The Role of Blood Microbiome in the Development of Thyroid Cancer in Breast Cancer Survivors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. DNA Extraction and Next-Generation Sequencing
2.3. Microbiome Analysis
2.4. Statistical Analysis
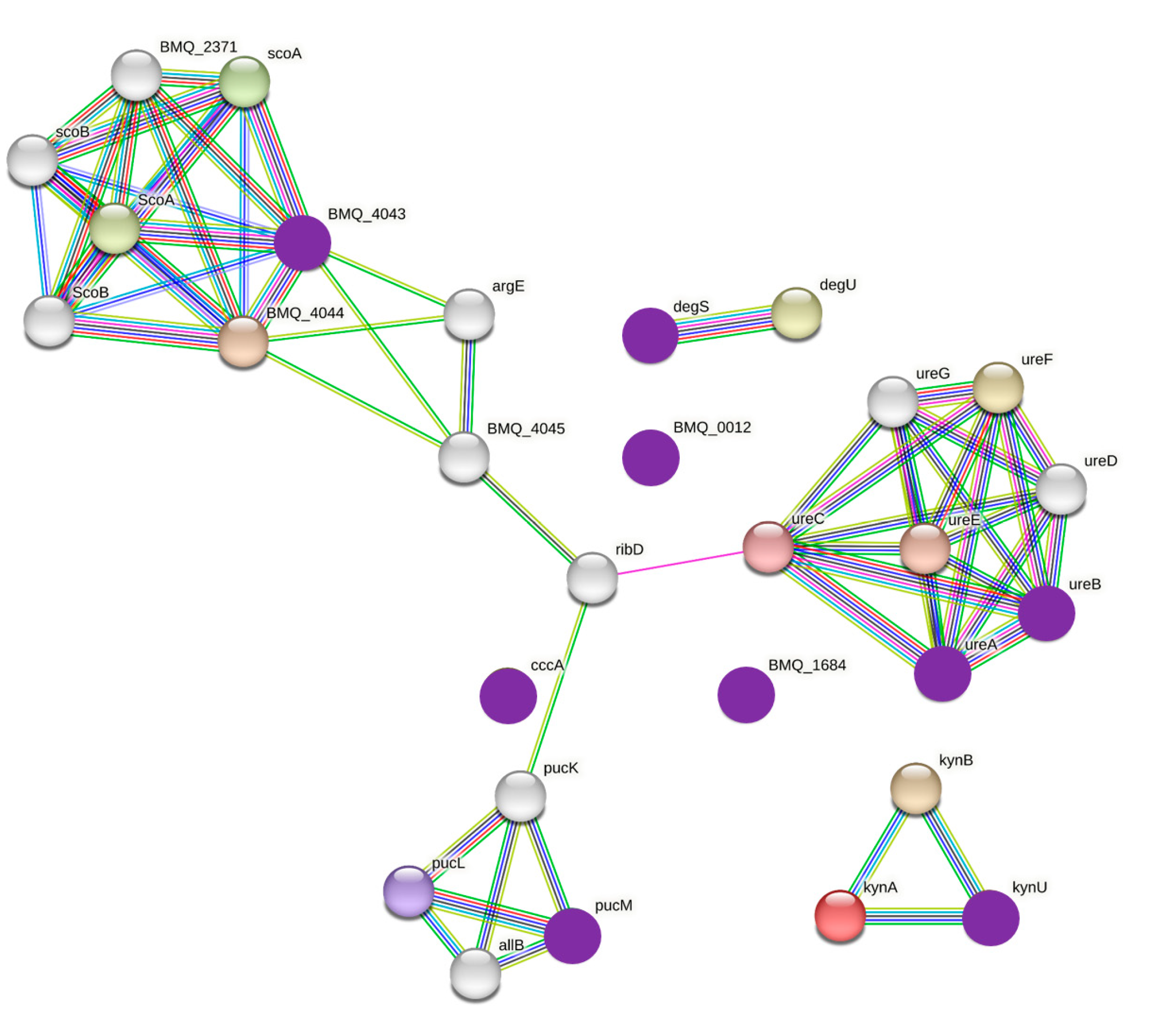
3. Results
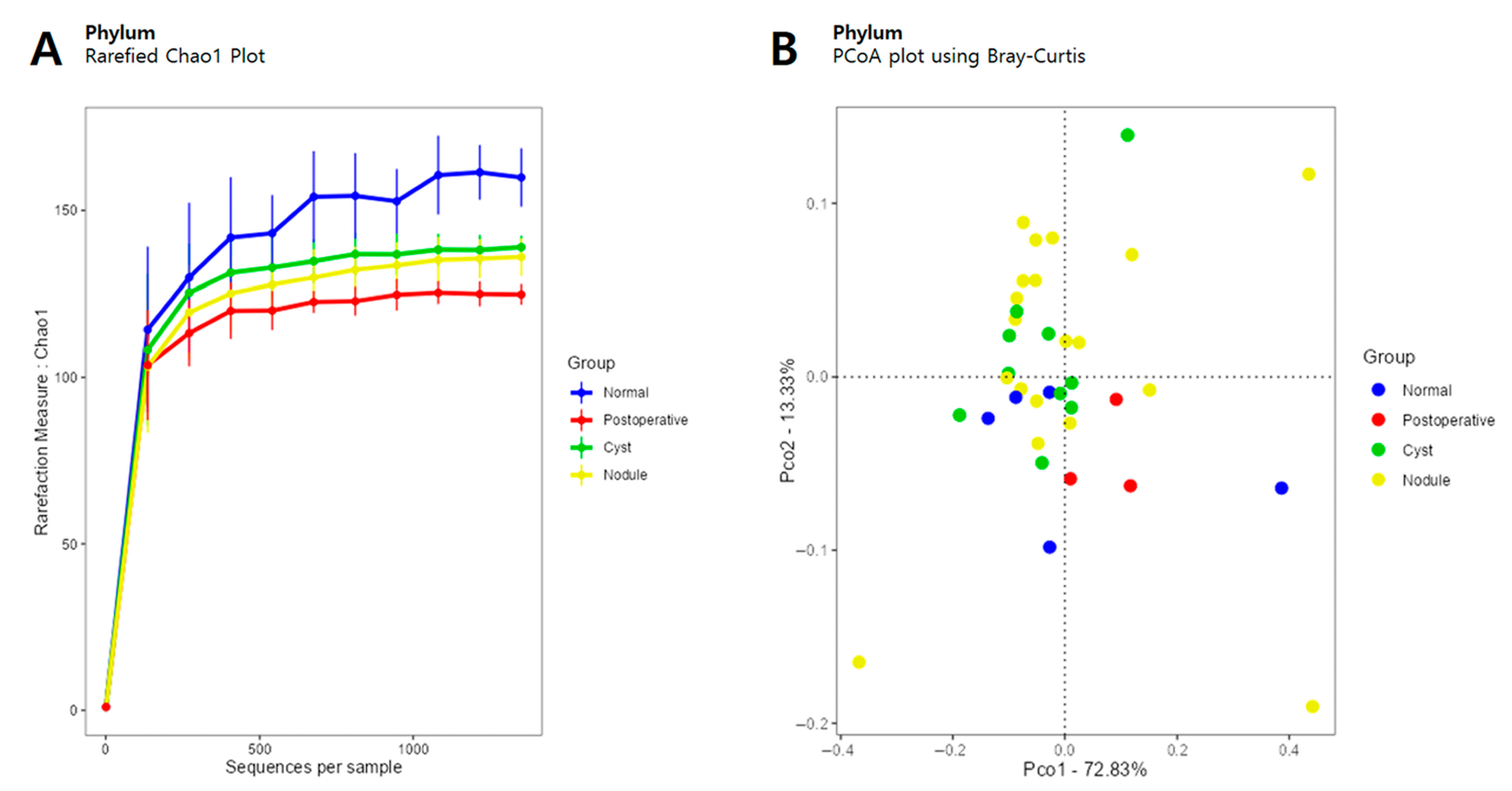
3.1. Diversity
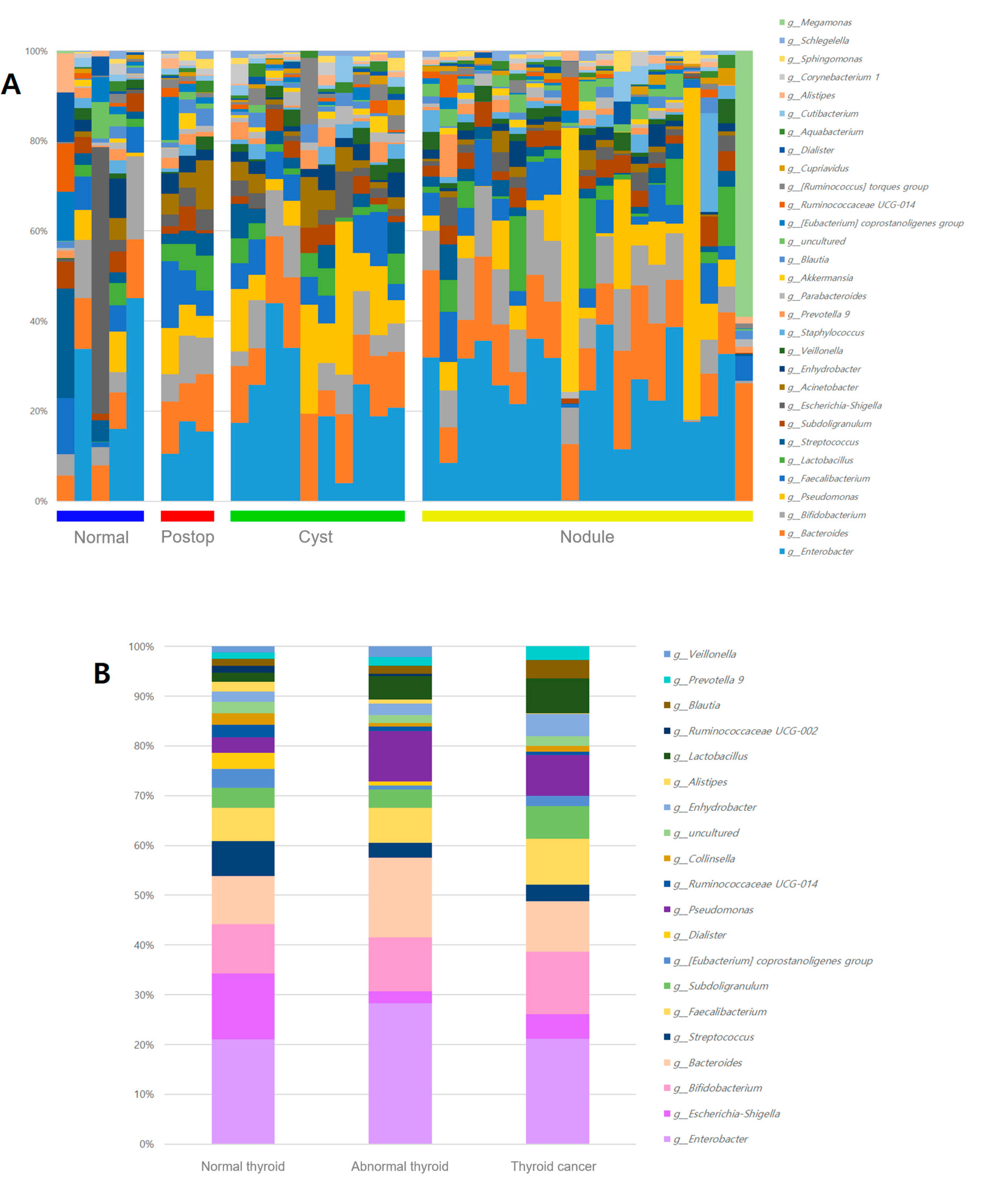
3.2. Microbiome Analysis According to Thyroid Ultrasonography
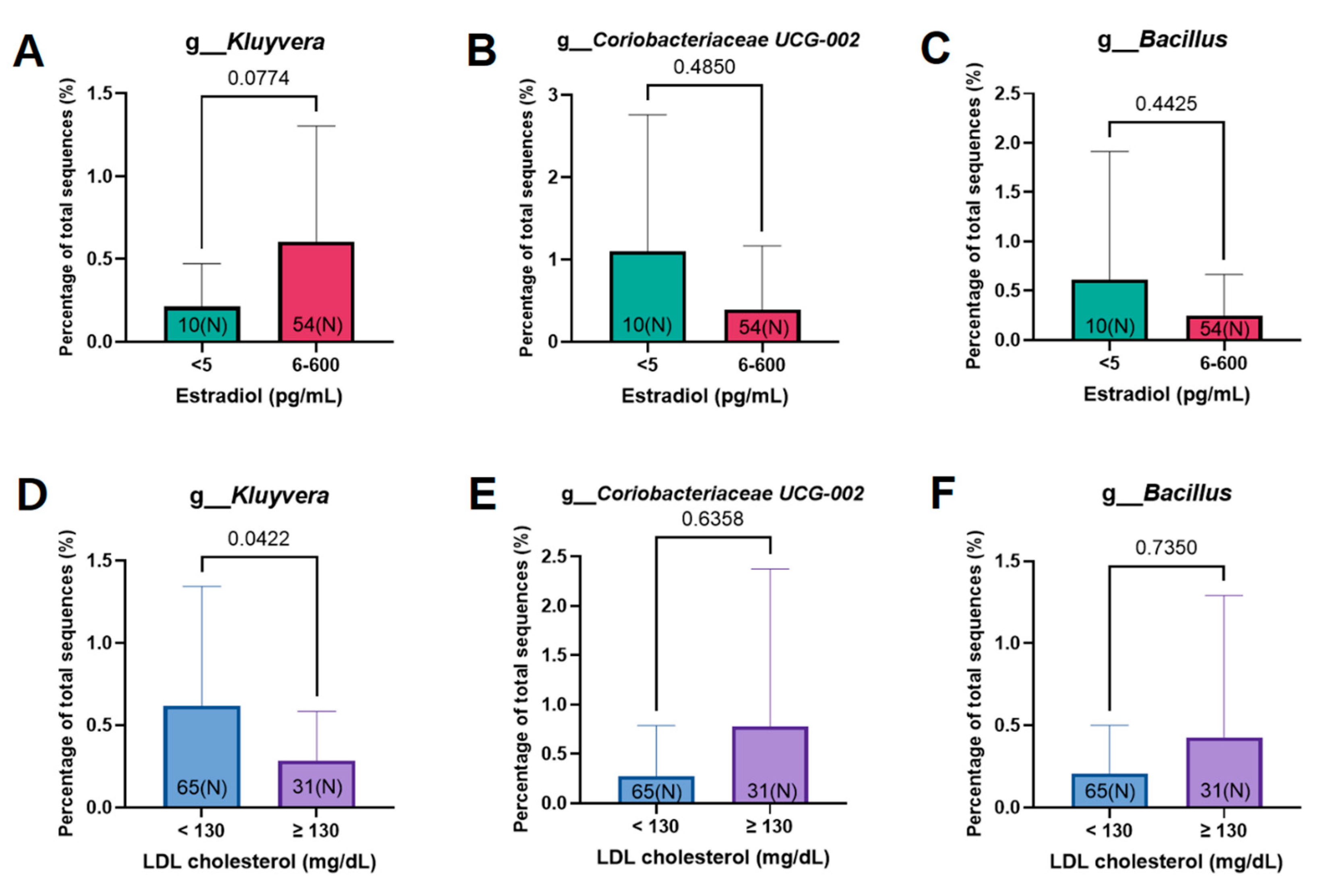
3.3. Estrogen-Related Microbiome According to Microbiome Abundance
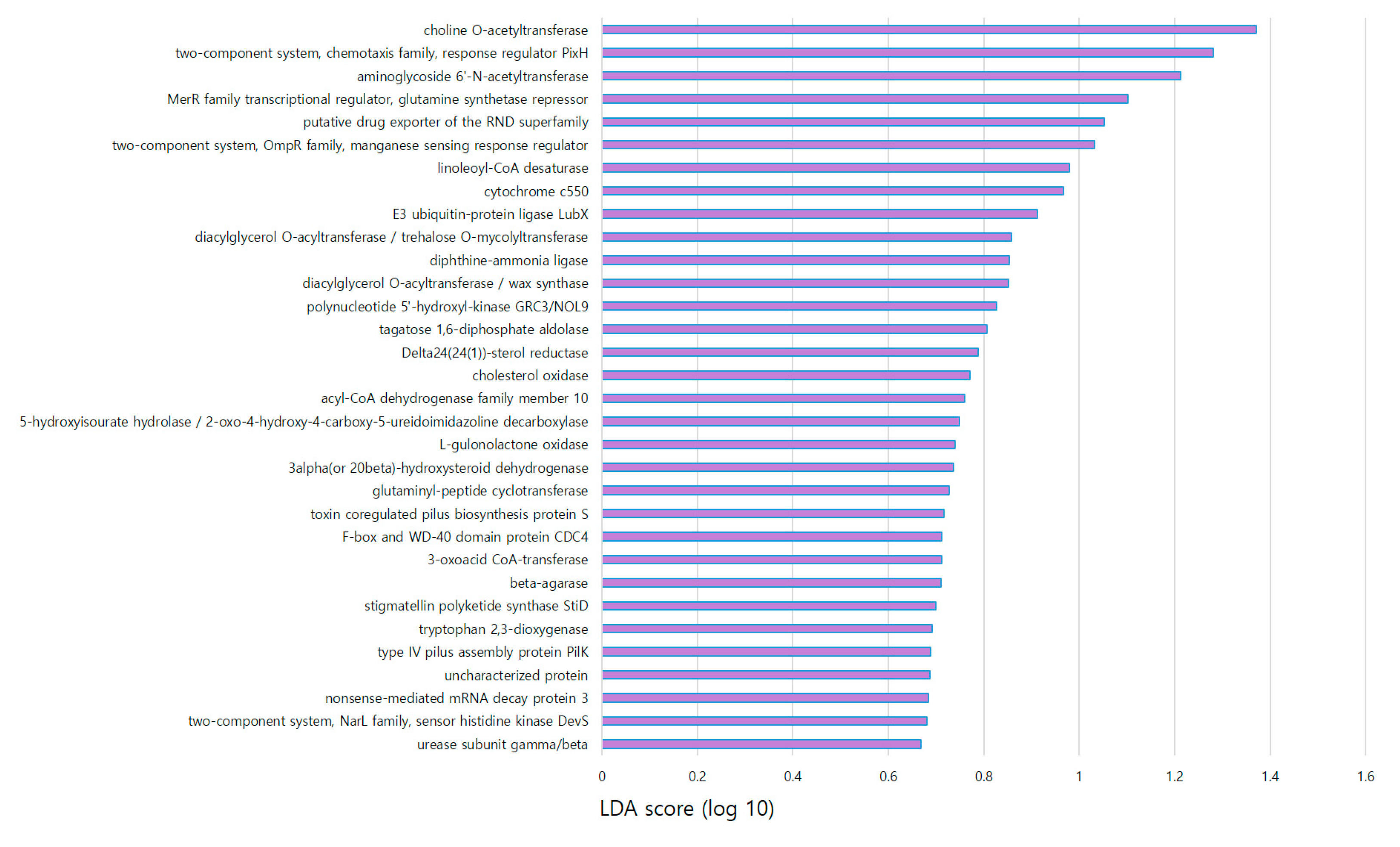
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kang, M.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of cancer incidence and mortality in Korea. Cancer Res. Treat. 2022, 54, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Bolf, E.L.; Sprague, B.L.; Carr, F.E. A linkage between thyroid and breast cancer: A common etiology? Cancer Epidemiol. Biomark. Prev. 2019, 28, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; White, M.G.; Hong, S.; Aschebrook-Kilfoy, B.; Kaplan, E.L.; Angelos, P.; Kulkarni, S.A.; Olopade, O.I.; Grogan, R.H. The breast-thyroid cancer link: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 231–238. [Google Scholar] [CrossRef]
- Kim, Y.A.; Cho, S.W.; Song, Y.S.; Min, H.S.; Park, I.A.; Park, D.J.; Hwang, K.T.; Park, Y.J. Increased expression of thyroid hormone receptor alpha and estrogen receptor alpha in breast cancer associated with thyroid cancer. Eur. J. Surg. Oncol. 2021, 47, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kwon, H.; Lim, W.; Moon, B.I. Staphylococcus aureus-derived extracellular vesicles enhance the efficacy of endocrine therapy in breast cancer cells. J. Clin. Med. 2022, 11, 2030. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, S.P.; Wong, D.M.K.; Koo, W.L.Y.; Wong, S.H.; Tan, N.S. The blood microbiome and health: Current evidence, controversies, and challenges. Int. J. Mol. Sci. 2023, 24, 5633. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- An, J.; Yang, J.; Kwon, H.; Lim, W.; Kim, Y.K.; Moon, B.I. Prediction of breast cancer using blood microbiome and identification of foods for breast cancer prevention. Sci. Rep. 2023, 13, 5110. [Google Scholar] [CrossRef]
- An, J.; Kim, J.B.; Yang, E.Y.; Kim, H.O.; Lee, W.H.; Yang, J.; Kwon, H.; Paik, N.S.; Lim, W.; Kim, Y.K.; et al. Bacterial extracellular vesicles affect endocrine therapy in MCF7 cells. Medicine 2021, 100, e25835. [Google Scholar] [CrossRef]
- Bogović Crnčić, T.; Ilić Tomaš, M.; Girotto, N.; Grbac Ivanković, S. Risk factors for thyroid cancer: What do we know so far? Acta. Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Lauro, A.; Tripodi, D.; Pironi, D.; Amabile, M.I.; Ferent, I.C.; Lori, E.; Gagliardi, F.; Bellini, M.I.; Forte, F.; et al. Thyroid diseases and breast cancer. J. Pers. Med. 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Bellynda, M.; Kamil, M.R.; Yarso, K.Y. Radiofrequency ablation for benign thyroid nodule treatment: New solution in our center. Int. J. Surg. Case Rep. 2022, 97, 107418. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.C.; Marqusee, E.; Kim, M.I.; Frates, M.C.; Ritner, J.; Peters, H.; Benson, C.B.; Doubilet, P.M.; Cibas, E.S.; Barletta, J.; et al. Thyroid nodule size and prediction of cancer. J. Clin. Endocrinol. Metab. 2013, 98, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xi, Z.; Xiao, Y.; Zhao, X.; Li, J.; Feng, N.; Hu, L.; Zheng, R.; Zhang, N.; Wang, S.; et al. TSH-TSHR axis promotes tumor immune evasion. J. Immunother. Cancer 2022, 10, e004049. [Google Scholar] [CrossRef]
- Puzziello, A.; Guerra, A.; Murino, A.; Izzo, G.; Carrano, M.; Angrisani, E.; Zeppa, P.; Marotta, V.; Faggiano, A.; Vitale, M. Benign thyroid nodules with RAS mutation grow faster. Clin. Endocrinol. 2016, 84, 736–740. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Thu, M.S.; Chotirosniramit, K.; Nopsopon, T.; Hirankarn, N.; Pongpirul, K. Human gut, breast, and oral microbiome in breast cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1144021. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Zhao, C.; Xu, Q.; Liang, C.; Yang, Y.; Wang, H.; Shang, Y.; Wang, Y.; Mu, X.; et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 2019, 64, 564–574. [Google Scholar] [CrossRef]
- Jiao, J.; Zheng, Y.; Zhang, Q.; Xia, D.; Zhang, L.; Ma, N. Saliva microbiome changes in thyroid cancer and thyroid nodules patients. Front. Cell Infect. Microbiol. 2022, 12, 989188. [Google Scholar] [CrossRef]
- Halada, S.; Casado-Medrano, V.; Baran, J.A.; Lee, J.; Chinmay, P.; Bauer, A.J.; Franco, A.T. Hormonal crosstalk between thyroid and breast cancer. Endocrinology 2022, 163, bqac075. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, F.; Baldini, E.; Lori, E.; Cardarelli, S.; Pironi, D.; Lauro, A.; Tripodi, D.; Palumbo, P.; D’Armiento, E.; Cavallaro, G.; et al. Insights on the association between thyroid diseases and colorectal cancer. J. Clin. Med. 2023, 12, 2234. [Google Scholar] [CrossRef]
- Guetta, V.; Cannon, R.O., 3rd. Cardiovascular effects of estrogen and lipid-lowering therapies in postmenopausal women. Circulation 1996, 93, 1928–1937. [Google Scholar] [CrossRef]
- Inukai, T.; Takanashi, K.; Takebayashi, K.; Tayama, K.; Aso, Y.; Takiguchi, Y.; Takemura, Y. Estrogen markedly increases LDL-receptor activity in hypercholesterolemic patients. J. Med. 2000, 31, 247–261. [Google Scholar]
- Tan, H.; Wang, S.; Huang, F.; Tong, Z. Association between breast cancer and thyroid cancer risk: A two-sample Mendelian randomization study. Front. Endocrinol. 2023, 14, 1138149. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.J., 3rd; Fanning, G.R.; Huntley-Carter, G.P.; Holmes, B.; Hickman, F.W.; Richard, C.; Brenner, D.J. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: Identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J. Clin. Microbiol. 1981, 13, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Bhatt-Wessel, B.; Jordan, T.W.; Miller, J.H.; Peng, L. Role of DGAT enzymes in triacylglycerol metabolism. Arch. Biochem. Biophys. 2018, 655, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rezapour-Firouzi, S. Chapter 24–Herbal oil supplement with hot-nature diet for multiple sclerosis. In Nutrition and Lifestyle in Neurological Autoimmune Diseases; Watson, R.R., Killgore, W.D.S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 229–245. [Google Scholar]
- El-Naggar, N.E.-A. Chapter 11–Streptomyces-based cell factories for production of biomolecules and bioactive metabolites. In Microbial Cell Factories Engineering for Production of Biomolecules; Singh, V., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 183–234. [Google Scholar]
- Zhang, C.; Gao, X.; Han, Y.; Teng, W.; Shan, Z. Correlation between thyroid nodules and metabolic syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2021, 12, 730279. [Google Scholar] [CrossRef]
- Miltiadou, D.; Hager-Theodorides, A.L.; Symeou, S.; Constantinou, C.; Psifidi, A.; Banos, G.; Tzamaloukas, O. Variants in the 3’ untranslated region of the ovine acetyl-coenzyme A acyltransferase 2 gene are associated with dairy traits and exhibit differential allelic expression. J. Dairy Sci. 2017, 100, 6285–6297. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Chen, J. Isolation and characteristics of 17beta-estradiol-degrading Bacillus spp. strains from activated sludge. Biodegradation 2010, 21, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Peng, H.; Chen, X.; Wu, X.; Wang, B. Hyperlipidemia and hypothyroidism. Clin. Chim. Acta. 2022, 527, 61–70. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Total |
|---|---|
| Female n (%) | 96 (100%) |
| Age (year) ± SD | 51.5 ± 11.1 |
| Stage of breast cancer n (%) | |
| 0 | 3 (3.1%) |
| I | 44 (45.8%) |
| II | 36 (37.5%) |
| III | 13 (13.5%) |
| BMI (kg/m2) | 23.2 ± 3.5 |
| LDL cholesterol (mg/dL) | 50.9 ± 13.2 (94.8%) |
| Estradiol (pg/mL) | 109.1 ± 143.4 (66.7%) |
| Thyroid function tests | |
| TSH (µIU/mL) ± SD | 3.0 ± 5.9 (100%) |
| T3 (ng/dL) ± SD | 112.8 ± 21.2 (100%) |
| T4 (μg/dL) ± SD | 8.1 ± 2.0 (70.8%) |
| Free T4 (ng/dL) ± SD | 1.4 ± 0.9 (75%) |
| Thyroid US n (%) | |
| Normal | 5 (5.2%) |
| Cyst | 10 (10.5%) |
| Nodule | 19 (20%) |
| Post-operative | 3 (3.1%) |
| (thyroid lobectomy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Kwon, H.; Kim, Y.J. The Role of Blood Microbiome in the Development of Thyroid Cancer in Breast Cancer Survivors. Cancers 2023, 15, 4492. https://doi.org/10.3390/cancers15184492
An J, Kwon H, Kim YJ. The Role of Blood Microbiome in the Development of Thyroid Cancer in Breast Cancer Survivors. Cancers. 2023; 15(18):4492. https://doi.org/10.3390/cancers15184492
Chicago/Turabian StyleAn, Jeongshin, Hyungju Kwon, and Young Ju Kim. 2023. "The Role of Blood Microbiome in the Development of Thyroid Cancer in Breast Cancer Survivors" Cancers 15, no. 18: 4492. https://doi.org/10.3390/cancers15184492
APA StyleAn, J., Kwon, H., & Kim, Y. J. (2023). The Role of Blood Microbiome in the Development of Thyroid Cancer in Breast Cancer Survivors. Cancers, 15(18), 4492. https://doi.org/10.3390/cancers15184492






