and relatively unpredictable pyrolytic organic reactions [132].
Different oily substances were used in many ELs as thickening agents and diluents. Vitamin E acetate (VEA) was used as oil in ECs, mainly cannabinoid-based e-liquids [133]. Recent studies reveal that pyrolysis of cannabinoid and nicotine E-cigarette mixtures can produce hazardous toxicants whose synergistic actions potentially drive acute lung injury upon inhalation [133]. Emerging evidence indicates a remarkable rise in E-cigarette or vaping-associated lung injury (EVALI) among cannabinoid-based vaping users, characterized by acute lung injury or organizing pneumonia [134]. The US Centers for Disease Control and Prevention (CDC) documented 2807 EVALI cases in hospitals and sixty-eight deaths in the US [135]. Generally, VEA is a relatively safe biologically inert compound; however, several EVALI cases have recently been documented [136]. Notably, VEA has been determined in the bronchoalveolar lavage fluid of 29 patients diagnosed with EVALI. It is suggested that vitamin E acetate and other ECS compounds play a significant role in the pathogenesis of this injury [137]. Wu et al. proved that the thermal decomposition of VEA produces highly toxic and irritant ketene gas (via elimination of the aryl acetate group) along with several other toxic ROS with noticeable carcinogenic activity, including benzene and various alkenes [138]. The aforementioned thermal decomposition of VEA plays an important role in lung carcinogenesis. Wu and O’Shea’s study demonstrated that vaping VEA can lead to exposure to the toxic gas ketene [138]. In animal experiments, severe, acute lung damage was observed after 24 days of ketene exposure. The Acute Exposure Guideline Level (lethal) 10-min exposure value for ketene is 0.24 ppm [139]. Table 1 summarizes the common additives and pollutants in vaping fluid, their mechanisms of pulmonary damage, or related toxicities.

Table 1.
The common additives and pollutants in vaping liquid, their mechanisms of pulmonary damage, or related toxicities.
Increased use of E-cigarettes and ECs has raised numerous adverse health concerns involving the risks of heavy metals exposure via Els and vapors [145]. Current studies have confirmed that many heavy metals are present in both EC liquids and vapors at potentially harmful levels, which endangers both user and passive vaping [146]. Several metal levels have been detected in ECs, ELs, and human biological samples collected from vaping users [147]. The most commonly found metals were arsenic (As), copper (Cu), cadmium (Cd), chromium (Cr), lead (Pb), nickel (Ni), iron (Fe), and zinc (Zn) [148]. The source of these metals is commonly from the metal coils incorporated in the clearomizer of the ECs device or from e-liquids [149]. Mikheev et al. found that the previous metal presence in nanoparticle size was less than 2.5 µm [150]. Hence, the ultrafine size range is more dangerous to the lungs than larger ones due to their ready access to the alveolar region and rapid absorption systemically [151]. Heavy metal exposure in ECs is linked to significant health threats, such as neurotoxic and carcinogenic effects [152]. Chronic inhalation of lead nanoparticles is linked with respiratory and central nervous system pathological changes [153]. Co-exposure to several heavy metals in ECs caused oxidative stress as indicated by increases in the generation of ROS and the expression of ferritin light chain mRNA and heme oxygenase-1 mRNA and protein [141]. Heavy metals prompt apoptosis and evoke oxidative stress and DNA damage in lung cells [141].
The potential harmful consequences of E-cigarettes are also linked to respiratory system damage [154]. The published data regarding heavy metal exposure in ECs and the risk of LC is scarce. Because E-cigarette use is still relatively new, there may not have been enough opportunity to observe long-term impacts, including LC [154]. Significantly, Ni is classified as a respiratory carcinogen [54]., and the lung represents the most sensitive target of Ni toxicity [155]. Fowles et al. determined Cr and Ni in Els and aerosols and stated that prolonged exposure to Ni could substantially enhance the carcinogenesis process [154]. Hess et al. documented high levels of metal concentrations up to 400-fold in ECs, particularly Cd, Cr, Pb, and Ni in Els [156,157].
6. Environmental Toxicants and Lung Cancer
Human exposure to environmental toxic substances with different mechanisms of action is a growing concern [35]. Even though tobacco smoking is a potent lung carcinogen, a significant percentage of lung cancer mortality occurs in non-smokers. Other risk factors besides smoking can contribute to 15–25% of all LC of non-smokers; however, its epidemiology is poorly established [158,159]. It is recognized that various chemicals in environmental contexts have been proposed to affect health [160]. Exposure to known and probable respiratory carcinogens, such as metals and organic toxicants, is an essential enhancer of carcinogenesis [161]. Chronic pulmonary inflammation is a significant risk factor for LC tumorigenesis [162]. The association between environmental toxicants and LC in epidemiological evidence is poorly established [35]. However, numerous experimental investigations have demonstrated that several substances, such as heavy metals, ionizing radiation, pesticides, dust and fibers, household coal, arsenic, asbestos, and polycyclic aromatic hydrocarbons, can cause cellular and molecular changes that can facilitate the development of cancer [54]. The lifelong exposure to various toxicants, dosing, confounding variables, and human physiological diversity are essential issues in LC development [12,39,45]. Recently, the adverse effects of environmental toxicants on the lungs have been an area of intense investigation. Figure 4 summarizes the potential mechanisms by which the environmental toxicants can induce LC. However, it is worth noting that the environmental, dietary, and life habit factors could influence the molecular pathologies and pathogenic processes of LC [163,164,165,166]. For example, by reducing oxidative stress levels, DNA oxidative damage, and modulating epigenetics, some nutrients and phytochemicals could have antioxidant/anti-inflammatory properties that can affect or prevent LC pathogenesis [167,168]. It was evident that the use of dietary antioxidants and/or the nutritional supplements of vitamins and minerals in patients with cancer, including LC, has reduced the cancer growth/mutation rates and induced differentiation/de-differentiation [163]. A link between smoking and specific mutational signatures has been well known. Smoking could increase cancer risk by increasing the somatic mutation load, including base substitutions, indels, and copy number changes associated with the misreplication of DNA damage [169,170].
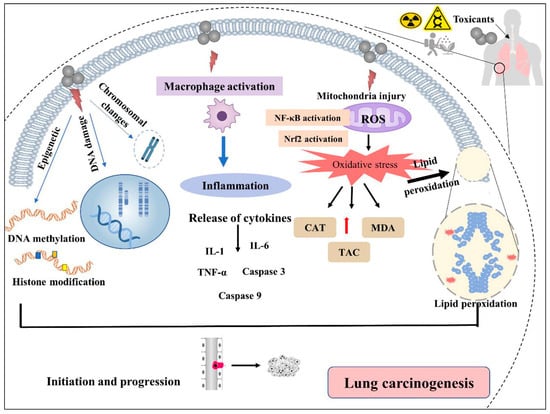
Figure 4.
Mechanisms of environmental toxicants causing lung cancer. DNAdsb: double-strand DNA breaks, ROS: reactive oxygen species, NF-κB: nuclear factor kappa B, Nrf2: nuclear factor erythroid 2–related factor 2, IL-1/6 interleukin-1/6, TNF-α: tumor necrosis factor-alpha, Caspase: cysteine-dependent aspartate-directed proteases, CAT: Catalase, MDA: malondialdehyde, TAC: Total antioxidant capacity.
Additionally, the interplay between the mutational signature landscape of the tumor and other outdoor/indoor exposures in increasing the risk of lung cancer in never-smokers was confirmed in the “Sherlock-Lung study; 2018–ongoing” [171]. Collectively, these studies highlight the limitation of dependence on just one aspect in determining the risk, diagnosis, and therapeutic approach to patients with cancers, including lung cancer, in the era of precision medicine/personalized treatment [172,173].
6.1. Radiation
Exposure to radiation causes cancer; in particular, LC has been well-established in the literature. Previous research has demonstrated that all ionizing radiation groups are carcinogenic to humans [54]. The main radiation types linked to lung cancer include X-rays, γ-rays, and α-particles [174]. Regarding the source of radiation, medically related procedures account for 48% of the average individual’s radiation exposure in the US [175]. Current research revealed that exposure to diagnostic radiography was associated with an increased risk of LC.
6.1.1. Radon
Radon is recognized as an essential natural environmental lung carcinogen (group 1), and the exposure occurs in occupational and outside the workplace [41]. Radon is a colorless, radioactive, odorless gas produced in the uranium-238 decay chain [176]. Radon is found outdoors, in soil, mines, dissolved radon in water, and in-built environments, such as offices, homes, and schools. Indoor radon is the most significant source of natural radiation to which humans are exposed (approximately 50%) [177]. Radon released from the soil into the atmosphere depends on the Uranium-238 content of the geological substrate on which these buildings are settled, soil parameters (porosity, density, and humidity), and weather conditions (wind, rain, and humidity, etc.) [178,179]. Importantly, radon is the second LC risk factor after smoking and may be responsible for 3–14% of LC cases, based on the WHO report [180]. Several previous publications documented a significant increase in LC mortality with cumulative exposure to radon and its decay products [181]. In Europe, nearly 21,000 deaths (2%) from cancer were attributed to radon [180,182]. Radon exposure in mining was linked to LC risk, as recognized by numerous investigations of non-smoking underground mine workers [183,184,185]. A study done by Darby et al. from 13 European case-controlled studies on 7148 cases confirmed a statistically linear increase of 16% (range, 5–31%) of LC risk per 100 Bq/m3 of indoor radon [182].
Radon emits α-ionizing particles, such as polonium-218 (218Po) and polonium-214 (214Po), associated with various genotoxic and cytotoxic effects. Although radon leads to DNA damage and genomic instability, the particular carcinogenesis mechanism in LC remains unidentified [186]. Alpha radiation releases energy, more than gamma and beta radiation. The released energy interacts with respiratory epithelium DNA differently, inducing DNA breaking, deletions, mutations, substitutions, and chromosomal changes [187,188]. The outcomes of genomic instability include cell cycle alteration, cytokine dysregulation, cell cycle regulation-related protein overexpression, apoptosis, and carcinogenesis [187]. Additionally, radon leads to oxidative stress and the release of ROS and hydroxyl radical attack [181,189]. Lim et al. detected genomic instability among high radon tumors in the forms of DNA damage and repair, such as ATRX, ATR, RAD50, BARD1, TP53, and SMARCA4 [190]. In chronic radon exposure, Chen et al. found that mutant KRAS was overexpressed in bronchial epithelial cells, linked to oxidative damage and let-7 downregulation [191]. It is observed that high radon levels cause chromosomal arrangements and micronuclei in miners [187]. Moreover, epigenetic influences play a significant role in radon carcinogenesis, including DNA methylation, alteration of histones, and microRNA dysregulation [192]. On a molecular basis, the miRNA dysregulation associated with radon exposure involved the upregulation of microRNA-15 (miR-15), mirR-19, miR-16, and miR-23, as well as the downregulation of miR-369, miR-373, let-7, miR-124, miR-194, miR-652, and mirR-146 [192,193,194,195]. Consequently, these miRNA dysregulations result in altered DNA methylation, oxidative stress, cell cycle, inflammation, and malignant transformation in patients with LC exposed to radon [181]. Identification of the cellular and molecular basis of radon-induced LC is essential and would provide significant assistance in the reduction of radon-induced carcinogenesis. [187,192].
6.1.2. Medical Radiation
Medical ionizing radiation exposure from diagnostic X-rays and radiation therapy γ-rays has been linked to different cancers. According to the IARC report, X-rays and γ-rays are classified as Group 1 lung carcinogens [54]. It is observed that stomach cancer is the first leading type of cancer, followed by LC, based on the Lifespan Study (LSS) of atomic bomb survivors in Nagasaki and Hiroshima, Japan [196]. Several studies found that risk estimates on second LC. Among patients who were treated between 1961 and 2007, 40% of them received radiotherapy; there was a significant association between radiotherapy and second LC with a RR of 1.23 (95% CI 1.07–1.43) [197]. A study explored the risk of radiation-induced LC in 10 years among women diagnosed with breast cancer who received radiation therapy. It is reported that the risk of LC overall was raised in females who underwent irradiation compared with those who were not irradiated, with a relative risk of 2.0 (95% confidence interval, 1.0–4.3) [198]. However, the evidence for ionizing radiation and LC relationship is inconclusive. Further studies that investigate the genetic radiation-molecular signature are recommended.
6.2. Air Pollution
The respiratory tract is a sensitive organ in contact with the external atmosphere and is liable to be exposed to air pollutants daily. Thus, a chronic inflammatory response and oxidative damage were provoked to deal with these foreign toxic particles, raising the risk of LC development [199,200]. Environmental exposures to harmful particles and gases, such as sulfur dioxide, ozone, and particulate matter (PM), have raised questions about the carcinogenesis of prolonged exposure to LC [201,202]. The WHO has concluded that there is an increased risk of lung cancer, with an estimated 250,000 worldwide deaths per year attributable to atmospheric pollution [203]. Although the association between air pollution and LC risk was studied in several epidemiological prospective research, this association was debatable, and other risk factors could be shared in LC [201]. A recent study discovered that high PM2.5 exposure, high genetic risk, and smoking were strongly linked to LC occurrence [48].
Airborne Particulate Matter (PM)
A complete understanding of how exposure to environmental air pollution provoked cancer development is lacking [204]. Air pollution exposure assessment has been challenging since several indoor and outdoor activities yield various air pollutants. Cooking and incense burning were considered significant indoor air pollutants linked to LC [205]. The “airborne particulate matter” (PM) terminology refers to a complex mixture of liquid and solid particles with varying compositions and sizes. PM is a significant component of air pollution and has been demonstrated to raise the risk of cancers and other diseases [206,207]. PM is categorized according to the diameter of the particle into coarse (2.5–10 μm, PM10), fine (0.1–2.5 μm, PM2.5), and ultrafine (≤0.1 μm, PM1). Different sources and human activities produce PM of various sizes, and PM has been extensively utilized in monitoring air pollution [208]. Long-term exposure to air pollution, especially particles with aerodynamic diameters ≤2.5 μm (PM2.5), has been a significant environmental risk factor for LC. Exposure to high levels of PM2.5 for a long time may cause inflammatory reactions and recurrent particle deposition, which can alter lung cell functions and increase the risk of LC [209]. According to IARC, PM was categorized as human carcinogenic (Group 1) in 2013 [54]. The adverse health effect of PM2.5 on LC has been well established, and a significant relationship between PM and incident LC has been observed in many studies [206,207,210,211]. In Rome, a 9-prospective study reported that the rise of each 10 μg/m3 PM2.5 level resulted in increases in LC Hazard ratio (HR) mortality of up to 6.2% [212], and these results were consistent with a US cohort research with an average follow-up of 8 years (HR = 1.33 for per 5 μg/m3) [211].
The association between LC of different histological subtypes and PM2.5 is yet underdeveloped, and future research focusing on this aspect is needed. One European Study of Cohorts for Air Pollution Effects (ESCAPE) determined that the HRs for LUAD and LUSC for the studied groups who did not change residence during the research period were 1.65 and 0.65 per 5 μg/m3 [213]. Similarly, a Canadian study observed that the HRs of LUAD and LUSC among females were 1.44 (95 % CI: 1.06, 1.97) and 1.28 (95 % CI: 0.74, 2.23) with a 10 μg/m3 increase in PM2.5 concentration [214]. In contrast, another study discovered no significant associations between PM2.5 and LC subtypes [215]. Numerous factors could play a role in PM2.5 carcinogenesis, such as the variable composition of PM2.5 in various locations, various exposure measurement techniques, different susceptibility to PM2.5, and durations of the study [48].
Many biological processes could be responsible for LC development, such as activating particular oncogenes mediated by microRNAs (miRs) leading to LC. It is suggested that PM2.5 encourages LC by acting on pre-existing oncogenic mutations in healthy lung cells [216]. Hill et al. reported a significant association between PM2.5 levels and the incidence of LC in 32,957 EGFR-driven LC cases. They investigated the sequences of tumor DNA samples from non-smokers living in polluted areas and reported that the sequences reveal few genetic point mutations, which can stimulate genes established to drive cancer growth [204]. Another study found that PM2.5 caused upregulation of the expression of three target oncogenes, namely serpin family B member 2, solute carrier family 30, member 1, and aldo-keto reductase family 1 member C1, which are significantly expressed in human LUSC [217].
6.3. Heavy Metals
Heavy metals are naturally existing metals having elemental densities larger than 5 gcm−3 and atomic numbers more than 20 [218]. Global industrialization, rapid urbanization, and mismanagement of wastes have imported heavy metals into the living environment [219]. Importantly, improper, uncontrolled disposal of industrial effluents leads to the presence of these heavy metals in water, land, and air [220]. Heavy metals are significant environmental toxicants globally, and their toxicity is a major public health concern [221]. Health adverse effects from metals in food, water, and air have been reported [1,2,3,4,5,6,7,8,9]. Heavy metals include industrial or electronic wastes, gasoline and diesel engine exhausts, pesticides, paints, incinerators, and agricultural products; they can be easily absorbed via ingestion, inhalation, or dermatological routes [6]. A large quantity of metal is used in electronic products, such as nickel (Ni), cadmium (Cd), chromium (Cr), and arsenic (As), which increases the risk of LC [222]. As a result, there is a possibility that the high metal concentrations released from e-waste could deteriorate health [223]. Although many essential metals, such as iron, magnesium, copper, and zinc, are required in recommended concentrations for human body biological processes, higher concentrations may be harmful [224]. Contrarily, other heavy metals, namely As, Cd, Cr, and Ni, have no detectable effect on biological activities and have been associated with the increased risk of LC [225,226,227].
6.3.1. Cadmium (Cd)
Cadmium is a category I pulmonary carcinogen that is a ubiquitous environmental pollutant with a highly toxic impact on human beings. Cadmium targets the liver, testes, lungs, and kidneys following acute and chronic intoxication and also stimulates tumorigenesis in prolonged exposures [228]. Exposure to Cd occurs through the inhalation of tobacco smoke or polluted air and the ingestion of contaminated water and food [229]. It is known that smoking cigarettes is the most common source of Cd exposure, and Mona et al. observed a significant increase in Cd concentration in smokers’ urine and serum compared to non-smokers [230]. Environmental and occupational Cd exposure has been associated with breast, nasopharynx, lung, prostate, urinary bladder, and pancreas cancers [231]. Lee et al. observed that participants who lived in highly polluted zones suffered from an increased prevalence of LC and a nearly 1.25-fold rise in the incidence of the risk of LC for each 1 μg/g-creatinine increase in the urine Cd level. Also, they found that patients with LC had significantly higher urinary Cd levels with a worse prognosis [227]. Another meta-analytical study stated that an estimated 68 percent increase (p < 0.0001) in the relative risk of LC was observed with a doubling of urinary Cd [232]. The association between Cd levels and Lc histological types is unclear; however, Demir et al. detected high Cd levels in patients with advanced stages of squamous and large cell LC compared with remaining LC types [233]. Another study reported that patients with squamous cell LC had greater urinary Cd concentration [227].
It has been known that Cd increases oxidative stress in ROS generation and provokes inflammatory cytokine production, contributing to respiratory tract irritation and pulmonary edema. Furthermore, Cd-induced apoptosis could be induced by Ca2+ accumulation, ROS production, Bcl-2 reduction, and apoptotic genes (e.g., Bcl-2, P53, Bax, Caspase 3, and Caspase 9) dysregulation [228,234]. In addition, Cd altered mitochondria functions in various pathologies, including oxidative stress and producing ROS, triggering apoptosis, altering gene expression, mutating mtDNA, insufficiency ATP release, and lipid peroxidation [235]. In animal studies, Cd leads to genotoxicity via DNA strand breaks, mutations, chromosomal damage, impaired DNA repair, and cell transformation [236].
6.3.2. Arsenic (As)
Arsenic is a highly toxic category I carcinogen metal responsible for different toxicity mechanisms and harmful effects on human organs, particularly the lungs [237]. Arsenic exposure can occur through ingestion and inhalation, respectively, in the form of soluble arsenite and particulate arsenic trioxide. Soluble arsenite has been proven to induce LC via both routes [238]. Long-term inhalation of inorganic As can cause chronic arsenic poisoning, which can cause hyperkeratosis, skin lesions, and, in some cases, bladder and lung cancer. Long-term inhalation of As can cause chronic As poisoning, which can cause hyperkeratosis, skin lesions, and bladder and lung cancer [239]. Smoking cigarettes and occupational exposure contributed to the elevated levels of inorganic As exposure [240]. The synergic interaction between smoking and As exposure could result in a significantly higher risk of LC [241].
The American Conference of Governmental Industrial Hygienists (ACGIH) demarcated the Threshold Limit Values (TLV-TWA) as 0.01 mg/m3 for As and inorganic compounds and 0.005 ppm for arsine. At the same time, the National Institute for Occupational Safety and Health (NIOSH) determined that the recommended exposure limit (REL) for arsine is 0.002 mg As/m3 [242]. The metabolism of As most likely contributes significantly to its carcinogenicity [243]. Arsenic is mainly metabolized by methylation into trivalent and pentavalent forms, considered toxic reactive metabolites. Reduced methylation capacity could result in a rise in LC risk, particularly in the presence of high levels of As [244].
The adverse health effect of inorganic As exposure could be delayed for up to four decades [245], and various studies suggest that exposure to As during gestation and childhood could result in LC in adulthood [246,247]. Several possible pathologies are linked to As-induced carcinogenesis, including oxidative epigenetic modification, DNA damage, genetic instability, and immunomodulation [237]. It is commonly accepted that oxidative stress can lead to the development of oxidative DNA damage, which is a factor in carcinogenesis [248]. Recently, Islam et al. found that miR-218-5p and EGFR were significantly downregulated and upregulated in arsenic-induced transformed (As-T) cells, respectively. It is suggested that tumor-suppressive miR-218-5p suppresses cancer proliferation, migration, and angiogenesis. In addition, miR-218-5p specifically targeted EGFR by attaching to its 3′-untranslated region (UTR) [249]. Overexpression or mutation of EGFR has a critical role in carcinogenesis in NSCLC [250]. Hence, EGFR is a known cancer biomarker frequently expressed in LC, while miR-218-5p possesses antitumor activity through EGFR. The miR-218-5p/EGFR signaling pathway plays a pivotal role in the increased risk of LC [251]. Recent research suggests impaired host immunity, specifically T cell anti-tumor immunity, may be crucial for cancer development [237,252,253]. A recent study reported that prolonged exposure to As in drinking water up-regulated programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) (PD-1/PD-L1), increased regulatory T cells (Tregs), decreased the CD8/Treg ratio, and these changes in mice’s lungs enhanced the formation of LC. CD8 acts as an anti-tumor immunosuppressive, while PD-1 and its ligand, PD-L1, are known as T cell inhibitory receptors [252]. It is proven that inhalation of particulate arsenic trioxide produces significant DNA damage in lung epithelial cells via strand breaks, oxidative damage, and superoxide formation [254].
6.4. Asbestos
Asbestos is a significant environmental carcinogen connected to lung cancer and has been identified by the IARC as one of the lung carcinogens group 1 [54]. According to WHO estimates, 125 million individuals are globally exposed to asbestos, which can lead to LC, laryngeal cancer, and mesothelioma development [255]. In the last few years, there has been a growing interest in asbestos-induced lung cancer. Asbestos fibers are naturally occurring silicate mineral fibers that have been heavily used in manufacturing due to their extraordinary properties. In buildings and construction, asbestos has been used as a thermal insulator in ceilings, flooring, shingles roofing, fire-retardant coatings, and water pipes and as an additive for asphalt concrete to improve the road surface’s stability [54]. Globally, asbestos-related lung disease is a severe public health issue, and inhaling asbestos fibers increases the risk of developing LC and malignant mesothelioma [256,257]. High levels of asbestos are the primary cause of between 5% and 7% of all LC-reported cases worldwide, chiefly due to occupational exposure [258]. Asbestos is responsible for around half of the occupational cancer deaths among workers in the asbestos sector [259]. Not only does occupational asbestos exposure lead to LC but also household exposure is responsible for thousands of deaths yearly due to the wide use of asbestos and asbestos-containing fibers in the home [260,261].
Additionally, it has been demonstrated that asbestos fibers and cigarette smoke exposure considerably raise the risk of LC. The danger increases when a person smokes more [262]. Interestingly, asbestos fibers trapped tobacco particulates, justifying the synergistic effect of asbestos with tobacco use on LC, with a cohort study reporting a 14.4-fold increased risk [263].
Several factors play an essential role in asbestosis and the risk of lung cancer, such as pulmonary functions, type and concentration of fiber, duration of exposure, genetic sustainability, and individual immunity. Long-term asbestos exposure can accumulate fibers in the lung tissues, causing chronic bronchitis, fibrosis, and pneumoconiosis (silicatoses) [228]. Additionally, various molecular abnormalities that may develop from asbestos exposure contribute to the worsening of the diagnosis and prognosis of LC [264]. Importantly, asbestos generates a considerable amount of ROS, which starts the genotoxicity cascade [24,25]. Although the precise methods by which asbestos damages DNA and induces apoptosis are not well understood, some of the pathways that have been suggested include the alteration in mitochondrial function, generation of ROS, reactive nitrogen species (RNS), and activation of the death receptor pathway [265].
Furthermore, oxidative stress may encourage cell death, gene mutations, and chromosomal abnormalities, ending in cell transformation. The sources of ROS production are attributed to the immune system’s response, fiber surface reactivity, and mitochondrial dysfunction [266]. Inflammation is another primary source of ROS synthesis. Asbestos triggers the production of ROS by alveolar macrophages and neutrophils during phagocytosis, a process that results in the secretion of proteases, chemokines, and cytokines, which mediate the inflammatory response [257].
It is well-recognized that microRNAs regulate a wide range of biological functions, including cell division, proliferation, and differentiation. In addition, changes in the expression profile of microRNAs are an essential epigenetic mechanism that may be involved in the pathogenesis of asbestos-induced LC [261]. Santarelli et al. stated that four serum miRNAs, namely miR-205, miR-520g, miR-126, and miR-222, were discovered to be associated with asbestos-related malignant diseases, and the authors proposed that these miRNAs are possibly contributing to LC linked to asbestos. Their expression reveals potential pathogenic mechanisms for asbestos-induced carcinogenesis [267].
A comparison of data from asbestos-exposed and MM subjects found that the most promising candidates for a multimarker signature were circulating miR-126-3p, miR-103a-3p, and miR-625-3p combined with mesothelin. The most consistently described tissue miRNAs, miR-16-5p, miR-126-3p, miR-143-3p, miR-145-5p, miR-192-5p, miR-193a-3p, miR-200b-3p, miR-203a-3p, and miR-652-3p, were also found to provide a diagnostic signature and should be further investigated as possible therapeutic targets. Similarly, Micolucci et al. found that asbestos increased some circulating miR-103a-3p, miR-126-3p, and miR-625-3p in combination with mesothelin, which were the most promising biological cancer markers; they also observed that asbestos significantly dysregulated the expression of miR-16-5p, miRNAs, miR-143-3p, miR-652-3p, miR-203a-3p, miR-126-3p, miR-192-5p, miR-145-5p, miR-193a-3p, and miR-200b-3p in LC [268]. Conclusively, these microRNAs increased in response to genotoxic stress, and their expressions aid in the diagnosis, follow-up, prognosis, and targeted therapy.
6.5. Pesticides
Pesticides, including insecticides, herbicides, fumigants, fungicides, and rodenticides, are crucial chemicals that are frequently employed in agriculture and other sectors. Organophosphate pesticides (OPPs) such as parathion, malathion, chlorpyrifos (CP), monocrotophos (MCP), and others have been extensively used in public health, agricultural, industrial, veterinary, and household contexts [269]. Molecular and epidemiological studies have demonstrated that OPPs are linked with increased cancer risk; however, the underlying mechanisms are not yet well developed [270]. Although many nations ban their use, OPPs are still used worldwide due to their cheapness, availability, and high efficacy. The improper use of huge uncontrolled applications has been identified in the environment as a pollutant [271]. Most OPPs feature a phosphorothioate group (P = S), which is safer than those with the P = O linkage because it has less reactivity with biomolecules and is hydrolytically stable [272]. Although many studies have identified the link between exposure to OPPs and the risk of LC, the precise carcinogenesis of these compounds is not yet well known [270,273,274,275]. A prospective study that was a component of the Agricultural Health Study (AHS) offered more proof in favor of a connection between lung cancer risk and pesticide use [274]. Jones et al. observed an increased LC incidence among men dealing with pesticides with high exposure over their lifetime days (diazinon: rate ratio, 1.60; 95%) [276]. Pesatori et al. found positive relationships between pesticide exposure and LC in a small, case-control study of structural pesticide applicators in Florida (odds ratio, 2.4; 95% CI, 1.0 to 5.9) [277]. Similarly, a French farmer cohort research found links between exposure to pesticides and the incidence of small-cell lung cancer (HR, 2.38; 95% CI, 1.07 to 5.28) [278].
Environmental and in vivo evidence indicates that OPPs can change from thionates to oxons, and thus, they become more toxic and reactive following exposure after inhalation, ingestion, and absorption from the skin [279]. The OPP action is based on irreversible acetylcholinesterase (AChE) inhibition in muscle and nerve tissues, causing the accumulation of acetylcholine in postsynaptic muscarinic and nicotinic choline receptors [280]. Several mechanisms are proposed in the literature to explore the pathogenesis of OPPs-induced LC. It is proven that OPPs play an essential role in oxidative stress, the generation of ROS, and reduced antioxidant enzymes, which consequently lead to oxidative DNA damage, altered DNA repair, and stimulated apoptosis [273]. Oxidative stress and oxidative DNA damage facilitate apoptotic signaling cascades in the mitochondria, further contributing to the release and activation of mitochondrial proteins such as apoptosis-inducing factor (AIF) and caspase-3 [281,282].
7. Conclusions
This article reviews how environmental risk factors and vaping may raise the risk of lung cancer. Epidemiological and experimental studies have shown that numerous chemical groups found in both vaping and environmental may adversely disrupt pulmonary functions and initiate carcinogenesis in an additive or synergistic manner. There is strong evidence that tobacco and flavoring compounds contribute to LC. Exposure to many toxicants found in vaping products and the environment, such as certain metals, suggests that these substances may also pose a risk for tumorigenesis. Risk factors for LC include hazardous geographic conditions such as places exposed to air pollution and natural radiation. Prenatal exposure to environmental toxicants during pregnancy or childhood may play a role in determining the development and severity of lung cancer in adulthood. Oxidative stress, the generation of ROS, oxidative DNA damage, apoptosis, and dysregulation of inflammatory proteins are the most common mechanisms that enhance lung carcinogenesis. The gene expression of several microRNAs plays a significant role in diagnosis, follow-up, and targeted therapy. However, more investigation is necessary to comprehend the mechanisms that result in lung cancer entirely and, ultimately, to identify the exact environmental toxicants that raise the risk of disease in individuals. The increased use of ECs and cannabis vaping, especially among young individuals, females, and nonsmokers, is a significant public health concern. Environmental effects from improper e-waste disposal and recycling are increasing worldwide. Electronic waste handling and disposal exposes people to highly toxic compounds, such as heavy metals. Air pollution, mainly PM2.5 from global industrialization and vehicle exhaust emissions, has been linked to LC. Consequently, reducing PM2.5 concentrations in the general population could be an effective preventative measure against LC. Even though occupational LC may have decreased recently, preventive measures are still required to lessen the exposures’ carcinogenic consequences.
Future epidemiologic research and review on the relationship between lung cancer risk and prolonged use of E-cigarettes and exposure to other environmental factors with their molecular pathology and clinical consequences is highly recommended. These “molecular pathologic epidemiology” studies will enrich our understanding of “how a specific exposure could impact the process of carcinogenesis, somatic molecular alterations, and tumor biomarkers”, as proposed by others [283,284].
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, drafting the article or revising it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number “NBU-FFR-2023-0035”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Winter, H.; Eichhorn, M.E.; Roesch, R.M.; Taber, S.; Christopoulos, P.; Wiegering, A.; Lenzi, J. Trends in Age- and Sex-Specific Lung Cancer Mortality in Europe and Northern America: Analysis of Vital Registration Data from the WHO Mortality Database between 2000 and 2017. Eur. J. Cancer 2022, 171, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sharma, R. Mapping of Global, Regional and National Incidence, Mortality and Mortality-to-Incidence Ratio of Lung Cancer in 2020 and 2050. Int. J. Clin. Oncol. 2022, 27, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol. 2021, 25, 45. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365. [Google Scholar] [CrossRef]
- Araghi, M.; Fidler-Benaoudia, M.; Arnold, M.; Rutherford, M.; Bardot, A.; Ferlay, J.; Bucher, O.; De, P.; Engholm, G.; Gavin, A.; et al. International Differences in Lung Cancer Survival by Sex, Histological Type and Stage at Diagnosis: An ICBP SURVMARK-2 Study. Thorax 2022, 77, 378–390. [Google Scholar] [CrossRef]
- Smok-Kalwat, J.; Mertowska, P.; Mertowski, S.; Smolak, K.; Kozińska, A.; Koszałka, F.; Kwaśniewski, W.; Grywalska, E.; Góźdź, S. The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer. Int. J. Mol. Sci. 2023, 24, 1506. [Google Scholar] [CrossRef]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and Occupational Determinants of Lung Cancer. Transl. Lung Cancer Res. 2019, 8, S31–S49. [Google Scholar] [CrossRef]
- Benusiglio, P.R.; Fallet, V.; Sanchis-Borja, M.; Coulet, F.; Cadranel, J. Lung Cancer Is Also a Hereditary Disease. Eur. Respir. Rev. 2021, 30. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Bailey-Wilson, J.E.; Amos, C.I. 6—Genetic Susceptibility to Lung Cancer. In IASLC Thoracic Oncology, 2nd ed.; Pass, H.I., Ball, D., Scagliotti, G.V., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 46–51.e2. ISBN 978-0-323-52357-8. [Google Scholar]
- Weir, B.A.; Woo, M.S.; Getz, G.; Perner, S.; Ding, L.; Beroukhim, R.; Lin, W.M.; Province, M.A.; Kraja, A.; Johnson, L.A.; et al. Characterizing the Cancer Genome in Lung Adenocarcinoma. Nature 2007, 450, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive Genomic Characterization of Squamous Cell Lung Cancers. Nature 2012, 489, 519–525. [CrossRef]
- Bracken-Clarke, D.; Kapoor, D.; Baird, A.M.; Buchanan, P.J.; Gately, K.; Cuffe, S.; Finn, S.P. Vaping and Lung Cancer—A Review of Current Data and Recommendations. Lung Cancer 2021, 153, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Vogelmeier, C.F.; Halpin, D.M.G. Tackling the Global Burden of Lung Disease through Prevention and Early Diagnosis. Lancet Respir. Med. 2022, 10, 1013–1015. [Google Scholar] [CrossRef]
- Kuditipudi, A.D.; Dirisanala, S.; Ganti, N.; Laller, S.; Williams, I.; Taj, S.; Arora, K.S.; Hameed, A.; Lei, Y.W.; Venkata, V.S.; et al. 247: Epidemiology and Prevalenceof Lung Disease Among E-Cigarette Users in the Usa: A National Study. Crit. Care Med. 2023, 51, 108. [Google Scholar] [CrossRef]
- Cullen, K.A. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students—United States, 2011–2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67. [Google Scholar] [CrossRef]
- Cornelius, M.E. Tobacco Product Use Among Adults—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69. [Google Scholar] [CrossRef]
- Tsai, J.; Walton, K.; Coleman, B.N.; Sharapova, S.R.; Johnson, S.E.; Kennedy, S.M.; Caraballo, R.S. Reasons for Electronic Cigarette Use Among Middle and High School Students—National Youth Tobacco Survey, United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 196–200. [Google Scholar] [CrossRef]
- Gentzke, A.S. Tobacco Product Use Among Middle and High School Students—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Kite-Powell, A.; Patel, M.T.; Haag, B.L.; Sheppard, M.J.; Dias, T.P.; King, B.A.; Melstrom, P.C.; Ritchey, M.D.; Stein, Z.; et al. Syndromic Surveillance for E-Cigarette, or Vaping, Product Use–Associated Lung Injury. N. Engl. J. Med. 2020, 382, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Rizzo, S.; Masiero, M.; Marzorati, C.; Casiraghi, M.; Bertolaccini, L.; Mazzella, A.; Pravettoni, G.; Spaggiari, L. Clinical Impact of Vaping on Cardiopulmonary Function and Lung Cancer Development: An Update. Eur. J. Cancer Prev. 2023. [Google Scholar] [CrossRef]
- Muthumalage, T.; Friedman, M.R.; McGraw, M.D.; Ginsberg, G.; Friedman, A.E.; Rahman, I. Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; LeBouf, R.F.; Ranpara, A.C.; Leonard, S.S. Toxicology of Flavoring- and Cannabis-Containing e-Liquids Used in Electronic Delivery Systems. Pharmacol. Ther. 2021, 224, 107838. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, N.; Gray, N.; Pappas, R.S.; Halstead, M.; Lewis, E.; Valentin-Blasini, L.; Watson, C.; Blount, B. Analysis of Toxic Metals in Aerosols from Devices Associated with Electronic Cigarette, or Vaping, Product Use Associated Lung Injury. Toxics 2021, 9, 240. [Google Scholar] [CrossRef]
- Khlystov, A.; Samburova, V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Fiore, M.; Oliveri Conti, G.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M.A.; Ferrante, M. Role of Emerging Environmental Risk Factors in Thyroid Cancer: A Brief Review. Int. J. Environ. Res. Public Health 2019, 16, 1185. [Google Scholar] [CrossRef]
- Kruger, E.; Toraih, E.A.; Hussein, M.H.; Shehata, S.A.; Waheed, A.; Fawzy, M.S.; Kandil, E. Thyroid Carcinoma: A Review for 25 Years of Environmental Risk Factors Studies. Cancers 2022, 14, 6172. [Google Scholar] [CrossRef]
- du Plessis, M.; Fourie, C.; Stone, W.; Engelbrecht, A.-M. The Impact of Endocrine Disrupting Compounds and Carcinogens in Wastewater: Implications for Breast Cancer. Biochimie 2023, 209, 103–115. [Google Scholar] [CrossRef]
- Valdez-Flores, C.; Erraguntla, N.; Budinsky, R.; Cagen, S.; Kirman, C.R. An Updated Lymphohematopoietic and Bladder Cancers Risk Evaluation for Occupational and Environmental Exposures to 1,3-Butadiene. Chem.-Biol. Interact. 2022, 366, 110077. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental Arsenic Exposure and Its Contribution to Human Diseases, Toxicity Mechanism and Management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Jaafarzadeh, N.; Poormohammadi, A.; Almasi, H.; Ghaedrahmat, Z.; Rahim, F.; Zahedi, A. Arsenic in Drinking Water and Kidney Cancer: A Systematic Review. Rev. Environ. Health 2022, 38, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Moubarz, G.; Saad-Hussein, A.; Shahy, E.M.; Mahdy-Abdallah, H.; Mohammed, A.M.F.; Saleh, I.A.; Abo-Zeid, M.A.M.; Abo-Elfadl, M.T. Lung Cancer Risk in Workers Occupationally Exposed to Polycyclic Aromatic Hydrocarbons with Emphasis on the Role of DNA Repair Gene. Int. Arch. Occup. Environ. Health 2023, 96, 313–329. [Google Scholar] [CrossRef]
- Lagoa, R.; Marques-da-Silva, D.; Diniz, M.; Daglia, M.; Bishayee, A. Molecular Mechanisms Linking Environmental Toxicants to Cancer Development: Significance for Protective Interventions with Polyphenols. Semin. Cancer Biol. 2022, 80, 118–144. [Google Scholar] [CrossRef] [PubMed]
- Farland, W.H. The US Environmental Protection Agency’s Risk Assessment Guidelines: Current Status and Future Directions. Toxicol. Ind. Health 1992, 8, 205–212. [Google Scholar]
- International Agency for Research on Cancer IARC Monographs on the Identification of Carcinogenic Hazards to Humans. IARC Monogr. Meet. 2019, 124, 1–4.
- Wang, Z.; Yang, C. Metal Carcinogen Exposure Induces Cancer Stem Cell-like Property through Epigenetic Reprograming: A Novel Mechanism of Metal Carcinogenesis. Semin. Cancer Biol. 2019, 57, 95–104. [Google Scholar] [CrossRef]
- Bade, B.C.; Cruz, C.S.D. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar]
- Marshall, G.; Ferreccio, C.; Yuan, Y.; Bates, M.N.; Steinmaus, C.; Selvin, S.; Liaw, J.; Smith, A.H. Fifty-Year Study of Lung and Bladder Cancer Mortality in Chile Related to Arsenic in Drinking Water. J. Natl. Cancer Inst. 2007, 99, 920–928. [Google Scholar] [CrossRef]
- Lantz, P.M.; Mendez, D.; Philbert, M.A. Radon, Smoking, and Lung Cancer: The Need to Refocus Radon Control Policy. Am. J. Public Health 2013, 103, 443–447. [Google Scholar] [CrossRef]
- Zou, K.; Sun, P.; Huang, H.; Zhuo, H.; Qie, R.; Xie, Y.; Luo, J.; Li, N.; Li, J.; He, J.; et al. Etiology of Lung Cancer: Evidence from Epidemiologic Studies. J. Natl. Cancer Cent. 2022, 2, 216–225. [Google Scholar] [CrossRef]
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Malhotra, J.; Boffetta, P. Epidemiology of Occupational Lung Cancer. In Occupational Cancers; Anttila, S., Boffetta, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 287–294. ISBN 978-3-030-30766-0. [Google Scholar]
- Ezzati, M.; Lopez, A.D. Estimates of Global Mortality Attributable to Smoking in 2000. Lancet 2003, 362, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Spyratos, D.; Zarogoulidis, P.; Porpodis, K.; Tsakiridis, K.; Machairiotis, N.; Katsikogiannis, N.; Kougioumtzi, I.; Dryllis, G.; Kallianos, A.; Rapti, A.; et al. Occupational Exposure and Lung Cancer. J. Thorac. Dis. 2013, 5, S440. [Google Scholar]
- Wang, X.; Wang, T.; Hua, J.; Cai, M.; Qian, Z.; Wang, C.; Li, H.; McMillin, S.E.; Aaron, H.E.; Xie, C.; et al. Histological Types of Lung Cancer Attributable to Fine Particulate, Smoking, and Genetic Susceptibility. Sci. Total Environ. 2023, 858, 159890. [Google Scholar] [CrossRef]
- Larsson, S.C.; Carter, P.; Kar, S.; Vithayathil, M.; Mason, A.M.; Michaëlsson, K.; Burgess, S. Smoking, Alcohol Consumption, and Cancer: A Mendelian Randomisation Study in UK Biobank and International Genetic Consortia Participants. PLoS Med. 2020, 17, e1003178. [Google Scholar] [CrossRef]
- Lipfert, F.W.; Wyzga, R.E. Longitudinal Relationships between Lung Cancer Mortality Rates, Smoking, and Ambient Air Quality: A Comprehensive Review and Analysis. Crit. Rev. Toxicol. 2019, 49, 790–818. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100 Pt E, 1–538. [Google Scholar]
- Burkhardt, T.; Scherer, M.; Scherer, G.; Pluym, N.; Weber, T.; Kolossa-Gehring, M. Time Trend of Exposure to Secondhand Tobacco Smoke and Polycyclic Aromatic Hydrocarbons between 1995 and 2019 in Germany—Showcases for Successful European Legislation. Environ. Res. 2023, 216, 114638. [Google Scholar] [CrossRef]
- Mourino, N.; Pérez-Ríos, M.; Yolton, K.; Lanphear, B.P.; Chen, A.; Buckley, J.P.; Kalkwarf, H.J.; Cecil, K.M.; Braun, J.M. Pre- and Postnatal Exposure to Secondhand Tobacco Smoke and Cardiometabolic Risk at 12 Years: Periods of Susceptibility. Environ. Res. 2023, 224, 115572. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Nitrobenzenes and other Industrial Chemicals. Lyon (FR): International Agency for Research on Cancer; 2020. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 123.). Available online: https://www.ncbi.nlm.nih.gov/books/NBK561907/ (accessed on 21 June 2023).
- de Oliveira Alves, N.; Martins Pereira, G.; Di Domenico, M.; Costanzo, G.; Benevenuto, S.; de Oliveira Fonoff, A.M.; de Souza Xavier Costa, N.; Ribeiro Júnior, G.; Satoru Kajitani, G.; Cestari Moreno, N.; et al. Inflammation Response, Oxidative Stress and DNA Damage Caused by Urban Air Pollution Exposure Increase in the Lack of DNA Repair XPC Protein. Environ. Int. 2020, 145, 106150. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and Molecular Classification of Lung Cancer. In Lung Cancer: Treatment and Research; Reckamp, K.L., Ed.; Cancer Treatment and Research; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–46. ISBN 978-3-319-40389-2. [Google Scholar]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef]
- Hynds, R.E.; Frese, K.K.; Pearce, D.R.; Grönroos, E.; Dive, C.; Swanton, C. Progress towards Non-Small-Cell Lung Cancer Models That Represent Clinical Evolutionary Trajectories. Open Biol. 2021, 11, 200247. [Google Scholar] [CrossRef]
- Curado, M.-P.; Edwards, B.; Shin, H.R.; Storm, H.; Ferlay, J.; Heanue, M.; Boyle, P. Cancer Incidence in Five Continents, Volume IX; IARC Press, International Agency for Research on Cancer: Lyon, France, 2007; ISBN 92-832-2160-5. [Google Scholar]
- Zeng, Z.; Wang, Z.; Li, Y.; Ye, D.; Zeng, J.; Hu, J.; Chen, P.; Xiao, J.; Zou, J.; Li, Z. Nuclear Factor Erythroid 2 (NF-E2)-Related Factor 2 (Nrf2) in Non-Small Cell Lung Cancer. Life Sci. 2020, 254, 117325. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Budkevich, I.N.; Zhivotovsky, B. Cell Death-Based Treatment of Lung Adenocarcinoma. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Marra, A.; Sui, J.S.Y.; Flynn, J.; Yang, S.-R.; Falcon, C.J.; Selenica, P.; Schoenfeld, A.J.; Rekhtman, N.; Gomez, D.; et al. Molecular Biomarkers of Disease Outcomes and Mechanisms of Acquired Resistance to First-Line Osimertinib in Advanced EGFR-Mutant Lung Cancers. J. Thorac. Oncol. 2023, 18, 463–475. [Google Scholar] [CrossRef]
- Panani, A.D.; Roussos, C. Cytogenetic and Molecular Aspects of Lung Cancer. Cancer Lett. 2006, 239, 1–9. [Google Scholar] [CrossRef]
- Ngamwong, Y.; Tangamornsuksan, W.; Lohitnavy, O.; Chaiyakunapruk, N.; Scholfield, C.N.; Reisfeld, B.; Lohitnavy, M. Additive Synergism between Asbestos and Smoking in Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135798. [Google Scholar] [CrossRef]
- Mauderly, J.L.; Seilkop, S.K.; Barr, E.B.; Gigliotti, A.P.; Hahn, F.F.; Hobbs, C.H.; Finch, G.L. Carcinogenic Interactions between a Single Inhalation of 239PuO2 and Chronic Exposure to Cigarette Smoke in Rats. Radiat. Res. 2010, 173, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, Y.; Xia, T.; Zhu, Y. Effects of Electronic Cigarettes on Indoor Air Quality and Health. Annu. Rev. Public Health 2020, 41, 363–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Sumner, W.; Chen, D.-R. In Vitro Particle Size Distributions in Electronic and Conventional Cigarette Aerosols Suggest Comparable Deposition Patterns. Nicotine Tob. Res. 2013, 15, 501–508. [Google Scholar] [CrossRef]
- Ruprecht, A.A.; De Marco, C.; Saffari, A.; Pozzi, P.; Mazza, R.; Veronese, C.; Angellotti, G.; Munarini, E.; Ogliari, A.C.; Westerdahl, D.; et al. Environmental Pollution and Emission Factors of Electronic Cigarettes, Heat-Not-Burn Tobacco Products, and Conventional Cigarettes. Aerosol Sci. Technol. 2017, 51, 674–684. [Google Scholar] [CrossRef]
- Nguyen, C.; Li, L.; Sen, C.A.; Ronquillo, E.; Zhu, Y. Fine and Ultrafine Particles Concentrations in Vape Shops. Atmos. Environ. 2019, 211, 159–169. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Qian, Y.; Li, X.; Guo, C.; Wang, Z.; Wei, Y. Influence of Metals from E-Waste Dismantling on Telomerelength and Mitochondrial DNA Copy Number in People Living near Recycling Sites. Environ. Int. 2020, 140, 105769. [Google Scholar] [CrossRef]
- Beutel, M.W.; Harmon, T.C.; Novotny, T.E.; Mock, J.; Gilmore, M.E.; Hart, S.C.; Traina, S.; Duttagupta, S.; Brooks, A.; Jerde, C.L.; et al. A Review of Environmental Pollution from the Use and Disposal of Cigarettes and Electronic Cigarettes: Contaminants, Sources, and Impacts. Sustainability 2021, 13, 12994. [Google Scholar] [CrossRef]
- Hendlin, Y.H. Alert: Public Health Implications of Electronic Cigarette Waste. Am. J. Public Health 2018, 108, 1489. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. E-Cigarette Use among Youth and Young Adults: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538680/ (accessed on 21 June 2023).
- Centers for Disease Control and Prevention. E-Cigarette, or Vaping, Products Visual Dictionary; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. Available online: https://stacks.cdc.gov/view/cdc/103783 (accessed on 21 June 2023).
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T. Nicotine Replacement Therapy versus Control for Smoking Cessation. Cochrane Database Syst. Rev. 2018, 5, CD000146. [Google Scholar] [CrossRef]
- Do, E.K.; O’Connor, K.; Perks, S.N.; Soule, E.K.; Eissenberg, T.; Amato, M.S.; Graham, A.L.; Martin, C.K.; Höchsmann, C.; Fuemmeler, B.F. E-Cigarette Device and Liquid Characteristics and E-Cigarette Dependence: A Pilot Study of Pod-Based and Disposable E-Cigarette Users. Addict. Behav. 2022, 124, 107117. [Google Scholar] [CrossRef]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Felicione, N.J.; Kaiser, L.; Leigh, N.J.; Page, M.K.; Block, A.C.; Schurr, B.E.; O’Connor, R.J.; Goniewicz, M.L. Comparing POD and MOD ENDS Users’ Product Characteristics, Use Behaviors, and Nicotine Exposure. Nicotine Tob. Res. 2023, 25, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Bals, R. Basic Science of Electronic Cigarettes: Assessment in Cell Culture and in Vivo Models. Respir. Res. 2016, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The Rise of E-Cigarettes, Pod Mod Devices, and JUUL among Youth: Factors Influencing Use, Health Implications, and Downstream Effects. Drug Alcohol. Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef]
- Sears, C.G.; Hart, J.L.; Walker, K.L.; Robertson, R.M. Generally Recognized as Safe: Uncertainty Surrounding E-Cigarette Flavoring Safety. Int. J. Environ. Res. Public Health 2017, 14, 1274. [Google Scholar] [CrossRef]
- Hamberger, E.S.; Halpern-Felsher, B. Vaping in Adolescents: Epidemiology and Respiratory Harm. Curr. Opin. Pediatr. 2020, 32, 378–383. [Google Scholar] [CrossRef]
- Glasser, A.M.; Johnson, A.L.; Niaura, R.S.; Abrams, D.B.; Pearson, J.L. Youth Vaping and Tobacco Use in Context in the United States: Results From the 2018 National Youth Tobacco Survey. Nicotine Tob. Res. 2021, 23, 447–453. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, S.-H.; Weng, M.; Wang, H.-T.; Huang, W.C.; Lepor, H.; Wu, X.-R.; Chen, L.-C.; Tang, M. E-Cigarette Smoke Damages DNA and Reduces Repair Activity in Mouse Lung, Heart, and Bladder as Well as in Human Lung and Bladder Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar] [CrossRef]
- Waldum, H.L.; Nilsen, O.G.; Nilsen, T.; Rørvik, H.; Syversen, U.; Sandvik, A.K.; Haugen, O.A.; Torp, S.H.; Brenna, E. Long-Term Effects of Inhaled Nicotine. Life Sci. 1996, 58, 1339–1346. [Google Scholar] [CrossRef]
- Sundar, I.K.; Mullapudi, N.; Yao, H.; Spivack, S.D.; Rahman, I. Lung Cancer and Its Association with Chronic Obstructive Pulmonary Disease: Update on Nexus of Epigenetics. Curr. Opin. Pulm. Med. 2011, 17, 279. [Google Scholar] [CrossRef]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-Cigarette Flavored Pods Induce Inflammation, Epithelial Barrier Dysfunction, and DNA Damage in Lung Epithelial Cells and Monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araújo, A.; Medeiros, R. The Role of Inflammation in Lung Cancer. In Inflammation and Cancer; Aggarwal, B.B., Sung, B., Gupta, S.C., Eds.; Advances in Experimental Medicine and Biology; Springer: Basel, Switzerland, 2014; pp. 1–23. ISBN 978-3-0348-0837-8. [Google Scholar]
- Uchiyama, S.; Noguchi, M.; Sato, A.; Ishitsuka, M.; Inaba, Y.; Kunugita, N. Determination of Thermal Decomposition Products Generated from E-Cigarettes. Chem. Res. Toxicol. 2020, 33, 576–583. [Google Scholar] [CrossRef]
- Roe, F.J.C.; Wood, D. Review: Acetaldehyde and Formaldehyde: Is There a Cancer Risk for Man? Indoor Environ. 1992, 1, 8–15. [Google Scholar] [CrossRef]
- Matsumoto, M.; Yamano, S.; Senoh, H.; Umeda, Y.; Hirai, S.; Saito, A.; Kasai, T.; Aiso, S. Carcinogenicity and Chronic Toxicity of Acrolein in Rats and Mice by Two-Year Inhalation Study. Regul. Toxicol. Pharmacol. 2021, 121, 104863. [Google Scholar] [CrossRef] [PubMed]
- Smets, J.; Baeyens, F.; Chaumont, M.; Adriaens, K.; Van Gucht, D. When Less Is More: Vaping Low-Nicotine vs. High-Nicotine E-Liquid Is Compensated by Increased Wattage and Higher Liquid Consumption. Int. J. Environ. Res. Public Health 2019, 16, 723. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, S.; Seng, S. Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN. Cancers 2014, 6, 1138–1156. [Google Scholar] [CrossRef]
- Tang, M.; Wu, X.-R.; Lee, H.-W.; Xia, Y.; Deng, F.-M.; Moreira, A.L.; Chen, L.-C.; Huang, W.C.; Lepor, H. Electronic-Cigarette Smoke Induces Lung Adenocarcinoma and Bladder Urothelial Hyperplasia in Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 21727–21731. [Google Scholar] [CrossRef]
- Stepanov, I.; Carmella, S.G.; Han, S.; Pinto, A.; Strasser, A.A.; Lerman, C.; Hecht, S.S. Evidence for Endogenous Formation of N′-Nitrosonornicotine in Some Long-Term Nicotine Patch Users. Nicotine Tob. Res. 2009, 11, 99–105. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. In Nicotine Psychopharmacology; Henningfield, J.E., London, E.D., Pogun, S., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–60. ISBN 978-3-540-69248-5. [Google Scholar]
- Shahab, L.; Goniewicz, M.L.; Blount, B.C.; Brown, J.; McNeill, A.; Alwis, K.U.; Feng, J.; Wang, L.; West, R. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users. Ann. Intern. Med. 2017, 166, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, Y.; Li, D.; Gao, N. Emission and Gas/Particle Partitioning Characteristics of Nicotine in Aerosols for Electronic Cigarettes. Chem. Res. Toxicol. 2022, 35, 890–897. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Propylene Glycol. 1997. Available online: http://medbox.iiab.me/modules/en-cdc/www.atsdr.cdc.gov/substances/toxsubstance.asp_toxid=240 (accessed on 21 June 2023).
- Cao, D.J.; Aldy, K.; Hsu, S.; McGetrick, M.; Verbeck, G.; De Silva, I.; Feng, S. Review of Health Consequences of Electronic Cigarettes and the Outbreak of Electronic Cigarette, or Vaping, Product Use-Associated Lung Injury. J. Med. Toxicol. 2020, 16, 295–310. [Google Scholar] [CrossRef]
- Kosmider, L.; Sobczak, A.; Fik, M.; Knysak, J.; Zaciera, M.; Kurek, J.; Goniewicz, M.L. Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. [Google Scholar] [CrossRef]
- Woodall, M.; Jacob, J.; Kalsi, K.K.; Schroeder, V.; Davis, E.; Kenyon, B.; Khan, I.; Garnett, J.P.; Tarran, R.; Baines, D.L. E-Cigarette Constituents Propylene Glycol and Vegetable Glycerin Decrease Glucose Uptake and Its Metabolism in Airway Epithelial Cells in Vitro. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L957–L967. [Google Scholar] [CrossRef]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Foulds, J.; Muscat, J.; Elias, R.J.; Richie, J.P., Jr. Effects of Solvent and Temperature on Free Radical Formation in Electronic Cigarette Aerosols. Chem. Res. Toxicol. 2018, 31, 4–12. [Google Scholar] [CrossRef]
- Escobar, Y.-N.H.; Nipp, G.; Cui, T.; Petters, S.S.; Surratt, J.D.; Jaspers, I. In Vitro Toxicity and Chemical Characterization of Aerosol Derived from Electronic Cigarette Humectants Using a Newly Developed Exposure System. Chem. Res. Toxicol. 2020, 33, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.D.; Chung, S.; Baumlin, N.; Sun, L.; Silswal, N.; Dennis, J.S.; Yoshida, M.; Sabater, J.; Horrigan, F.T.; Salathe, M. E-Cigarette Aerosols of Propylene Glycol Impair BK Channel Activity and Parameters of Mucociliary Function. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 324, L468–L479. [Google Scholar] [CrossRef] [PubMed]
- Bellier, J.; Nokin, M.-J.; Lardé, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcène, A. Methylglyoxal, a Potent Inducer of AGEs, Connects between Diabetes and Cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Bellahcène, A.; Nokin, M.-J.; Castronovo, V.; Schalkwijk, C. Methylglyoxal-Derived Stress: An Emerging Biological Factor Involved in the Onset and Progression of Cancer. Semin. Cancer Biol. 2018, 49, 64–74. [Google Scholar] [CrossRef]
- Huynh, D.; Huang, J.; Le, L.T.T.; Liu, D.; Liu, C.; Pham, K.; Wang, H. Electronic Cigarettes Promotes the Lung Colonization of Human Breast Cancer in NOD-SCID-Gamma Mice. Int. J. Clin. Exp. Pathol. 2020, 13, 2075–2081. [Google Scholar]
- Effah, F.; Taiwo, B.; Baines, D.; Bailey, A.; Marczylo, T. Pulmonary Effects of E-Liquid Flavors: A Systematic Review. J. Toxicol. Environ. Health Part B 2022, 25, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Gillman, I.G.; Pennington, A.S.C.; Humphries, K.E.; Oldham, M.J. Determining the Impact of Flavored E-Liquids on Aldehyde Production during Vaping. Regul. Toxicol. Pharmacol. 2020, 112, 104588. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-A.; Reisinger, S.A.; Freudenheim, J.L.; Brasky, T.M.; Mathé, E.A.; McElroy, J.P.; Nickerson, Q.A.; Weng, D.Y.; Wewers, M.D.; Shields, P.G. Effects of Electronic Cigarette Constituents on the Human Lung: A Pilot Clinical Trial. Cancer Prev. Res. 2020, 13, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ogunwale, M.A.; Li, M.; Ramakrishnam Raju, M.V.; Chen, Y.; Nantz, M.H.; Conklin, D.J.; Fu, X.-A. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2017, 2, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, S.; Muthumalage, T.; Rahman, I. Comparative Reactive Oxygen Species (ROS) Content among Various Flavored Disposable Vape Bars, Including Cool (Iced) Flavored Bars. Toxics 2021, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Rahman, I. Current Concepts on Oxidative/Carbonyl Stress, Inflammation and Epigenetics in Pathogenesis of Chronic Obstructive Pulmonary Disease. Toxicol. Appl. Pharmacol. 2011, 254, 72–85. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Martin, E.M.; Clapp, P.W.; Rebuli, M.E.; Pawlak, E.A.; Glista-Baker, E.; Benowitz, N.L.; Fry, R.C.; Jaspers, I. E-Cigarette Use Results in Suppression of Immune and Inflammatory-Response Genes in Nasal Epithelial Cells Similar to Cigarette Smoke. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 311, L135–L144. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, S.; Li, C.; Loor, J.J.; Jiang, Q.; Yang, Y.; Feng, X.; Liu, S.; He, J.; Wang, K.; et al. Free Fatty Acids Promote Degranulation of Azurophil Granules in Neutrophils by Inducing Production of NADPH Oxidase–Derived Reactive Oxygen Species in Cows with Subclinical Ketosis. J. Dairy Sci. 2022, 105, 2473–2486. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Phandthong, R.; Chaili, A.; Remark, G.; Talbot, P. Epithelial-to-Mesenchymal Transition of A549 Lung Cancer Cells Exposed to Electronic Cigarettes. Lung Cancer 2018, 122, 224–233. [Google Scholar] [CrossRef]
- Nair, V.; Tran, M.; Behar, R.Z.; Zhai, S.; Cui, X.; Phandthong, R.; Wang, Y.; Pan, S.; Luo, W.; Pankow, J.F.; et al. Menthol in Electronic Cigarettes: A Contributor to Respiratory Disease? Toxicol. Appl. Pharmacol. 2020, 407, 115238. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.A.; Yapa, K.T.D.S.; Monteith, G.R. Altered Calcium Signaling in Cancer Cells. Biochim. Biophys. Acta (BBA)—Biomembr. 2015, 1848, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.W.; Lavrich, K.S.; van Heusden, C.A.; Lazarowski, E.R.; Carson, J.L.; Jaspers, I. Cinnamaldehyde in Flavored E-Cigarette Liquids Temporarily Suppresses Bronchial Epithelial Cell Ciliary Motility by Dysregulation of Mitochondrial Function. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L470–L486. [Google Scholar] [CrossRef] [PubMed]
- Borodovsky, J.T.; Lee, D.C.; Crosier, B.S.; Gabrielli, J.L.; Sargent, J.D.; Budney, A.J. U.S. Cannabis Legalization and Use of Vaping and Edible Products among Youth. Drug Alcohol. Depend. 2017, 177, 299–306. [Google Scholar] [CrossRef]
- Jett, J.; Stone, E.; Warren, G.; Cummings, K.M. Cannabis Use, Lung Cancer, and Related Issues. J. Thorac. Oncol. 2018, 13, 480–487. [Google Scholar] [CrossRef]
- Fataar, F.; Hammond, D. The Prevalence of Vaping and Smoking as Modes of Delivery for Nicotine and Cannabis among Youth in Canada, England and the United States. Int. J. Environ. Res. Public Health 2019, 16, 4111. [Google Scholar] [CrossRef]
- Pomahacova, B.; Van der Kooy, F.; Verpoorte, R. Cannabis Smoke Condensate III: The Cannabinoid Content of Vaporised Cannabis Sativa. Inhal. Toxicol. 2009, 21, 1108–1112. [Google Scholar] [CrossRef]
- Love, C.A.; Kim, H.-Y.H.; Tallman, K.A.; Clapp, P.W.; Porter, N.A.; Jaspers, I. Vaping Induced Cannabidiol (CBD) Oxidation Product CBD Quinone Forms Protein Adducts with KEAP1 and Activates KEAP1-Nrf2 Genes. Chem. Res. Toxicol. 2023. [Google Scholar] [CrossRef]
- Leigh, N.J.; Goniewicz, M.L. Acute Effect of Electronic Cigarette-Generated Aerosol from Flavored CBD-Containing Refill Solutions on Human Bronchial Epithelial Cells. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Muthumalage, T.; Rahman, I. Cannabidiol Differentially Regulates Basal and LPS-Induced Inflammatory Responses in Macrophages, Lung Epithelial Cells, and Fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef]
- Zhang, L.R.; Morgenstern, H.; Greenland, S.; Chang, S.-C.; Lazarus, P.; Teare, M.D.; Woll, P.J.; Orlow, I.; Cox, B.; Cannabis and Respiratory Disease Research Group of New Zealand; et al. Cannabis Smoking and Lung Cancer Risk: Pooled Analysis in the International Lung Cancer Consortium. Int. J. Cancer 2015, 136, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; Lefever, T.W.; Cortes, R.A.; Grabenauer, M.; Kovach, A.L.; Cox, A.O.; Patel, P.R.; Pollard, G.T.; Marusich, J.A.; Kevin, R.C.; et al. Thermolytic Degradation of Synthetic Cannabinoids: Chemical Exposures and Pharmacological Consequences. J. Pharmacol. Exp. Ther. 2017, 361, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, A.; Singh, D.; Christiani, D.; Demokritou, P. E-Cigarette (E-Cig) Liquid Composition and Operational Voltage Define the In Vitro Toxicity of Δ8Tetrahydrocannabinol/Vitamin E Acetate (Δ8THC/VEA) E-Cig Aerosols. Toxicol. Sci. Off. J. Soc. Toxicol. 2022, 187. [Google Scholar] [CrossRef] [PubMed]
- Hooper, R.W.; Garfield, J.L. An Emerging Crisis: Vaping-Associated Pulmonary Injury. Ann. Intern. Med. 2020, 172, 57–58. [Google Scholar] [CrossRef]
- Ellington, S. Update: Product, Substance-Use, and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use–Associated Lung Injury—United States, August 2019–January 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef]
- Sund, L.J.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M. E-Cigarette or Vaping-Associated Lung Injury (EVALI): A Review of International Case Reports from Outside the United States of America. Clin. Toxicol. 2023, 61, 91–97. [Google Scholar] [CrossRef]
- Wu, D.; O’Shea, D.F. Potential for Release of Pulmonary Toxic Ketene from Vaping Pyrolysis of Vitamin E Acetate. Proc. Natl. Acad. Sci. USA 2020, 117, 6349–6355. [Google Scholar] [CrossRef]
- Committee on Acute Exposure Guideline Levels; Committee on Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 16; National Academies Press (US): Washington, DC, USA, 2014; ISBN 978-0-309-30096-4.
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Elias, R.J.; Silakov, A.; Foulds, J.; Muscat, J.; Richie, J.P. Effect of Flavoring Chemicals on Free Radical Formation in Electronic Cigarette Aerosols. Free Radic. Biol. Med. 2018, 120, 72–79. [Google Scholar] [CrossRef]
- Durrani, K.; El Din, S.-M.A.; Sun, Y.; Rule, A.M.; Bressler, J. Ethyl Maltol Enhances Copper Mediated Cytotoxicity in Lung Epithelial Cells. Toxicol. Appl. Pharmacol. 2021, 410, 115354. [Google Scholar] [CrossRef]
- Weng, M.; Lee, H.-W.; Park, S.-H.; Hu, Y.; Wang, H.-T.; Chen, L.-C.; Rom, W.N.; Huang, W.C.; Lepor, H.; Wu, X.-R.; et al. Aldehydes Are the Predominant Forces Inducing DNA Damage and Inhibiting DNA Repair in Tobacco Smoke Carcinogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E6152–E6161. [Google Scholar] [CrossRef] [PubMed]
- Canchola, A.; Ahmed, C.M.S.; Chen, K.; Chen, J.Y.; Lin, Y.-H. Formation of Redox-Active Duroquinone from Vaping of Vitamin E Acetate Contributes to Oxidative Lung Injury. Chem. Res. Toxicol. 2022, 35, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Lucas, J.H.; Wang, Q.; Lamb, T.; McGraw, M.D.; Rahman, I. Pulmonary Toxicity and Inflammatory Response of Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI. Toxics 2020, 8, 46. [Google Scholar] [CrossRef]
- Jităreanu, A.; Cara, I.G.; Sava, A.; Mârțu, I.; Caba, I.-C.; Agoroaei, L. The Impact of the Storage Conditions and Type of Clearomizers on the Increase of Heavy Metal Levels in Electronic Cigarette Liquids Retailed in Romania. Toxics 2022, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Din, A.E.; Mohsen, S.-M. The Flavoring Ethyl Maltol, Found in E-Cigarettes, Mediates Metal Cellular Toxicity. Ph.D. Thesis, Johns Hopkins University, Baltimore, MD, USA, 2020. [Google Scholar]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and Quantitative Determination of Heavy Metals in Electronic Cigarette Refill Liquids Using Total Reflection X-Ray Fluorescence Spectrometry. Food Chem. Toxicol. 2018, 116, 233–237. [Google Scholar] [CrossRef]
- Gray, N.; Halstead, M.; Gonzalez-Jimenez, N.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of Toxic Metals in Liquid from Electronic Cigarettes. Int. J. Environ. Res. Public Health 2019, 16, 4450. [Google Scholar] [CrossRef]
- Na, C.-J.; Jo, S.-H.; Kim, K.-H.; Sohn, J.-R.; Son, Y.-S. The Transfer Characteristics of Heavy Metals in Electronic Cigarette Liquid. Environ. Res. 2019, 174, 152–159. [Google Scholar] [CrossRef]
- Mikheev, V.B.; Brinkman, M.C.; Granville, C.A.; Gordon, S.M.; Clark, P.I. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob. Res. 2016, 18, 1895–1902. [Google Scholar] [CrossRef]
- Mo, Y.; Jiang, M.; Zhang, Y.; Wan, R.; Li, J.; Zhong, C.-J.; Li, H.; Tang, S.; Zhang, Q. Comparative Mouse Lung Injury by Nickel Nanoparticles with Differential Surface Modification. J. Nanobiotechnol. 2019, 17, 2. [Google Scholar] [CrossRef]
- Re, D.B.; Hilpert, M.; Saglimbeni, B.; Strait, M.; Ilievski, V.; Coady, M.; Talayero, M.; Wilmsen, K.; Chesnais, H.; Balac, O.; et al. Exposure to E-Cigarette Aerosol over Two Months Induces Accumulation of Neurotoxic Metals and Alteration of Essential Metals in Mouse Brain. Environ. Res. 2021, 202, 111557. [Google Scholar] [CrossRef]
- Wiener, R.C.; Bhandari, R. Association of Electronic Cigarette Use with Lead, Cadmium, Barium, and Antimony Body Burden: NHANES 2015–2016. J. Trace Elem. Med. Biol. 2020, 62, 126602. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Barreau, T.; Wu, N. Cancer and Non-Cancer Risk Concerns from Metals in Electronic Cigarette Liquids and Aerosols. Int. J. Environ. Res. Public Health 2020, 17, 2146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Aravindakshan, A.; Hilpert, M.; Olmedo, P.; Rule, A.M.; Navas-Acien, A.; Aherrera, A. Metal/Metalloid Levels in Electronic Cigarette Liquids, Aerosols, and Human Biosamples: A Systematic Review. Environ. Health Perspect. 2020, 128, 036001. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wise, J.T.F.; Wang, L.; Schumann, K.; Zhang, Z.; Shi, X. Dual Roles of Oxidative Stress in Metal Carcinogenesis. JEP(T) 2017, 36. [Google Scholar] [CrossRef]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-Cigarettes as a Source of Toxic and Potentially Carcinogenic Metals. Environ. Res. 2017, 152, 221–225. [Google Scholar] [CrossRef]
- Blair, A.; Freeman, L.B. Lung Cancer among Nonsmokers. Epidemiology 2006, 17, 601–603. [Google Scholar] [CrossRef]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung Cancer in Never Smokers: Clinical Epidemiology and Environmental Risk Factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, L.; Zhang, Y.; Zhao, Y.; Liu, Y. Air Pollution: A Culprit of Lung Cancer. J. Hazard. Mater. 2022, 434, 128937. [Google Scholar] [CrossRef]
- Aalami, A.H.; Hoseinzadeh, M.; Hosseini Manesh, P.; Jiryai Sharahi, A.; Kargar Aliabadi, E. Carcinogenic Effects of Heavy Metals by Inducing Dysregulation of MicroRNAs: A Review. Mol. Biol. Rep. 2022, 49, 12227–12238. [Google Scholar] [CrossRef]
- Brenner, D.R.; Boffetta, P.; Duell, E.J.; Bickeböller, H.; Rosenberger, A.; McCormack, V.; Muscat, J.E.; Yang, P.; Wichmann, H.-E.; Brueske-Hohlfeld, I.; et al. Previous Lung Diseases and Lung Cancer Risk: A Pooled Analysis from the International Lung Cancer Consortium. Am. J. Epidemiol. 2012, 176, 573–585. [Google Scholar] [CrossRef]
- Porro, C.; La Torre, M.E.; Tartaglia, N.; Benameur, T.; Santini, M.; Ambrosi, A.; Messina, G.; Cibelli, G.; Fiorelli, A.; Polito, R.; et al. The Potential Role of Nutrition in Lung Cancer Establishment and Progression. Life 2022, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gümüş, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Tu, Z.; Chen, H.; Liu, Z. Identifying Modifiable Risk Factors of Lung Cancer: Indications from Mendelian Randomization. PLoS ONE 2021, 16, e0258498. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.B.; Lang, J.J.; Compton, K.; Xu, R.; Acheson, A.R.; Henrikson, H.J.; Kocarnik, J.M.; Penberthy, L.; Aali, A.; Abbas, Q.; et al. The Global Burden of Cancer Attributable to Risk Factors, 2010–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Zhai, T.; Li, S.; Hu, W.; Li, D.; Leng, S. Potential Micronutrients and Phytochemicals against the Pathogenesis of Chronic Obstructive Pulmonary Disease and Lung Cancer. Nutrients 2018, 10, 813. [Google Scholar] [CrossRef]
- Dogan, S.; Shen, R.; Ang, D.C.; Johnson, M.L.; D’Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.G.; Zakowski, M.F.; et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012, 18, 6169–6177. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational Signatures Associated with Tobacco Smoking in Human Cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef]
- Landi, M.T.; Synnott, N.C.; Rosenbaum, J.; Zhang, T.; Zhu, B.; Shi, J.; Zhao, W.; Kebede, M.; Sang, J.; Choi, J.; et al. Tracing Lung Cancer Risk Factors Through Mutational Signatures in Never-Smokers: The Sherlock-Lung Study. Am. J. Epidemiol. 2021, 190, 962–976. [Google Scholar] [CrossRef]
- Ernst, S.M.; Mankor, J.M.; van Riet, J.; von der Thüsen, J.H.; Dubbink, H.J.; Aerts, J.G.J.V.; de Langen, A.J.; Smit, E.F.; Dingemans, A.-M.C.; Monkhorst, K. Tobacco Smoking-Related Mutational Signatures in Classifying Smoking-Associated and Nonsmoking-Associated NSCLC. J. Thorac. Oncol. 2023, 18, 487–498. [Google Scholar] [CrossRef]
- Inamura, K.; Hamada, T.; Bullman, S.; Ugai, T.; Yachida, S.; Ogino, S. Cancer as Microenvironmental, Systemic and Environmental Diseases: Opportunity for Transdisciplinary Microbiomics Science. Gut 2022, 71, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A Review of Human Carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.A.; Linton, O.W. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, Medical Exposure—Are We Doing Less with More, and Is There a Role for Health Physicists? Health Phys. 2009, 97, 1. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.E. Radon Gas: A Geologic Hazard in Arizona; Arizona Geological Survey Down-to-Earth Series DTE-2; University of Arizona: Tucson, AZ, USA, 1992; 24p, Available online: http://hdl.handle.net/10150/628821 (accessed on 21 June 2023).
- Report No. 160—Ionizing Radiation Exposure of the Population of the United States (2009); NCRP: Bethesda, MD, USA; Available online: https://ncrponline.org/shop/reports/report-no-160-ionizing-radiation-exposure-of-the-population-of-the-united-states/ (accessed on 12 April 2023).
- Akerblom, G.; Wilson, C. Radon—Geological Aspects of an Environmental Problem: Environmental Radon Investigation in Sweden—Regional Environmental Documentation of Natural Radiation in Sweden; Sveriges Geologiska Undersoekning: Uppsala, Sweden, 1982. [Google Scholar]
- Kreienbrock, L.; Kreuzer, M.; Gerken, M.; Dingerkus, G.; Wellmann, J.; Keller, G.; Erich Wichmann, H. Case-Control Study on Lung Cancer and Residential Radon in Western Germany. Am. J. Epidemiol. 2001, 153, 42–52. [Google Scholar] [CrossRef]
- Zeeb, H.; Shannoun, F.; World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, Switzerland, 2009; Available online: https://apps.who.int/iris/handle/10665/44149 (accessed on 21 June 2023).
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in Homes and Risk of Lung Cancer: Collaborative Analysis of Individual Data from 13 European Case-Control Studies. BMJ 2005, 330, 223. [Google Scholar] [CrossRef]
- Samet, J.M.; Kutvirt, D.M.; Waxweiler, R.J.; Key, C.R. Uranium Mining and Lung Cancer in Navajo Men. N. Engl. J. Med. 1984, 310, 1481–1484. [Google Scholar] [CrossRef]
- Greenberg, M.; Selikoff, I.J. Lung Cancer in the Schneeberg Mines: A Reappraisal of the Data Reported by Harting and Hesse in 1879. Ann. Occup. Hyg. 1993, 37, 5–14. [Google Scholar] [CrossRef]
- Grosche, B.; Kreuzer, M.; Kreisheimer, M.; Schnelzer, M.; Tschense, A. Lung Cancer Risk among German Male Uranium Miners: A Cohort Study, 1946–1998. Br. J. Cancer 2006, 95, 1280–1287. [Google Scholar] [CrossRef]
- Lorenzo-González, M.; Ruano-Ravina, A.; Torres-Durán, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Leiro-Fernández, V.; Vidal-García, I.; Castro-Añón, O.; Martínez, C.; et al. Residential Radon, Genetic Polymorphisms in DNA Damage and Repair-Related. Lung Cancer 2019, 135, 10–15. [Google Scholar] [CrossRef]
- Robertson, A.; Allen, J.; Laney, R.; Curnow, A. The Cellular and Molecular Carcinogenic Effects of Radon Exposure: A Review. Int. J. Mol. Sci. 2013, 14, 14024–14063. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.A.; Hei, T.K.; Hukku, B.; McRaven, J.A.; Willey, J.C. Cytogenetic and Molecular Genetic Analysis of Tumorigenic Human Bronchial Epithelial Cells Induced by Radon Alpha Particles. Carcinogenesis 1997, 18, 1251–1257. [Google Scholar] [CrossRef]
- Meenakshi, C.; Sivasubramanian, K.; Venkatraman, B. Nucleoplasmic Bridges as a Biomarker of DNA Damage Exposed to Radon. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017, 814, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Choi, J.W.; Hong, M.H.; Jung, D.; Lee, C.Y.; Park, S.Y.; Shim, H.S.; Sheen, S.; Kwak, K.I.; Kang, D.R.; et al. Indoor Radon Exposure Increases Tumor Mutation Burden in Never-Smoker Patients with Lung Adenocarcinoma. Lung Cancer 2019, 131, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, D.; Gu, C.; Liu, X.; Pei, W.; Li, J.; Cao, Y.; Jiao, Y.; Tong, J.; Nie, J. Down-Regulation of Let-7 MicroRNA Increased K-Ras Expression in Lung Damage Induced by Radon. Environ. Toxicol. Pharmacol. 2015, 40, 541–548. [Google Scholar] [CrossRef]
- Bersimbaev, R.; Pulliero, A.; Bulgakova, O.; Kussainova, A.; Aripova, A.; Izzotti, A. Radon Biomonitoring and MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2020, 21, 2154. [Google Scholar] [CrossRef]
- Czochor, J.R.; Glazer, P.M. MicroRNAs in Cancer Cell Response to Ionizing Radiation. Antioxid. Redox Signal. 2014, 21, 293–312. [Google Scholar] [CrossRef]
- Wei, W.; Dong, Z.; Gao, H.; Zhang, Y.-Y.; Shao, L.-H.; Jin, L.-L.; Lv, Y.-H.; Zhao, G.; Shen, Y.-N.; Jin, S.-Z. MicroRNA-9 Enhanced Radiosensitivity and Its Mechanism of DNA Methylation in Non-Small Cell Lung Cancer. Gene 2019, 710, 178–185. [Google Scholar] [CrossRef]
- Nie, J.; Wu, J.; Chen, Z.; Jiao, Y.; Zhang, J.; Tian, H.; Li, J.; Tong, J. Expression Profiles of Long Non-Coding RNA in Mouse Lung Tissue Exposed to Radon. J. Toxicol. Environ. Health Part A 2019, 82, 854–861. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Polychlorinated Dibenzo-para-dioxins and Polychlorinated Dibenzofurans. Lyon (FR): International Agency for Research on Cancer; 1997. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 69.). Available online: https://www.ncbi.nlm.nih.gov/books/NBK409980/ (accessed on 21 June 2023).
- Grantzau, T.; Overgaard, J. Risk of Second Non-Breast Cancer after Radiotherapy for Breast Cancer: A Systematic Review and Meta-Analysis of 762,468 Patients. Radiother. Oncol. 2015, 114, 56–65. [Google Scholar] [CrossRef]
- Neugut, A.I.; Lee, W.C.; Murray, T.; Robinson, E.; Karwoski, K.; Kutcher, G.J. Lung Cancer after Radiation Therapy for Breast Cancer. Cancer 1993, 71, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Li, Z.; Yue, J.; Xu, M.; Zhang, Y.; Yung, K.K.L.; Li, R. Fine Particulate Matter Induces Mitochondrial Dysfunction and Oxidative Stress in Human SH-SY5Y Cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Losacco, C.; Perillo, A. Particulate Matter Air Pollution and Respiratory Impact on Humans and Animals. Environ. Sci. Pollut. Res. 2018, 25, 33901–33910. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, X.; Zhu, Y.; Li, D.; Jing, D.; Su, X.; Pan, P.; Liu, H.; Zhang, Y. Association of Outdoor Air Pollution, Lifestyle, Genetic Factors with the Risk of Lung Cancer: A Prospective Cohort Study. Environ. Res. 2023, 218, 114996. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Tsuang, B.-J.; Chiang, C.-J.; Ku, K.-C.; Tseng, J.-S.; Yang, T.-Y.; Hsu, K.-H.; Chen, K.-C.; Yu, S.-L.; Lee, W.-C.; et al. The Relationship Between Air Pollution and Lung Cancer in Nonsmokers in Taiwan. J. Thorac. Oncol. 2019, 14, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-Year Trends of the Global Burden of Disease Attributable to Ambient Air Pollution: An Analysis of Data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Hill, W.; Lim, E.L.; Weeden, C.E.; Lee, C.; Augustine, M.; Chen, K.; Kuan, F.-C.; Marongiu, F.; Evans, E.J.; Moore, D.A.; et al. Lung Adenocarcinoma Promotion by Air Pollutants. Nature 2023, 616, 159–167. [Google Scholar] [CrossRef]
- Mu, L.; Liu, L.; Niu, R.; Zhao, B.; Shi, J.; Li, Y.; Swanson, M.; Scheider, W.; Su, J.; Chang, S.-C.; et al. Indoor Air Pollution and Risk of Lung Cancer among Chinese Female Non-Smokers. Cancer Causes Control 2013, 24, 439–450. [Google Scholar] [CrossRef]
- Guo, H.; Li, W.; Wu, J. Ambient PM2.5 and Annual Lung Cancer Incidence: A Nationwide Study in 295 Chinese Counties. Int. J. Environ. Res. Public Health 2020, 17, 1481. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Severi, G.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Boutron-Ruault, M.-C.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; et al. Long-Term Low-Level Ambient Air Pollution Exposure and Risk of Lung Cancer—A Pooled Analysis of 7 European Cohorts. Environ. Int. 2021, 146, 106249. [Google Scholar] [CrossRef]
- Stavroulas, I.; Grivas, G.; Michalopoulos, P.; Liakakou, E.; Bougiatioti, A.; Kalkavouras, P.; Fameli, K.M.; Hatzianastassiou, N.; Mihalopoulos, N.; Gerasopoulos, E. Field Evaluation of Low-Cost PM Sensors (Purple Air PA-II) Under Variable Urban Air Quality Conditions, in Greece. Atmosphere 2020, 11, 926. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, D.; Cui, B.; Ding, R.; Shi, X.; He, P. Association between Particulate Matter Air Pollution and Lung Cancer. Thorax 2020, 75, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Yang, J.; He, J.; Yang, X.; Hubbard, R.; Ji, D. An Investigation into the Impact of Variations of Ambient Air Pollution and Meteorological Factors on Lung Cancer Mortality in Yangtze River Delta. Sci. Total Environ. 2021, 779, 146427. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, G.; Badaloni, C.; Gariazzo, C.; Stafoggia, M.; Sozzi, R.; Davoli, M.; Forastiere, F. Long-Term Exposure to Urban Air Pollution and Mortality in a Cohort of More than a Million Adults in Rome. Environ. Health Perspect. 2013, 121, 324–331. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air Pollution and Lung Cancer Incidence in 17 European Cohorts: Prospective Analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Tomczak, A.; Miller, A.B.; Weichenthal, S.A.; To, T.; Wall, C.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Villeneuve, P.J. Long-Term Exposure to Fine Particulate Matter Air Pollution and the Risk of Lung Cancer among Participants of the Canadian National Breast Screening Study. Int. J. Cancer 2016, 139, 1958–1966. [Google Scholar] [CrossRef]
- Hart, J.E.; Spiegelman, D.; Beelen, R.; Hoek, G.; Brunekreef, B.; Schouten, L.J.; van den Brandt, P. Long-Term Ambient Residential Traffic–Related Exposures and Measurement Error–Adjusted Risk of Incident Lung Cancer in the Netherlands Cohort Study on Diet and Cancer. Environ. Health Perspect. 2015, 123, 860–866. [Google Scholar] [CrossRef]
- Balmain, A. Air Pollution’s Role in the Promotion of Lung Cancer. Nature 2023, 616, 35–36. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; Cheng, X.; Shao, M.; Wu, C.; Wang, S.; Li, H.; Wei, L.; Gao, Y.; Tan, W.; et al. Exposure to Airborne PM2.5 Suppresses MicroRNA Expression and Deregulates Target Oncogenes That Cause Neoplastic Transformation in NIH3T3 Cells. Oncotarget 2015, 6, 29428–29439. [Google Scholar] [CrossRef][Green Version]
- Ali, H.; Khan, E. What Are Heavy Metals? Long-Standing Controversy over the Scientific Use of the Term ‘Heavy Metals’—Proposal of a Comprehensive Definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.I.; Ong, H.C.; Mofijur, M. Heavy Metal Toxicity, Sources, and Remediation Techniques for Contaminated Water and Soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Xu, X.; Liao, W.; Lin, Y.; Dai, Y.; Shi, Z.; Huo, X. Blood Concentrations of Lead, Cadmium, Mercury and Their Association with Biomarkers of DNA Oxidative Damage in Preschool Children Living in an e-Waste Recycling Area. Environ. Geochem. Health 2018, 40, 1481–1494. [Google Scholar] [CrossRef]
- Alabi, O.A.; Adeoluwa, Y.M.; Huo, X.; Xu, X.; Bakare, A.A. Environmental Contamination and Public Health Effects of Electronic Waste: An Overview. J. Environ. Health Sci. Eng. 2021, 19, 1209–1227. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Ducatman, A. Association between Six Environmental Chemicals and Lung Cancer Incidence in the United States. J. Environ. Public Health 2011, 2011, e463701. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Lee, N.-W.; Wang, H.-Y.; Du, C.-L.; Yuan, T.-H.; Chen, C.-Y.; Yu, C.-J.; Chan, C.-C. Air-Polluted Environmental Heavy Metal Exposure Increase Lung Cancer Incidence and Mortality: A Population-Based Longitudinal Cohort Study. Sci. Total Environ. 2022, 810, 152186. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2010; Available online: www.who.int/ipcs/features/cadmium.pdf (accessed on 13 January 2015).
- Taha, M.M.; Mahdy-Abdallah, H.; Shahy, E.M.; Ibrahim, K.S.; Elserougy, S. Impact of Occupational Cadmium Exposure on Bone in Sewage Workers. Int. J. Occup. Environ. Health 2018, 24, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzóska, M.M. Environmental Exposure to Cadmium—A Risk for Health of the General Population in Industrialized Countries and Preventive Strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Martens, D.S.; Hara, A.; Plusquin, M.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Association of Total Cancer and Lung Cancer with Environmental Exposure to Cadmium: The Meta-Analytical Evidence. Cancer Causes Control 2015, 26, 1281–1288. [Google Scholar] [CrossRef]
- Demir, N.; Enon, S.; Turksoy, V.A.; Kayaalti, Z.; Kaya, S.; Cangir, A.K.; Soylemezoglu, T.; Savas, I. Association of Cadmium but Not Arsenic Levels in Lung Cancer Tumor Tissue with Smoking, Histopathological Type and Stage. Asian Pac. J. Cancer Prev. 2014, 15, 2965–2970. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A. Combined Effect of Single-Walled Carbon Nanotubes and Cadmium on Human Lung Cancer Cells. Environ. Sci. Pollut. Res. 2022, 29, 87844–87857. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of Oxidative Stress in Cadmium Toxicity and Carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and Cellular Mechanisms of Cadmium Carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Rao, C.V.; Pal, S.; Mohammed, A.; Farooqui, M.; Doescher, M.P.; Asch, A.S.; Yamada, H.Y. Biological Effects and Epidemiological Consequences of Arsenic Exposure, and Reagents That Can Ameliorate Arsenic Damage in Vivo. Oncotarget 2017, 8, 57605–57621. [Google Scholar] [CrossRef]
- Smith, A.H.; Ercumen, A.; Yuan, Y.; Steinmaus, C.M. Increased Lung Cancer Risks Are Similar Whether Arsenic Is Ingested or Inhaled. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 343–348. [Google Scholar] [CrossRef]
- Monteiro De Oliveira, E.C.; Caixeta, E.S.; Santos, V.S.V.; Pereira, B.B. Arsenic Exposure from Groundwater: Environmental Contamination, Human Health Effects, and Sustainable Solutions. J. Toxicol. Environ. Health Part B 2021, 24, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Folesani, G.; Galetti, M.; Petronini, P.G.; Mozzoni, P.; La Monica, S.; Cavallo, D.; Corradi, M. Interaction between Occupational and Non-Occupational Arsenic Exposure and Tobacco Smoke on Lung Cancerogenesis: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4167. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Picciotto, I.; Smith, A.H.; Holtzman, D.; Lipsett, M.; Alexeeff, G. Synergism between Occupational Arsenic Exposure and Smoking in the Induction of Lung Cancer. Epidemiology 1992, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- CDC. Immediately Dangerous to Life or Health Concentrations (IDLH): Arsine—NIOSH Publications and Products. Available online: https://www.cdc.gov/niosh/idlh/7784421.html (accessed on 15 April 2023).
- Thomas, D.J. Molecular Processes in Cellular Arsenic Metabolism. Toxicol. Appl. Pharmacol. 2007, 222, 365–373. [Google Scholar] [CrossRef]
- Lewis, A.S.; Beyer, L.A.; Zu, K. Considerations in Deriving Quantitative Cancer Criteria for Inorganic Arsenic Exposure via Inhalation. Environ. Int. 2015, 74, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.M.; Ferreccio, C.; Romo, J.A.; Yuan, Y.; Cortes, S.; Marshall, G.; Moore, L.E.; Balmes, J.R.; Liaw, J.; Golden, T.; et al. Drinking Water Arsenic in Northern Chile: High Cancer Risks 40 Years after Exposure Cessation. Cancer Epidemiol. Biomark. Prev. 2013, 22, 623–630. [Google Scholar] [CrossRef]
- Dauphiné, D.C.; Ferreccio, C.; Guntur, S.; Yuan, Y.; Hammond, S.K.; Balmes, J.; Smith, A.H.; Steinmaus, C. Lung Function in Adults Following in Utero and Childhood Exposure to Arsenic in Drinking Water: Preliminary Findings. Int. Arch. Occup. Environ. Health 2011, 84, 591–600. [Google Scholar] [CrossRef]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Yuan, Y.; Liaw, J.; Durán, V.; Cuevas, S.; García, J.; Meza, R.; Valdés, R.; et al. Increased Lung and Bladder Cancer Incidence in Adults after In Utero and Early-Life Arsenic Exposure. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1529–1538. [Google Scholar] [CrossRef]
- Georgakilas, A.G. Oxidative Stress, DNA Damage and Repair in Carcinogenesis: Have We Established a Connection? Cancer Lett. 2012, 327, 3–4. [Google Scholar] [CrossRef]
- Islam, R.; Zhao, L.; Zhang, X.; Liu, L.-Z. MiR-218-5p/EGFR Signaling in Arsenic-Induced Carcinogenesis. Cancers 2023, 15, 1204. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, Q.; Fang, S.; Han, X.; Wang, Z. Anti-Tumor Activity of High-Dose EGFR Tyrosine Kinase Inhibitor and Sequential Docetaxel in Wild Type EGFR Non-Small Cell Lung Cancer Cell Nude Mouse Xenografts. Oncotarget 2016, 8, 9134–9143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Ding, H.; Wang, W.; Liao, Z.; Fu, Z.; Hong, Y.; Zhou, Y.; Zhang, C.-Y.; Chen, X. Tumor-Suppressive MiR-218-5p Inhibits Cancer Cell Proliferation and Migration via EGFR in Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 28075–28085. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, J.; Wu, L.; He, C.; Chen, G. The Role of PD-1/PD-L1 Checkpoint in Arsenic Lung Tumorigenesis. Toxicol. Appl. Pharmacol. 2021, 426, 115633. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, L.; Liu, Y.; Yi, M.; Chu, Q.; Jiao, D.; Wu, K. Metabolic Profiles of Regulatory T Cells and Their Adaptations to the Tumor Microenvironment: Implications for Antitumor Immunity. J. Hematol. Oncol. 2022, 15, 104. [Google Scholar] [CrossRef]
- Cooper, K.L.; Liu, R.; Zhou, X. Particulate Arsenic Trioxide Induces Higher DNA Damage and Reactive Oxygen Species than Soluble Arsenite in Lung Epithelial Cells. Toxicol. Appl. Pharmacol. 2022, 457, 116320. [Google Scholar] [CrossRef]
- Kameda, T.; Takahashi, K.; Kim, R.; Jiang, Y.; Movahed, M.; Park, E.-K.; Rantanen, J. Asbestos: Use, Bans and Disease Burden in Europe. Bull. World Health Organ. 2014, 92, 790–797. [Google Scholar] [CrossRef]
- Norbet, C.; Joseph, A.; Rossi, S.S.; Bhalla, S.; Gutierrez, F.R. Asbestos-Related Lung Disease: A Pictorial Review. Curr. Probl. Diagn. Radiol. 2015, 44, 371–382. [Google Scholar] [CrossRef]
- Huang, S.X.L.; Jaurand, M.-C.; Kamp, D.W.; Whysner, J.; Hei, T.K. Role of Mutagenicity in Asbestos Fiber-Induced Carcinogenicity and Other Diseases. J. Toxicol. Environ. Health Part B 2011, 14, 179–245. [Google Scholar] [CrossRef]
- Ospina, D.; Villegas, V.E.; Rodríguez-Leguizamón, G.; Rondón-Lagos, M. Analyzing Biological and Molecular Characteristics and Genomic Damage Induced by Exposure to Asbestos. Cancer Manag. Res. 2019, 11, 4997–5012. [Google Scholar] [CrossRef]
- Wolff, H.; Vehmas, T.; Oksa, P.; Rantanen, J.; Vainio, H. Asbestos, Asbestosis, and Cancer, the Helsinki Criteria for Diagnosis and Attribution 2014: Recommendations. Scand. J. Work Environ. Health 2015, 41, 5–15. [Google Scholar] [CrossRef]
- Donovan, E.P.; Donovan, B.L.; McKinley, M.A.; Cowan, D.M.; Paustenbach, D.J. Evaluation of Take Home (Para-Occupational) Exposure to Asbestos and Disease: A Review of the Literature. Crit. Rev. Toxicol. 2012, 42, 703–731. [Google Scholar] [CrossRef] [PubMed]
- Bersimbaev, R.; Bulgakova, O.; Aripova, A.; Kussainova, A.; Ilderbayev, O. Role of MicroRNAs in Lung Carcinogenesis Induced by Asbestos. J. Pers. Med. 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, Smoking and Lung Cancer: An Update. Int. J. Environ. Res. Public Health 2020, 17, 258. [Google Scholar] [CrossRef]
- Markowitz, S.B.; Levin, S.M.; Miller, A.; Morabia, A. Asbestos, Asbestosis, Smoking, and Lung Cancer. New Findings from the North American Insulator Cohort. Am. J. Respir. Crit. Care Med. 2013, 188, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S. Asbestos-Related Lung Cancer and Malignant Mesothelioma of the Pleura: Selected Current Issues. Semin. Respir. Crit. Care Med. 2015, 36, 334–346. [Google Scholar] [CrossRef]
- Upadhyay, D.; Kamp, D.W. Asbestos-Induced Pulmonary Toxicity: Role of DNA Damage and Apoptosis. Exp. Biol. Med. 2003, 228, 650–659. [Google Scholar] [CrossRef]
- Liu, G.; Cheresh, P.; Kamp, D.W. Molecular Basis of Asbestos-Induced Lung Disease. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 161–187. [Google Scholar] [CrossRef]
- Santarelli, L.; Gaetani, S.; Monaco, F.; Bracci, M.; Valentino, M.; Amati, M.; Rubini, C.; Sabbatini, A.; Pasquini, E.; Zanotta, N.; et al. Four-MiRNA Signature to Identify Asbestos-Related Lung Malignancies. Cancer Epidemiol. Biomark. Prev. 2019, 28, 119–126. [Google Scholar] [CrossRef]
- Micolucci, L.; Akhtar, M.M.; Olivieri, F.; Rippo, M.R.; Procopio, A.D. Diagnostic Value of MicroRNAs in Asbestos Exposure and Malignant Mesothelioma: Systematic Review and Qualitative Meta-Analysis. Oncotarget 2016, 7, 58606–58637. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–269. [Google Scholar]
- Thakur, S.; Sarkar, B.; Dhiman, M.; Mantha, A.K. Organophosphate-Pesticides Induced Survival Mechanisms and APE1-Mediated Nrf2 Regulation in Non-Small-Cell Lung Cancer Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22640. [Google Scholar] [CrossRef]
- Ecobichon, D.J. Pesticide Use in Developing Countries. Toxicology 2001, 160, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Prins, J.M.; George, K.M.; Thompson, C.M. Thionate versus Oxon: Comparison of Stability, Uptake, and Cell Toxicity of (14CH3O)2-Labeled Methyl Parathion and Methyl Paraoxon with SH-SY5Y Cells. J. Agric. Food Chem. 2010, 58, 8460–8466. [Google Scholar] [CrossRef] [PubMed]
- Paz-Trejo, C.; Gómez-Arroyo, S. Genotoxic Evaluation of Common Commercial Pesticides in Human Peripheral Blood Lymphocytes. Toxicol. Ind. Health 2017, 33, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Freeman, L.E.B.; Hoppin, J.A.; Koutros, S.; Sandler, D.P.; Lynch, C.F.; Hines, C.J.; Thomas, K.; Blair, A.; Alavanja, M.C. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 544–551. [Google Scholar] [CrossRef]
- Kim, B.; Park, E.Y.; Kim, J.; Park, E.; Oh, J.-K.; Lim, M.K. Occupational Exposure to Pesticides and Lung Cancer Risk: A Propensity Score Analyses. Cancer Res. Treat. 2021, 54, 130–139. [Google Scholar] [CrossRef]
- Jones, R.R.; Barone-Adesi, F.; Koutros, S.; Lerro, C.C.; Blair, A.; Lubin, J.; Heltshe, S.L.; Hoppin, J.A.; Alavanja, M.C.R.; Freeman, L.E.B. Incidence of Solid Tumours among Pesticide Applicators Exposed to the Organophosphate Insecticide Diazinon in the Agricultural Health Study: An Updated Analysis. Occup. Environ. Med. 2015, 72, 496–503. [Google Scholar] [CrossRef]
- Pesatori, A.C.; Sontag, J.M.; Lubin, J.H.; Consonni, D.; Blair, A. Cohort Mortality and Nested Case-Control Study of Lung Cancer among Structural Pest Control Workers in Florida (United States). Cancer Causes Control 1994, 5, 310–318. [Google Scholar] [CrossRef]
- Boulanger, M.; Tual, S.; Lemarchand, C.; Guizard, A.-V.; Delafosse, P.; Marcotullio, E.; Pons, R.; Piel, C.; Pouchieu, C.; Baldi, I.; et al. Lung Cancer Risk and Occupational Exposures in Crop Farming: Results from the AGRIculture and CANcer (AGRICAN) Cohort. Occup. Environ. Med. 2018, 75, 776–785. [Google Scholar] [CrossRef]
- Bajgar, J. Organophosphates/Nerve Agent Poisoning: Mechanism of Action, Diagnosis, Prophylaxis, And Treatment. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2004; Volume 38, pp. 151–216. [Google Scholar]
- Costa, L.G. Current Issues in Organophosphate Toxicology. Clin. Chim. Acta 2006, 366, 1–13. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Riemer, J. Apoptosis Inducing Factor and Mitochondrial NADH Dehydrogenases: Redox-Controlled Gear Boxes to Switch between Mitochondrial Biogenesis and Cell Death. Biol. Chem. 2021, 402, 289–297. [Google Scholar] [CrossRef]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental Toxicity, Oxidative Stress and Apoptosis: Ménage à Trois. Mutat. Res. 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.E.; Simons, C.C.J.M.; van den Brandt, P.A.; van Engeland, M.; Weijenberg, M.P. Lifestyle, Diet, and Colorectal Cancer Risk According to (Epi)Genetic Instability: Current Evidence and Future Directions of Molecular Pathological Epidemiology. Curr. Color. Cancer Rep. 2017, 13, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Chan, A.T.; Fuchs, C.S.; Giovannucci, E. Molecular Pathological Epidemiology of Colorectal Neoplasia: An Emerging Transdisciplinary and Interdisciplinary Field. Gut 2011, 60, 397–411. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).