Macroscopic Evaluation of Colon Cancer Resection Specimens
Abstract
Simple Summary
Abstract
1. Introduction
2. Paving the Way: Jamieson and Dobson
3. Contemporary CME with CVL
4. Oncological Outcomes and Benefits
5. The Pathologist, a Gatekeeper to Quality Control
6. Pathological Assessment of Mesocolic Integrity
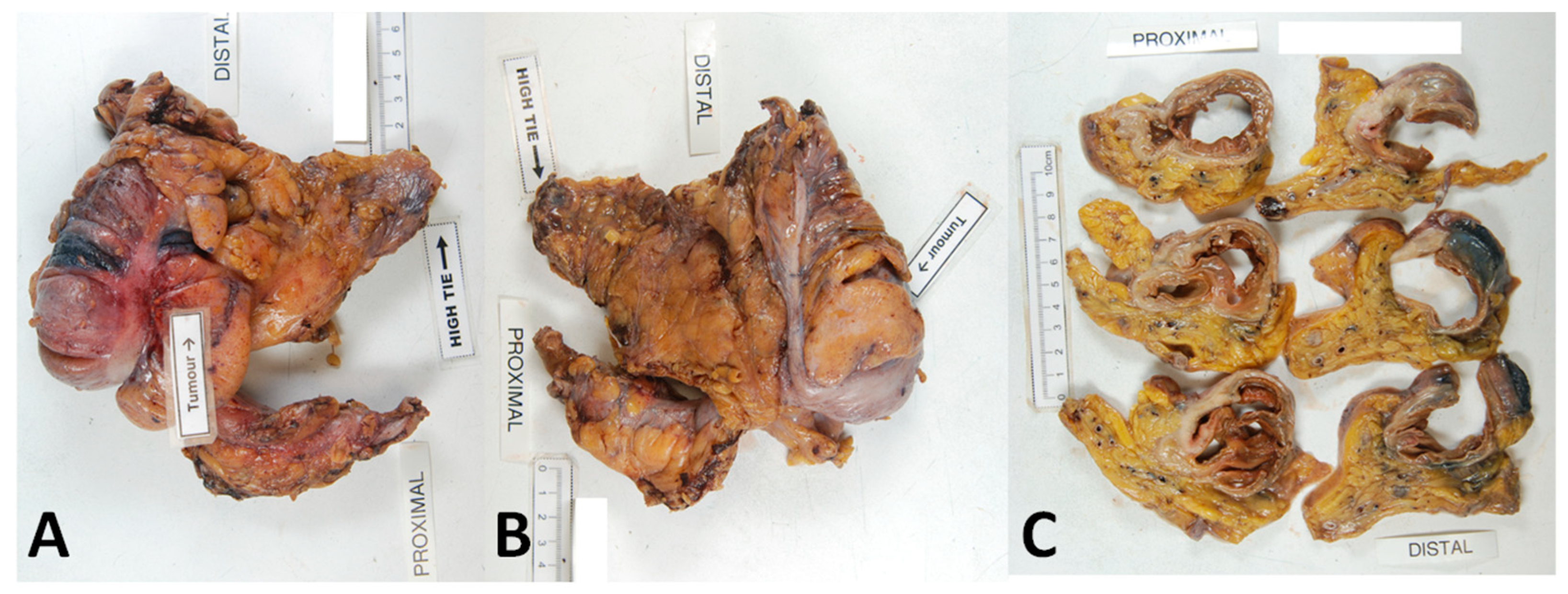
7. Distance between the Tumour and Point of Central Arterial Ligation
8. Length of Bowel Resection
9. Lymph Node Yield
10. Photography
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund. Worldwide Cancer Data: Global Cancer Statistics for the Most Common Cancers. 2022. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data (accessed on 2 January 2022).
- American Cancer Society. Key Statistics for Colorectal Cancer. 2022. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html (accessed on 2 January 2022).
- National Cancer Institute. Colon Cancer Treatment. 2022. Available online: https://www.cancer.gov/types/colorectal/patient/colon-treatment-pdq (accessed on 4 January 2022).
- Heald, R.; Husband, E.; Ryall, R. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Sebag-Montefiore, D.; Steele, R.; Khanna, S.; Monson, J.; Holliday, A.; Thompson, L.; Griffiths, G.; Stephens, R. Local recurrence after rectal cancer resection is strongly related to the plane of surgical dissection and is further reduced by pre-operative short course radiotherapy. Preliminary results of the Medical Research Council (MRC) CR07 trial. J. Clin. Oncol. 2006, 24 (Suppl. S18), 3512. [Google Scholar] [CrossRef]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation--technical notes and outcome. Color. Dis. 2009, 11, 354–364, discussion 355–364. [Google Scholar] [CrossRef] [PubMed]
- Storli, K.; Søndenaa, K.; Furnes, B.; Nesvik, I.; Gudlaugsson, E.; Bukholm, I.; Eide, G. Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II. Tech. Coloproctol. 2014, 18, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, C.A.; Neuenschwander, A.U.; Jansen, J.E.; Wilhelmsen, M.; Kirkegaard-Klitbo, A.; Tenma, J.R.; Bols, B.; Ingeholm, P.; Rasmussen, L.A.; Jepsen, L.V. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: A retrospective, population-based study. Lancet Oncol. 2015, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Merkel, S.; Weber, K.; Matzel, K.; Agaimy, A.; Göhl, J.; Hohenberger, W. Prognosis of patients with colonic carcinoma before, during and after implementation of complete mesocolic excision. Br. J. Surg. 2016, 103, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Lieto, E.; De Vita, F.; Ferraraccio, F.; Zamboli, A.; Mabilia, A.; Auricchio, A.; Castellano, P.; Napolitano, V.; Orditura, M. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int. J. Color. Dis. 2014, 29, 89–97. [Google Scholar] [CrossRef]
- Culligan, K.; Remzi, F.; Soop, M.; Coffey, J. Review of nomenclature in colonic surgery–proposal of a standardised nomenclature based on mesocolic anatomy. Surgeon 2013, 11, 1–5. [Google Scholar] [CrossRef]
- Abdelkhalek, M.; Setit, A.; Bianco, F.; Belli, A.; Denewer, A.; Youssef, T.F.; Falato, A.; Romano, G.M. Complete Mesocolic Excision with Central Vascular Ligation in Comparison with Conventional Surgery for Patients with Colon Cancer—The Experiences at Two Centers. Ann. Coloproctol. 2018, 34, 180–186. [Google Scholar] [CrossRef]
- Ng, K.-S.; West, N.P.; Scott, N.; Holzgang, M.; Quirke, P.; Jayne, D.G. What factors determine specimen quality in colon cancer surgery? A cohort study. Int. J. Color. Dis. 2020, 35, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, J.K.; Dobson, J.F. The Lymphatics of the Colon. Proc. R. Soc. Med. Surg. Sect. 1909, 2, 149–174. [Google Scholar] [CrossRef]
- Byrnes, K.G.; Walsh, D.; Lewton-Brain, P.; McDermott, K.; Coffey, J.C. Anatomy of the mesentery: Historical development and recent advances. Semin. Cell Dev. Biol. 2019, 92, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Walsh, D.; Byrnes, K.G.; Hohenberger, W.; Heald, R.J. Mesentery—A ‘New’ organ. Emerg. Top. Life Sci. 2020, 4, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.; Coffey, J.C.; Kiran, R.P.; Kalady, M.; Lavery, I.C.; Remzi, F.H. The mesocolon: A prospective observational study. Color. Dis. 2012, 14, 421–428, discussion 428–430. [Google Scholar] [CrossRef]
- Vandamme, J.-P.; Bonte, J. Vascular Anatomy in Abdominal Surgery; Thieme Medical Publishers: New York, NY, USA, 1990. [Google Scholar]
- Kijima, S.; Sasaki, T.; Nagata, K.; Utano, K.; Lefor, A.T.; Sugimoto, H. Preoperative evaluation of colorectal cancer using CT colonography, MRI, and PET/CT. World J. Gastroenterol. WJG 2014, 20, 16964. [Google Scholar] [CrossRef]
- Hirai, K.; Yoshinari, D.; Ogawa, H.; Nakazawa, S.; Takase, Y.; Tanaka, K.; Miyamae, Y.; Takahashi, N.; Tsukagoshi, H.; Toya, H. Three-dimensional computed tomography for analyzing the vascular anatomy in laparoscopic surgery for right-sided colon cancer. Surg. Laparosc. Endosc. Percutaneous Tech. 2013, 23, 536–539. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- West, N.; Hohenberger, W.; Finan, P.; Quirke, P. Mesocolic plane surgery: An old but forgotten technique? Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2009, 11, 988–989. [Google Scholar] [CrossRef]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colonic cancer surgery: Comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J. Clin. Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef]
- Kobayashi, H.; West, N.P.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Quirke, P.; Sugihara, K. Quality of surgery for stage III colon cancer: Comparison between England, Germany, and Japan. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), S398–S404. [Google Scholar] [CrossRef] [PubMed]
- Toyota, S.; Ohta, H.; Anazawa, S. Rationale for extent of lymph node dissection for right colon cancer. Dis. Colon Rectum 1995, 38, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, E.; Yasutomi, M.; Shindou, K.; Matsuda, T.; Mori, N.; Hida, J.; Kubo, R.; Kitaoka, M.; Nakamura, M.; Fujimoto, K.; et al. Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis. Colon Rectum 1994, 37, 219–223. [Google Scholar] [CrossRef]
- Tan, K.Y.; Kawamura, Y.J.; Mizokami, K.; Sasaki, J.; Tsujinaka, S.; Maeda, T.; Nobuki, M.; Konishi, F. Distribution of the first metastatic lymph node in colon cancer and its clinical significance. Color. Dis. 2010, 12, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Baek, S.J.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.K. Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: Long-term outcomes and prognostic factors. Ann. Surg. 2015, 261, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Koh, F.H.; Tan, K.-K. Complete mesocolic excision for colon cancer: Is it worth it? J. Gastrointest. Oncol. 2019, 10, 1215. [Google Scholar] [CrossRef]
- Yamamoto, S.; Inomata, M.; Katayama, H.; Mizusawa, J.; Etoh, T.; Konishi, F.; Sugihara, K.; Watanabe, M.; Moriya, Y.; Kitano, S. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann. Surg. 2014, 260, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.K.; Kim, H.C.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A.; Chun, H.-K. Laparoscopic modified mesocolic excision with central vascular ligation in right-sided colon cancer shows better short-and long-term outcomes compared with the open approach in propensity score analysis. Surg. Endosc. 2018, 32, 2721–2731. [Google Scholar] [CrossRef]
- Storli, K.E.; Søndenaa, K.; Furnes, B.; Eide, G.E. Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig. Surg. 2013, 30, 317–327. [Google Scholar] [CrossRef]
- Munkedal, D.; West, N.; Iversen, L.; Hagemann-Madsen, R.; Quirke, P.; Laurberg, S. Implementation of complete mesocolic excision at a university hospital in Denmark: An audit of consecutive, prospectively collected colon cancer specimens. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 1494–1501. [Google Scholar] [CrossRef]
- Bae, S.U.; Saklani, A.P.; Lim, D.R.; Kim, D.W.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann. Surg. Oncol. 2014, 21, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Inomata, M.; Mizusawa, J.; Katayama, H.; Watanabe, M.; Yamamoto, S.; Ito, M.; Saito, S.; Fujii, S.; Konishi, F.; et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2017, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Petz, W.; Ribero, D.; Bertani, E.; Borin, S.; Formisano, G.; Esposito, S.; Spinoglio, G.; Bianchi, P. Suprapubic approach for robotic complete mesocolic excision in right colectomy: Oncologic safety and short-term outcomes of an original technique. Eur. J. Surg. Oncol. 2017, 43, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Spinoglio, G.; Bianchi, P.P.; Marano, A.; Priora, F.; Lenti, L.M.; Ravazzoni, F.; Petz, W.; Borin, S.; Ribero, D.; Formisano, G. Robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: Perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann. Surg. Oncol. 2018, 25, 3580–3586. [Google Scholar] [CrossRef]
- Yozgatli, T.K.; Aytac, E.; Ozben, V.; Bayram, O.; Gurbuz, B.; Baca, B.; Balik, E.; Hamzaoglu, I.; Karahasanoglu, T.; Bugra, D. Robotic complete mesocolic excision versus conventional laparoscopic hemicolectomy for right-sided colon cancer. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 671–676. [Google Scholar] [CrossRef]
- Widmar, M.; Keskin, M.; Strombom, P.; Beltran, P.; Chow, O.S.; Smith, J.J.; Nash, G.M.; Shia, J.; Russell, D.; Garcia-Aguilar, J. Lymph node yield in right colectomy for cancer: A comparison of open, laparoscopic and robotic approaches. Color. Dis. 2017, 19, 888–894. [Google Scholar] [CrossRef]
- Tagliacozzo, S.; Tocchi, A. Extended mesenteric excision in right hemicolectomy for carcinoma of the colon. Int. J. Color. Dis. 1997, 12, 272–275. [Google Scholar] [CrossRef]
- Kotake, K.; Kobayashi, H.; Asano, M.; Ozawa, H.; Sugihara, K. Influence of extent of lymph node dissection on survival for patients with pT2 colon cancer. Int. J. Color. Dis. 2015, 30, 813–820. [Google Scholar] [CrossRef]
- Olofsson, F.; Buchwald, P.; Elmståhl, S.; Syk, I. No benefit of extended mesenteric resection with central vascular ligation in right-sided colon cancer. Color. Dis. 2016, 18, 773–778. [Google Scholar] [CrossRef]
- Mazzarella, G.; Maria Muttillo, E.; Picardi, B.; Rossi, S.; Angelo Muttillo, I. Complete mesocolic excision and D3 lymphadenectomy with central vascular ligation in right-sided colon cancer: A systematic review of postoperative outcomes, tumor recurrence and overall survival. Surg. Endosc. 2021, 35, 4945–4955. [Google Scholar] [CrossRef]
- Karachun, A.; Petrov, A.; Panaiotti, L.; Voschinin, Y.; Ovchinnikova, T. Protocol for a multicentre randomized clinical trial comparing oncological outcomes of D2 versus D3 lymph node dissection in colonic cancer (COLD trial). BJS Open 2019, 3, 288. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Y.; Xu, L.; Xue, H.-D.; Zhou, W.-X.; Xu, T.; Qiu, H.-Z.; Wu, B.; Lin, G.-L.; Xiao, Y. The Radical Extent of lymphadenectomy—D2 dissection versus complete mesocolic excision of LAparoscopic Right Colectomy for right-sided colon cancer (RELARC) trial: Study protocol for a randomized controlled trial. Trials 2016, 17, 582. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Morris, E.J.; Rotimi, O.; Cairns, A.; Finan, P.J.; Quirke, P. Pathology grading of colon cancer surgical resection and its association with survival: A retrospective observational study. Lancet Oncol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vico, T.; Fernández-Hevia, M.; Suárez-Sánchez, A.; García-Gutiérrez, C.; Mihic-Góngora, L.; Fernández-Martínez, D.; Antonio Álvarez-Pérez, J.; Luis Otero-Díez, J.; Electo Granero-Trancón, J.; Joaquín García-Flórez, L. Complete Mesocolic Excision and D3 Lymphadenectomy versus Conventional Colectomy for Colon Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2021, 28, 8823–8837. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Sutton, K.M.; Ingeholm, P.; Hagemann-Madsen, R.H.; Hohenberger, W.; Quirke, P. Improving the quality of colon cancer surgery through a surgical education program. Dis. Colon Rectum 2010, 53, 1594–1603. [Google Scholar] [CrossRef]
- Emmanuel, A.; Haji, A. Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: Is it worth that extra effort? A review of the literature. Int. J. Color. Dis. 2016, 31, 797–804. [Google Scholar] [CrossRef]
- Enker, W.E.; Laffer, U.T.; Block, G.E. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann. Surg. 1979, 190, 350. [Google Scholar] [CrossRef]
- Bokey, E.; Chapuis, P.; Dent, O.; Mander, B.; Bissett, I.; Newland, R. Surgical technique and survival in patients having a curative resection for colon cancer. Dis. Colon Rectum 2003, 46, 860–866. [Google Scholar] [CrossRef]
- Sheehan-Dare, G.E.; Marks, K.M.; Tinkler-Hundal, E.; Ingeholm, P.; Bertelsen, C.A.; Quirke, P.; West, N.P. The effect of a multidisciplinary regional educational programme on the quality of colon cancer resection. Color. Dis. 2018, 20, 105–115. [Google Scholar] [CrossRef]
- Guo, P.; Ye, Y.; Jiang, K.; Gao, Z.; Wang, T.; Yin, M.; Wang, Y.; Xie, Q.; Yang, X.; Qu, J. Learning curve of complete mesocolic excision for colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi = Chin. J. Gastrointest. Surg. 2012, 15, 28. [Google Scholar]
- Nagtegaal, I.D.; van de Veld, C.; Worp, E.v.d.; Kapiteijn, E.; Quirke, P.; van Krieken, J.H.J. Macroscopic evaluation of rectal cancer resection specimen: Clinical significance of the pathologist in quality control. J. Clin. Oncol. 2002, 20, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef]
- Munkedal, D.L.; Laurberg, S.; Hagemann-Madsen, R.; Stribolt, K.J.; Krag, S.R.; Quirke, P.; West, N.P. Significant Individual Variation between Pathologists in the Evaluation of Colon Cancer Specimens After Complete Mesocolic Excision. Dis. Colon Rectum 2016, 59, 953–961. [Google Scholar] [CrossRef]
- FOxTROT Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: The pilot phase of a randomised controlled trial. Lancet Oncol. 2012, 13, 1152–1160. [Google Scholar] [CrossRef]
- Siani, L.; Pulica, C. Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: Long-term oncologic outcome between mesocolic and non-mesocolic planes of surgery. Scand. J. Surg. 2015, 104, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Munkedal, D.; Rosenkilde, M.; Nielsen, D.; Sommer, T.; West, N.; Laurberg, S. Radiological and pathological evaluation of the level of arterial division after colon cancer surgery. Color. Dis. 2017, 19, O238–O245. [Google Scholar] [CrossRef] [PubMed]
- Spasojevic, M.; Stimec, B.V.; Gronvold, L.B.; Nesgaard, J.-M.; Edwin, B.; Ignjatovic, D. The anatomical and surgical consequences of right colectomy for cancer. Dis. Colon Rectum 2011, 54, 1503–1509. [Google Scholar] [CrossRef]
- Kaye, T.L.; West, N.P.; Jayne, D.G.; Tolan, D.J. CT assessment of right colonic arterial anatomy pre and post cancer resection–a potential marker for quality and extent of surgery? Acta Radiol. 2016, 57, 394–400. [Google Scholar] [CrossRef]
- Munkedal, D.L.E.; Rosenkilde, M.; West, N.P.; Laurberg, S. Routine CT scan one year after surgery can be used to estimate the level of central ligation in colon cancer surgery. Acta Oncol. 2019, 58, 469–471. [Google Scholar] [CrossRef]
- Shiozawa, M.; Ueno, H.; Shiomo, A.; Kim, N.; Kim, J.; Tsarkov, P.; Grützmann, R.; Dulskas, A.; Liang, J.; Samalavičius, N. Study protocol for an International Prospective Observational Cohort Study for Optimal Bowel Resection Extent and Central Radicality for Colon Cancer (T-REX Study). Jpn. J. Clin. Oncol. 2020, 51, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, N.; Yang, M.; Wong-Chong, N.; Liberman, A.S.; Charlebois, P.; Stein, B.; Fried, G.M.; Lee, L. Comparison between conventional colectomy and complete mesocolic excision for colon cancer: A systematic review and pooled analysis. Surg. Endosc. 2019, 33, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, M.B.; Quirke, P.; Shepherd, N.A. Standards and Datasets for Reporting Cancers: Dataset for Histopathological Reporting of Colorectal Cancer (V4, December 2017). The Royal College of Pathologists Website. 2018. Available online: https://www.rcpath.org/profession/publications/cancer-datasets.html (accessed on 2 January 2022).
- Jepsen, R.K.; Ingeholm, P.; Lund, E.L. Upstaging of early colorectal cancers following improved lymph node yield after methylene blue injection. Histopathology 2012, 61, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Inamori, K.; Togashi, Y.; Fukuoka, S.; Akagi, K.; Ogasawara, K.; Irie, T.; Motooka, D.; Kobayashi, Y.; Sugiyama, D.; Kojima, M.; et al. Importance of lymph node immune responses in MSI-H/dMMR colorectal cancer. JCI Insight 2021, 6, e137365. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.A.; Maughan, N.J.; Forman, D.; Quirke, P. Identifying stage III colorectal cancer patients: The influence of the patient, surgeon, and pathologist. J. Clin. Oncol. 2007, 25, 2573–2579. [Google Scholar] [CrossRef]
- Søndenaa, K.; Quirke, P.; Hohenberger, W.; Sugihara, K.; Kobayashi, H.; Kessler, H.; Brown, G.; Tudyka, V.; D’Hoore, A.; Kennedy, R.H.; et al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery: Proceedings of a consensus conference. Int. J. Color. Dis. 2014, 29, 419–428. [Google Scholar] [CrossRef]
- Nakajima, K.; Inomata, M.; Akagi, T.; Etoh, T.; Sugihara, K.; Watanabe, M.; Yamamoto, S.; Katayama, H.; Moriya, Y.; Kitano, S. Quality control by photo documentation for evaluation of laparoscopic and open colectomy with D3 resection for stage II/III colorectal cancer: Japan Clinical Oncology Group Study JCOG 0404. Jpn. J. Clin. Oncol. 2014, 44, 799–806. [Google Scholar] [CrossRef][Green Version]
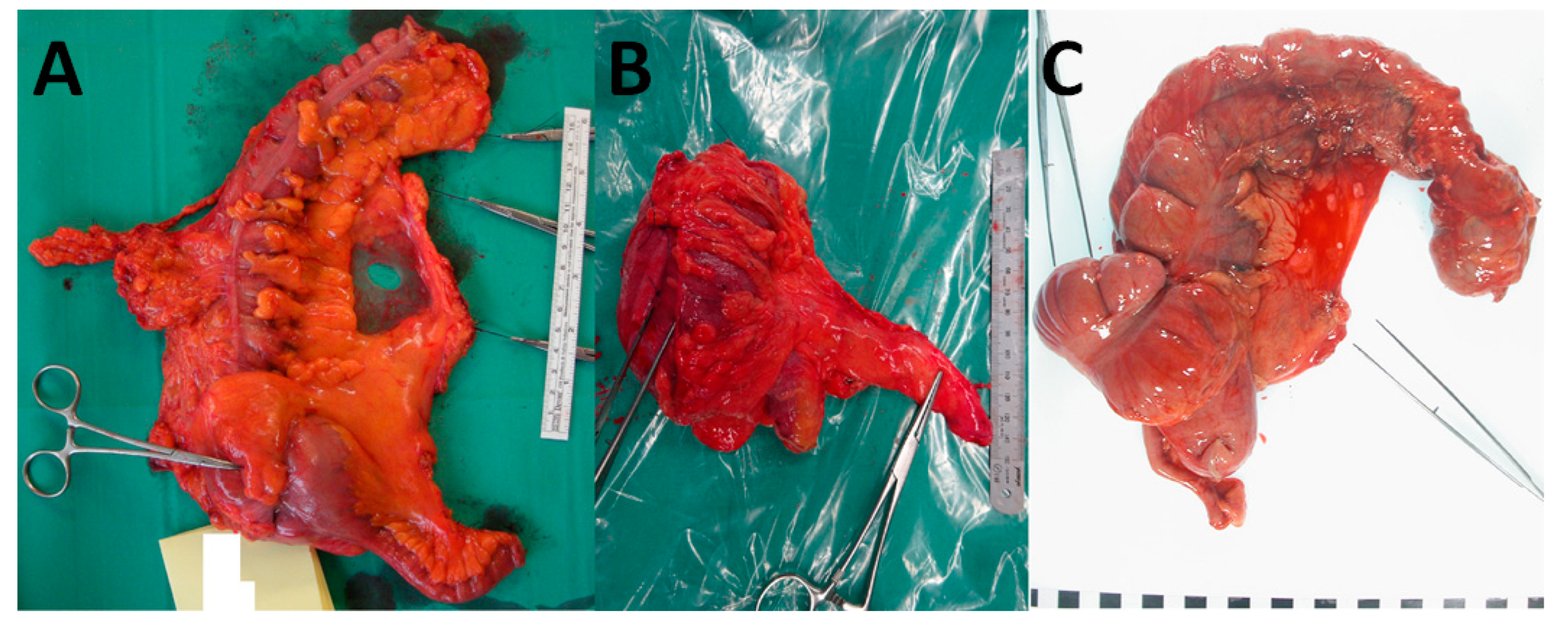

| Plane | Description |
|---|---|
| Mesocolic plane | Mesocolon intact and covered by peritoneum and fascia (where relevant). Defects measure no more than 5 mm in maximum size. |
| Intramesocolic plane | Mesocolic defects greater than 5 mm in size that do not extend down to the muscularis propria. |
| Muscularis propria plane | Substantial mesocolic defects that extend down on to the muscularis propria or beyond, e.g., perforation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrett, R.; West, N.P. Macroscopic Evaluation of Colon Cancer Resection Specimens. Cancers 2023, 15, 4116. https://doi.org/10.3390/cancers15164116
Jarrett R, West NP. Macroscopic Evaluation of Colon Cancer Resection Specimens. Cancers. 2023; 15(16):4116. https://doi.org/10.3390/cancers15164116
Chicago/Turabian StyleJarrett, Ross, and Nicholas P. West. 2023. "Macroscopic Evaluation of Colon Cancer Resection Specimens" Cancers 15, no. 16: 4116. https://doi.org/10.3390/cancers15164116
APA StyleJarrett, R., & West, N. P. (2023). Macroscopic Evaluation of Colon Cancer Resection Specimens. Cancers, 15(16), 4116. https://doi.org/10.3390/cancers15164116






