Insights into Facilitated Subcutaneous Immunoglobulin Use in Patients with Secondary Immunodeficiency Diseases: A FIGARO Subgroup Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Study Endpoints
2.4. Statistical Analyses
3. Results
3.1. Patients’ Demographics and Clinical Characteristics at Inclusion
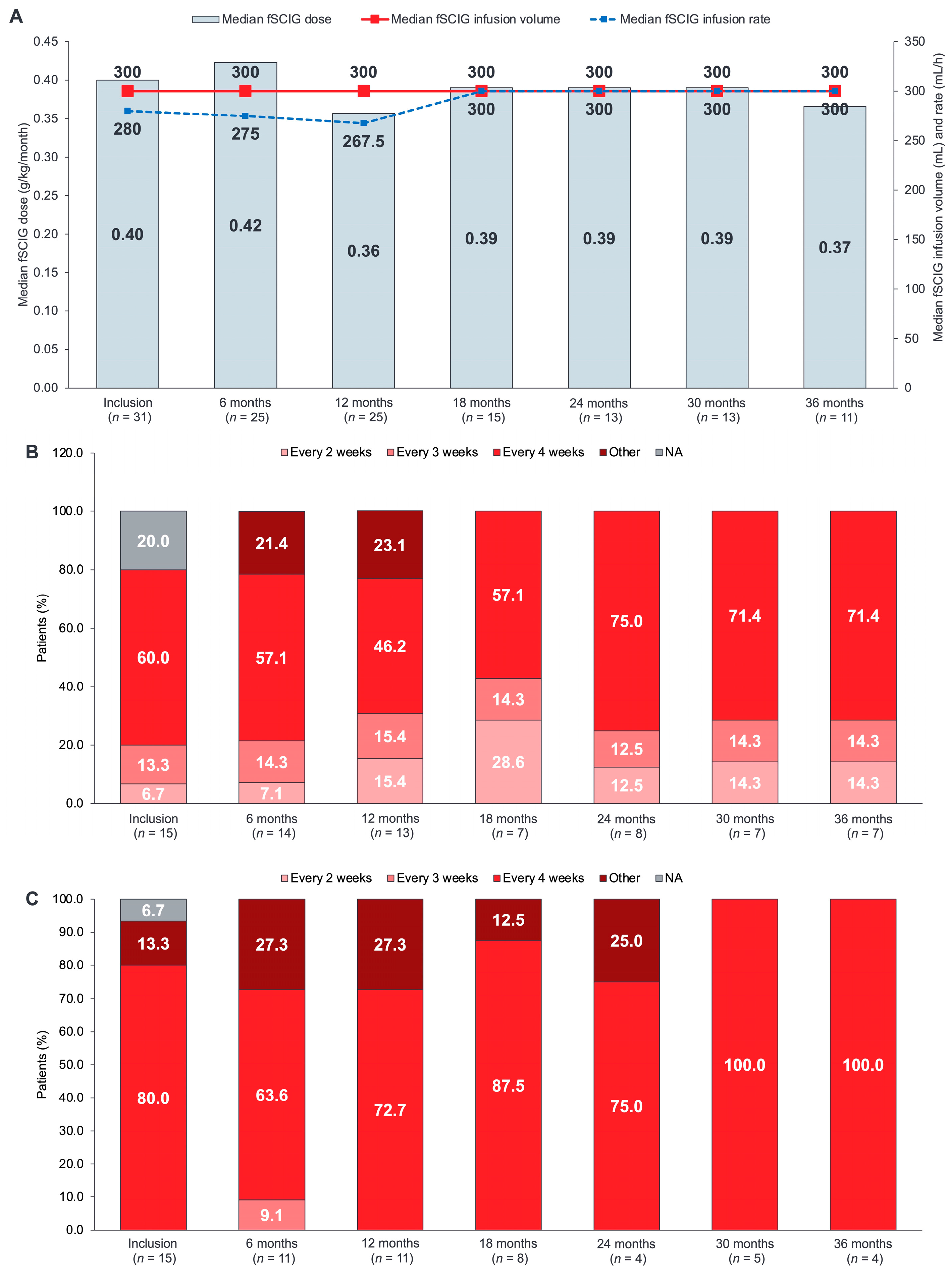
3.2. fSCIG Dose and Administration
3.3. fSCIG Infusion Parameters
3.4. Safety
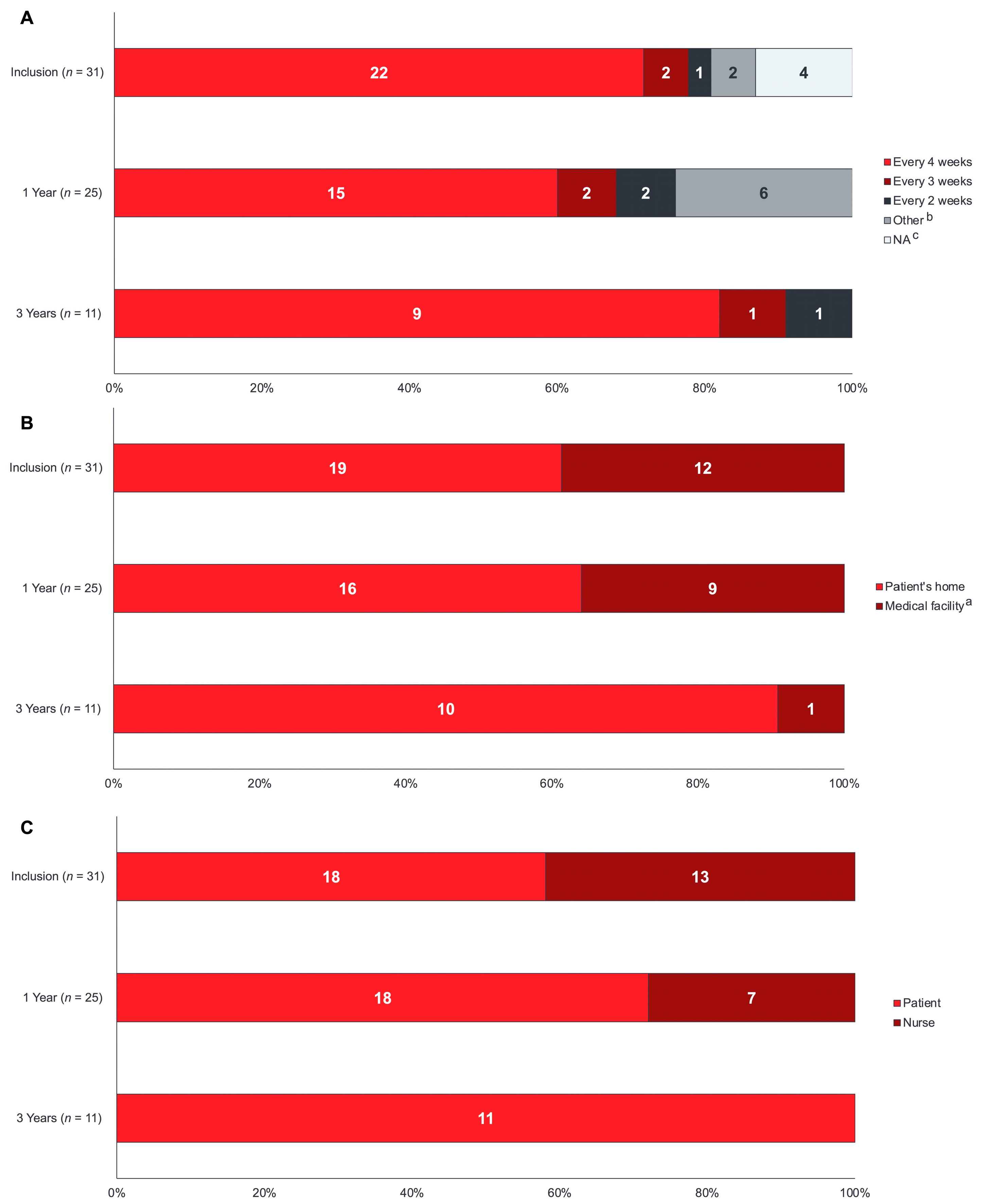
3.5. Training and Administration Health Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.Y.; Carbone, J.; Jolles, S. The expanding field of secondary antibody deficiency: Causes, diagnosis, and management. Front. Immunol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Duraisingham, S.S.; Buckland, M.; Dempster, J.; Lorenzo, L.; Grigoriadou, S.; Longhurst, H.J. Primary vs. secondary antibody deficiency: Clinical features and infection outcomes of immunoglobulin replacement. PLoS ONE 2014, 9, e100324. [Google Scholar] [CrossRef]
- Na, I.K.; Buckland, M.; Agostini, C.; Edgar, J.D.M.; Friman, V.; Michallet, M.; Sánchez-Ramón, S.; Scheibenbogen, C.; Quinti, I. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur. J. Haematol. 2019, 102, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Wood, P. Secondary antibody deficiency—Causes and approach to diagnosis. Clin. Med. 2016, 16, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Blau, I.W.; Conlon, N.; Petermann, R.; Nikolov, N.; Plesner, T. Facilitated subcutaneous immunoglobulin administration (fSCIg): A new treatment option for patients with secondary immune deficiencies. Expert Rev. Clin. Immunol. 2016, 12, 705–711. [Google Scholar] [CrossRef]
- Jolles, S.; Smith, B.D.; Vinh, D.C.; Mallick, R.; Espinoza, G.; DeKoven, M.; Divino, V. Risk factors for severe infections in secondary immunodeficiency: A retrospective US administrative claims study in patients with hematological malignancies. Leuk. Lymphoma 2022, 63, 64–73. [Google Scholar] [CrossRef]
- Jolles, S.; Chapel, H.; Litzman, J. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: A practical approach. Clin. Exp. Immunol. 2017, 188, 333–341. [Google Scholar] [CrossRef]
- Katragkou, A.; Roilides, E.; Walsh, T.J. Role of immunoglobulin therapy to prevent and treat infections. Manag. Infect. Immunocompromised Host 2018, 19, 339–358. [Google Scholar]
- Borte, M.; Hanitsch, L.G.; Mahlaoui, N.; Fasshauer, M.; Huscher, D.; Speletas, M.; Dimou, M.; Kamieniak, M.; Hermann, C.; Pittrow, D.; et al. Facilitated subcutaneous immunoglobulin treatment in patients with immunodeficiencies: The FIGARO study. J. Clin. Immunol. 2023, 43, 1259–1271. [Google Scholar] [CrossRef]
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.; Dorsey, M.; El-Gamal, Y.; Harville, T.O.; Hossny, E.; Mazer, B.; et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Murphy, E.; Riley, P.; Bergman, G.E.; Investigators, V.T. Improved quality of life, immunoglobulin G levels, and infection rates in patients with primary immunodeficiency diseases during self-treatment with subcutaneous immunoglobulin G. South. Med. J. 2010, 103, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Ness, S. Differentiating characteristics and evaluating intravenous and subcutaneous immunoglobulin. Am. J. Manag. Care 2019, 25 (Suppl. 6), S98–S104. [Google Scholar] [PubMed]
- Wasserman, R.L. Recombinant human hyaluronidase-facilitated subcutaneous immunoglobulin infusion in primary immunodeficiency diseases. Immunotherapy 2017, 9, 1035–1050. [Google Scholar] [CrossRef]
- Bonet, C.M.; Waser, N.; Cheng, K.; Tzivelekis, S.; Edgar, J.D.M.; Sánchez-Ramón, S. A systematic literature review of the effects of immunoglobulin replacement therapy on the burden of secondary immunodeficiency diseases associated with hematological malignancies and stem cell transplants. Expert Rev. Clin. Immunol. 2020, 16, 911–921. [Google Scholar] [CrossRef]
- Agostini, C.; Blau, I.W.; Kimby, E.; Plesner, T. Prophylactic immunoglobulin therapy in secondary immune deficiency—An expert opinion. Expert Rev. Clin. Immunol. 2016, 12, 921–926. [Google Scholar] [CrossRef]
- Jolles, S. Hyaluronidase facilitated subcutaneous immunoglobulin in primary immunodeficiency. Immunotargets Ther. 2013, 2, 125–133. [Google Scholar] [CrossRef]
- European Public Assessment Report (EPAR). HyQvia Product Information. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/hyqvia-epar-product-information_en.pdf (accessed on 6 June 2023).
- Bookbinder, L.H.; Hofer, A.; Haller, M.F.; Zepeda, M.L.; Keller, G.A.; Lim, J.E.; Edgington, T.S.; Shepard, H.M.; Patton, J.S.; Frost, G.I. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J. Control. Release 2006, 114, 230–241. [Google Scholar] [CrossRef]
- Frost, G.I. Recombinant human hyaluronidase (rHuPH20): An enabling platform for subcutaneous drug and fluid administration. Expert Opin. Drug Deliv. 2007, 4, 427–440. [Google Scholar] [CrossRef]
- Wasserman, R.L.; Melamed, I.; Stein, M.R.; Engl, W.; Sharkhawy, M.; Leibl, H.; Puck, J.; Rubinstein, A.; Kobrynski, L.; Gupta, S.; et al. Long-term tolerability, safety, and efficacy of recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulin for primary immunodeficiency. J. Clin. Immunol. 2016, 36, 571–582. [Google Scholar] [CrossRef]
- Wasserman, R.L.; Melamed, I.; Kobrynski, L.; Strausbaugh, S.D.; Stein, M.R.; Sharkhawy, M.; Engl, W.; Leibl, H.; Sobolevsky, L.; Gelmont, D.; et al. Efficacy, safety, and pharmacokinetics of a 10% liquid immune globulin preparation (GAMMAGARD LIQUID, 10%) administered subcutaneously in subjects with primary immunodeficiency disease. J. Clin. Immunol. 2011, 31, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.L.; Melamed, I.; Kobrynski, L.; Puck, J.; Gupta, S.; Doralt, J.; Sharkhawy, M.; Engl, W.; Leibl, H.; Gelmont, D.; et al. Recombinant human hyaluronidase facilitated subcutaneous immunoglobulin treatment in pediatric patients with primary immunodeficiencies: Long-term efficacy, safety and tolerability. Immunotherapy 2016, 8, 1175–1186. [Google Scholar] [CrossRef]
- Wasserman, R.L.; Melamed, I.; Stein, M.R.; Gupta, S.; Puck, J.; Engl, W.; Leibl, H.; McCoy, B.; Empson, V.G.; Gelmont, D.; et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J. Allergy Clin. Immunol. 2012, 130, 951–957.e11. [Google Scholar] [CrossRef]
- Reiser, M.; Borte, M.; Huscher, D.; Baumann, U.; Pittrow, D.; Sommer, C.; Stangel, M.; Fasshauer, M.; Gold, R.; Hensel, M. Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: Long-term data of the SIGNS study. Eur. J. Haematol. 2017, 99, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Borte, M.; Anderson-Smits, C.; Hermann, C. Update on real-world use of facilitated subcutaneous immunoglobulin and immune globulin subcutaneous (human) 20% solution in patients with immunodeficiencies: Poster extracts from the 19th Biennial Meeting of the European Society for Immunodeficiencies. Expert Rev. Clin. Immunol. 2021, 17 (Suppl. 1), 7–8. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Home-Based Subcutaneous Infusion of Immunoglobulin for Primary and Secondary Immunodeficiencies: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2017, 17, 1–86. [Google Scholar]
- Ponsford, M.; Carne, E.; Kingdon, C.; Joyce, C.; Price, C.; Williams, C.; El-Shanawany, T.; Williams, P.; Jolles, S. Facilitated subcutaneous immunoglobulin (fSCIg) therapy–practical considerations. Clin. Exp. Immunol. 2015, 182, 302–313. [Google Scholar] [CrossRef]
- Dimou, M.; Iliakis, T.; Maltezas, D.; Bitsani, A.; Kalyva, S.; Koudouna, A.; Kotsanti, S.; Petsa, P.; Papaioannou, P.; Kyrtsonis, M.C.; et al. Efficacy-safety of facilitated subcutaneous immunoglobulin in immunodeficiency due to hematological malignancies. A single-center retrospective analysis. Anticancer Res. 2018, 38, 4187–4191. [Google Scholar] [CrossRef]
- Petersson, C.; Fust, R.; Hagstedt, C.; Wågström, P.; Nilsdotter-Augustinsson, Å. “Experiences of the burden of treatment”—Patient reports of facilitated subcutaneous immunoglobulin treatment in adults with immunodeficiency. J. Clin. Nurs. 2018, 27, 4270–4278. [Google Scholar] [CrossRef]
- Compagno, N.; Cinetto, F.; Semenzato, G.; Agostini, C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: A single-center experience in 61 patients. Haematologica 2014, 99, 1101–1106. [Google Scholar] [CrossRef]
- Li, Z.; Follman, K.; Freshwater, E.; Engler, F.; Yel, L. Integrated population pharmacokinetics of immunoglobulin G following intravenous or subcutaneous administration of various immunoglobulin products in patients with primary immunodeficiencies. Int. Immunopharmacol. 2022, 113 Pt A, 109331, Erratum in Int. Immunopharmacol. 2023, 114, 109555. [Google Scholar] [CrossRef] [PubMed]

| Parameter | SID Overall Population a (n = 31) | 18–64 Years (n = 15) | ≥65 Years (n = 15) |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 61.4 (17.8) | 52.5 (12) | 74.1 (6.0) |
| Male, n (%) | 19 (61.3) | 8 (53.3) | 10 (66.7) |
| Caucasian/White, n (%) | 29 (93.5) | 14 (93.3) | 14 (93.3) |
| Clinical characteristics | |||
| BMI (kg/m2), mean (SD) | 25.4 (4.1) | 25.5 (4.8) | 25.6 (3.3) |
| Indication for IGRT, n (%) | |||
| CLL | 20 (64.5) | 8 (53.3) | 12 (80.0) |
| Indolent lymphoma | 4 (12.9) | 1 (6.7) | 3 (20.0) |
| Other SID | 7 (22.6) | 6 (40) | - |
| B-NHL | 1 (14.3) | 1 (16.7) | - |
| Diffuse large B-cell lymphoma | 1 (14.3) | 1 (16.7) | - |
| Hodgkin’s disease | 1 (14.3) | 1 (16.7) | - |
| Hodgkin’s lymphoma after autologous transplantation | 1 (14.3) | 1 (16.7) | - |
| Lung transplantation, rituximab therapy | 1 (14.3) | - | - |
| Rituximab | 1 (14.3) | 1 (16.7) | - |
| Immunosuppressive therapy for autoimmune disorders | 1 (14.3) | 1 (16.7) | - |
| Received chemotherapy, immunosuppressive therapy or supportive therapy, n (%) | 29 (93.5) | 13 (86.7) | 15 (100.0) |
| Concomitant supportive therapy at initiation, n (%) | |||
| Antibiotics | 8 (25.8) | 4 (26.7) | 4 (26.7) |
| Corticosteroids | 4 (12.9) | 3 (20) | 1 (6.7) |
| Expectorants | 1 (3.2) | - | 1 (6.7) |
| Inhalation therapy | 4 (12.9) | 2 (13.3) | 2 (13.3) |
| PJP prophylaxis | 14 (45.2) | 4 (26.7) | 10 (66.7) |
| Virostatics | 13 (41.9) | 5 (33.3) | 8 (53.3) |
| Other supportive therapy | 14 (45.2) | 7 (46.7) | 7 (46.7) |
| IGRT history b | |||
| IG route of administration c, n (%) | |||
| IV | 17 (54.8) | 9 (60.0) | 7 (46.7) |
| SC | 31 (100.0) | 15 (100.0) | 15 (100.0) |
| Total monthly dose of past and current IGRT (g), mean (SD) | 23.1 (9.3) | 24 (9.7) | 23.2 (8.2) |
| Reason for prior treatment discontinuation and change to fSCIG therapy d, n (% of total discontinued treatments) | |||
| Tolerability | 5 (21.7) | 5 (38.5) | - |
| Patient request | 6 (26.1) | 2 (15.4) | 3 (33.3) |
| Administrative | 3 (13.0) | 3 (23.1) | - |
| Other | 9 (39.1) | 3 (23.1) | 6 (66.7) |
| Parameter, Median (IQR) | SID Overall Population a (n = 31) | SID Overall Population a (n = 11) | 18–64 Years (n = 15) | ≥65 Years (n = 15) |
|---|---|---|---|---|
| Inclusion | 36 Months | Inclusion | Inclusion | |
| fSCIG dose, g | 30 (25–30) | 30 (30–30) | 30 (20–30) | 30 (25–30) |
| fSCIG total monthly dose, g | 30 (25–30) | 30 (30–30) | 30 (25–30) | 30 (27.5–30) |
| fSCIG dose, g/kg/month | 0.400 (0.333–0.500) | 0.366 (0.348–0.462) | 0.390 (0.304–0.484) | 0.392 (0.350–0.501) |
| fSCIG infusion volume b, mL | 300 (250–300) | 300 (300–300) | 300 (250–300) | 300 (250–300) |
| Right upper abdomen | 300 (300–300) | 300 (300–300) | 300 (300–300) | 300 |
| Left upper abdomen | 300 (100–300) | 300 (300–300) | 300 (300–300) | 300 |
| Right lower abdomen | 300 (250–300) | 300 (300–300) | 300 (200–300) | 300 (250–300) |
| Left lower abdomen | 300 (200–300) | 200 | 300 | 250 (200–300) |
| Right thigh | 125 | - | 125 | - |
| Left thigh | 125 | - | 125 | - |
| fSCIG maximum infusion rate, mL/h | 280 (240–300) | 300 (300–300) | 240 (200–300) | 300 (240–300) |
| Infusion location, n (%) | ||||
| Lower abdomen | 12 (38.7) | 4 (31.8) | 3 (20.0) | 9 (60.0) |
| Upper abdomen | 9 (29.0) | 6 (50.0) | 7 (46.7) | 1 (6.7) |
| Thigh | 1 (3.2) | - | 1 (6.7) | - |
| Unknown | 9 (29.0) | 2 (18.2) | 4 (26.7) | 5 (33.3) |
| Infusion interval, n (%) | ||||
| Every 2 weeks | 1 (3.2) | 1 (9.1) | 1 (6.7) | - |
| Every 3 weeks | 2 (6.5) | 1 (9.1) | 2 (13.3) | - |
| Every 4 weeks | 22 (71.0) | 9 (81.8) | 9 (60.0) | 12 (80.0) |
| Other | 2 (6.5) | - | - | 2 (13.3) |
| NA c | 4 (12.9) | - | 3 (20.0) | 1 (6.7) |
| Needle length, mm, n (%) d | ||||
| 9 | 8 (42.1) | - | 5 (50.0) | 3 (33.3) |
| 12 | 10 (52.6) | 2 (100.0) | 5 (50.0) | 5 (55.6) |
| 20 | 1 (5.3) | - | - | 1 (11.1) |
| Missing data | 12 (38.7) | 9 (81.8) | 5 (33.3) | 6 (40.0) |
| Needle diameter, 24 gauge, n (%) d | 19 (100.0) | 9 (100.0) | 9 (100.0) | 10 (100.0) |
| IgG serum trough level, g/L | 5.4 (4.6–7.3) | 9.4 (7.5–9.5) | 6.7 (4.6–7.4) | 4.8 (4.3–5.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimou, M.; Speletas, M.; Milito, C.; Pyzik, A.; Huscher, D.; Kamieniak, M.; Pittrow, D.; Borte, M. Insights into Facilitated Subcutaneous Immunoglobulin Use in Patients with Secondary Immunodeficiency Diseases: A FIGARO Subgroup Analysis. Cancers 2023, 15, 4524. https://doi.org/10.3390/cancers15184524
Dimou M, Speletas M, Milito C, Pyzik A, Huscher D, Kamieniak M, Pittrow D, Borte M. Insights into Facilitated Subcutaneous Immunoglobulin Use in Patients with Secondary Immunodeficiency Diseases: A FIGARO Subgroup Analysis. Cancers. 2023; 15(18):4524. https://doi.org/10.3390/cancers15184524
Chicago/Turabian StyleDimou, Maria, Matthaios Speletas, Cinzia Milito, Aleksandra Pyzik, Dörte Huscher, Marta Kamieniak, David Pittrow, and Michael Borte. 2023. "Insights into Facilitated Subcutaneous Immunoglobulin Use in Patients with Secondary Immunodeficiency Diseases: A FIGARO Subgroup Analysis" Cancers 15, no. 18: 4524. https://doi.org/10.3390/cancers15184524
APA StyleDimou, M., Speletas, M., Milito, C., Pyzik, A., Huscher, D., Kamieniak, M., Pittrow, D., & Borte, M. (2023). Insights into Facilitated Subcutaneous Immunoglobulin Use in Patients with Secondary Immunodeficiency Diseases: A FIGARO Subgroup Analysis. Cancers, 15(18), 4524. https://doi.org/10.3390/cancers15184524







