Tolerance and Oncological Outcomes of In-Field Reirradiation for Locally Recurrent Breast Cancer: A Long-Term Single-Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Extraction and Collection
2.3. Statistical Analysis
3. Results
3.1. Description of the Cohort
3.1.1. Patient Characteristics at First Irradiation
3.1.2. Patient Characteristics at Second Irradiation
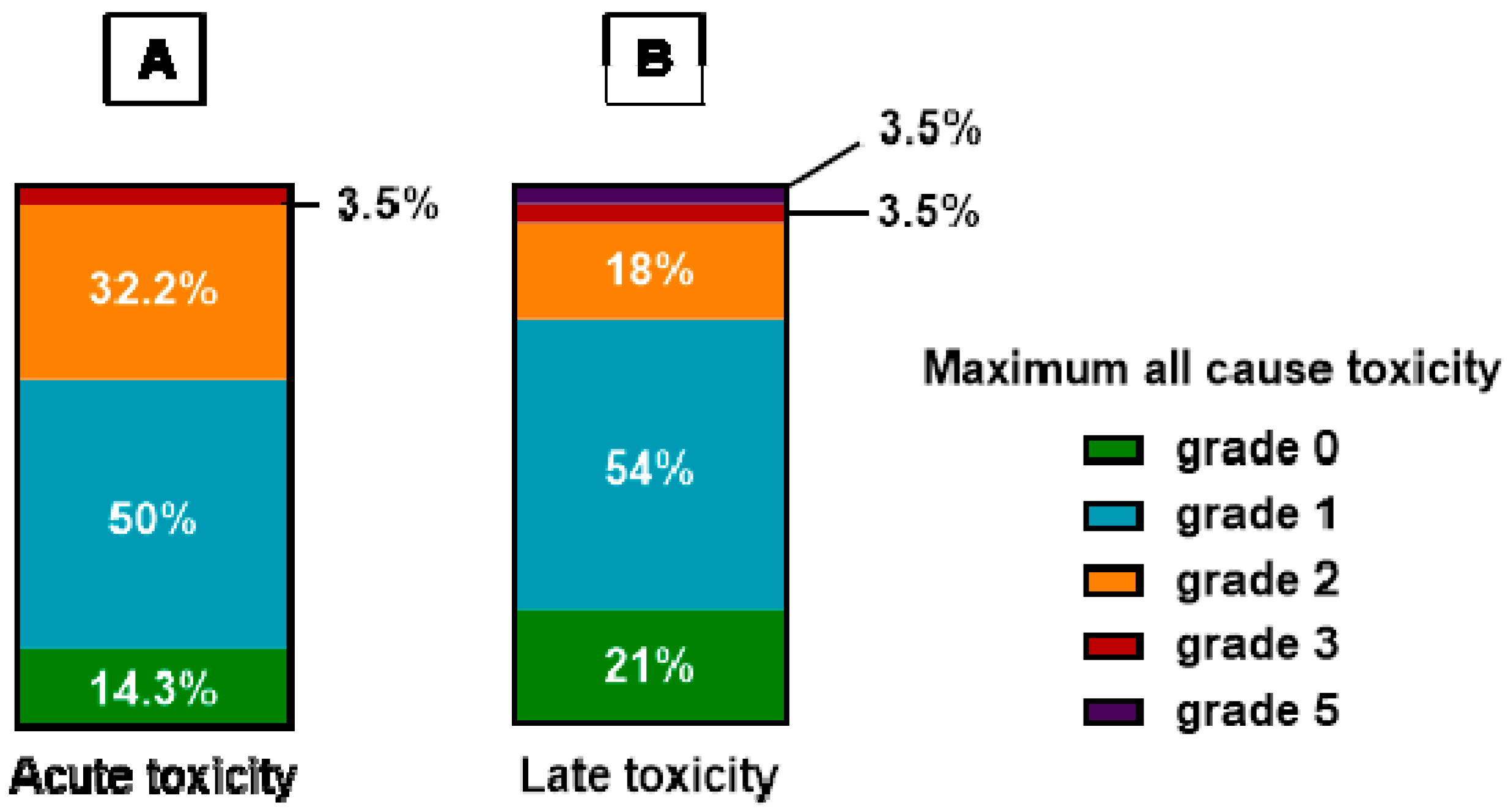
3.2. Reirradiation Toxicity Outcomes
3.3. Reirradiation Efficacy Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.W.; Winter, K.A.; Kuerer, H.M.; Haffty, B.; Cuttino, L.; Todor, D.A.; Anne, P.R.; Anderson, P.; Woodward, W.A.; McCormick, B.; et al. Effectiveness of Breast-Conserving Surgery and 3-Dimensional Conformal Partial Breast Reirradiation for Recurrence of Breast Cancer in the Ipsilateral Breast: The NRG Oncology/RTOG 1014 Phase 2 Clinical Trial. JAMA. Oncol. 2020, 6, 75–82. [Google Scholar] [CrossRef]
- Abeloos, C.H.; Purswani, J.M.; Galavis, P.; McCarthy, A.; Hitchen, C.; Choi, J.I.; Gerber, N.K. Different Re-Irradiation Techniques after Breast-Conserving Surgery for Recurrent or New Primary Breast Cancer. Curr. Oncol. 2023, 30, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.E.; Deutsch, M.; Bloomer, W.D. Salvage Radiotherapy for Local Failures of Lumpectomy and Breast Irradiation. Radiother. Oncol. 1997, 42, 25–29. [Google Scholar] [CrossRef]
- Deutsch, M. Repeat High-Dose External Beam Irradiation for in-Breast Tumor Recurrence after Previous Lumpectomy and Whole Breast Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Ahmed, S.K.; Park, S.S.; Petersen, I.A.; Shumway, D.A.; Stish, B.J.; Yan, E.S.; Remmes, N.B.; Mutter, R.W.; Corbin, K.S. Reirradiation for Locoregional Recurrent Breast Cancer. Adv. Radiat. Oncol. 2021, 6, 100640. [Google Scholar] [CrossRef]
- Oldenborg, S.; Griesdoorn, V.; van Os, R.; Kusumanto, Y.H.; Oei, B.S.; Venselaar, J.L.; Zum Vörde Sive Vörding, P.J.; Heymans, M.W.; Kolff, M.W.; Rasch, C.R.N.; et al. Reirradiation and Hyperthermia for Irresectable Locoregional Recurrent Breast Cancer in Previously Irradiated Area: Size Matters. Radiother. Oncol. 2015, 117, 223–228. [Google Scholar] [CrossRef]
- Linthorst, M.; van Geel, A.N.; Baaijens, M.; Ameziane, A.; Ghidey, W.; van Rhoon, G.C.; van der Zee, J. Re-Irradiation and Hyperthermia after Surgery for Recurrent Breast Cancer. Radiother. Oncol. 2013, 109, 188–193. [Google Scholar] [CrossRef]
- Janssen, S.; Rades, D.; Meyer, A.; Fahlbusch, F.B.; Wildfang, I.; Meier, A.; Schild, S.; Christiansen, H.; Henkenberens, C. Local Recurrence of Breast Cancer: Conventionally Fractionated Partial External Beam Re-Irradiation with Curative Intention. Strahlenther. Onkol. 2018, 194, 806–814. [Google Scholar] [CrossRef]
- Chen, I.; Botty Van den Bruele, A.M.; Gillespie, E.F.; Mueller, B.A.; Xu, A.J.; Cuaron, J.; Khan, A.J.; McCormick, B.; Cahlon, O.; Powell, S.N.; et al. Salvage of Locally Recurrent Breast Cancer with Repeat Breast Conservation Using 45 Gy Hyperfractionated Partial Breast Re-Irradiation. Breast Cancer Res. Treat. 2021, 188, 409–414. [Google Scholar] [CrossRef]
- Thorpe, C.S.; Niska, J.R.; Girardo, M.E.; Kosiorek, H.E.; McGee, L.A.; Hartsell, W.F.; Larson, G.L.; Tsai, H.K.; Rossi, C.J.; Rosen, L.R.; et al. Proton Beam Therapy Reirradiation for Breast Cancer: Multi-Institutional Prospective PCG Registry Analysis. Breast J. 2019, 25, 1160–1170. [Google Scholar] [CrossRef]
- LaRiviere, M.J.; Dreyfuss, A.; Taunk, N.K.; Freedman, G.M. Proton Reirradiation for Locoregionally Recurrent Breast Cancer. Adv. Radiat. Oncol. 2021, 6, 100710. [Google Scholar] [CrossRef] [PubMed]
- Hannoun-Levi, J.-M.; Gal, J.; Schiappa, R.; Chand, M.-E. 10-Year Oncological Outcome Report after Second Conservative Treatment for Ipsilateral Breast Tumor Event. Clin. Transl. Radiat. Oncol. 2023, 38, 71–76. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.; Barrett, J.M.; Barrett-Lee, P.J.; Bliss, J.M.; Brown, J.; Dewar, J.A.; Dobbs, H.J.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomised Trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar] [CrossRef]
- Massaccesi, M.; Fontana, A.; Palumbo, I.; Argenone, A.; De Santis, M.C.; Masiello, V.; Pontoriero, A.; Ciabattoni, A. Pattern of Practice of Re-Irradiation for Ipsilateral Breast Tumor Recurrence in Italy: A Survey by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Clin. Transl. Oncol. 2023. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.-M.; Gal, J.; Polgar, C.; Strnad, V.; Loessl, K.; Polat, B.; Kauer-Domer, D.; Schiappa, R.; Gutierrez, C. GEC-ESTRO Breast Cancer Working Group Second Conservative Treatment for Local Recurrence Breast Cancer: A GEC-ESTRO Oncological Outcome and Prognostic Factor Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2023, S0360-3016(23)02194-6. [Google Scholar] [CrossRef]
- Montagne, L.; Gal, J.; Chand, M.-E.; Schiappa, R.; Falk, A.T.; Kinj, R.; Gauthier, M.; Hannoun-Levi, J.-M. GEC-ESTRO APBI Classification as a Decision-Making Tool for the Management of 2nd Ipsilateral Breast Tumor Event. Breast Cancer Res. Treat. 2019, 176, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Schouten, D.; van Os, R.; Westermann, A.M.; Crezee, H.; van Tienhoven, G.; Kolff, M.W.; Bins, A.D. A Randomized Phase-II Study of Reirradiation and Hyperthermia versus Reirradiation and Hyperthermia plus Chemotherapy for Locally Recurrent Breast Cancer in Previously Irradiated Area. Acta. Oncol. 2022, 61, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Choi, J.I.; Khan, A.J.; Powell, S.N.; McCormick, B.; Lozano, A.J.; Del Rosario, G.; Mamary, J.; Liu, H.; Fox, P.; Gillespie, E.; et al. Proton Reirradiation for Recurrent or New Primary Breast Cancer in the Setting of Prior Breast Irradiation. Radiother. Oncol. 2021, 165, 142–151. [Google Scholar] [CrossRef]
- Loap, P.; Beddok, A.; Cao, K.I.; Goudjil, F.; Fourquet, A.; Dendale, R.; Kirova, Y. Clinical Practice of Breast Cancer Protontherapy: A Single-Centre Experience from Selection to Treatment. Cancer Radiother. 2021, 25, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Weill Medical College of Cornell University. Personalized Second Chance Breast Conservation (PSCBC): A Two Center Prospective Phase II Clinical Study. 2023. Available online: clinicaltrials.gov (accessed on 6 September 2023).
- Proton Collaborative Group. Phase II Protocol of Proton Therapy for Partial Breast Irradiation in Early Stage Breast Cancer. 2023. Available online: clinicaltrials.gov (accessed on 6 September 2023).
- Kirova, Y.; Tallet, A.; Aznar, M.C.; Loap, P.; Bouali, A.; Bourgier, C. Radio-Induced Cardiotoxicity: From Physiopathology and Risk Factors to Adaptation of Radiotherapy Treatment Planning and Recommended Cardiac Follow-Up. Cancer Radiother. 2020, 24, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kirova, Y.M.; Hijal, T.; Campana, F.; Fournier-Bidoz, N.; Stilhart, A.; Dendale, R.; Fourquet, A. Whole Breast Radiotherapy in the Lateral Decubitus Position: A Dosimetric and Clinical Solution to Decrease the Doses to the Organs at Risk (OAR). Radiother. Oncol. 2014, 110, 477–481. [Google Scholar] [CrossRef] [PubMed]
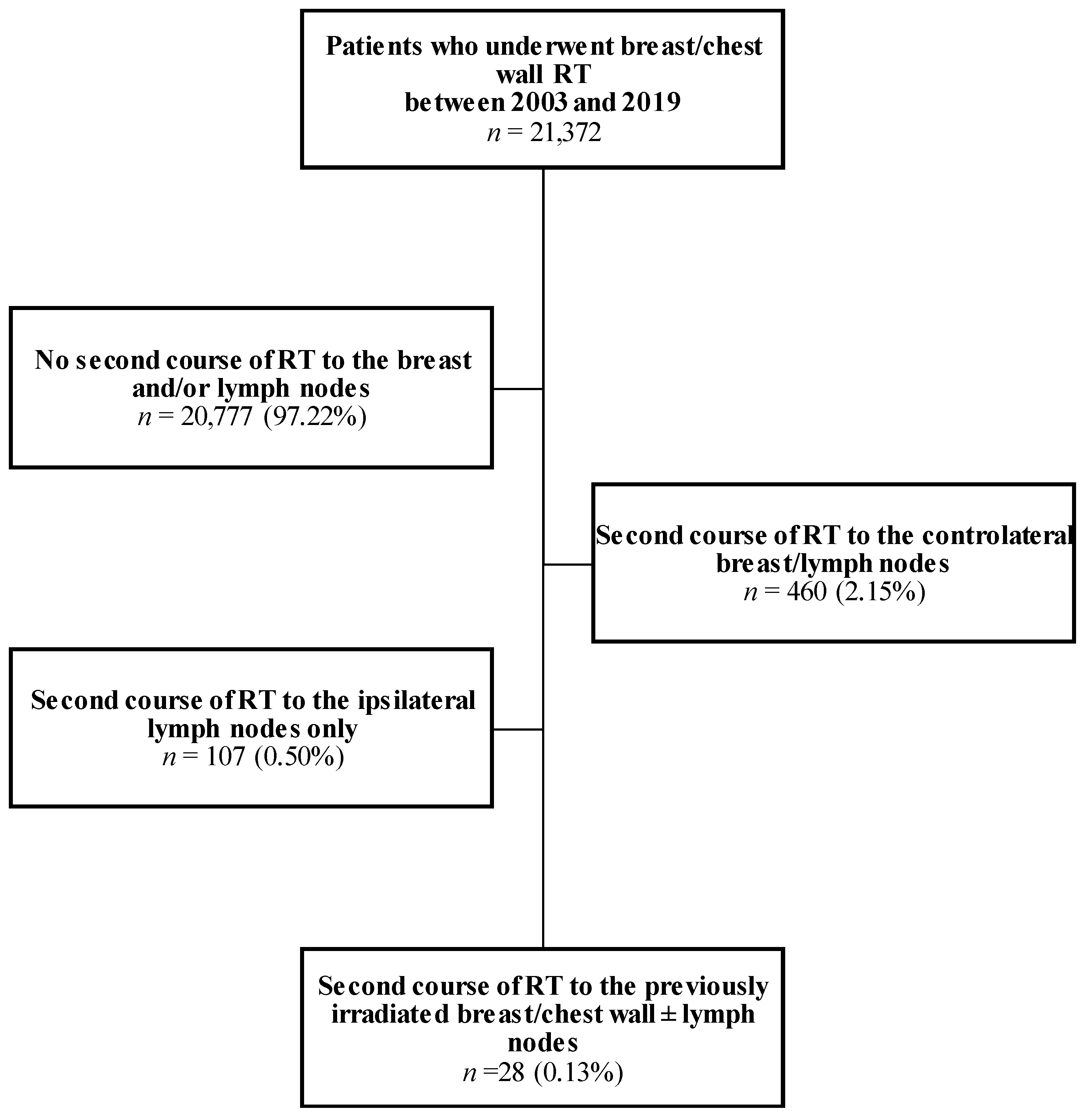

| n = 28 | First RT Course | Second RT Course |
|---|---|---|
| Age (years), median (IQR) | 57 (44–70.5) | 63 (49–78) |
| Primary surgery (%) | ||
| Mastectomy | 6 (22%) | 14 (50%) |
| Lumpectomy | 18 (64%) | 6 (21%) |
| Biopsy only | 4 (14%) | 3 (11%) |
| No surgery | 0 | 5 (18%) |
| Resection status (%) | ||
| R0 | 21 (75%) | 14 (70%) |
| R1 | 2 (7%) | 3 (15%) |
| NR | 5 (18%) | 3 (15%) |
| Axillary surgery (%) | ||
| Axillary lymph node dissection | 18 (64%) | 8 (29%) |
| Sentinel lymph node biopsy | 4 (14%) | 3 (11%) |
| No axillary surgery | 6 (22%) | 17 (61%) |
| Histology (%) | ||
| DCIS | 0 | 0 |
| Invasive ductal carcinoma | 22 (79%) | 15 (54%) |
| Invasive lobular carcinoma | 5 (18%) | 2 (7%) |
| Invasive micropapillary carcinoma | 1 (4%) | 5 (18%) |
| Clinical TNM classification (%) | N/A | |
| cTx | 1 (4%) | |
| cT1 | 3 (11%) | |
| cT2 | 15 (54%) | |
| cT3 | 2 (7%) | |
| cT4 | 7 (25%) | |
| cN0 | 17 (61%) | |
| cN1 | 10 (36%) | |
| cN2 | 1 (4%) | |
| cN3 | 0 | |
| Pathological TNM classification (%) | N/A | |
| pT0 | 2 (7%) | |
| pT1 | 12 (43%) | |
| pT2 | 7 (25%) | |
| pT3 | 0 | |
| pT4 | 2 (7%) | |
| N/A | 5 (18%) | |
| pN0 | 12 (43%) | |
| pN1 | 8 (29%) | |
| pN2 | 3 (11%) | |
| pN3 | 0 | |
| No axillary surgery | 5 (18%) | |
| AJCC 8th edition stage (cTNM) (%) | N/A | |
| I | 4 (14%) | |
| II | 16 (57%) | |
| III | 8 (29%) | |
| IV | 0 | |
| Hormone receptor and HER2 status (%) | ||
| HR+ HER2- (%) | 16 (57%) | 14 (50%) |
| HR+ HER2+ (%) | 1 (4%) | 2 (7%) |
| HR- HER2+ (%) | 3 (11%) | 2 (7%) |
| TN (%) | 3 (11%) | 2 (7%) |
| NR (%) | 5 (18%) | 8 (29%) |
| Chemotherapy (%) | ||
| Neoadjuvant | 8 (29%) | 2 (7%) |
| Adjuvant | 6 (21%) | 14 (50%) |
| Both | 4 (14%) | 0 |
| Without surgery | 0 | 1 (4%) |
| None | 10 (36%) | 11 (39%) |
| Hormone therapy (%) | ||
| Yes | 19 (68%) | 18 (64%) |
| None | 9 (32%) | 10 (36%) |
| Radiotherapy modality (%) | ||
| Photons | 4 (14%) | 8 (29%) |
| Electrons | 1 (4%) | 13 (46%) |
| Photons + electrons | 12 (43%) | 6 (21%) |
| 60Cobalt | 3 (11%) | 1 (4%) |
| 60Cobalt + electrons | 5 (18%) | 0 |
| NR | 3 (11%) | 0 |
| Radiotherapy fields (%) | ||
| Chest wall | 6 (21%) | 20 (71%) |
| Whole/partial breast | 22 (79%)/0 | 7 (25%)/1 (4%) |
| Berg II-IV | 22 (79%) | 6 (21%) |
| Internal mammary chain | 17 (61%) | 5 (18%) |
| Axillary region (Berg I) | 7 (25%) | 2 (7%) |
| No lymph node irradiation | 6 (21%) | 21 (75%) |
| Radiotherapy positioning (%) | ||
| Dorsal decubitus | 16 (57%) | 24 (86%) |
| Lateral decubitus | 3 (11%) | 2 (7%) |
| NR | 9 (32%) | 2 (7%) |
| Use of boost (%) | 14 (50%) | 1 (3.5%) |
| RT dose with boost (Gy2), median (IQR) | 60 (50–66) | 48 (30–50) |
| Time from first RT course (months), median (IQR) | 47 (22.75–109.5) | |
| Cumulative RT dose (Gy2), median (IQR) | 99 (90.6–114.3) | |
| Patient No. | Age at 2nd RT Course | Year of 2nd RT Course | Time from 1st RT Course (Months) | Site and Dose of Reirradiation (Gy) | Cumulative Dose to the Breast/Chest Wall (Gy2) | Treatments Besides RT | Intent | PFS (Months) | Progression after 2nd RT Course | OS (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | 2003 | 31 | Chest wall 38 | 104 | Mastectomy + adjuvant Capecitabine + Triptorelin + Letrozole | Palliative | 3 | Unique choroid metastasis | 15 |
| 2 | 80 | 2003 | 24 | Whole Breast 39.6 | 115.1 | Adjuvant Vinorelbin + Methotrexate | Palliative | 5 | Extensive cutaneous lymphangitis | 78 |
| 3 | 37 | 2003 | 18 | Chest wall 50 | 110 | Mastectomy + adjuvant Vinorelbine + Methotrexate + Enantone + Anastrozole | Curative | 15 | Breast cutaneous nodes | 46 |
| 4 | 78 | 2004 | 4 | Chest wall 30 | 80 | Continuing Exemestane | Curative | 60+ (DF) | 58 | |
| 5 | 61 | 2004 | 35 | Chest wall 52, Berg I 46 | 118 | Mastectomy + adjuvant Docetaxel + Fulvestrant | Curative | 68 | Multiple bone metastases | 114+ (A) |
| 6 | 77 | 2004 | 124 | Chest wall 50, Berg II- IV 48, CMI 48, Boost to the tumor bed 16 | 116 | Mastectomy | Curative | 9 | Chest wall recurrence | 34 |
| 7 | 79 | 2005 | 11 | Whole breast 20 | 70 | Adjuvant Capecitabine + Tamoxifen | Palliative | 1,0 | Out-of-field breast cutaneous nodes | 32 |
| 8 | 54 | 2005 | 98 | Chest wall 48 | 98 | Partial mastectomy | Palliative | 203+ (DF) | 203+ (A) | |
| 9 | 83 | 2005 | 10 | Whole breast 13 (2 × 6.5) | 86.5 | Concurrent and adjuvant Exemestane | Palliative | 126 | Metastatic pleurisy | 7 |
| 10 | 60 | 2005 | 45 | Chest wall 46 | 110 | Partial mastectomy + adjuvant Docetaxel + 5FU + Letrozole | Curative | 26 | Controlateral axillary adenopathies | 90 |
| 11 | 45 | 2008 | 130 | Chest wall 20 | 70 | Mastectomy + axillary lymph node resection + adjuvant Docetaxel + Bevacizumab | Curative | 3 | Inguinal adenopathy | 22 |
| 12 | 78 | 2009 | 14 | Whole breast 10 (2 × 5) | 49.6 | Adjuvant Navelbine + Trastuzumab | Palliative | 12 | Breast cutaneous nodes | 24 |
| 13 | 73 | 2009 | 19 | Chest wall 48 | 114 | Neoadjuvant Paclitaxel + Bevacizumab + mastectomy + axillary lymph node resection | Palliative | 11 | Lung metastases and multiple mediastinal adenopathies | 18 |
| 14 | 64 | 2010 | 94 | Chest wall 48 | 114 | Mastectomy + adjuvant Docetaxel + Cyclophosphamide | Palliative | 15 | Lung metastatis | 37 |
| 15 | 89 | 2010 | 67 | Chest wall 20 | 77.6 | Partial mastectomy + adjuvant Exemestane | Curative | 44 | Unique intramuscular metastasis of the trapezius | 45 |
| 16 | 78 | 2010 | 91 | Chest wall 50 | 115 | Partial mastectomy + adjuvant Anastrozole | Curative | 15 | Unique infield presternal cutaneous node | 42+ (A) |
| 17 | 75 | 2011 | 109 | Chest wall 50, Berg II- IV 46, CMI 48 | 101 | Mastectomy + adjuvant FEC100 then Docetaxel + Letrozole | Curative | 72+ (DF) | 72 (A) | |
| 18 | 61 | 2012 | 111 | Whole breast 30 | 96 | Axillary lymph node resection + adjuvant Docetaxel + Fulvestrant | Palliative | 6 | Breast recurrence | 13 * |
| 19 | 37 | 2013 | 34 | Chest wall 46 | 92 | Mastectomy + sentinel lymph node biopsy + adjuvant Docetaxel + Cyclophosphamide + Triptorelin | Curative | 103 | Multiple bone metastases | 109+ (A) |
| 20 | 50 | 2014 | 49 | Chest wall 30, Berg II- IV 46, CMI 46 | 96 | Mastectomy + adjuvant FEC100 then Docetaxel + Enantone + Letrozole | Curative | 86+ (DF) | 86+ (A) | |
| 21 | 51 | 2015 | 36 | Chest wall 48, Berg II-IV 40, CMI 40 | 96 | Mastectomy + axillary lymph node resection + adjuvant Trastuzumab + Pertuzumab | Curative | 21 | Breast recurrence and multiple mediastinal adenopathies | 85 |
| 22 | 62 | 2015 | 92 | Chest wall 45 | 97 | Partial mastectomy + adjuvant Docetaxel + Trastuzumab + Pertuzumab | Curative | 88+ (DF) | 88+ (A) | |
| 23 | 72 | 2016 | 38 | Chest wall 50 | 100 | Mastectomy + adjuvant Letrozole | Curative | 30 | Chest wall recurrence | 76+ (A) |
| 24 | 46 | 2017 | 138 | Whole breast 50 | 118 | Adjuvant Capecitabine + Trastuzumab + Pertuzumab + Tamoxifene | Palliative | 22 | Breast cutaneous nodes | 49 |
| 25 | 82 | 2017 | 149 | Chest wall 48, Berg I-IV 48 | 98 | Mastectomy + sentinel lymph node biopsy + adjuvant Exemestane | Curative | 18 | Chest wall recurrence | 63+ (A) |
| 26 | 42 | 2019 | 7 | Partial breast 26 | 76 | - | Curative | 43+ (DF) | 43+ (A) | |
| 27 | 42 | 2019 | 123 | Chest wall 50 | 116 | mastectomy + sentinel lymph node biopsy + adjuvant Tamoxifene | Curative | 18 | Multiple supra and infradiaphragmatic adenopathies | 37+ (A) |
| 28 | 71 | 2019 | 142 | Whole breast 50, Berg II- IV 50, CMI 50 | 116 | Partial mastectomy + adjuvant Letrozole | Curative | 42+ (DF) | 42+ (A) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baude, J.; Dendale, R.; Cao, K.; Fourquet, A.; Kirova, Y. Tolerance and Oncological Outcomes of In-Field Reirradiation for Locally Recurrent Breast Cancer: A Long-Term Single-Center Experience. Cancers 2023, 15, 4515. https://doi.org/10.3390/cancers15184515
Baude J, Dendale R, Cao K, Fourquet A, Kirova Y. Tolerance and Oncological Outcomes of In-Field Reirradiation for Locally Recurrent Breast Cancer: A Long-Term Single-Center Experience. Cancers. 2023; 15(18):4515. https://doi.org/10.3390/cancers15184515
Chicago/Turabian StyleBaude, Jérémy, Rémi Dendale, Kim Cao, Alain Fourquet, and Youlia Kirova. 2023. "Tolerance and Oncological Outcomes of In-Field Reirradiation for Locally Recurrent Breast Cancer: A Long-Term Single-Center Experience" Cancers 15, no. 18: 4515. https://doi.org/10.3390/cancers15184515
APA StyleBaude, J., Dendale, R., Cao, K., Fourquet, A., & Kirova, Y. (2023). Tolerance and Oncological Outcomes of In-Field Reirradiation for Locally Recurrent Breast Cancer: A Long-Term Single-Center Experience. Cancers, 15(18), 4515. https://doi.org/10.3390/cancers15184515






