Modern Approach to Melanoma Adjuvant Treatment with Anti-PD1 Immune Check Point Inhibitors or BRAF/MEK Targeted Therapy: Multicenter Real-World Report
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Inclusion Criteria
2.2. Collected Covariates
2.3. Analysis Plan
2.4. Statistical Methods
2.5. Ethical Statement
3. Results
3.1. Patient and Tumor Characteristics
3.2. Local Therapy
3.2.1. Surgical Treatment before Adjuvant Systemic Therapy
3.2.2. Radiotherapy
3.3. Systemic Treatment
3.4. Survival Analysis
3.4.1. Registered Events
3.4.2. Overall and Recurrence-Free Survival
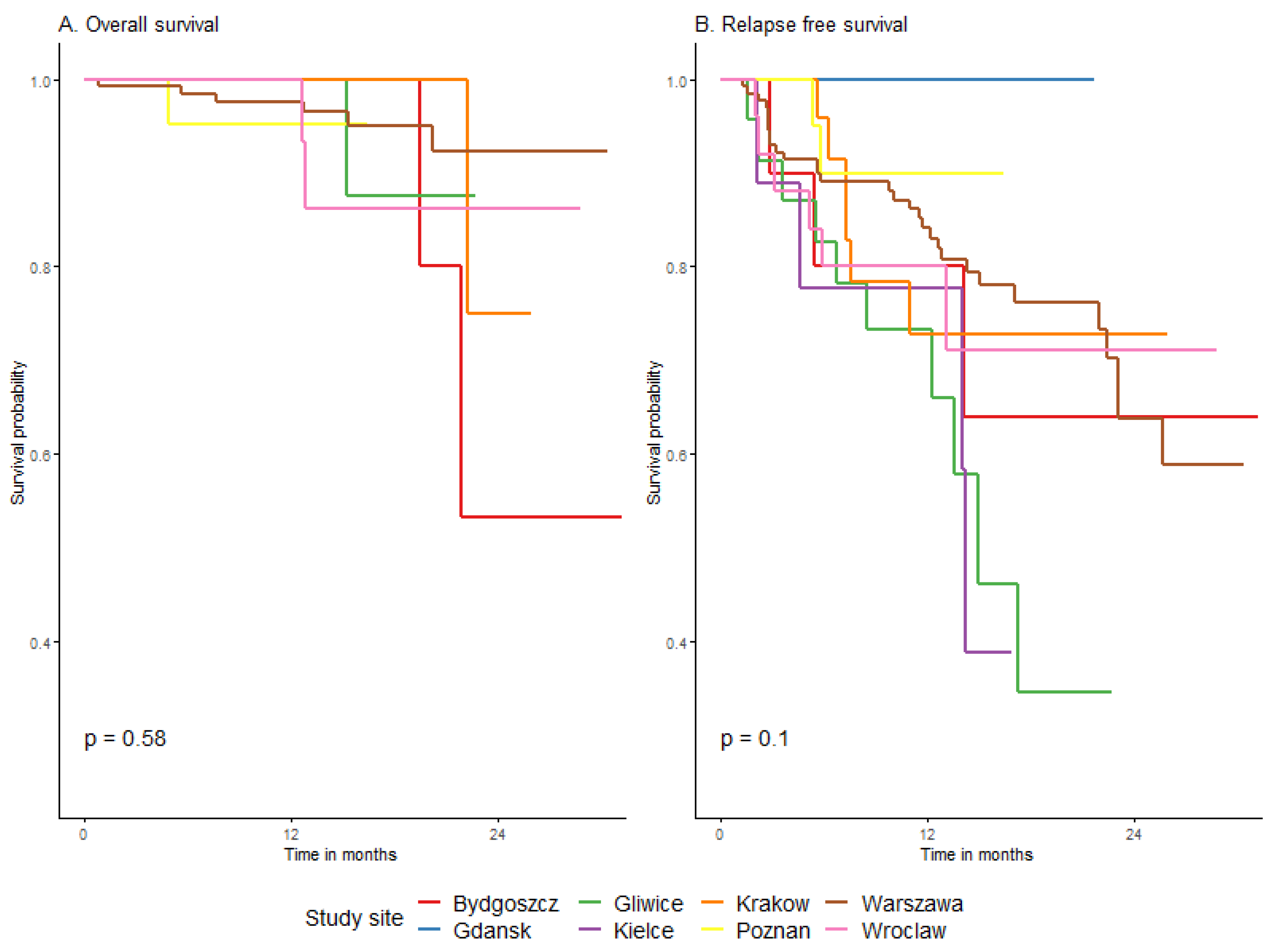
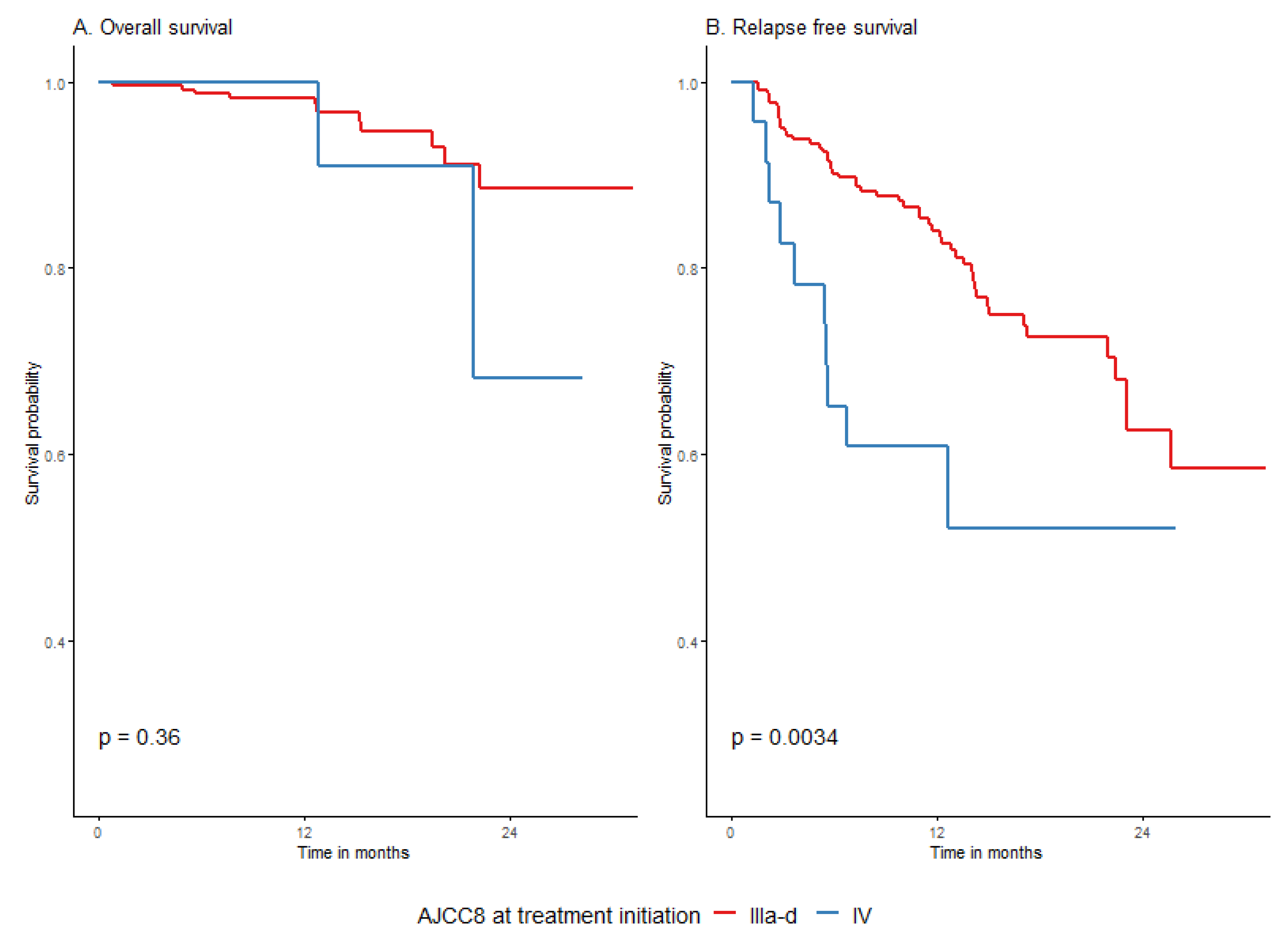
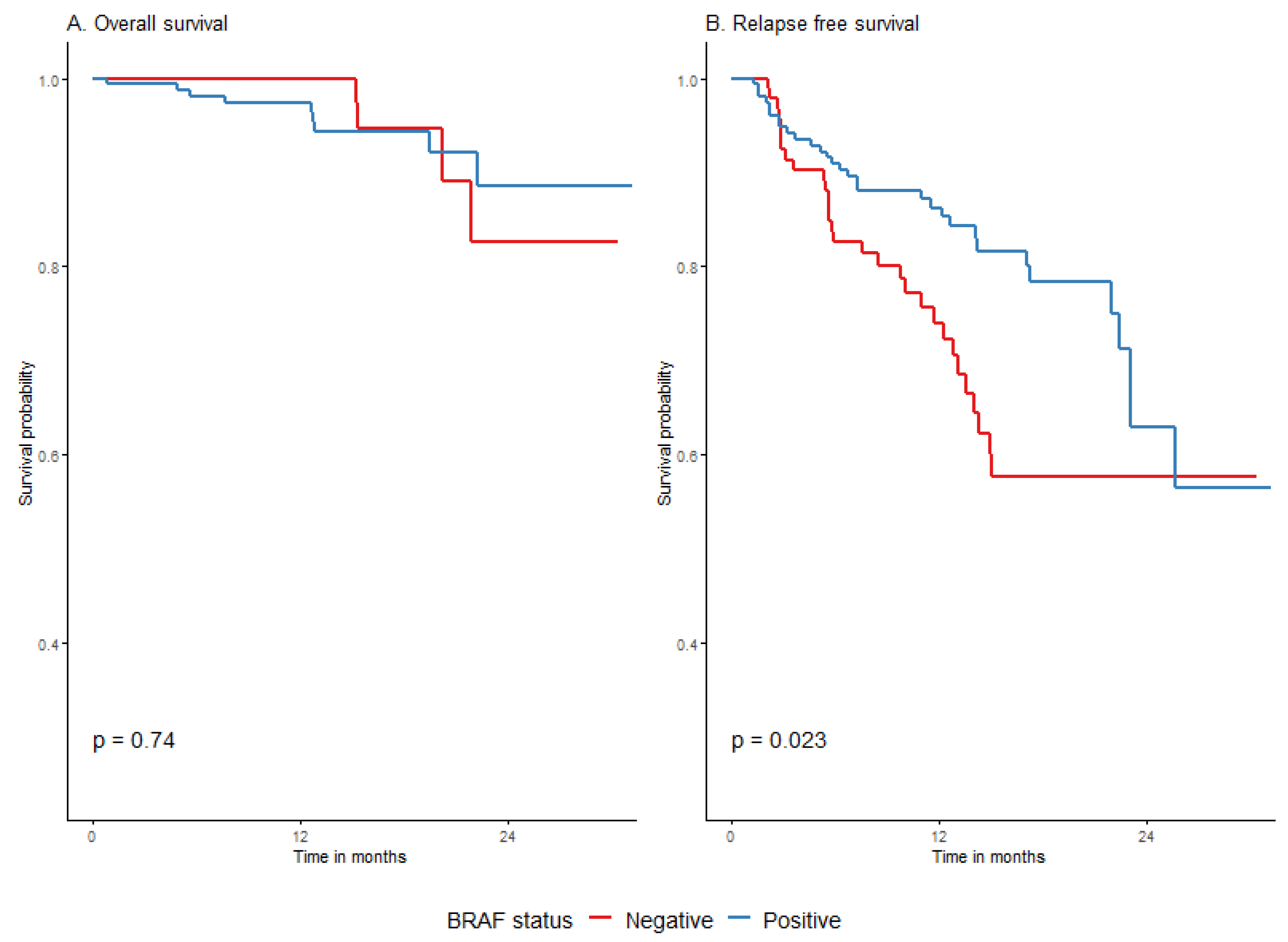
3.4.3. Survival Depending on Patient and Melanoma Characteristics
3.4.4. Survival Association with Surgical Procedure before Adjuvant and the Adjuvant Type
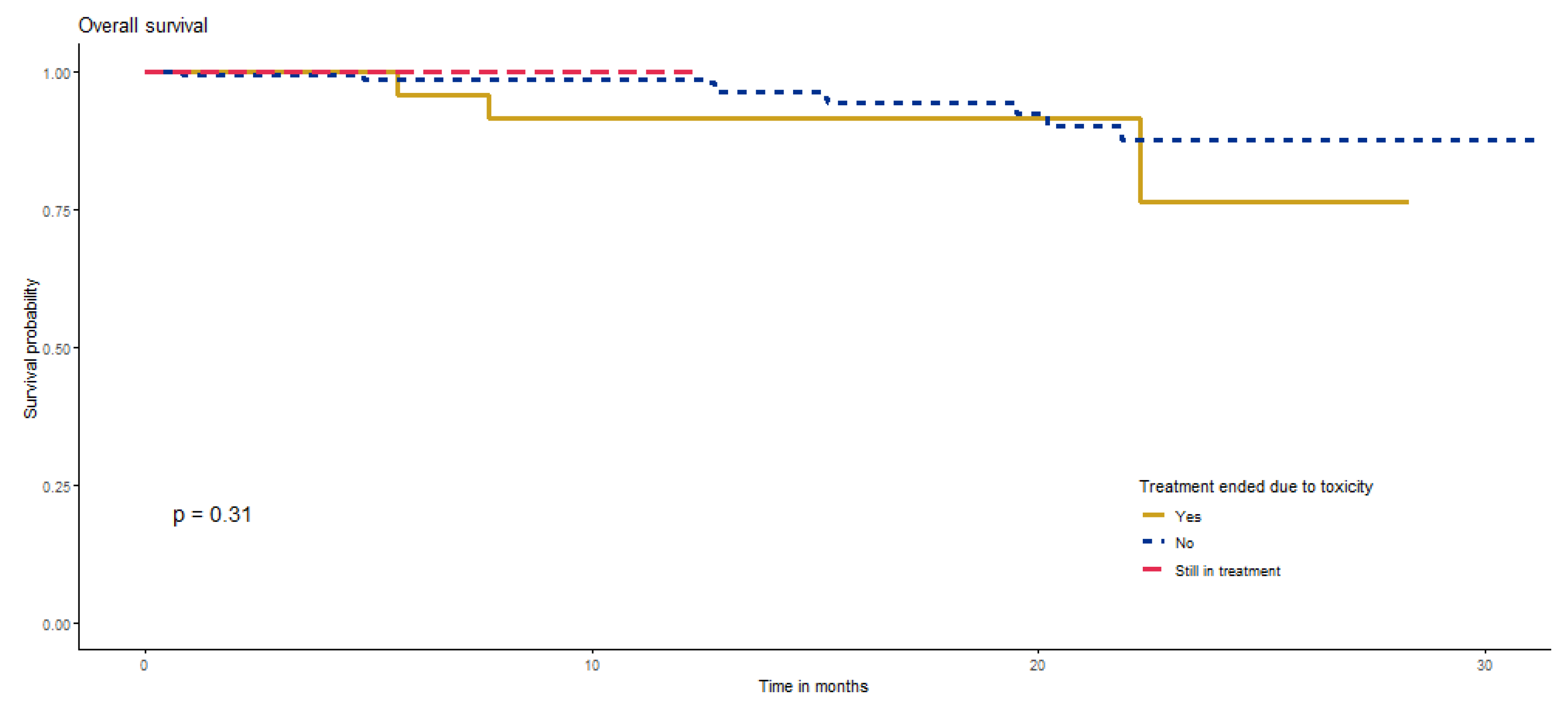
3.4.5. Survival According to Treatment-Related Adverse Events (TRAE)
3.5. Prognostic Model
3.6. Subgroup Analysis of Primary/LND Group
4. Discussion
4.1. Approach to Adjuvant Melanoma Treatment Inclusion Criteria
4.2. Prognostic and Predictive Tools
4.3. Systemic Treatment Impact
4.4. Real-World Analysis in Comparison to Registration Trials
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| BRAF/MEKi | BRAF/MEK inhibitors |
| CLND | completion lymphadenectomy |
| DMFS | distant-metastases-free survival |
| DT | dabrafenib plus trametinib |
| HR | hazard ratio |
| ICI | immune check point inhibitors |
| NSLN | nonsentinel lymph node metastases |
| OS | overall survival |
| RFS | relapse-free survival |
| SLNB | sentinel lymph node biopsy |
| TLND | therapeutic lymph node dissection |
| TRAE | treatment-related adverse events |
| WHO | World Health Organisation |
Appendix A
Appendix A.1. Prognostic Model for Relapse-free Survival
Appendix A.1.1. Model Building Strategy
| Factor | Level | HR | 95% HR-Lower | 95% HR-Upper | p-Value (for Significance of HR Not Equal 1) | p-Value (Overall Significance of the Factor) |
|---|---|---|---|---|---|---|
| Sex | M vs. F | 1.8 | 1 | 3.1 | 0.049 | 0.046 |
| Age group | 45–54 vs. <45 | 2.4 | 1 | 5.9 | 0.0607 | 0.3009 |
| 55–64 vs. <45 | 1.6 | 0.7 | 3.7 | 0.2948 | ||
| 65+ vs. <45 | 1.4 | 0.6 | 3.4 | 0.4412 | ||
| WHO performance status | 1 vs. 0 | 1.8 | 0.9 | 3.4 | 0.09 | 0.0927 |
| BRAF mutational status | Positive vs. Negative | 0.9 | 0.5 | 1.9 | 0.8785 | 0.8783 |
| AJCC8 at treatment initiation | IIIB vs. IIIA | 1.8 | 0.4 | 9.4 | 0.4606 | 0.6945 |
| IIIC vs. IIIA | 2.2 | 0.5 | 9.7 | 0.3085 | ||
| IIID vs. IIIA | 1.7 | 0.3 | 11.6 | 0.5671 | ||
| IV vs. IIIA | 7.9 | 1.5 | 40.3 | 0.0136 | ||
| Indication group for adjuvant treatment | M1 vs. Primary/LND | NA | NA | NA | NA | 0.6182 |
| Scar/In transit/LND vs. Primary/LND | 1.2 | 0.6 | 2.2 | 0.6182 | ||
| Adjuvant treatment | Anti-PD1 vs. BRAF/MEKi | 1.7 | 0.8 | 3.3 | 0.2031 | 0.2044 |
| Grade of TRAE | 1 vs. No TRAE | 0.5 | 0.2 | 1 | 0.0617 | 0.0036 |
| 2 vs. No TRAE | 0.4 | 0.2 | 0.8 | 0.0092 | ||
| 3 vs. No TRAE | 0.1 | 0 | 1 | 0.0494 |
| Chi-Square | df | p-Value | |
|---|---|---|---|
| Sex | 0.0439 | 1 | 0.834 |
| WHO performance status | 1.7243 | 1 | 0.189 |
| AJCC8 stage | 1.8538 | 1 | 0.173 |
| Adjuvant treatment | 4.7520 | 1 | 0.029 |
| Grade of TRAE | 0.5898 | 3 | 0.899 |
| GLOBAL | 8.9276 | 7 | 0.258 |
Appendix A.1.2. Final model
| Factor | Level | HR | 95% HR-Lower | 95% HR-Upper | p-Value (for Significance of HR Not Equal 1) | p-Value (Overall Significance of the Factor) |
|---|---|---|---|---|---|---|
| Sex | M vs. F | 1.9 | 1.1 | 3.3 | 0.0157 | 0.0138 |
| WHO status | 1 vs. 0 | 1.9 | 1 | 3.3 | 0.0372 | 0.0407 |
| AJCC8 stage | IV vs. IIIa–IIId | 3.2 | 1.5 | 6.8 | 0.0031 | 0.0067 |
| Grade of adverse events | G1 vs. none | 0.4 | 0.2 | 1 | 0.0382 | 0.0036 |
| G2 vs. none | 0.4 | 0.2 | 0.8 | 0.0102 | ||
| ≥G3 vs. none | 0.1 | 0 | 1 | 0.0514 | ||
| Class of adjuvant (by time since the beginning of treatment) | Anti-PD1 vs. BRAF/MEKi | 0.0096 | ||||
| <6 M | 5.8 | 1.3 | 24.9 | 0.0183 | ||
| 6–<12 M | 0.8 | 0.3 | 2.6 | 0.719 | ||
| 12–<18 M | 1.8 | 0.6 | 6 | 0.3052 | ||
| 18 M+ | 0.2 | 0 | 1.5 | 0.1123 |
| Chi-Square | df | p-Value | |
|---|---|---|---|
| Sex | 0.179 | 1 | 0.67 |
| WHO performance status | 0.692 | 1 | 0.41 |
| AJCC8 stage | 1.074 | 1 | 0.30 |
| Grade of TRAE | 0.305 | 3 | 0.96 |
| Adjuvant type: Time strata | 5.567 | 4 | 0.23 |
| GLOBAL | 9.334 | 10 | 0.50 |
Appendix B
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 116 | 23 | 24.8 (1.2) | NA (23.1–NA) | 84.8% (78.1–92.1%) | 76.9% (68.3–86.7%) | 62.7% (45.9–85.5%) | Ref. | 0.1696 |
| male | 132 | 38 | 22.7 (1.1) | NA (23.1–NA) | 79.2% (72.3–86.8%) | 65.9% (56.6–76.8%) | 59.3% (48–73.3%) | 1.4 (0.9–2.4) | ||
| Age group | <45 | 62 | 10 | 26.4 (1.4) | NA (NA–NA) | 90.3% (83.2–98%) | 81% (69.8–93.9%) | 73.6% (58–93.4%) | Ref. | 0.3518 |
| 45–54 | 41 | 12 | 22.6 (2) | 25.7 (25.7–NA) | 79.6% (67.8–93.4%) | 68.4% (53.9–86.7%) | 68.4% (53.9–86.7%) | 1.9 (0.8–4.4) | ||
| 55–64 | 64 | 18 | 22.2 (1.7) | NA (17.1–NA) | 80.1% (69.9–91.8%) | 58.8% (44–78.4%) | 50.4% (33.2–76.5%) | 1.9 (0.9–4.2) | ||
| 65+ | 81 | 21 | 23.7 (1.4) | NA (23.1–NA) | 77.8% (68.9–87.8%) | 74% (64.4–85%) | 59.2% (42.1–83.1%) | 1.7 (0.8–3.6) | ||
| Comorbidities | No | 114 | 27 | 24.4 (1.1) | NA (NA–NA) | 84.5% (77.9–91.6%) | 72.5% (63.4–82.9%) | 64.7% (52.5–79.6%) | Ref. | 0.5045 |
| Yes | 134 | 34 | 23.1 (1.2) | NA (23.1–NA) | 79.4% (72.3–87.1%) | 68.9% (59.3–80.1%) | 58.3% (44.2–76.9%) | 1.2 (0.7–2) | ||
| BRAF mutational status | negative | 92 | 30 | 21.9 (1.4) | NA (14.9–NA) | 74% (65–84.2%) | 57.7% (46.4–71.7%) | 57.7% (46.4–71.7%) | Ref. | 0.0237 |
| positive | 155 | 31 | 24.7 (1) | NA (23.1–NA) | 86.3% (80.8–92.1%) | 78.4% (70.8–86.8%) | 62.9% (49.4–80%) | 0.6 (0.3–0.9) | ||
| WHO performance status | 0 | 161 | 37 | 24.4 (1) | NA (25.7–NA) | 85.5% (80–91.4%) | 71.9% (63.7–81.1%) | 65.5% (54.9–78.2%) | Ref. | 0.1753 |
| 1 | 87 | 24 | 22.4 (1.5) | NA (22.5–NA) | 74.7% (65.5–85.2%) | 69.3% (58.5–82%) | 52% (33.7–80.2%) | 1.4 (0.9–2.4) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 2 | 27.6 (2.4) | NA (NA–NA) | 93.8% (82.6–100%) | 83.3% (64–100%) | 36.2% (15.7–83.7%) | Ref. | 0.0483 |
| IIIB | 56 | 11 | 23.5 (1.7) | 23.1 (22.5–NA) | 91.8% (84.2–100%) | 80.4% (67.6–95.7%) | 69.4% (60.6–79.5%) | 1.4 (0.3–6.4) | ||
| IIIC | 139 | 34 | 24.2 (1) | NA (NA–NA) | 81.5% (75–88.5%) | 69.4% (60.6–79.5%) | 66.3% (41–100%) | 1.8 (0.4–7.3) | ||
| IIID | 11 | 3 | 24 (3.5) | NA (11.5–NA) | 66.3% (41–100%) | 66.3% (41–100%) | 52.2% (33.4–81.5%) | 1.9 (0.3–11.7) | ||
| IV | 23 | 10 | 19 (2.9) | NA (5.6–NA) | 60.9% (43.9–84.5%) | 52.2% (33.4–81.5%) | 36.2% (15.7–83.7%) | 4.4 (1–20) | ||
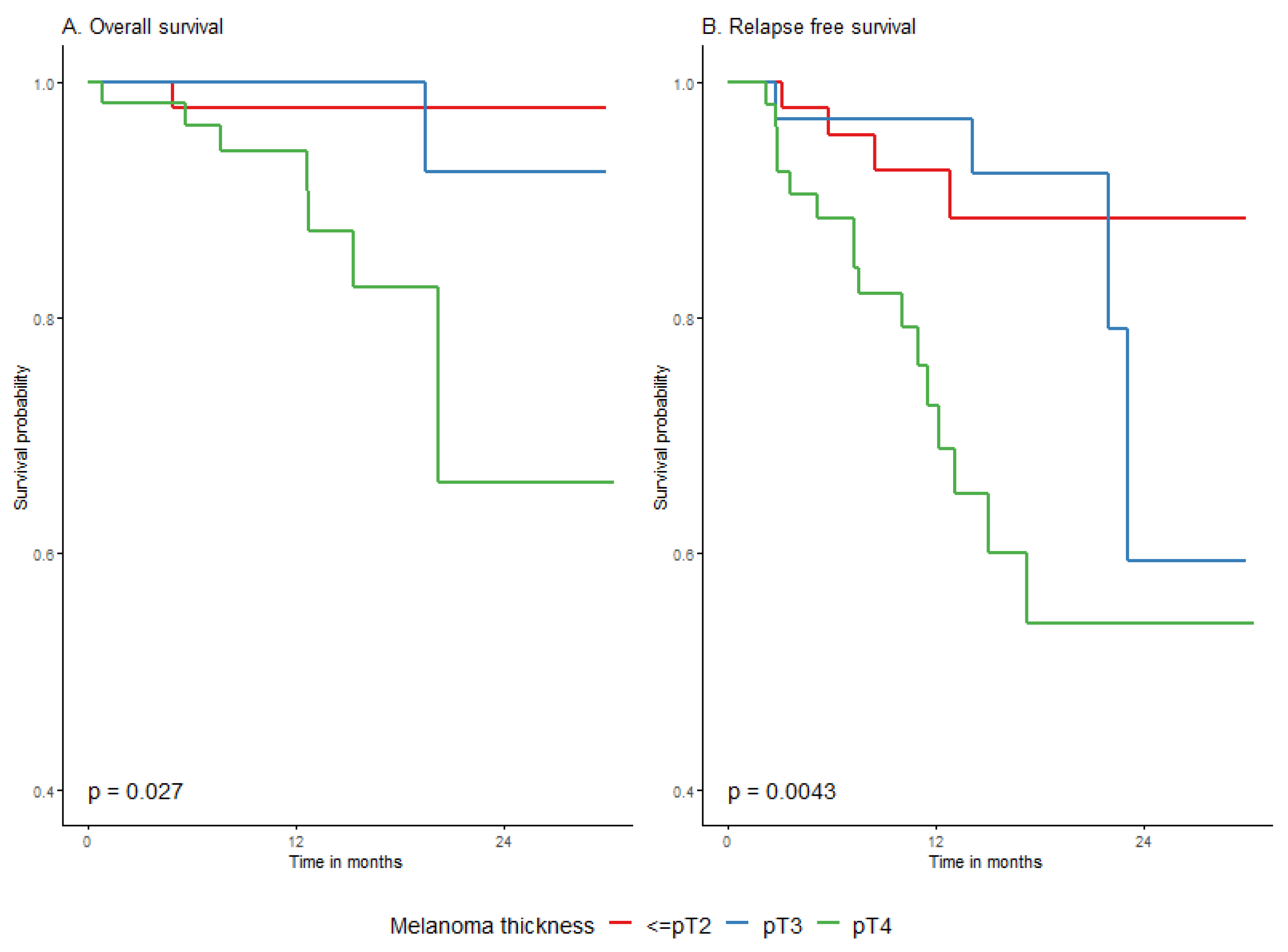
| AJCC8 stage primary tumor, pT | T0-T2 | 84 | 16 | 25 (1.4) | NA (25.7–NA) | 87.1% (79.8–95%) | 78% (67.8–89.7%) | 71.5% (57.3–89.2%) | Ref. | 0.012 |
| T3a-b | 62 | 12 | 25.2 (1.5) | NA (23.1–NA) | 91.9% (85.4–99%) | 82.4% (72.3–93.9%) | 58.7% (39–88.5%) | 0.9 (0.4–1.9) | ||
| T4a-b | 93 | 31 | 21.4 (1.4) | NA (17.1–NA) | 68.7% (59.1–79.8%) | 55.4% (43.3–70.9%) | 55.4% (43.3–70.9%) | 2 (1.1–3.7) | ||
| Group of indications for adjuvant | LND/Primary | 130 | 24 | 25.2 (1.1) | NA (NA–NA) | 85.9% (79.7–92.7%) | 76.4% (67.6–86.4%) | 65.4% (50.9–83.9%) | Ref. | 0.0065 |
| M1 | 23 | 10 | 19 (2.9) | NA (5.6–NA) | 60.9% (43.9–84.5%) | 52.2% (33.4–81.5%) | 52.2% (33.4–81.5%) | 3.2 (1.5–6.6) | ||
| Scar/In transit/LND | 95 | 27 | 23.1 (1.3) | NA (23.1–NA) | 81.1% (73.4–89.7%) | 68% (57.5–80.4%) | 58.8% (45.3–76.5%) | 1.4 (0.8–2.5) | ||
| Adjuvant type | Anti-PD1 | 147 | 46 | 21.7 (1.1) | NA (17.3–NA) | 74.3% (67.1–82.2%) | 59.4% (49.9–70.8%) | 56.1% (45.6–69.1%) | Ref. | <0.0001 |
| BRAF/MEKi | 101 | 15 | 26.1 (1.2) | NA (23.1–NA) | 92.3% (87–98%) | 85.2% (77.1–94.2%) | 65.9% (48.4–89.7%) | 0.4 (0.2–0.7) | ||
| TRAE | no | 131 | 40 | 21.8 (1.2) | NA (17.1–NA) | 75.2% (67.7–83.5%) | 56.6% (45.8–70%) | 56.6% (45.8–70%) | Ref. | 0.0031 |
| yes | 115 | 21 | 25.8 (1) | NA (25.7–NA) | 88.9% (83.1–95.1%) | 83.4% (76.1–91.4%) | 68.7% (55.5–84.9%) | 0.5 (0.3–0.8) | ||
| Highest grade toxicity | 1 | 37 | 9 | 23.9 (1.9) | NA (23.1–NA) | 83.8% (72.7–96.5%) | 75.2% (61.1–92.4%) | 64.4% (44.7–92.9%) | Ref. | 0.2286 |
| 2 | 52 | 10 | 25.3 (1.4) | NA (25.7–NA) | 89.7% (81.4–98.8%) | 83.8% (73.3–95.9%) | 70.8% (53.9–92.9%) | 0.7 (0.3–1.8) | ||
| 3 | 23 | 1 | 29.2 (1.2) | NA (NA–NA) | 93.8% (82.6–100%) | 93.8% (82.6–100%) | 93.8% (82.6–100%) | 0.2 (0–1.5) | ||
| Toxicities after immuno | no | 91 | 34 | 19.3 (1.4) | 17.3 (14–NA) | 67.2% (57.5–78.6%) | 48% (35.5–65%) | 48% (35.5–65%) | Ref. | 0.0286 |
| yes | 56 | 12 | 24.3 (1.5) | NA (NA–NA) | 85.7% (77–95.4%) | 75.8% (63.6–90.4%) | 68.9% (53.3–89.1%) | 0.5 (0.3–0.9) | ||
| Toxicities after BRAF/MEKi | no | 40 | 6 | 26.4 (1.8) | NA (NA–NA) | 92.3% (84.3–100%) | 74.6% (56.9–97.9%) | 74.6% (56.9–97.9%) | Ref. | 0.4709 |
| yes | 59 | 9 | 26.4 (1.3) | NA (23.1–NA) | 92.4% (85.5–99.9%) | 89.8% (81.5–98.9%) | 65.8% (45.6–95%) | 0.7 (0.2–2) | ||
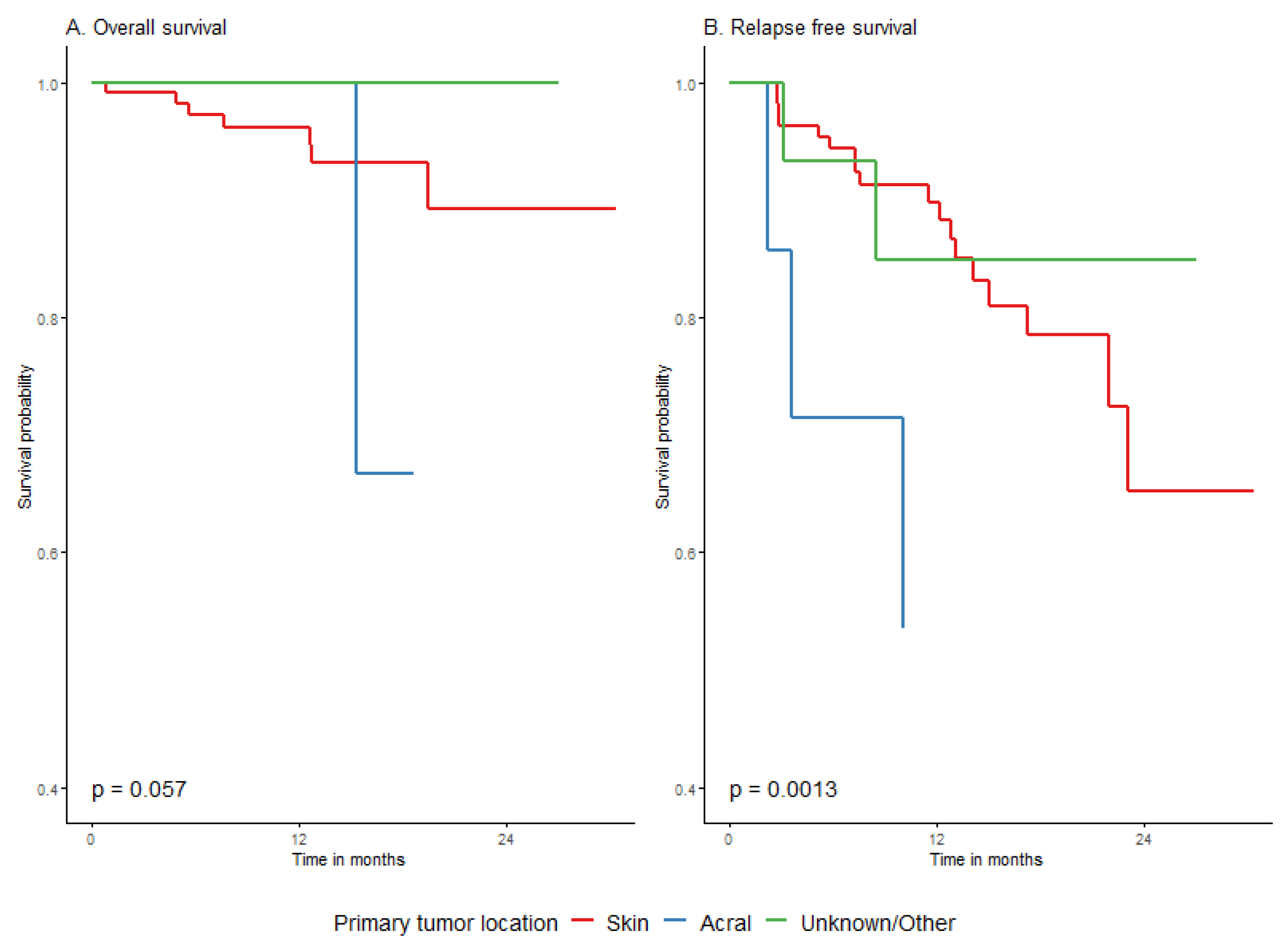
| Primary melanoma type | cutaneous | 215 | 49 | 24.1 (0.9) | NA (25.7–NA) | 84.7% (79.8–89.9%) | 72.7% (65.5–80.6%) | 61.3% (50.4–74.7%) | Ref. | <0.0001 |
| acral | 8 | 5 | 15.0 (4.1) | 10.0 (9.8–NA) | 30% (9.5–94.3%) | NA | NA | 4.2 (1.7–10.8) | ||
| mucosal | 3 | 3 | 4.1 (0.7) | 3.6 (2.9–NA) | NA | NA | NA | 13.6 (4–45.5) | ||
| FPI | 22 | 4 | 26.0 (2.3) | NA (NA–NA) | 85.2% (71–100%) | 78.1% (60.8–100%) | 78.1% (60.8–100%) | 0.7 (0.3–2.1) |
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 116 | 6 | 29.1 (0.9) | NA (NA–NA) | 98.3% (95.9–100%) | 94.7% (89.5–100%) | 84.2% (70.7–100%) | Ref. (-) | 0.8392 |
| male | 132 | 7 | 29.5 (0.6) | NA (NA–NA) | 98.4% (96.3–100%) | 94.2% (89.3–99.5%) | 88.1% (78.9–98.3%) | 0.9 (0.3–2.7) | ||
| Age group | <45 | 62 | 2 | 30.2 (0.7) | NA (NA–NA) | 98.1% (94.5–100%) | 98.1% (94.5–100%) | 93.2% (83.7–100%) | Ref. (-) | 0.6666 |
| 45–54 | 41 | 2 | 29.1 (1.5) | NA (NA–NA) | 100% (100–100%) | 96.3% (89.4–100%) | 80.2% (55.7–100%) | 1.6 (0.2–11.7) | ||
| 55–64 | 64 | 5 | 28 (1.3) | NA (NA–NA) | 98.4% (95.4–100%) | 91.4% (82.1–100%) | 74.8% (55.5–100%) | 2.7 (0.5–13.8) | ||
| 65+ | 81 | 4 | 29.9 (0.7) | NA (NA–NA) | 97.5% (94.2–100%) | 93.7% (87.8–100%) | 93.7% (87.8–100%) | 1.7 (0.3–9.1) | ||
| Comorbidities | No | 114 | 4 | 29.9 (0.6) | NA (NA–NA) | 100% (100–100%) | 98.6% (95.8–100%) | 88.4% (77.7–100%) | Ref. (-) | 0.1566 |
| Yes | 134 | 9 | 29 (0.8) | NA (NA–NA) | 97% (94.1–99.9%) | 90.5% (83.9–97.7%) | 86% (75.8–97.5%) | 2.3 (0.7–7.5) | ||
| BRAF mutational status | negative | 92 | 4 | 29.2 (1) | NA (NA–NA) | 100% (100–100%) | 94.6% (87.6–100%) | 82.7% (67.5–100%) | Ref. (-) | 0.7446 |
| positive | 155 | 9 | 29.4 (0.6) | NA (NA–NA) | 97.4% (94.8–99.9%) | 94.4% (90.3–98.6%) | 88.6% (80.1–97.9%) | 1.2 (0.4–4) | ||
| WHO performance status | 0 | 161 | 7 | 29.6 (0.6) | NA (NA–NA) | 99.3% (97.9–100%) | 97% (93.5–100%) | 86.5% (76.7–97.5%) | Ref. (-) | 0.2101 |
| 1 | 87 | 6 | 29.1 (0.9) | NA (NA–NA) | 96.6% (92.8–100%) | 89.5% (81.2–98.7%) | 89.5% (81.2–98.7%) | 2 (0.7–6) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 0 | 31.3 (0) | NA (NA–NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.5055 |
| IIIB | 56 | 1 | 30.8 (0.5) | NA (NA–NA) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 9074122.1 (0-Inf) | ||
| IIIC | 139 | 9 | 29 (0.7) | NA (NA–NA) | 97.8% (95.3–100%) | 94% (89.3–99%) | 84.2% (73.5–96.6%) | 32889264.9 (0-Inf) | ||
| IIID | 11 | 1 | 28.1 (2.9) | NA (NA–NA) | 100% (100–100%) | 80% (51.6–100%) | 80% (51.6–100%) | 48063018.4 (0-Inf) | ||
| IV | 23 | 2 | 27.5 (2.3) | NA (21.9–NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 50160099.4 (0-Inf) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 84 | 2 | 30.6 (0.5) | NA (NA–NA) | 98.8% (96.5–100%) | 96.7% (92.2–100%) | 96.7% (92.2–100%) | Ref. (-) | 0.0204 |
| T3a-b | 62 | 2 | 30.3 (0.7) | NA (NA–NA) | 100% (100–100%) | 97.2% (92–100%) | 92.6% (82.9–100%) | 1.1 (0.2–8.1) | ||
| T4a-b | 93 | 9 | 27.1 (1.3) | NA (22.3–NA) | 96.6% (93–100%) | 90% (82.2–98.6%) | 68% (48.8–94.8%) | 5 (1.1–23.1) | ||
| Group of indications for adjuvant | LND/Primary | 130 | 9 | 28.8 (0.8) | NA (NA–NA) | 96.8% (93.8–99.9%) | 92.4% (86.8–98.4%) | 85.1% (74.6–97%) | Ref. (-) | 0.1128 |
| M1 | 23 | 2 | 27.5 (2.3) | NA (21.9–NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 1.3 (0.3–5.9) | ||
| Scar/In transit/LND | 95 | 2 | 30.5 (0.5) | NA (NA–NA) | 100% (100–100%) | 97.8% (93.7–100%) | 92.9% (83.3–100%) | 0.2 (0.1–1.1) | ||
| Adjuvant type | Anti-PD1 | 147 | 8 | 29.1 (0.7) | NA (NA–NA) | 98.5% (96.6–100%) | 92.5% (86.6–98.8%) | 85.3% (74.9–97.2%) | Ref. (-) | 0.6318 |
| Inh BRAF/MEK | 101 | 5 | 29.6 (0.7) | NA (NA–NA) | 98% (95.3–100%) | 96.7% (93–100%) | 87.8% (76.2–100%) | 0.8 (0.2–2.3) | ||
| TRAE | no | 131 | 8 | 28.7 (0.9) | NA (NA–NA) | 99.2% (97.8–100%) | 91% (84–98.6%) | 82.8% (71.1–96.5%) | Ref. (-) | 0.1207 |
| yes | 115 | 4 | 30.2 (0.5) | NA (NA–NA) | 98.2% (95.8–100%) | 98.2% (95.8–100%) | 91.2% (82–100%) | 0.4 (0.1–1.3) | ||
| Highest grade toxicity | 1 | 37 | 0 | 30.4 (0) | NA (NA–NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.004 |
| 2 | 52 | 1 | 29.8 (0.5) | NA (NA–NA) | 100% (100–100%) | 100% (100–100%) | 93.8% (82.6–100%) | 186760662.3 (0-Inf) | ||
| 3 | 23 | 3 | 25.8 (2.4) | NA (22.3–NA) | 90.9% (79.5–100%) | 90.9% (79.5–100%) | 60.6% (26.9–100%) | 2022430985.8 (0-Inf) | ||
| Toxicities after immuno | no | 91 | 6 | 27.7 (1) | NA (NA–NA) | 98.9% (96.8–100%) | 88.6% (79.2–99.1%) | 81.8% (67.4–99.2%) | Ref. (-) | 0.253 |
| yes | 56 | 2 | 29.3 (0.7) | NA (NA–NA) | 98% (94.2–100%) | 98% (94.2–100%) | 91% (78.3–100%) | 0.4 (0.1–2) | ||
| Toxicities after BRAF/MEKi | no | 40 | 2 | 29.3 (1.3) | NA (NA–NA) | 100% (100–100%) | 96.3% (89.4–100%) | 85.6% (67.2–100%) | Ref. (-) | 0.4335 |
| yes | 59 | 2 | 30.2 (0.7) | NA (NA–NA) | 98.3% (95.1–100%) | 98.3% (95.1–100%) | 91.3% (78.6–100%) | 0.5 (0.1–3.4) | ||
| Primary melanoma type | cutaneous | 215 | 10 | 29.6 (0.5) | NA (NA–NA) | 98.1% (96.2–100%) | 95.5% (92.1–99.0%) | 88.2% (80.0–97.3%) | Ref. (-) | 0.0396 |
| acral | 8 | 2 | 18.6 (1.3) | 20.2 (15.3–NA) | 100% (100–100%) | 66.7% (30.0–100%) | NA | 6.8 (1.4–31.9) | ||
| mucosal | 3 | 0 | 31.3 (NA) | NA (NA–NA) | NA | NA | NA | 0 (0-Inf) | ||
| FPI | 22 | 1 | 29.8 (1.4) | NA (NA–NA) | 100% (100–100%) | 92.3% (78.9–100%) | 92.3% (78.9–100%) | 0.8 (0.1–6.5) |
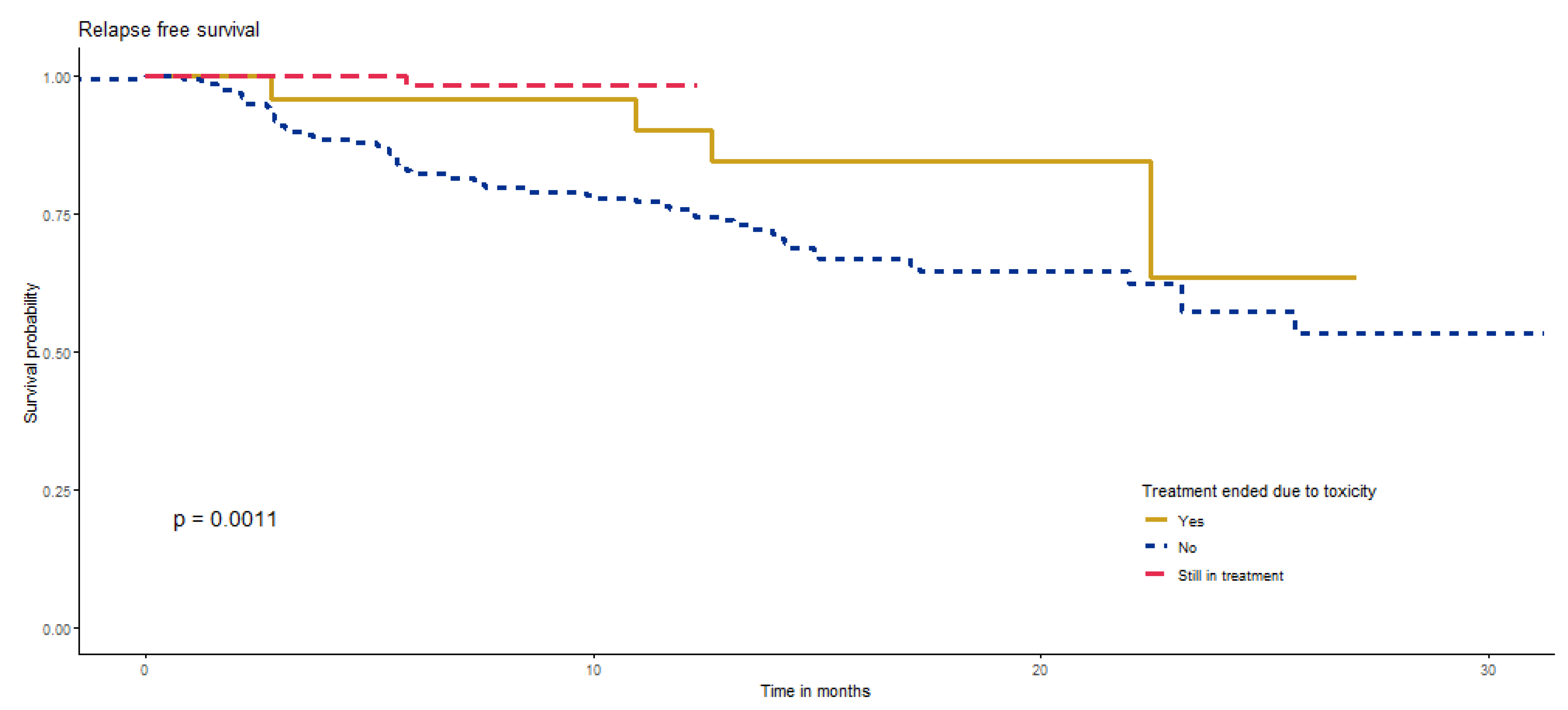

| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 54 | 6 | 27.2 (1.2) | (−) | 86% (75.9–97.4%) | 86% (75.9–97.4%) | 86% (75.9–97.4%) | Ref. (-) | 0.1303 |
| male | 76 | 18 | 23.3 (1.4) | (22−) | 85.9% (78.1–94.5%) | 70.2% (57.9–85%) | 56.1% (39–80.8%) | 2 (0.8–5.1) | ||
| Age group | <45 | 36 | 3 | 27.1 (1.7) | (22−) | 100% (100–100%) | 87.7% (73–100%) | 73.1% (48.9–100%) | Ref. (-) | 0.0765 |
| 45–54 | 27 | 4 | 26.5 (1.8) | (−) | 92.3% (82.5–100%) | 80.7% (64.8–100%) | 80.7% (64.8–100%) | 1.7 (0.4–7.8) | ||
| 55–64 | 30 | 5 | 24.9 (2.2) | (17.3−) | 82.3% (67.4–100%) | 70.5% (49.1–100%) | 70.5% (49.1–100%) | 2.3 (0.5–9.7) | ||
| 65+ | 37 | 12 | 21.5 (2.1) | (23.1−) | 70.6% (56.7–87.9%) | 66.4% (51.7–85.2%) | 53.1% (32.1–88%) | 4.2 (1.2–14.9) | ||
| Comorbidities | No | 64 | 11 | 25.1 (1.4) | (22−) | 92.6% (85.8–99.9%) | 76% (63.5–90.8%) | 65.1% (45.8–92.5%) | Ref. (-) | 0.4491 |
| Yes | 66 | 13 | 24.4 (1.5) | (23.1−) | 79.1% (69–90.8%) | 79.1% (69–90.8%) | 65.9% (44.9–96.7%) | 1.4 (0.6–3.1) | ||
| BRAF mutational status | negative | 35 | 13 | 20.6 (2.1) | (13.1−) | 69.1% (54.6–87.3%) | 56% (40.1–78.4%) | 56% (40.1–78.4%) | Ref. (-) | 0.0041 |
| positive | 94 | 11 | 25.7 (1.4) | (23.1−) | 92.5% (86.8–98.6%) | 84.9% (75.4–95.6%) | 61.9% (39.1–98%) | 0.3 (0.1–0.7) | ||
| WHO performance status | 0 | 90 | 14 | 25.4 (1.2) | (−) | 91.5% (85.7–97.8%) | 79.9% (69.6–91.9%) | 67.1% (50.7–88.8%) | Ref. (-) | 0.0964 |
| 1 | 40 | 10 | 23.3 (1.9) | (−) | 73% (58.9–90.4%) | 68.4% (53.4–87.7%) | 68.4% (53.4–87.7%) | 2 (0.9–4.5) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 15 | 1 | 28.4 (1.8) | (−) | 100% (100–100%) | 88.9% (70.6–100%) | 88.9% (70.6–100%) | Ref. (-) | 0.2252 |
| IIIB | 23 | 2 | 25.2 (2.1) | 23.1 (22−) | 100% (100–100%) | 100% (100–100%) | 33.3% (6.7–100%) | 1.2 (0.1–13.3) | ||
| IIIC | 87 | 19 | 24.2 (1.2) | (−) | 82.5% (74.5–91.4%) | 70.9% (59.8–84%) | 70.9% (59.8–84%) | 3.3 (0.4–24.4) | ||
| IIID | 5 | 2 | 17.6 (5.2) | 11.5 (11−) | 33.3% (6.7–100%) | 33.3% (6.7–100%) | 33.3% (6.7–100%) | 5.9 (0.5–66.2) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 45 | 4 | 27.8 (1.2) | (−) | 92.4% (84.4–100%) | 88.4% (78–100%) | 88.4% (78–100%) | Ref. (-) | 0.0043 |
| T3a-b | 32 | 4 | 26.2 (1.8) | (23.1−) | 96.9% (91–100%) | 92.3% (82.3–100%) | 59.3% (30.9–100%) | 1.1 (0.3–4.3) | ||
| T4a-b | 53 | 16 | 21 (1.8) | (15−) | 72.5% (59.8–87.8%) | 54% (37.8–77.1%) | 54% (37.8–77.1%) | 3.9 (1.3–11.8) | ||
| Primary melanoma type | cutaneous | 108 | 18 | 25 (1.1) | (23.1−) | 89.7% (83.8–96.1%) | 78.5% (69–89.3%) | 65.2% (48.8–87.1%) | Ref. (-) | <0.0001 |
| acral | 6 | 3 | 17.4 (5) | 11 (10−) | 41.7% (14.7–100%) | 41.7% (14.7–100%) | 41.7% (14.7–100%) | 4.7 (1.3–16.3) | ||
| mucosal | 1 | 1 | 3.6 (0) | 3.6 (−) | 0% (NA–NA%) | 0% (NA–NA%) | 0% (NA–NA%) | 23.5 (2.8–198.2) | ||
| FPI | 15 | 2 | 26.7 (2.4) | (−) | 84.8% (67.4–100%) | 84.8% (67.4–100%) | 84.8% (67.4–100%) | 0.8 (0.2–3.4) | ||
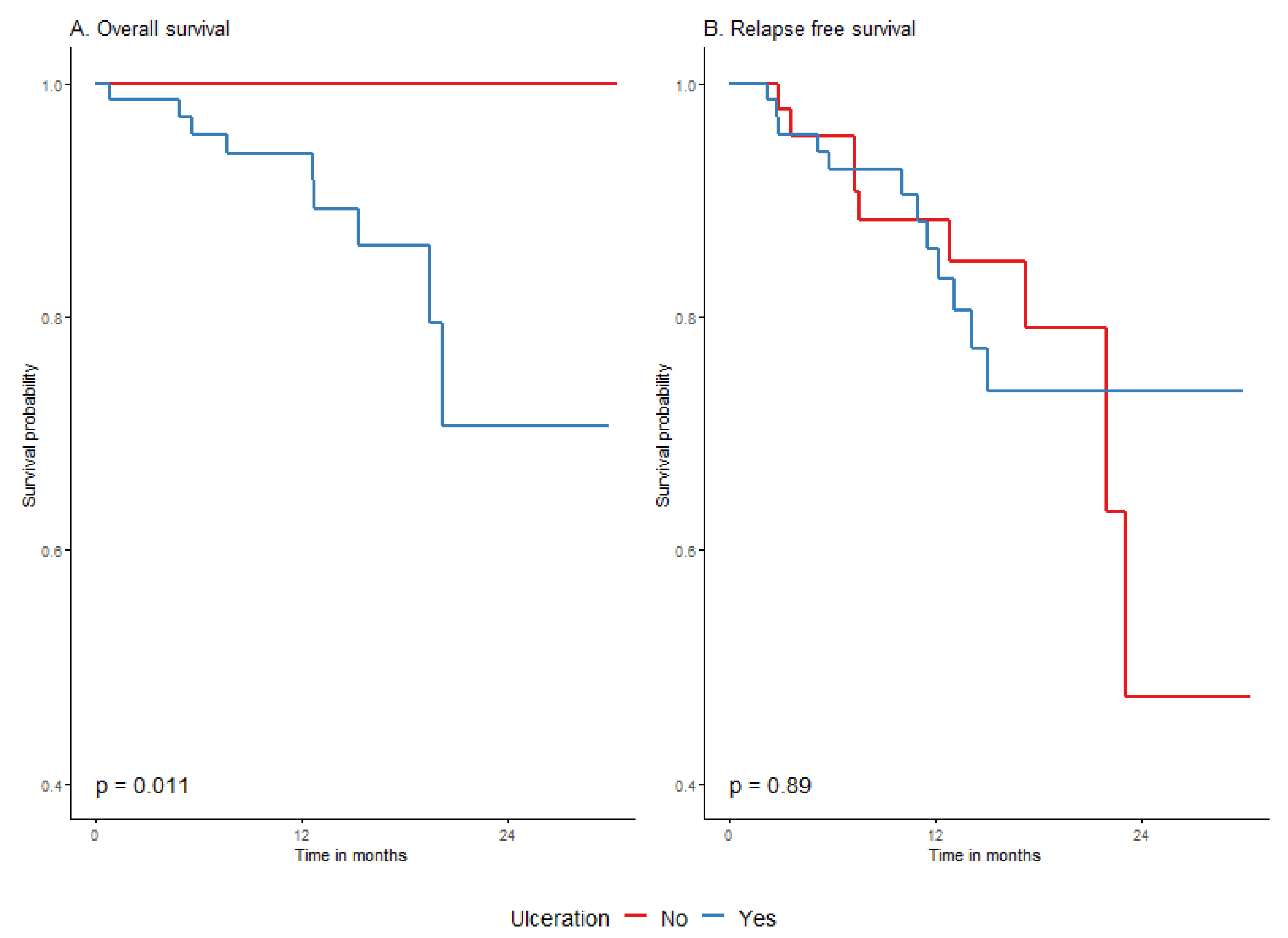
| Ulceration | No | 44 | 9 | 23.6 (1.8) | 23.1 (22−) | 88.2% (79–98.5%) | 79.1% (65.3–95.8%) | 47.4% (22.6–99.5%) | Ref. (-) | 0.8919 |
| Yes | 69 | 12 | 25 (1.4) | (−) | 85.8% (76.8–95.7%) | 73.6% (60.9–88.8%) | 73.6% (60.9–88.8%) | 0.9 (0.4–2.2) | ||
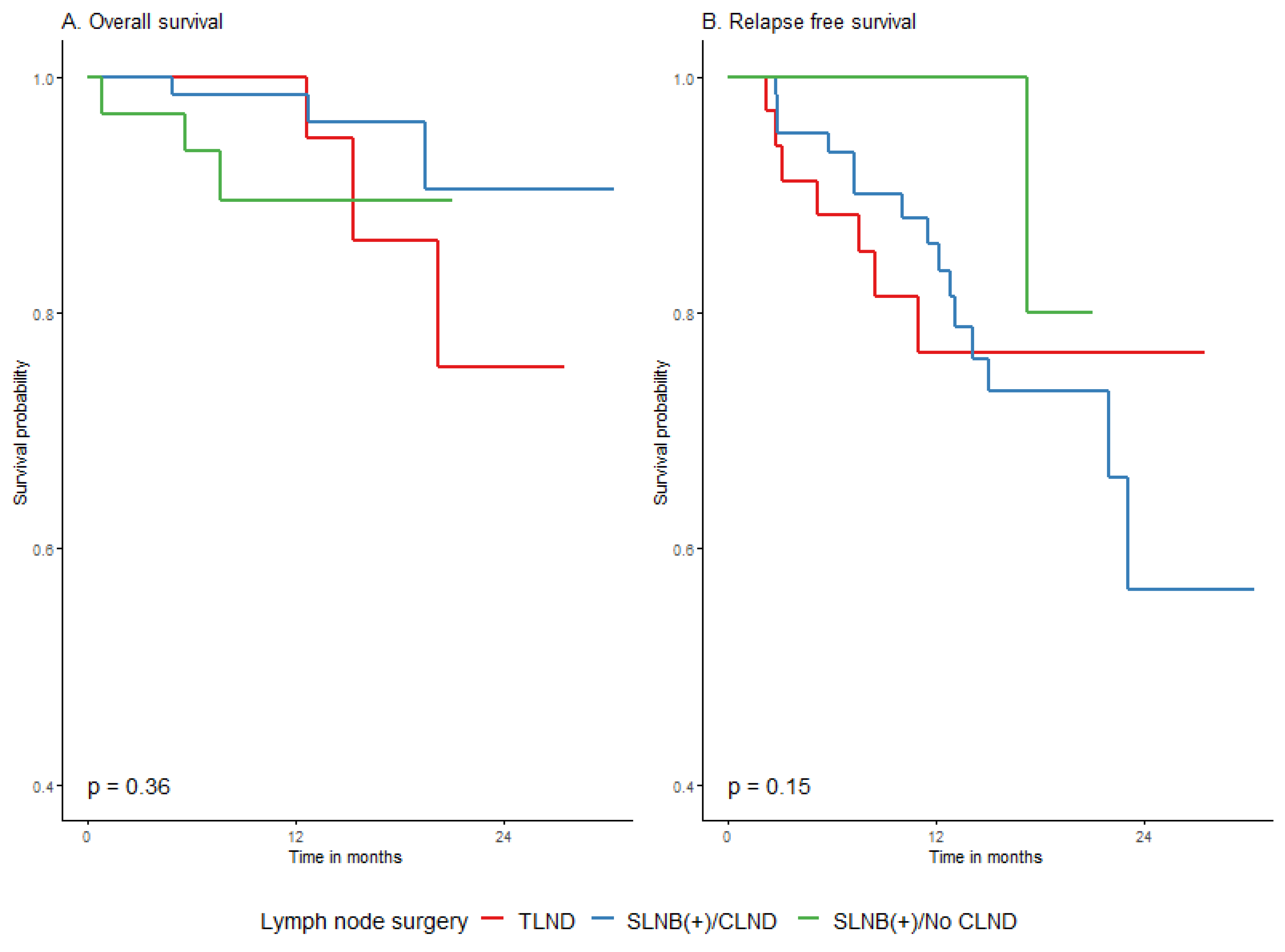
| LND group | No SLNB/TLND | 34 | 7 | 24.7 (1.9) | (−) | 76.6% (62.4–94%) | 76.6% (62.4–94%) | 76.6% (62.4–94%) | Ref. (-) | 0.1489 |
| SLNB(+)/CLND | 62 | 15 | 23.6 (1.5) | (23.1−) | 85.8% (77.1–95.5%) | 73.3% (61.5–87.4%) | 56.5% (37.7–84.9%) | 1 (0.4–2.5) | ||
| SLNB(+)/No CLND | 32 | 1 | 27.8 (2.3) | (−) | 100% (100–100%) | 80% (51.6–100%) | 80% (51.6–100%) | 0.2 (0–1.4) | ||
| Adjuvant type | Anti-PD1 | 67 | 18 | 22.1 (1.6) | (17.3−) | 77.7% (67.3–89.6%) | 63.5% (49.5–81.3%) | 56.4% (40.2–79.2%) | Ref. (-) | 0.0056 |
| Inh BRAF/MEK | 63 | 6 | 26.9 (1.4) | (23.1−) | 94.3% (88.2–100%) | 88.9% (79.9–98.9%) | 71.1% (45.3–100%) | 0.3 (0.1–0.7) | ||
| TRAE | no | 67 | 15 | 23 (1.6) | (17.3−) | 80.3% (70.3–91.9%) | 63% (47.5–83.6%) | 63% (47.5–83.6%) | Ref. (-) | 0.0599 |
| yes | 61 | 9 | 26.2 (1.2) | (−) | 91.2% (84–99%) | 86.4% (77.3–96.5%) | 71.2% (53.3–95.2%) | 0.5 (0.2–1.1) | ||
| Highest grade toxicity | 1 | 21 | 5 | 24.7 (2.2) | (23.1−) | 90.5% (78.8–100%) | 77% (59.2–100%) | 64.2% (41.1–100%) | Ref. (-) | 0.2152 |
| 2 | 22 | 4 | 24.2 (2.6) | (22−) | 83.9% (68.4–100%) | 83.9% (68.4–100%) | 55.9% (24.5–100%) | 1.1 (0.3–4.1) | ||
| 3 | 15 | 0 | 30.4 (0) | (−) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | 0 (0-Inf) | ||
| Toxicities after immuno | no | 43 | 12 | 21.5 (2.1) | (15−) | 71.5% (57.3–89.1%) | 57.2% (39.1–83.6%) | 57.2% (39.1–83.6%) | Ref. (-) | 0.5184 |
| yes | 24 | 6 | 23.4 (2.4) | (22−) | 87.5% (75.2–100%) | 72.9% (54.3–97.9%) | 60.8% (38.2–96.6%) | 0.7 (0.3–1.9) | ||
| Toxicities after BRAF/MEKi | no | 24 | 3 | 25.8 (2.2) | (−) | 95.5% (87.1–100%) | 76.6% (55.3–100%) | 76.6% (55.3–100%) | Ref. (-) | 0.2003 |
| yes | 37 | 3 | 27.4 (1.4) | (23.1−) | 93.8% (85.7–100%) | 93.8% (85.7–100%) | 75% (47.9–100%) | 0.3 (0.1–2) |
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 116 | 6 | 29.1 (0.9) | NA (NA-NA) | 98.3% (95.9–100%) | 94.7% (89.5–100%) | 84.2% (70.7–100%) | Ref. (-) | 0.8392 |
| male | 132 | 7 | 29.5 (0.6) | NA (NA-NA) | 98.4% (96.3–100%) | 94.2% (89.3–99.5%) | 88.1% (78.9–98.3%) | 0.9 (0.3–2.7) | ||
| Age group | <45 | 62 | 2 | 30.2 (0.7) | NA (NA-NA) | 98.1% (94.5–100%) | 98.1% (94.5–100%) | 93.2% (83.7–100%) | Ref. (-) | 0.6666 |
| 45–54 | 41 | 2 | 29.1 (1.5) | NA (NA-NA) | 100% (100–100%) | 96.3% (89.4–100%) | 80.2% (55.7–100%) | 1.6 (0.2–11.7) | ||
| 55–64 | 64 | 5 | 28 (1.3) | NA (NA-NA) | 98.4% (95.4–100%) | 91.4% (82.1–100%) | 74.8% (55.5–100%) | 2.7 (0.5–13.8) | ||
| 65+ | 81 | 4 | 29.9 (0.7) | NA (NA-NA) | 97.5% (94.2–100%) | 93.7% (87.8–100%) | 93.7% (87.8–100%) | 1.7 (0.3–9.1) | ||
| Comorbidities | No | 114 | 4 | 29.9 (0.6) | NA (NA-NA) | 100% (100–100%) | 98.6% (95.8–100%) | 88.4% (77.7–100%) | Ref. (-) | 0.1566 |
| Yes | 134 | 9 | 29 (0.8) | NA (NA-NA) | 97% (94.1–99.9%) | 90.5% (83.9–97.7%) | 86% (75.8–97.5%) | 2.3 (0.7–7.5) | ||
| BRAF mutational status | negative | 92 | 4 | 29.2 (1) | NA (NA-NA) | 100% (100–100%) | 94.6% (87.6–100%) | 82.7% (67.5–100%) | Ref. (-) | 0.7446 |
| positive | 155 | 9 | 29.4 (0.6) | NA (NA-NA) | 97.4% (94.8–99.9%) | 94.4% (90.3–98.6%) | 88.6% (80.1–97.9%) | 1.2 (0.4–4) | ||
| WHO performance status | 0 | 161 | 7 | 29.6 (0.6) | NA (NA-NA) | 99.3% (97.9–100%) | 97% (93.5–100%) | 86.5% (76.7–97.5%) | Ref. (-) | 0.2101 |
| 1 | 87 | 6 | 29.1 (0.9) | NA (NA-NA) | 96.6% (92.8–100%) | 89.5% (81.2–98.7%) | 89.5% (81.2–98.7%) | 2 (0.7–6) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 0 | 31.3 (0) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.5055 |
| IIIB | 56 | 1 | 30.8 (0.5) | NA (NA-NA) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 9074122.1 (0-Inf) | ||
| IIIC | 139 | 9 | 29 (0.7) | NA (NA-NA) | 97.8% (95.3–100%) | 94% (89.3–99%) | 84.2% (73.5–96.6%) | 32889264.9 (0-Inf) | ||
| IIID | 11 | 1 | 28.1 (2.9) | NA (NA-NA) | 100% (100–100%) | 80% (51.6–100%) | 80% (51.6–100%) | 48063018.4 (0-Inf) | ||
| IV | 23 | 2 | 27.5 (2.3) | NA (21.9-NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 50160099.4 (0-Inf) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 84 | 2 | 30.6 (0.5) | NA (NA-NA) | 98.8% (96.5–100%) | 96.7% (92.2–100%) | 96.7% (92.2–100%) | Ref. (-) | 0.0204 |
| T3a-b | 62 | 2 | 30.3 (0.7) | NA (NA-NA) | 100% (100–100%) | 97.2% (92–100%) | 92.6% (82.9–100%) | 1.1 (0.2–8.1) | ||
| T4a-b | 93 | 9 | 27.1 (1.3) | NA (22.3-NA) | 96.6% (93–100%) | 90% (82.2–98.6%) | 68% (48.8–94.8%) | 5 (1.1–23.1) | ||
| Group of indications for adjuvant | LND/Primary | 130 | 9 | 28.8 (0.8) | NA (NA-NA) | 96.8% (93.8–99.9%) | 92.4% (86.8–98.4%) | 85.1% (74.6–97%) | Ref. (-) | 0.1128 |
| M1 | 23 | 2 | 27.5 (2.3) | NA (21.9-NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 1.3 (0.3–5.9) | ||
| Scar/In transit/LND | 95 | 2 | 30.5 (0.5) | NA (NA-NA) | 100% (100–100%) | 97.8% (93.7–100%) | 92.9% (83.3–100%) | 0.2 (0.1–1.1) | ||
| Adjuvant type | Anti-PD1 | 147 | 8 | 29.1 (0.7) | NA (NA-NA) | 98.5% (96.6–100%) | 92.5% (86.6–98.8%) | 85.3% (74.9–97.2%) | Ref. (-) | 0.6318 |
| Inh BRAF/MEK | 101 | 5 | 29.6 (0.7) | NA (NA-NA) | 98% (95.3–100%) | 96.7% (93–100%) | 87.8% (76.2–100%) | 0.8 (0.2–2.3) | ||
| TRAE | no | 131 | 8 | 28.7 (0.9) | NA (NA-NA) | 99.2% (97.8–100%) | 91% (84–98.6%) | 82.8% (71.1–96.5%) | Ref. (-) | 0.1207 |
| yes | 115 | 4 | 30.2 (0.5) | NA (NA-NA) | 98.2% (95.8–100%) | 98.2% (95.8–100%) | 91.2% (82–100%) | 0.4 (0.1–1.3) | ||
| Highest grade toxicity | 1 | 37 | 0 | 30.4 (0) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.004 |
| 2 | 52 | 1 | 29.8 (0.5) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 93.8% (82.6–100%) | 186760662.3 (0-Inf) | ||
| 3 | 23 | 3 | 25.8 (2.4) | NA (22.3-NA) | 90.9% (79.5–100%) | 90.9% (79.5–100%) | 60.6% (26.9–100%) | 2022430985.8 (0-Inf) | ||
| Toxicities after immuno | no | 91 | 6 | 27.7 (1) | NA (NA-NA) | 98.9% (96.8–100%) | 88.6% (79.2–99.1%) | 81.8% (67.4–99.2%) | Ref. (-) | 0.253 |
| yes | 56 | 2 | 29.3 (0.7) | NA (NA-NA) | 98% (94.2–100%) | 98% (94.2–100%) | 91% (78.3–100%) | 0.4 (0.1–2) | ||
| Toxicities after BRAF/MEKi | no | 40 | 2 | 29.3 (1.3) | NA (NA-NA) | 100% (100–100%) | 96.3% (89.4–100%) | 85.6% (67.2–100%) | Ref. (-) | 0.4335 |
| yes | 59 | 2 | 30.2 (0.7) | NA (NA-NA) | 98.3% (95.1–100%) | 98.3% (95.1–100%) | 91.3% (78.6–100%) | 0.5 (0.1–3.4) | ||
| Primary melanoma type | cutaneous | 215 | 10 | 29.6 (0.5) | NA (NA-NA) | 98.1% (96.2–100%) | 95.5% (92.1–99.0%) | 88.2% (80.0–97.3%) | Ref. (-) | 0.0396 |
| acral | 8 | 2 | 18.6 (1.3) | 20.2 (15.3-NA) | 100% (100–100%) | 66.7% (30.0–100%) | NA | 6.8 (1.4–31.9) | ||
| mucosal | 3 | 0 | 31.3 (NA) | NA (NA-NA) | NA | NA | NA | 0 (0-Inf) | ||
| FPI | 22 | 1 | 29.8 (1.4) | NA (NA-NA) | 100% (100–100%) | 92.3% (78.9–100%) | 92.3% (78.9–100%) | 0.8 (0.1–6.5) |
| Operation Group | Occurrence of TRAE | n | n Events | Restricted Mean (SD) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Relapse-free survival | |||||||||
| LND/Primary | Overall | 130 | 24 | 20.5 (0.6) | NA (NA-NA) | 85.9% (79.7–92.7%) | 76.4% (67.6–86.4%) | 65.4% (50.9–83.9%) | |
| No TRAE | 67 | 15 | 19 (1.1) | NA (17.3-NA) | 80.3% (70.3–91.9%) | 63% (47.5–83.6%) | 63% (47.5–83.6%) | 0.0598 | |
| TRAE | 61 | 9 | 21.7 (0.8) | NA (NA-NA) | 91.2% (84–99%) | 86.4% (77.3–96.5%) | 71.2% (53.3–95.2%) | ||
| Scar/In transit/LND | Overall | 95 | 27 | 19.2 (0.8) | NA (23.1-NA) | 81.1% (73.4–89.7%) | 68% (57.5–80.4%) | 58.8% (45.3–76.5%) | |
| No TRAE | 53 | 19 | 17.4 (1.2) | NA (14.4-NA) | 74.7% (63.6–87.7%) | 54.2% (39.6–74.2%) | 54.2% (39.6–74.2%) | 0.05 | |
| TRAE | 42 | 8 | 21.5 (1) | NA (23.1-NA) | 89.4% (80–100%) | 85.4% (73.9–98.6%) | 67.2% (46.7–96.7%) | ||
| M1 | Overall | 23 | 10 | 15.2 (2.1) | NA (5.6-NA) | 60.9% (43.9–84.5%) | 52.2% (33.4–81.5%) | 52.2% (33.4–81.5%) | |
| No TRAE | 11 | 6 | 13 (3.1) | 6.7 (5.6-NA) | 45.5% (23.8–86.8%) | 45.5% (23.8–86.8%) | 45.5% (23.8–86.8%) | 0.208 | |
| TRAE | 12 | 4 | 17.6 (2.6) | NA (12.7-NA) | 75% (54.1–100%) | 62.5% (38.5–100%) | 62.5% (38.5–100%) | ||
| Overall survival | |||||||||
| LND/Primary | Overall | 130 | 9 | 22.6 (0.4) | NA (NA-NA) | 96.8% (93.8–99.9%) | 92.4% (86.8–98.4%) | 85.1% (74.6–97%) | |
| No TRAE | 67 | 6 | 21.8 (0.8) | NA (20.2-NA) | 98.5% (95.6–100%) | 88.3% (77.5–100%) | 66.2% (42.9–100%) | 0.0735 | |
| TRAE | 61 | 2 | 23.4 (0.4) | NA (NA-NA) | 96.6% (92.1–100%) | 96.6% (92.1–100%) | 96.6% (92.1–100%) | ||
| Scar/In transit/LND | Overall | 95 | 2 | 23.7 (0.2) | NA (NA-NA) | 100% (100–100%) | 97.8% (93.7–100%) | 92.9% (83.3–100%) | |
| No TRAE | 53 | 1 | 23.6 (0.4) | NA (NA-NA) | 100% (100–100%) | 95.7% (87.7–100%) | 95.7% (87.7–100%) | 0.9435 | |
| TRAE | 42 | 1 | 23.8 (0.2) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 90.9% (75.4–100%) | ||
| M1 | Overall | 23 | 2 | 22.5 (1) | NA (21.9-NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | |
| No TRAE | 11 | 1 | 20.3 (3) | NA (12.8-NA) | 100% (100–100%) | 66.7% (30–100%) | 66.7% (30–100%) | 0.1025 | |
| TRAE | 12 | 1 | 23.5 (0.5) | NA (21.9-NA) | 100% (100–100%) | 100% (100–100%) | 75% (42.6–100%) | ||
Appendix C
| No TRAE | TRAE | Total | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Overall | Dt * | 31 | 24.6 | 55 | 47.8 | 86 | 35.7 | <0.001 |
| q14 | 14 | 11.1 | 18 | 15.7 | 32 | 13.3 | ||
| q21 | 27 | 21.4 | 22 | 19.1 | 49 | 20.3 | ||
| q28 | 42 | 33.3 | 17 | 14.8 | 59 | 24.5 | ||
| q42 | 12 | 9.5 | 3 | 2.6 | 15 | 6.2 | ||
| Anti-PD1 group | q14 | 14 | 16.1 | 18 | 32.1 | 32 | 22.4 | 0.023 |
| q21 | 27 | 31 | 22 | 39.3 | 49 | 34.3 | ||
| q28 | 34 | 39.1 | 13 | 23.2 | 47 | 32.9 | ||
| q42 | 12 | 13.8 | 3 | 5.4 | 15 | 10.5 | ||
| BRAF/MEK inhibitor group | dt | 31 | 79.5 | 55 | 93.2 | 86 | 87.8 | 0.086 |
| q28 | 8 | 20.5 | 4 | 6.8 | 12 | 12.2 |
| No TRAE | TRAE | Total | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Overall | ||||||||
| Treatment termination as intended | Yes | 53 | 42.1 | 56 | 48.7 | 109 | 45.2 | 0.306 |
| No | 36 | 28.6 | 35 | 30.4 | 71 | 29.5 | ||
| (Treatment ongoing) | 37 | 29.4 | 24 | 20.9 | 61 | 25.3 | ||
| Dose reduction | No | 119 | 94.4 | 43 | 37.4 | 162 | 67.2 | <0.001 |
| Yes | 7 | 5.6 | 72 | 62.6 | 79 | 32.8 | ||
| Percent of intended dose received | (Treatment ongoing) | 36 | 28.6 | 24 | 20.9 | 60 | 24.9 | 0.014 |
| <50% | 21 | 16.7 | 28 | 24.3 | 49 | 20.3 | ||
| 50 – <100% | 24 | 19 | 34 | 29.6 | 58 | 24.1 | ||
| ≥100% | 40 | 31.7 | 20 | 17.4 | 60 | 24.9 | ||
| 5 | 4 | 9 | 7.8 | 14 | 5.8 | |||
| Anti-PD1 group | ||||||||
| Treatment termination as intended | Yes | 31 | 35.6 | 19 | 33.9 | 50 | 35 | 0.97 |
| No | 31 | 35.6 | 21 | 37.5 | 52 | 36.4 | ||
| (Treatment ongoing) | 25 | 28.7 | 16 | 28.6 | 41 | 28.7 | ||
| Dose reduction | No | 83 | 95.4 | 34 | 60.7 | 117 | 81.8 | <0.001 |
| Yes | 4 | 4.6 | 22 | 39.3 | 26 | 18.2 | ||
| Percent of intended dose received | (Treatment ongoing) | 25 | 28.7 | 16 | 28.6 | 41 | 28.7 | 0.857 |
| <50% | 19 | 21.8 | 14 | 25 | 33 | 23.1 | ||
| 50–<100% | 17 | 19.5 | 10 | 17.9 | 27 | 18.9 | ||
| ≥100% | 25 | 28.7 | 14 | 25 | 39 | 27.3 | ||
| 1 | 1.1 | 2 | 3.6 | 3 | 2.1 | |||
| BRAF/MEK inhibitor group | ||||||||
| Treatment termination as intended | Yes | 22 | 56.4 | 37 | 62.7 | 59 | 60.2 | 0.082 |
| No | 5 | 12.8 | 14 | 23.7 | 19 | 19.4 | ||
| (Treatment ongoing) | 12 | 30.8 | 8 | 13.6 | 20 | 20.4 | ||
| Dose reduction | No | 36 | 92.3 | 9 | 15.3 | 45 | 45.9 | <0.001 |
| Yes | 3 | 7.7 | 50 | 84.7 | 53 | 54.1 | ||
| Percent of intended dose received | (Treatment ongoing) | 11 | 28.2 | 8 | 13.6 | 19 | 19.4 | <0.001 |
| <50% | 2 | 5.1 | 14 | 23.7 | 16 | 16.3 | ||
| 50–<100% | 7 | 17.9 | 24 | 40.7 | 31 | 31.6 | ||
| ≥100% | 15 | 38.5 | 6 | 10.2 | 21 | 21.4 | ||
| 4 | 10.3 | 7 | 11.9 | 11 | 11.2 |
References
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schulte, K.-W.; Lehmann, P.; et al. Complete Lymph Node Dissection versus No Dissection in Patients with Sentinel Lymph Node Biopsy Positive Melanoma (DeCOG-SLT): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2016, 17, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Cochran, A.J.; Thompson, J.F. Re: “Time to Reconsider the Role of Sentinel Lymph Node Biopsy in Melanoma”. J. Am. Acad. Dermatol. 2023, 88, e25–e26. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; van Akkooi, A.; Lorigan, P.; Ascierto, P.A.; Dummer, R.; Robert, C.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO Consensus Conference Recommendations on the Management of Locoregional Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1449–1461. [Google Scholar] [CrossRef]
- Lao, C.D.; Khushalani, N.I.; Angeles, C.; Petrella, T.M. Current State of Adjuvant Therapy for Melanoma: Less Is More, or More Is Better? Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 738–744. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma (EORTC 1325-MG/KEYNOTE-054): Distant Metastasis-Free Survival Results from a Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Downs, J.S.; Subramaniam, S.; Henderson, M.A.; Paton, E.; Spillane, A.J.; Mathy, J.A.; Gyorki, D.E. A Survey of Surgical Management of the Sentinel Node Positive Melanoma Patient in the Post-MSLT2 Era. J. Surg. Oncol. 2021, 124, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, A.A.G.; Spillane, A.J.; Stretch, J.R.; Saw, R.P.M.; Menzies, A.M.; Uren, R.F.; Thompson, J.F.; Nieweg, O.E. Current Management of Patients with Melanoma Who Are Found to Be Sentinel Node-positive. ANZ J. Surg. 2020, 90, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Keim, U.; Suciu, S.; Amaral, T.; Eigentler, T.K.; Gesierich, A.; Hauschild, A.; Heinzerling, L.; Kiecker, F.; Schadendorf, D.; et al. Prognosis of Patients With Stage III Melanoma According to American Joint Committee on Cancer Version 8: A Reassessment on the Basis of 3 Independent Stage III Melanoma Cohorts. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.E. The Role of Clinical Prediction Tools to Risk Stratify Patients with Melanoma After a Positive Sentinel Lymph Node Biopsy. Ann. Surg. Oncol. 2021, 28, 4082–4083. [Google Scholar] [CrossRef]
- Broman, K.K.; Bettampadi, D.; Pérez-Morales, J.; Sun, J.; Kirichenko, D.; Carr, M.J.; Eroglu, Z.; Tarhini, A.A.; Khushalani, N.; Schabath, M.B.; et al. Surveillance of Sentinel Node Positive Melanoma Patients Who Receive Adjuvant Therapy without Undergoing Completion Lymph Node Dissection. Ann. Surg. Oncol. 2021, 28, 6978–6985. [Google Scholar] [CrossRef]
- Eroglu, Z.; Broman, K.K.; Thompson, J.F.; Nijhuis, A.; Hieken, T.J.; Kottschade, L.; Farma, J.M.; Hotz, M.; Deneve, J.; Fleming, M.; et al. Outcomes with Adjuvant Anti-PD-1 Therapy in Patients with Sentinel Lymph Node-Positive Melanoma without Completion Lymph Node Dissection. J. Immunother. Cancer 2022, 10, e004417. [Google Scholar] [CrossRef]
- Mitra, D.; Ologun, G.; Keung, E.Z.; Goepfert, R.P.; Amaria, R.N.; Ross, M.I.; Gershenwald, J.E.; Lucci, A.; Fisher, S.B.; Davies, M.A.; et al. Nodal Recurrence Is a Primary Driver of Early Relapse for Patients with Sentinel Lymph Node Positive Melanoma in the Modern Therapeutic Era. Ann. Surg. Oncol. 2021, 28, 3480–3489. [Google Scholar] [CrossRef]
- Livingstone, E.; Zimmer, L.; Hassel, J.C.; Fluck, M.; Eigentler, T.K.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant Nivolumab plus Ipilimumab or Nivolumab Alone versus Placebo in Patients with Resected Stage IV Melanoma with No Evidence of Disease (IMMUNED): Final Results of a Randomised, Double-Blind, Phase 2 Trial. Lancet 2022, 400, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Multicenter Selective Lymphadenectomy Trials Study Group. Therapeutic Value of Sentinel Lymph Node Biopsy in Patients With Melanoma: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 835–842. [Google Scholar] [CrossRef]
- Rhodin, K.E.; Beasley, G.M.; Tyler, D.S. Prognostic or Therapeutic—The Role of Sentinel Lymph Node Biopsy in Contemporary Practice. JAMA Surg. 2022, 157, 843. [Google Scholar] [CrossRef] [PubMed]
- Rentroia-Pacheco, B.; Tjien-Fooh, F.J.; Quattrocchi, E.; Kobic, A.; Wever, R.; Bellomo, D.; Meves, A.; Hieken, T.J. Clinicopathologic Models Predicting Non-Sentinel Lymph Node Metastasis in Cutaneous Melanoma Patients: Are They Useful for Patients with a Single Positive Sentinel Node? J. Surg. Oncol. 2022, 125, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Bertolli, E.; Franke, V.; Calsavara, V.F.; de Macedo, M.P.; Pinto, C.A.L.; van Houdt, W.J.; Wouters, M.W.J.M.; Duprat Neto, J.P.; van Akkooi, A.C.J. Validation of a Nomogram for Non-Sentinel Node Positivity in Melanoma Patients, and Its Clinical Implications: A Brazilian–Dutch Study. Ann. Surg. Oncol. 2019, 26, 395–405. [Google Scholar] [CrossRef]
- Isaksson, K.; Katsarelias, D.; Mikiver, R.; Carneiro, A.; Ny, L.; Olofsson Bagge, R. A Population-Based Comparison of the AJCC 7th and AJCC 8th Editions for Patients Diagnosed with Stage III Cutaneous Malignant Melanoma in Sweden. Ann. Surg. Oncol. 2019, 26, 2839–2845. [Google Scholar] [CrossRef]
- Tonella, L.; Pala, V.; Ponti, R.; Rubatto, M.; Gallo, G.; Mastorino, L.; Avallone, G.; Merli, M.; Agostini, A.; Fava, P.; et al. Prognostic and Predictive Biomarkers in Stage III Melanoma: Current Insights and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 4561. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Baade, P.D.; Olsen, C.M. More People Die from Thin Melanomas (≤1 Mm) than from Thick Melanomas (>4 Mm) in Queensland, Australia. J. Investig. Dermatol. 2015, 135, 1190–1193. [Google Scholar] [CrossRef]
- Dizier, B.; Callegaro, A.; Debois, M.; Dreno, B.; Hersey, P.; Gogas, H.J.; Kirkwood, J.M.; Vansteenkiste, J.F.; Sequist, L.V.; Atanackovic, D.; et al. A Th1/IFNγ Gene Signature Is Prognostic in the Adjuvant Setting of Resectable High-Risk Melanoma but Not in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1725–1735. [Google Scholar] [CrossRef]
- Gastman, B.R.; Gerami, P.; Kurley, S.J.; Cook, R.W.; Leachman, S.; Vetto, J.T. Identification of Patients at Risk of Metastasis Using a Prognostic 31-Gene Expression Profile in Subpopulations of Melanoma Patients with Favorable Outcomes by Standard Criteria. J. Am. Acad. Dermatol. 2019, 80, 149–157.e4. [Google Scholar] [CrossRef]
- Hsueh, E.C.; DeBloom, J.R.; Lee, J.; Sussman, J.J.; Covington, K.R.; Middlebrook, B.; Johnson, C.; Cook, R.W.; Slingluff, C.L.; McMasters, K.M. Interim Analysis of Survival in a Prospective, Multi-Center Registry Cohort of Cutaneous Melanoma Tested with a Prognostic 31-Gene Expression Profile Test. J. Hematol. Oncol. 2017, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ramón, J.L.; Gardeazabal, J.; Izu, R.M.; Garrote, E.; Rasero, J.; Apraiz, A.; Penas, C.; Seijo, S.; Lopez-Saratxaga, C.; de la Peña, P.M.; et al. Melanoma Clinical Decision Support System: An Artificial Intelligence-Based Tool to Diagnose and Predict Disease Outcome in Early-Stage Melanoma Patients. Cancers 2023, 15, 2174. [Google Scholar] [CrossRef]
- Luskin, M.R.; Murakami, M.A.; Manalis, S.R.; Weinstock, D.M. Targeting Minimal Residual Disease: A Path to Cure? Nat. Rev. Cancer 2018, 18, 255–263. [Google Scholar] [CrossRef]
- Menon, D.R.; Das, S.; Krepler, C.; Vultur, A.; Rinner, B.; Schauer, S.; Kashofer, K.; Wagner, K.; Zhang, G.; Rad, E.B.; et al. A Stress-Induced Early Innate Response Causes Multidrug Tolerance in Melanoma. Oncogene 2015, 34, 4545. [Google Scholar] [CrossRef] [PubMed]
- Rabbie, R.; Ferguson, P.; Molina-Aguilar, C.; Adams, D.J.; Robles-Espinoza, C.D. Melanoma Subtypes: Genomic Profiles, Prognostic Molecular Markers and Therapeutic Possibilities. J. Pathol. 2019, 247, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Rambow, F.; Rogiers, A.; Marin-Bejar, O.; Aibar, S.; Femel, J.; Dewaele, M.; Karras, P.; Brown, D.; Chang, Y.H.; Debiec-Rychter, M.; et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell 2018, 174, 843–855.e19. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.S.; Lawless, A.; Woodford, R.; Fa’ak, F.; Tipirneni, A.; Patrinely, J.R.; Yeoh, H.L.; Rapisuwon, S.; Haydon, A.; Osman, I.; et al. Extended Follow-Up of Chronic Immune-Related Adverse Events Following Adjuvant Anti–PD-1 Therapy for High-Risk Resected Melanoma. JAMA Netw. Open 2023, 6, e2327145. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.; Kicinski, M.; Suciu, S. Association of Selected (Immune-Related) Adverse Events and Outcome in Two Adjuvant Phase III Trials, Checkmate-238 and EORTC1325/KEYNOTE-054. J. Immunother. Cancer 2022, 10, e004272. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, E.; Malissen, N.; Roger, A.; Lebbé, C.; Deilhes, F.; Frénard, C.; Dréno, B.; Meyer, N.; Grob, J.-J.; Tétu, P.; et al. Which Adjuvant Treatment for Patients with BRAF(V600)-Mutant Cutaneous Melanoma? Ann. Dermatol. Venereol. 2021, 148, 145–155. [Google Scholar] [CrossRef]
- Suciu, S.; Eggermont, A.M.M.; Lorigan, P.; Kirkwood, J.M.; Markovic, S.N.; Garbe, C.; Cameron, D.; Kotapati, S.; Chen, T.-T.; Wheatley, K.; et al. Relapse-Free Survival as a Surrogate for Overall Survival in the Evaluation of Stage II-III Melanoma Adjuvant Therapy. J. Natl. Cancer Inst. 2018, 110, 87–96. [Google Scholar] [CrossRef]
- Signorovitch, J.; Moshyk, A.; Zhao, J.; Le, T.K.; Burns, L.; Gooden, K.; Hamilton, M. Overall Survival in the Real-World and Clinical Trials: A Case Study Validating External Controls in Advanced Melanoma. Future Oncol. 2022, 18, 1321–1331. [Google Scholar] [CrossRef]
- de Meza, M.M.; Ismail, R.K.; Rauwerdink, D.; van Not, O.J.; van Breeschoten, J.; Blokx, W.A.M.; de Boer, A.; van Dartel, M.; Hilarius, D.L.; Ellebaek, E.; et al. Adjuvant Treatment for Melanoma in Clinical Practice-Trial versus Reality. Eur. J. Cancer 2021, 158, 234–245. [Google Scholar] [CrossRef]
- Farrow, N.E.; Raman, V.; Williams, T.P.; Nguyen, K.Y.; Tyler, D.S.; Beasley, G.M. Adjuvant Therapy Is Effective for Melanoma Patients with a Positive Sentinel Lymph Node Biopsy Who Forego Completion Lymphadenectomy. Ann. Surg. Oncol. 2020, 27, 5121–5125. [Google Scholar] [CrossRef] [PubMed]
- Torphy, R.J.; Friedman, C.; Ho, F.; Leonard, L.D.; Thieu, D.; Lewis, K.D.; Medina, T.M.; Robinson, W.A.; Gonzalez, R.C.; Stewart, C.L.; et al. Adjuvant Therapy for Stage III Melanoma Without Immediate Completion Lymph Node Dissection. Ann. Surg. Oncol. 2022, 29, 806–815. [Google Scholar] [CrossRef] [PubMed]






| Covariate | Group | Total | |
|---|---|---|---|
| n | % | ||
| Sex | female | 116 | 46.8 |
| male | 132 | 53.2 | |
| Age group | <45 | 62 | 25 |
| 45–54 | 41 | 16.5 | |
| 55–64 | 64 | 25.8 | |
| 65+ | 81 | 32.7 | |
| Melanoma type | skin | 215 | 86.7 |
| acral | 8 | 3.2 | |
| mucosal | 3 | 1.2 | |
| unknown | 22 | 8.9 | |
| BRAF mutational status | negative | 92 | 37.1 |
| positive | 155 | 62.5 | |
| undocumented | 1 | 0.4 | |
| AJCC8 primary tumor characteristics, pT | T0 | 21 | 8.5 |
| T1a–b | 20 | 8.0 | |
| T2a–b | 43 | 17.3 | |
| T3a–b | 62 | 25 | |
| T4a–b | 93 | 37.5 | |
| NA | 9 | 3.6 | |
| Covariate | Group | Total | |
|---|---|---|---|
| n | % | ||
| WHO performance status at treatment initiation | 0 | 161 | 64.9 |
| 1 | 86 | 34.7 | |
| 2 | 1 | 0.4 | |
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 6.5 |
| IIIB | 56 | 22.6 | |
| IIIC | 139 | 56 | |
| IIID | 11 | 4.4 | |
| IIIx | 3 | 1.2 | |
| IV | 23 | 9.3 | |
| Group of indications for adjuvant | Primary surgery (primary melanoma and nodes) | 130 | 52.4 |
| Resected distant metastases | 23 | 9.3 | |
| Resected local recurrence (scar/in transit/nodal) | 95 | 38.3 | |
| Node surgery in the primary surgery group | SLNB (+), no CLND | 34 | 26.2 |
| SLNB (+), CLND | 63 | 48.5 | |
| TLND | 33 | 25.4 | |
| Adjuvant type | dabrafenib plus trametinib | 101 | 40.7 |
| pembrolizumab | 65 | 26.2 | |
| nivolumab | 82 | 33.1 | |
| Adjuvant treatment ended | no | 64 | 25.8 |
| yes | 184 | 74.2 | |
| Reason for ending of adjuvant treatment | (Treatment ongoing) | 64 | 25.8 |
| As planned | 111 | 44.8 | |
| Other | 4 | 1.6 | |
| Progression | 39 | 15.7 | |
| Toxicity | 23 | 9.3 | |
| Undocumented | 7 | 2.8 | |
| Covariate | Group | Anti-PD1 | DT | Total | p-Value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Toxicities after treatment | Yes | 56 (38.1) | 59 (58.4) | 115 (46.4) | 0.001 |
| No | 91 (61.9) | 40 (39.6) | 131 (52.8) | ||
| Undocumented | 0 | 2 (2.0) | 2 (0.8) | ||
| Treatment ended due to toxicity | Yes | 13 (8.8) | 11 (10.9) | 24 (9.7) | 0.302 |
| No | 91 (61.9) | 68 (67.3) | 159 (64.1) | ||
| (Treatment ongoing) | 43 (29.3) | 21 (20.8) | 64 (25.8) | ||
| Undocumented | 0 | 1 (1.0) | 1 (0.4) | ||
| Highest grade toxicity if toxicity was reported (n = 115) | 1 | 23 (41.1) | 14 (23.7) | 37 (32.2) | 0.041 |
| 2 | 25 (44.6) | 26 (44.1) | 51 (44.3) | ||
| 3 | 8 (14.3) | 15 (25.4) | 23 (20.0) | ||
| Undocumented | 0 | 4 (6.8) | 4 (3.5) |
| n | n Events ** | Restricted Mean * (SD) | 24-Month Survival (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| Recurrence-free survival (RFS) | ||||||
| Overall | 248 | 61 | 19.5 (0.5) | 61.4% (51.8–72.8%) | ||
| Indication group | LND/Primary | 130 | 24 | 20.5 (0.6) | 65.4% (50.9–83.9%) | 0.0064 |
| M1 | 23 | 10 | 15.2 (2.1) | 52.2% (33.4–81.5%) | ||
| Scar/In transit/LND | 95 | 27 | 19.2 (0.8) | 58.8% (45.3–76.5%) | ||
| Adjuvant group | Anti-PD1 | 147 | 46 | 17.7 (0.8) | 56.1% (45.6–69.1%) | <0.0001 |
| BRAF/MEKi | 101 | 15 | 21.8 (0.6) | 65.9% (48.4–89.7%) | ||
| Overall survival (OS) | ||||||
| Overall | 248 | 13 | 23.1 (0.3) | 86.7% (78.8–95.3%) | ||
| Indication group | LND/Primary | 130 | 9 | 22.6 (0.4) | 85.1% (74.6–97%) | 0.1128 |
| M1 | 23 | 2 | 22.5 (1) | 68.2% (37.6–100%) | ||
| Scar/In transit/LND | 95 | 2 | 23.7 (0.2) | 92.9% (83.3–100%) | ||
| Adjuvant group | Anti-PD1 | 147 | 8 | 22.9 (0.4) | 85.3% (74.9–97.2%) | 0.6318 |
| BRAF/MEKi | 101 | 5 | 23.2 (0.4) | 87.8% (76.2–100%) | ||
| Distant-metastases-free survival (DMFS) | ||||||
| Overall | 248 | 49 | 20.3 (0.5) | 70.2% (61.3–80.4%) | ||
| Indication group | LND/Primary | 130 | 22 | 20.7 (0.6) | 72.4% (60.5–86.7%) | 0.0525 |
| M1 | 23 | 8 | 16.7 (2.1) | 60.9% (41.8–88.7%) | ||
| Scar/In transit/LND | 95 | 19 | 20.6 (0.7) | 70.2% (56.4–87.3%) | ||
| Adjuvant group | Anti-PD1 | 147 | 37 | 18.9 (0.7) | 64.8% (54.4–77.2%) | 0.003 |
| BRAF/MEKi | 101 | 12 | 22.2 (0.5) | 76.5% (61.2–95.5%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Placzke, J.; Rosińska, M.; Sobczuk, P.; Ziętek, M.; Kempa-Kamińska, N.; Cybulska-Stopa, B.; Kamińska-Winciorek, G.; Bal, W.; Mackiewicz, J.; Galus, Ł.; et al. Modern Approach to Melanoma Adjuvant Treatment with Anti-PD1 Immune Check Point Inhibitors or BRAF/MEK Targeted Therapy: Multicenter Real-World Report. Cancers 2023, 15, 4384. https://doi.org/10.3390/cancers15174384
Placzke J, Rosińska M, Sobczuk P, Ziętek M, Kempa-Kamińska N, Cybulska-Stopa B, Kamińska-Winciorek G, Bal W, Mackiewicz J, Galus Ł, et al. Modern Approach to Melanoma Adjuvant Treatment with Anti-PD1 Immune Check Point Inhibitors or BRAF/MEK Targeted Therapy: Multicenter Real-World Report. Cancers. 2023; 15(17):4384. https://doi.org/10.3390/cancers15174384
Chicago/Turabian StylePlaczke, Joanna, Magdalena Rosińska, Paweł Sobczuk, Marcin Ziętek, Natasza Kempa-Kamińska, Bożena Cybulska-Stopa, Grażyna Kamińska-Winciorek, Wiesław Bal, Jacek Mackiewicz, Łukasz Galus, and et al. 2023. "Modern Approach to Melanoma Adjuvant Treatment with Anti-PD1 Immune Check Point Inhibitors or BRAF/MEK Targeted Therapy: Multicenter Real-World Report" Cancers 15, no. 17: 4384. https://doi.org/10.3390/cancers15174384
APA StylePlaczke, J., Rosińska, M., Sobczuk, P., Ziętek, M., Kempa-Kamińska, N., Cybulska-Stopa, B., Kamińska-Winciorek, G., Bal, W., Mackiewicz, J., Galus, Ł., Las-Jankowska, M., Jankowski, M., Dziura, R., Drucis, K., Borkowska, A., Świtaj, T., Rogala, P., Kozak, K., Klimczak, A., ... Rutkowski, P. (2023). Modern Approach to Melanoma Adjuvant Treatment with Anti-PD1 Immune Check Point Inhibitors or BRAF/MEK Targeted Therapy: Multicenter Real-World Report. Cancers, 15(17), 4384. https://doi.org/10.3390/cancers15174384





