Management of Small Bowel Neuroendocrine Tumours: 10 Years’ Experience at a Tertiary Referral Centre
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnostics
2.3. Staging and Grading
2.4. Surgical Procedures
2.5. Non-Surgical Treatments
2.6. Monitoring of Response to Treatment and Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Surgical Treatment
3.2. Non-Surgical Treatment
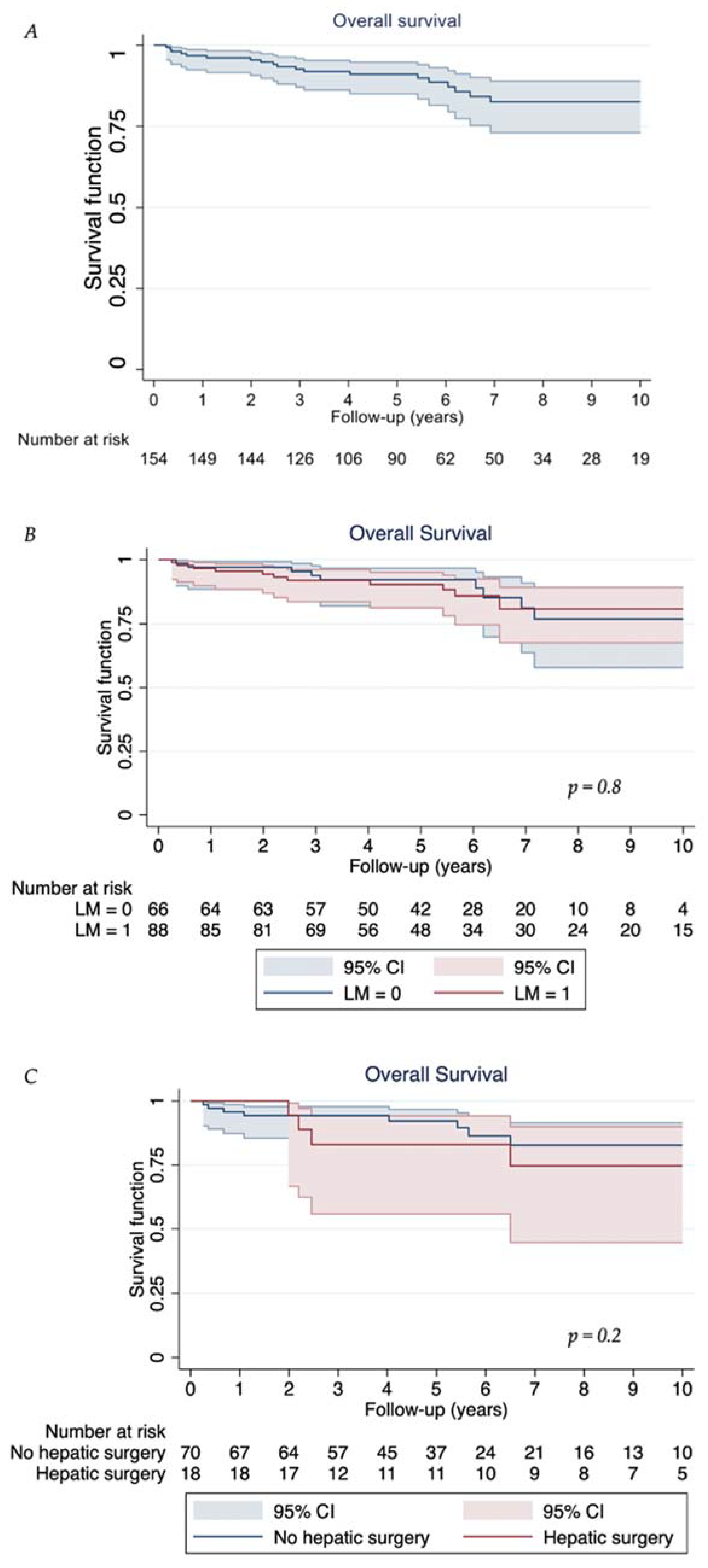
3.3. Follow-Up and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Sandor, A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997, 79, 813–829. [Google Scholar] [CrossRef]
- Keck, K.J.; Maxwell, J.E.; Menda, Y.; Bellizzi, A.; Dillon, J.; O’Dorisio, T.M.; Howe, J.R. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery 2017, 161, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Siegel, E.; Barmparas, G.; Lo, S.; Jamil, L.H.; Hendifar, A.; Nissen, N.N.; Wolin, E.M.; Amersi, F. Multifocality in Small Bowel Neuroendocrine Tumors. J. Gastrointest. Surg. 2018, 22, 303–309. [Google Scholar] [CrossRef]
- Choi, A.B.; Maxwell, J.E.; Keck, K.J.; Bellizzi, A.J.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. Is Multifocality an Indicator of Aggressive Behavior in Small Bowel Neuroendocrine Tumors? Pancreas 2017, 46, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Clift, A.K.; Faiz, O.; Al-Nahhas, A.; Bockisch, A.; Liedke, M.O.; Schloericke, E.; Wasan, H.; Martin, J.; Ziprin, P.; Moorthy, K.; et al. Role of Staging in Patients with Small Intestinal Neuroendocrine Tumours. J. Gastrointest. Surg. 2016, 20, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Sotiropoulos, G.C.; Radtke, A.; Malago, M.; Bockisch, A.; Kuehl, H.; Li, J.; Broelsch, C.E. The Impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann. Surg. 2010, 252, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, S.M.; Neychev, V.; Millo, C.; Shih, J.; Nilubol, N.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Kebebew, E. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J. Clin. Oncol. 2016, 34, 588–597. [Google Scholar] [CrossRef]
- Zaidi, M.Y.; Lopez-Aguiar, A.G.; Dillhoff, M.; Beal, E.; Poultsides, G.; Makris, E.; Rocha, F.; Crown, A.; Idrees, K.; Marincola Smith, P.; et al. Prognostic Role of Lymph Node Positivity and Number of Lymph Nodes Needed for Accurately Staging Small-Bowel Neuroendocrine Tumors. JAMA Surg. 2019, 154, 134–140. [Google Scholar] [CrossRef]
- Pasquer, A.; Walter, T.; Hervieu, V.; Forestier, J.; Scoazec, J.-Y.; Lombard-Bohas, C.; Poncet, G. Surgical Management of Small Bowel Neuroendocrine Tumors: Specific Requirements and Their Impact on Staging and Prognosis. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S742–S749. [Google Scholar] [CrossRef] [PubMed]
- Norlén, O.; Stålberg, P.; Öberg, K.; Eriksson, J.; Hedberg, J.; Hessman, O. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J. Surg. 2012, 36, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.P.; Mramba, L.K.; Bishnoi, R.; Unnikrishnan, A.; Duff, J.M.; Chandana, S.R. Survival trends of metastatic small intestinal neuroendocrine tumor: A population-based analysis of SEER database. J. Gastrointest. Oncol. 2019, 10, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.R.; Cardona, K.; Fraker, D.L.; Kebebew, E.; Untch, B.R.; Wang, Y.Z.; Law, C.H.; Liu, E.H.; Kim, M.K.; Menda, Y.; et al. The surgical management of small bowel neuroendocrine tumors. Pancreas 2017, 46, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.; Hallet, J.; Pasieka, J.; Koea, J.; Meyer-Rochow, W. Role of Primary Tumor Resection for Metastatic Small Bowel Neuroendocrine Tumors. World J. Surg. 2021, 45, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Turner, G.; King, B.; Jones, L.; Culliford, D.; McCance, D.; Ardill, J.; Johnston, B.T.; Poston, G.; Rees, M.; et al. Midgut neuroendocrine tumours with liver metastases: Results of the UKINETS study. Endocr. Relat. Cancer 2009, 16, 885–894. [Google Scholar] [CrossRef]
- Fairweather, M.; Swanson, R.; Wang, J.; Brais, L.K.; Dutton, T.; Kulke, M.H.; Clancy, T.E. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann. Surg. Oncol. 2017, 24, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Linecker, M.; Kambakamba, P.; Raptis, D.A.; Malagó, M.; Ratti, F.; Aldrighetti, L.; Robles-Campos, R.; Lehwald-Tywuschik, N.; Knoefel, W.; Balci, D.; et al. ALPPS in neuroendocrine liver metastases not amenable for conventional resection—Lessons learned from an interim analysis of the International ALPPS Registry. HPB 2020, 22, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aimnossadati, B.; Pape, U.-F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Hulke, M.H.; Jacene, H.; et al. Phase 3 Trial of177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Clift, A.K.; Kidd, M.; Bodei, L.; Toumpanakis, C.; Baum, R.P.; Oberg, K.; Modlin, I.M.; Frilling, A. At the Cutting Edge Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology 2020, 110, 444–476. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Couvelard, A.; Komminoth, P.; Körner, M.; Lopes, J.M.; McNicol, A.-M.; Nilsson, O.; Perren, A.; Scarpa, A.; et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2007, 451, 757–762. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Kloppel, G.; Rosai, J.; World Health Organization; International Agency for Research on Cancer. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 10. [Google Scholar]
- Klimstra, D.S. Classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; p. 16. [Google Scholar]
- Ohrvall, U.; Eriksson, B.; Juhlin, C.; Karacagil, S.; Rastad, J.; Hellman, P.; Akerstrom, G. Method for dissection of mesenteric metastases in mid-gut carcinoid tumors. World J. Surg. 2000, 24, 1402–1408. [Google Scholar] [CrossRef]
- Strasberg, S.M. Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Surg. 2005, 12, 351–355. [Google Scholar] [CrossRef]
- Fouché, M.; Bouffard, Y.; Le Goff, M.C.; Prothet, J.; Malavieille, F.; Sagnard, P.; Christin, F.; Hayi-Slayman, D.; Pasquer, A.; Poncet, G.; et al. Intraoperative carcinoid syndrome during small-bowel neuroendocrine tumour surgery. Endocr. Connect. 2018, 7, 1245–1250. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K.; Frampton, A.E.; Bomanji, J.; Kaemmerer, D.; Al-Nahhas, A.; Alsafi, A.; Kidd, M.; Modlin, I.M.; Horsch, D.; et al. A combination of surgery, theranostics, and liquid biopsy—A personalised oncologic approach to treatment of patients with advanced metastatic neuroendocrine neoplasms. Int. J. Med. Sci. 2021, 18, 2166–2175. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K.; Braat, A.J.A.T.; Alsafi, A.; Wasan, H.S.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]- fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Jakobsen, L.H.; Andersson, T.M.L.; Biccler, J.L.; Poulsen, L.; Severinsen, M.T.; El-Galaly, T.C.; Bogsted, M. On estimating the time to statistical cure. BMC Med. Res. Methodol. 2020, 20, 71. [Google Scholar] [CrossRef]
- Clift, A.K.; Giele, H.; Reddy, S.; MacEdo, R.; Al-Nahhas, A.; Wasan, H.S.; Gondolesi, G.E.; Vianna, R.M.; Friend, P.; Vaidya, A.; et al. Neoadjuvant peptide receptor radionuclide therapy and modified multivisceral transplantation for an advanced small intestinal neuroendocrine neoplasm: An updated case report. Innov. Surg. Sci. 2017, 2, 247–253. [Google Scholar] [CrossRef]
- Wu, L.; Fu, J.; Wan, L.; Pan, J.; Lai, S.; Zhong, J.; Chung, D.C.; Wang, L. Survival outcomes and surgical intervention of small intestinal neuroendocrine tumors: A population based retrospective study. Oncotarget 2017, 8, 4935–4947. [Google Scholar] [CrossRef]
- Habbe, N.; Fendrich, V.; Heverhagen, A.; Ramaswamy, A.; Bartsch, D.K. Outcome of surgery for ileojejunal neuroendocrine tumors. Surg. Today 2013, 43, 1168–1174. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Wang, Y.-Z.; Diebold, A.E.; Frey, D.J.; Anthony, L.; Uhlhorn, A.P.; Ryan, P.; Woltering, E.A. A single institution’s experience with surgical cytoreduction of stage IV, well-differentiated, small bowel neuroendocrine tumors. J. Am. Coll. Surg. 2014, 218, 837–844. [Google Scholar] [CrossRef]
- Burke, A.P.; Sobin, L.H.; Federspiel, B.H.; Shekitka, K.M.; Helwig, E.B. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch. Pathol. Lab. Med. 1990, 114, 700–704. [Google Scholar]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef]
- Van Den Heede, K.; Chidambaram, S.; Van Slycke, S.; Brusselaers, N.; Warfvinge, C.F.; Ohlsson, H.; Gistafsson, R.; Nordenstrom, E.; Almquist, M. Long-term survival of metastatic small intestine neuroendocrine tumors: A meta-analysis. Endocr. Relat. Cancer 2022, 29, 163–173. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.; et al. 177 Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Elias, E.; Ardalan, A.; Lindberg, M.; Reinsbach, S.E.; Muth, A.; Nilsson, O.; Arvisson, Y.; Larsson, E. Independent somatic evolution underlies clustered neuroendocrine tumors in the human small intestine. Nat. Commun. 2021, 12, 6367. [Google Scholar] [CrossRef]
- Wonn, S.M.; Limbach, K.E.; Pommier, S.E.J.; Ratzlaff, A.N.; Leon, E.J.; McCully, B.H.; Pommier, R.F. Outcomes of cytoreductive operations for peritoneal carcinomatosis with or without liver cytoreduction in patients with small bowel neuroendocrine tumors. Surgery 2021, 169, 168–174. [Google Scholar] [CrossRef]
- Fata, C.R.; Gonzalez, R.S.; Liu, E.; Cates, J.M.; Shi, C. Mesenteric Tumor Deposits in Midgut Small Intestinal Neuroendocrine Tumors Are a Stronger Indicator Than Lymph Node Metastasis for Liver Metastasis and Poor Prognosis. Am. J. Surg. Pathol. 2017, 41, 128–133. [Google Scholar] [CrossRef]
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J. Surg. 2014, 38, 1353–1361. [Google Scholar] [CrossRef]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.-F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef]
- Reissman, P.; Shmailov, S.; Grozinsky-Glasberg, S.; Gross, D.J. Laparoscopic resection of primary midgut carcinoid tumors. Surg. Endosc. 2013, 27, 3678–3682. [Google Scholar] [CrossRef]
- Figueiredo, M.N.; Maggiori, L.; Gaujoux, S.; Couvelard, A.; Guedj, N.; Ruszniewski, P.; Panis, Y. Surgery for small-bowel neuroendocrine tumors: Is there any benefit of the laparoscopic approach? Surg. Endosc. 2014, 28, 1720–1726. [Google Scholar] [CrossRef]
- Kaçmaz, E.; Van Eeden, S.; Koppes, J.C.C.; Klümpen, H.J.; Bemelman, W.A.; Nieveen Van Dijkum, E.J.M.; Engelsman, A.F.; Tanis, P.J. Value of Laparoscopy for Resection of Small-Bowel Neuroendocrine Neoplasms Including Central Mesenteric Lymphadenectomy. Dis. Colon. Rectum. 2021, 64, 1240–1248. [Google Scholar] [CrossRef]
- Kasai, Y.; Mahuron, K.; Hirose, K.; Corvera, C.U.; Kim, G.E.; Hope, T.A.; Shih, B.E.; Warren, R.S.; Bergsland, E.K.; Nakakura, E.K. A novel stratification of mesenteric mass involvement as a predictor of challenging mesenteric lymph node dissection by minimally invasive approach for ileal neuroendocrine tumors. J. Surg. Oncol. 2020, 122, 204–211. [Google Scholar] [CrossRef]
- Kaçmaz, E.; Sarasqueta, A.F.; van Eeden, S.; Dreijerink, K.M.A.; Klümpen, H.J.; Tanis, P.J.; Nieveen van Dikjum, E.J.M.; Engelsman, A.F. Update on Incidence, Prevalence, Treatment and Survival of Patients with Small Bowel Neuroendocrine Neoplasms in the Netherlands. World J. Surg. 2021, 45, 2482–2491. [Google Scholar] [CrossRef]
- Pasquer, A.; Walter, T.; Rousset, P.; Hervieu, V.; Forestier, J.; Lombard-Bohas, C.; Poncet, G. Lymphadenectomy during Small Bowel Neuroendocrine Tumor Surgery: The Concept of Skip Metastases. Ann. Surg. Oncol. 2016, 23, 804–808. [Google Scholar] [CrossRef]
- Lardière-Deguelte, S.; De Mestier, L.; Appéré, F.; Vullierme, M.P.; Zappa, M.; Hoeffel, C.; Noaves, M.; Brixi, H.; Hentic, O.; Ruszniewski, P.; et al. Toward a preoperative classification of lymph node metastases in patients with small intestinal neuroendocrine tumors in the era of intestinal-sparing surgery. Neuroendocrinology 2016, 103, 552–559. [Google Scholar] [CrossRef]
- Motz, B.M.; Lorimer, P.D.; Boselli, D.; Hill, J.S.; Salo, J.C. Optimal Lymphadenectomy in Small Bowel Neuroendocrine Tumors: Analysis of the NCDB. J. Gastrointest. Surg. 2018, 22, 117–123. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C. Extent of Lymph Node Dissection for Small Bowel Neuroendocrine Tumors. World J. Surg. 2021, 45, 197–202. [Google Scholar] [CrossRef]
- Deguelte, S.; Hammoutene, C.; Poncet, G.; Brunaud, L.; Perrier, M.; Kianmanesh, R.; Cadiot, G. Concept of reintervention with thorough lymphadenectomy after suboptimal resection of small-intestine neuroendocrine neoplasms: A multicentre preliminary study. J. Neuroendocrinol. 2022, 34, e13117. [Google Scholar] [CrossRef]
- Saxena, A.; Chua, T.C.; Perera, M.; Chu, F.; Morris, D.L. Surgical resection of hepatic metastases from neuroendocrine neoplasms: A systematic review. Surg. Oncol. 2012, 21, e131–e141. [Google Scholar] [CrossRef]
- Frilling, A.; Li, J.; Malamutmann, E.; Schmid, K.-W.K.-W.; Bockisch, A.; Broelsch, C.E. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br. J. Surg. 2009, 96, 175–184. [Google Scholar] [CrossRef]
- Yu, X.; Gu, J.; Wu, H.; Fu, D.; Li, J.; Jin, C. Resection of Liver Metastases: A Treatment Provides a Long-Term Survival Benefit for Patients with Advanced Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Oncol. 2018, 2018, 6273847. [Google Scholar] [CrossRef]
- Addeo, P.; Bertin, J.B.; Imperiale, A.; Averous, G.; Terrone, A.; Goichot, B.; Bachellier, P. Outcomes of Simultaneous Resection of Small Bowel Neuroendocrine Tumors with Synchronous Liver Metastases. World J. Surg. 2020, 44, 2377–2384. [Google Scholar] [CrossRef]
- Spolverato, G.; Bagante, F.; Aldrighetti, L.; Poultsides, G.A.; Bauer, T.W.; Fields, R.C.; Kumar Maithel, S.; Marques, H.P.; Weiss, M.; Pawlik, T.M. Management and outcomes of patients with recurrent neuroendocrine liver metastasis after curative surgery: An international multi-institutional analysis. J. Surg. Oncol. 2017, 116, 298–306. [Google Scholar] [CrossRef]
- Mayo, S.C.; de Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinski, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Scigliano, S.; Lebtahi, R.; Maire, F.; Stievenart, J.L.; Kianmanesh, R.; Sauvanet, A.; Vullierme, M.P.; Coulevard, A.; Belghiti, J.; Ruszniewski, P.; et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: A 15-year monocentric experience. Endocr. Relat. Cancer 2009, 16, 977–990. [Google Scholar] [CrossRef]
- Glazer, E.S.; Tseng, J.F.; Al-Refaie, W.; Solorzano, C.C.; Liu, P.; Willborn, K.A.; Abdalla, E.K.; Vauthey, J.-N.; Curley, S.A. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB 2010, 12, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Graff-Baker, A.N.; Sauer, D.A.; Pommier, S.J.; Pommier, R.F. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery 2014, 156, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, J.M.; Heywood, G.; Rubin, J.; Ilstrup, D.M.; Nagorney, D.M.; Que, F.G. Surgical treatment of neuroendocrine metastases to the liver: A plea for resection to increase survival. J. Am. Coll. Surg. 2003, 197, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.E.; Sherman, S.K.; O’Dorisio, T.M.; Bellizzi, A.M.; Howe, J.R. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery 2016, 159, 320–335. [Google Scholar] [CrossRef]
- Scott, A.T.; Breheny, P.J.; Keck, K.J.; Bellizzi, A.M.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 2019, 165, 166–175. [Google Scholar] [CrossRef]
- Van Den Heede, K.; Chidambaram, S.; Van Slycke, S.; Brusselaers, N.; Warfvinge, C.F.; Ohlsson, H.; Nordenstrom, E.; Almquist, M. Effect of primary tumour resection without curative intent in patients with metastatic neuroendocrine tumours of the small intestine and right colon: Meta-analysis. Br. J. Surg. 2022, 109, 191–199. [Google Scholar] [CrossRef]
- Bennett, S.; Coburn, N.; Law, C.; Mahar, A.; Zhao, H.; Singh, S.; Zuk, V.; Myrehaug, S.; Gupta, V.; Levy, J.; et al. Upfront Small Bowel Resection for Small Bowel Neuroendocrine Tumors with Synchronous Metastases: A Propensity-Score Matched Comparative Population-Based Analysis. Ann. Surg. 2020, 276, e450–e458. [Google Scholar] [CrossRef]
- Daskalakis, K.; Karakatsanis, A.; Hessman, O.; Stuart, H.C.; Welin, S.; Tiensuu Janson, E.; Oberg, K.; Hellman, P.; Norlen, O.; Stalberg, P. Association of a Prophylactic Surgical Approach to Stage IV Small Intestinal Neuroendocrine Tumors With Survival. JAMA Oncol. 2018, 4, 183–189. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Twrznik, M.; Kulkarni, H.R.; Hörsch, D.; Sehner, S.; Baum, R.P.; Hommann, M. Prior Resection of the Primary Tumor Prolongs Survival After Peptide Receptor Radionuclide Therapy of Advanced Neuroendocrine Neoplasms. Ann. Surg. 2021, 274, e45–e53. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Bertani, E.; Bartolomei, M.; Perali, C.; Muffatti, F.; Grana, C.M.; Lena, S.M.; Doglioni, C.; Crippa, S.; Farzio, N.; et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery 2018, 163, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Parghane, R.V.; Bhandare, M.; Chaudhari, V.; Ostwal, V.; Ramaswamy, A.; Talole, S.; Shrikhande, S.V.; Basu, B. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant 177 Lu-DOTATATE PRRT for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2021, 62, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.T.; Titan, A.L.; Foster, D.S.; Worth, P.J.; Poultsides, G.A.; Visser, B.C.; Dua, M.M.; Norton, J.A. Management of Ileal Neuroendocrine Tumors with Liver Metastases. J. Gastrointest. Surg. 2020, 24, 1530–1539. [Google Scholar] [CrossRef]
- Karpathakis, A.; Dibra, H.; Pipinikas, C.; Feber, A.; Morris, T.; Francis, J.; Oukrif, D.; Mandair, D.; Pericleous, M.; Mohmaduvesh, M.; et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin. Cancer Res. 2016, 22, 250–258. [Google Scholar] [CrossRef]
- Scarpa, A. The landscape of molecular alterations in pancreatic and small intestinal neuroendocrine tumours. Ann. Endocrinol. 2019, 80, 153–158. [Google Scholar] [CrossRef]
- Kidd, M.; Modlin, I.M.; Drozdov, I. Gene network-based analysis identifies two potential subtypes of small intestinal neuroendocrine tumors. BMC Genom. 2014, 15, 595. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Frilling, A.; Falconi, M.; Filosso, P.L.; Malczewska, A.; Kitz, A. Molecular Genomic Assessment Using a Blood-based mRNA Signature (NETest) is Cost-effective and Predicts Neuroendocrine Tumor Recurrence with 94% Accuracy. Ann. Surg. 2021, 274, 481–490. [Google Scholar] [CrossRef]
- Malczewska, A.; Frampton, A.E.; Mato Prado, M.; Ameri, S.; Dabrowska, A.F.; Zagorac, S.; Clift, A.K.; Kos-Kudla, B.; Faiz, O.; Stebbing, J.; et al. Circulating MicroRNAs in Small-bowel Neuroendocrine Tumors: A Potential Tool for Diagnosis and Assessment of Effectiveness of Surgical Resection. Ann. Surg. 2021, 274, e1–e9. [Google Scholar] [CrossRef]
| Parameter | N (Column % unless Otherwise Specified) |
|---|---|
| Total patients | 154 |
| Age at initial diagnosis | Median 64 years (range 33–87) |
| <50 years | 24 |
| 50 to 59 years | 35 |
| 60 to 69 years | 50 |
| 70 to 74 years | 21 |
| 75+ years | 24 |
| Sex | |
| Female | 81 (52.6) |
| Male | 73 (47.4) |
| Stage at initial diagnosis (on imaging) | |
| Stage I/II—localised disease only | 19 (12.3) |
| Stage III—nodal metastases only | 44 (28.6) |
| Stage IV—distant metastases (with or without nodal metastases) | 91 (59.1) |
| Tumour grade | |
| G1 | 107 (69.5) |
| G2 | 35 (22.7) |
| G3 | 1 (0.7) |
| Not available | 11 (7.1) |
| Site of distant metastases | |
| Liver only | 70 (45.5) |
| Liver + bone | 7 (4.5) |
| Liver + mesenteric mass | 3 (2.0) |
| Liver + peritoneum or liver + omentum | 4 (2.6) |
| Peritoneum + bone | 2 (1.3) |
| Mesenteric mass | 2 (1.3) |
| Bone only | 2 (1.3) |
| Liver + lung + bone | 1 (0.6) |
| Primary tumour location | |
| Ileum | 154 (100) |
| Jejunum (all multifocal, also lesions in ileum) | 3 (1.9) |
| Carcinoid syndrome present | 46 (29.9) |
| Carcinoid heart disease present | 10 (6.5) |
| Surgery used as first line treatment | 125 (81.2) |
| Non-surgical treatment use (at least once during clinical course) | |
| Somatostatin analogues | 89 (57.8) |
| Peptide receptor radionuclide therapy | 45 (29.2) |
| Selective internal radiotherapy | 31 (20.1) |
| Radiofrequency ablation | 10 (6.5) |
| mTOR inhibitor | 5 (3.3) |
| Transarterial chemoembolisation | 2 (1.3) |
| Parameter | N (Column % unless Otherwise Specified) |
|---|---|
| Total patients undergoing surgical treatment | 125 |
| Type of surgery | |
| Small bowel resection | 76 (49.4) |
| Right hemicolectomy | 42 (27.3) |
| Multivisceral resection | 3 (2.0) |
| Incidental finding during other abdominal surgery | 4 (2.6) |
| Resection + modified multivisceral transplantation | 1 (0.7) |
| Mesenteric lymphadenectomy (complete) | 84 (67.2) |
| Mesenteric lymphadenectomy (incomplete) | 2 (1.4) |
| Repeat lymphadenectomy after lymph node recurrence | 2 (1.4) |
| Emergency surgery | |
| Emergency index surgery | 13 (10.4) |
| Exploratory laparotomy and biopsy only | 1 (1.4) |
| Tumour grade (as per ENETS-WHO system) | |
| G1 | 92 (73.6) |
| G1 | 28 (22.4) |
| G3 | 1 (0.8) |
| Not available * | 4 (3.2) |
| Primary tumour stage (as per ENETS-UICC system) | |
| pT1 | 3 (2.4) |
| pT2 | 15 (12.0) |
| pT3 | 26 (20.8) |
| pT4 | 42 (33.6) |
| Not available | 39 (31.2) |
| Lymph node metastases | |
| N0 | 35 (28.0) |
| N1 | 90 (72.0) |
| Multifocal primary tumour | 46 (36.8) |
| Mean number of lymph nodes resected per patient | 18 (range 7–46) |
| Mesenteric tumour deposits present | 6 (4.8) |
| Perineural invasion present | 60 (48.0) |
| Lympho-angioinvasion present | 85 (68.0) |
| 90-days surgical morbidity | 7 (5.6) |
| 90-days surgical mortality | 0 (0) |
| Overall Survival | Kaplan–Meier Estimate (95% CI) |
|---|---|
| 1-year | 96.8% (92.4 to 98.5) |
| 3-year | 92.6% (87.1 to 95.9) |
| 5-year | 91.0% (85.0 to 94.7) |
| 10-year | 82.5% (73.0 to 88.9) |
| Progression-free survival | |
| 1-year | 96.7% (92.4 to 98.6) |
| 3-year | 84.3% (77.5 to 89.2) |
| 5-year | 63.4% (55.0 to 70.6) |
| 10-year | 18.7% (12.4 to 26.1) |
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Parameter | Hazard Ratio (95% CI) | p-Value | Parameter | Hazard Ratio (95% CI) | p-Value |
| Age (per year) | 1.04 (1.00 to 1.08) | 0.038 | Age (per year) | 1.04 (1.00 to 1.10) | 0.099 |
| Sex (female vs. male) | 1.05 (0.43 to 2.59) | 0.916 | Sex (female vs. male) | 0.77 (0.29 to 2.02) | 0.591 |
| Stage | Stage | ||||
| Stage I/II | 1 (reference) | Stage I/II | 1 (reference) | ||
| Stage III | 1.24 (0.25 to 6.14) | 0.796 | Stage III | 0.65 (0.11 to 3.82) | 0.631 |
| Stage IV | 1.20 (0.29 to 5.40) | 0.808 | Stage IV | 0.53 (0.09 to 3.26) | 0.497 |
| Grade | Grade | ||||
| 1 | 1 (reference) | 1 | 1 (reference) | ||
| 2 | 2.79 (1.08 to 6.75) | 0.033 | 2 | 3.22 (1.20 to 8.62) | 0.020 |
| 3 | Not estimable | N/A | 3 | Not estimable | N/A |
| Multifocal primary | Multifocal primary | ||||
| No | 1 (reference) | No | 1 (reference) | ||
| Yes | 1.15 (0.44 to 2.99) | 0.779 | Yes | 1.03 (0.35 to 2.97) | 0.959 |
| Carcinoid syndrome | Carcinoid syndrome | ||||
| No | 1 (reference) | No | 1 (reference) | ||
| Yes | 0.93 (0.36 to 2.40) | 1.00 | Yes | 1.03 (0.35 to 2.99) | 0.958 |
| Surgery as 1st line treatment | Surgery as 1st line treatment | ||||
| No | 1 (reference) | No | 1 (reference) | ||
| Yes | 0.93 (0.31 to 2.77) | 0.897 | Yes | 0.57 (0.13 to 2.47) | 0.454 |
| Lymphovascular invasion | 0.94 (0.30 to 2.80) | 0.899 | Lymphovascular invasion | Not estimable | N/A |
| Perineural invasion | 1.74 (0.72 to 4.19) | 0.215 | Perineural invasion | 2.25 (0.69 to 7.29) | 0.177 |
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Parameter | Hazard Ratio (95% CI) | p-Value | Parameter | Hazard Ratio (95% CI) | p-Value |
| Age (per year) | 1.00 (1.00 to 1.02) | 0.188 | Age (per year) | 1.00 (0.99 to 1.02) | 0.369 |
| Sex (female vs. male) | 1.26 (0.88 to 1.88) | 0.205 | Sex (female vs. male) | 1.03 (0.69 to 1.54) | 0.867 |
| Stage | Stage | ||||
| Stage I/II | 1 (reference) | Stage I/II | 1 (reference) | ||
| Stage III | 1.17 (0.59 to 2.33) | 0.656 | Stage III | 1.10 (0.53 to 2.33) | 0.785 |
| Stage IV | 1.32 (0.70 to 2.49) | 0.389 | Stage IV | 1.32 (0.63 to 2.77) | 0.464 |
| Grade | Grade | ||||
| 1 | 1 (reference) | 1 | 1 (reference) | ||
| 2 | 1.61 (1.07 to 2.43) | 0.024 | 2 | 1.71 (1.10 to 2.67) | 0.018 |
| 3 | 1.17 (0.16 to 8.48) | 0.873 | 3 | 1.69 (0.22 to 13.18) | 0.615 |
| Multifocal primary | Multifocal primary | ||||
| No | 1 (reference) | No | 1 (reference) | ||
| Yes | 1.32 (0.89 to 1.97) | 0.159 | Yes | 1.37 (0.90 to 2.08) | 0.146 |
| Carcinoid syndrome | Carcinoid syndrome | ||||
| No | 1 (reference) | No | 1 (reference) | ||
| Yes | 1.07 (0.72 to 1.56) | 0.743 | Yes | 0.73 (0.45 to 1.17) | 0.190 |
| Surgery as 1st line treatment | Surgery as 1st line treatment | ||||
| No | 1 (reference) | No | 1 (reference) | ||
| Yes | 0.87 (0.56 to 1.34) | 0.519 | Yes | 0.95 (0.54 to 1.69) | 0.865 |
| Lymphovascular invasion | 0.80 (0.51 to 1.37) | 0.500 | Lymphovascular invasion | Not estimable | N/A |
| Perineural invasion | 0.95 (0.65 to 1.41) | 0.815 | Perineural invasion | 0.95 (0.59 to 1.52) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clift, A.K.; Drymousis, P.; von Roon, A.; Humphries, A.; Goldin, R.; Bomanji, J.; Leaman, S.; Wasan, H.; Habib, N.; Frilling, A. Management of Small Bowel Neuroendocrine Tumours: 10 Years’ Experience at a Tertiary Referral Centre. Cancers 2023, 15, 4438. https://doi.org/10.3390/cancers15184438
Clift AK, Drymousis P, von Roon A, Humphries A, Goldin R, Bomanji J, Leaman S, Wasan H, Habib N, Frilling A. Management of Small Bowel Neuroendocrine Tumours: 10 Years’ Experience at a Tertiary Referral Centre. Cancers. 2023; 15(18):4438. https://doi.org/10.3390/cancers15184438
Chicago/Turabian StyleClift, Ashley K., Panagiotis Drymousis, Alexander von Roon, Adam Humphries, Robert Goldin, Jamshed Bomanji, Sydney Leaman, Harpreet Wasan, Nagy Habib, and Andrea Frilling. 2023. "Management of Small Bowel Neuroendocrine Tumours: 10 Years’ Experience at a Tertiary Referral Centre" Cancers 15, no. 18: 4438. https://doi.org/10.3390/cancers15184438
APA StyleClift, A. K., Drymousis, P., von Roon, A., Humphries, A., Goldin, R., Bomanji, J., Leaman, S., Wasan, H., Habib, N., & Frilling, A. (2023). Management of Small Bowel Neuroendocrine Tumours: 10 Years’ Experience at a Tertiary Referral Centre. Cancers, 15(18), 4438. https://doi.org/10.3390/cancers15184438







